Abstract
This study presents a comprehensive optimization of algal oil extraction and transesterification for sustainable biodiesel production. Freshwater Spirogyra algae underwent Soxhlet extraction using n-hexane. response surface methodology (RSM), principal component analysis (PCA), and multivariate regression analysis (MRA) were employed to investigate the effects of biomass–solvent ratio (BSR), algae particle size (APS), and extraction-contact time (E-CT) on algal oil yield (AOY). The extracted oil was then converted to biodiesel via transesterification, and the impacts of the methanol–oil ratio (MOR) and transesterification-contact time (T-CT) on biodiesel conversion efficiency (BCE) were analyzed. Results demonstrate that optimal BSR, APS, and E-CT for maximal AOY are 1:7, 400 µm, and 3–4 h, respectively. For transesterification, a MOR of 12:1 and a T-CT of 4 h yielded the highest BCE. Predictive models exhibited exceptional accuracy, with R2 values of 0.916 and 0.950 for AOY and BCE, respectively. The produced biodiesel complied with ASTM D6751 and EN 14214, showcasing its potential for renewable energy applications.
1. Introduction
With the relentless surge in global crude oil demand reaching a staggering 101.6 million barrels per day in 2020 [1], the dependence on fossil fuels has become an undeniable reality of modern civilization. Each passing day witnesses a gradual escalation in the utilization of these non-renewable resources, amplifying concerns regarding energy security and environmental sustainability [2]. The juxtaposition of soaring population figures and rapid industrial expansion exacerbates these challenges, further driving up the demand and prices of fossil fuels [3]. In response to these pressing issues, the quest for renewable and eco-friendly alternatives has become imperative, propelling researchers towards the exploration of sustainable fuel sources [4].
Among the plethora of renewable energy options, biodiesel emerges as a promising contender, embodying the principles of sustainability, biodegradability, and non-toxicity [3,5]. Derived from diverse sources such as plant oils, animal fats, and notably, algae oil, biodiesel offers a viable pathway towards reducing the carbon footprint associated with conventional fossil fuels [6,7]. Notably, algae oil represents a particularly compelling avenue due to its high productivity and environmental benefits.
Microalgae, constituting a significant portion of the global algae biomass, holds immense potential as a sustainable energy resource [8,9]. These microscopic organisms, adept at harnessing solar energy through photosynthesis, efficiently convert carbon dioxide and water into energy-rich triacylglycerols (TAGs), the precursor to biodiesel [10]. Notably, microalgae possess a superior carbon capture efficiency, with large-scale cultivation capable of sequestering CO2 from industrial emissions with remarkable effectiveness [11].
Despite the tantalizing promise of microalgae-derived biodiesel, commercialization efforts have been hindered by formidable challenges, chiefly among them, the lack of robust process technologies [12]. High processing costs and energy requirements present significant barriers to scaling up microalgae biodiesel production to industrial levels [13]. Conventional transesterification processes, involving strong acids or alkalis as catalysts, entail substantial energy consumption, intricate purification steps, and environmental risks associated with chemical usage [14]. Consequently, there exists an urgent imperative to innovate and optimize biodiesel production processes, leveraging advanced methodologies to enhance recovery efficiency, intensify production, and minimize waste [15,16].
In response to these challenges, the present study delves into the optimization of algal oil extraction and transesterification parameters through the synergistic application of Response Surface Methodology (RSM), Principal Component Analysis (PCA), and Multivariate Regression Analysis (MRA). Freshwater Spirogyra algae, sourced from diverse locations within Tandojam, were subjected to meticulous experimentation to ascertain optimal extraction conditions. Variation in parameters such as biomass–solvent ratio (BSR), algae particle size (APS), and extraction-contact time (E-CT) were systematically explored to maximize algal oil yield (AOY). Subsequently, transesterification reactions were conducted using methanol and sodium hydroxide as catalysts, with varying methanol–oil ratio (MOR) and transesterification-contact time (T-CT), to elucidate their influence on biodiesel conversion efficiency (BCE).
This study’s novelty lies in the synergistic application of RSM, PCA, and MRA to optimize algal oil extraction and transesterification parameters. RSM allows for efficient experimental design and optimization [17], while PCA helps manage data complexity and potential multicollinearity [18]. MRA complements these by quantifying relationships between variables and responses [19]. This integrated approach leverages the strengths of each technique, enabling a comprehensive understanding of process parameters and their effects on algal oil yield and biodiesel conversion efficiency. By combining these methodologies, this study aims to overcome the challenges impeding the commercial viability of algae-derived biodiesel, advancing its potential as a sustainable energy alternative.
2. Results and Discussions
2.1. Dynamics of Algal Oil Extraction Parameters
2.1.1. Effects of Biomass–Solvent Ratio on Algal Oil Yield
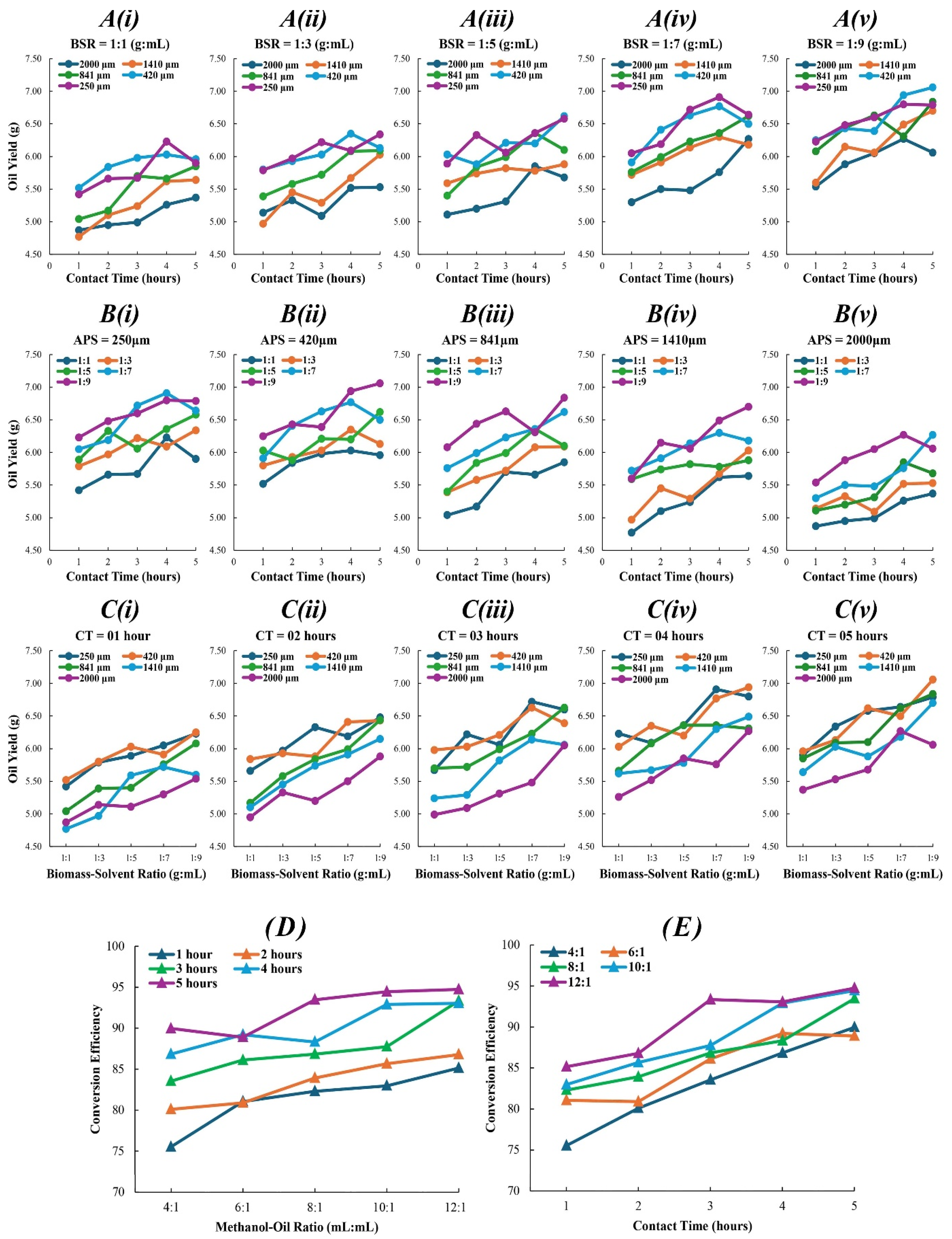
The analysis of biomass–solvent ratios (BSR) in algal oil extraction demonstrated a complex, non-linear relationship between the BSR and algal oil yield (AOY). Statistical evaluations, including analysis of variance (ANOVA) (F(4, 120) = 17.85, p < 0.001) and regression analysis (R2 = 0.372), indicate a marked increase in AOY with increasing BSR up to 1:7, beyond which a plateau is observed which can be seen in Figure 1A, indicating an optimal threshold for extraction efficiency, contradicting previous linear extrapolations, indicating that extraction efficiency does not consistently increase beyond specific concentrations. This observation aligns with the phenomenon noted in previous studies [20]. BSR strongly correlates (r = 0.610) with yield, emphasizing the need to optimize for efficient extraction, as demonstrated in recent studies [21]. This study extends the previously established understanding for cost-effective solvent reduction without compromising yield, supporting sustainable biofuel production [22,23].
Figure 1.
AOY as a function of (A) E-CT for various APS and BSR (i–v), (B) E-CT for various BSR and APS (i–v), (C) BSR for various APS and E-CT (i–v). BCE as a function of (D) MOR for various T-CT and (E) T-CT for various MOR.
2.1.2. Effects of Algae Particle Sizes on Algal Oil Yield
Algae particle size (APS) investigation revealed vital insights for optimizing algal oil extraction. Regression analysis (R2 = 0.327) confirms the correlation between APS and AOY, as seen in previous studies. It was observed that the AOY increases with decreasing APS until a significant plateau emerges after 400 µm, as seen in Figure 1B, indicating an optimal APS range, which aligns with the earlier research [24]. Further reduction beyond this threshold does not proportionally increase AOY. A strong negative correlation coefficient (r = −0.569) further supports this observation, echoing previous findings [25]. The plateau after 400 µm highlights a balance between solvent contact surface area and mechanical cell disruption limits, which suggests rethinking the energy-intensive practices and achieving an optimal balance between input energy and extraction efficiency to enhance economic viability and sustainability.
2.1.3. Effects of Extraction-Contact Time on Algal Oil Yield
Extraction-contact time (E-CT) investigation also revealed vital insights for optimizing biofuel production processes. Statistical analysis including ANOVA (F(4, 120) = 8.566, p < 0.001) revealed that varying E-CT significantly impact AOY, with longer periods increasing AOY, notably between 3 and 4 h, aligning with the earlier findings [26]. No added benefits were found beyond this range, supported by a regression model (R2 = 0.222). This study identifies a critical threshold for E-CT, illustrated in Figure 1C, emphasizing optimization of process parameters, and aligned with previous findings [27]. Correlation analysis (r = 0.463) shows a strong link between E-CT and AOY, as earlier noticed [28]. These insights are crucial for industrial biofuel production, impacting efficiency, as supported by a previously developed model [29].
2.2. Dynamics of Transesterification Parameters
2.2.1. Effects of Methanol–Oil Ratio on Biodiesel Conversion Efficiency
In biodiesel production, the methanol–oil ratio (MOR) is typically a crucial parameter effecting biodiesel conversion efficiency (BCE). However, in this study, ANOVA analysis (F(4, 20) = 1.924, p = 0.146) revealed no statistically significant differences among various MORs. This suggests a complex interplay rather than a linear correlation, as indicated in previous research [30]. Nonetheless, further regression analysis revealed a notable trend, in which at 12:1 MOR, there was a significant improvement in BCE (coefficient = 7.418, p = 0.021), aligning with earlier research [31]. This finding is supported by a significant positive correlation (r = 0.527), indicating a tendency for higher MOR to enhance BCE [32]. These findings imply that a uniform increase in MOR does not consistently enhance BCE, reflected in Figure 1D. Optimal MOR, leading to BCE peaks, offers refined insights into methanol’s role in biodiesel conversion [33]. This understanding can improve efficiency, cost-effectiveness, and sustainability in biodiesel production.
2.2.2. Effects of Transesterification-Contact Time on Biodiesel Conversion Efficiency
During transesterification, the transesterification-contact time (T-CT) is critical for BCE, which was confirmed by ANOVA (F(4, 20) = 10.29, p < 0.001) and regression analysis (R2 = 0.673) which revealed that varying T-CT significantly impact BCE, with longer T-CT leading to higher efficiencies, as shown in Figure 1E. This was further supported by a strong positive correlation (r = 0.816). Specifically, a T-CT of 3 to 4 h was identified as the most optimum, with extended T-CT inducing biodiesel degradation, potentially lowering fuel quality and negating BCE gains. This degradation might result from transesterification side reactions or thermal degradation [34]. Insights into T-CT are vital for large-scale biodiesel production, ensuring a balance between BCE and product quality. Previous studies consistently support the optimization of T-CT for improving biodiesel yield and production efficiency [35,36,37,38].
2.3. Synthesis of Algal Oil Extraction Parameters
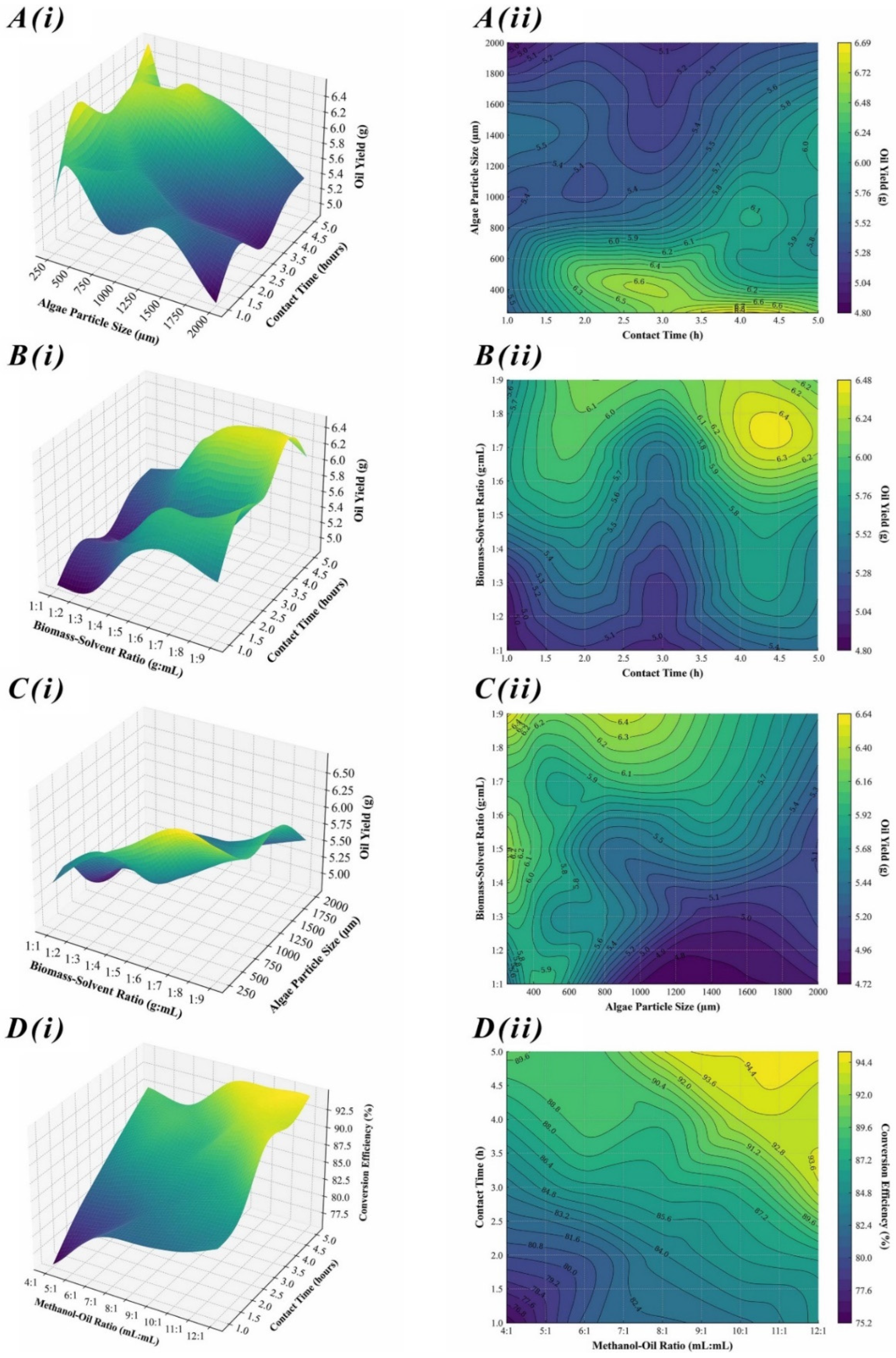
This study utilized RSM to analyze algal oil extraction parameters, revealing the complex interaction among BSR, APS, and E-CT. This produced a strong quadratic model with high accuracy (R2 = 0.916). The accuracy of the model confirms a strong correlation between these variables and AOY, echoing findings of previous studies [39]. It was particularly identified that BSR notably influences extraction efficiency [21]. Figure 2A–C visually represent data complexities with surface and contour plots, showing optimal and suboptimal extraction conditions. PCA, utilized previously, offered further quantitative insights [40]. Despite the atypical variance distribution (33% across the first two components), it emphasized each variable’s significance in system variance. This highlights a multidirectional impact of process parameters on AOY, suggesting a balanced interdependence rather than a hierarchical one. MRA also supported the RSM model, confirming its direct and inverse relationships, with a notable inverse correlation between APS and AOY (R2 = −0.2848), emphasizing surface area accessibility in enhancing solvent-extraction efficacy. This concept aligns with diffusion limitations discussed in algal bioprocessing [41]. This study integrates empirical data with advanced statistical methods to construct a model that not only predicts AOY with high accuracy but also provides a framework for optimizing extraction parameters, similar to the previous approaches [42].
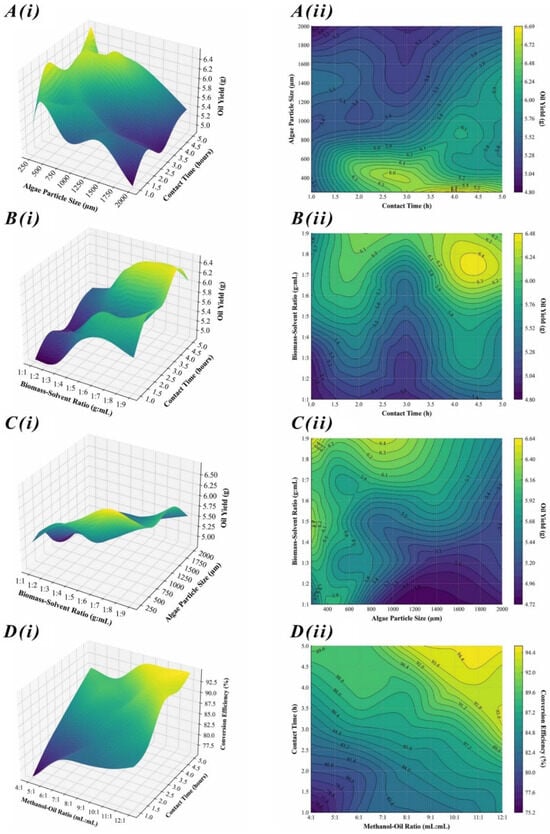
Figure 2.
Interpolated 3D response surface plots, i(A–D), and corresponding 2D contour plots, ii(A–D), illustrating optimization of AOY and BCE by evaluating effects of varying parameters.
2.4. Synthesis of Transesterification Parameters
RSM was utilized to analyze the optimization of transesterification parameters. The RSM-generated model showed a significant coefficient of determination (R2 = 0.950), indicating a strong correlation among MOR, T-CT, and BCE. The MOR notably boosted BCE from the 4:1 baseline to an optimal 12:1 MOR, peaking at 92.5%. This aligns with reaction equilibrium principles in biofuel kinetics studies [38]. Figure 2D illustrates this rise, showing that higher MOR and longer T-CT progressively enhance BCE, where MOR above 9:1 consistently achieved efficiencies surpassing 90%, extending the previously suggested ideas [43]. PCA revealed an even variance distribution across components, unlike the typical dominance of one. This highlights each parameter’s vital role in the conversion process and underscores the complexity and interdependence of transesterification parameters. MRA quantification revealed a 0.92% BCE increase per unit rise in MOR and a 2.84% rise for every additional hour of T-CT. These coefficients emphasize the critical interplay between methanol quantity and reaction duration, aligning with mass transfer and reaction kinetics models [44]. The precision of this model represents a substantial contribution to optimization of biodiesel production, promising advancements in the efficiency and sustainability of renewable energy resources [45].
2.5. Development and Validation of Predictive Models
2.5.1. Predictive Model for Algal Oil Yield
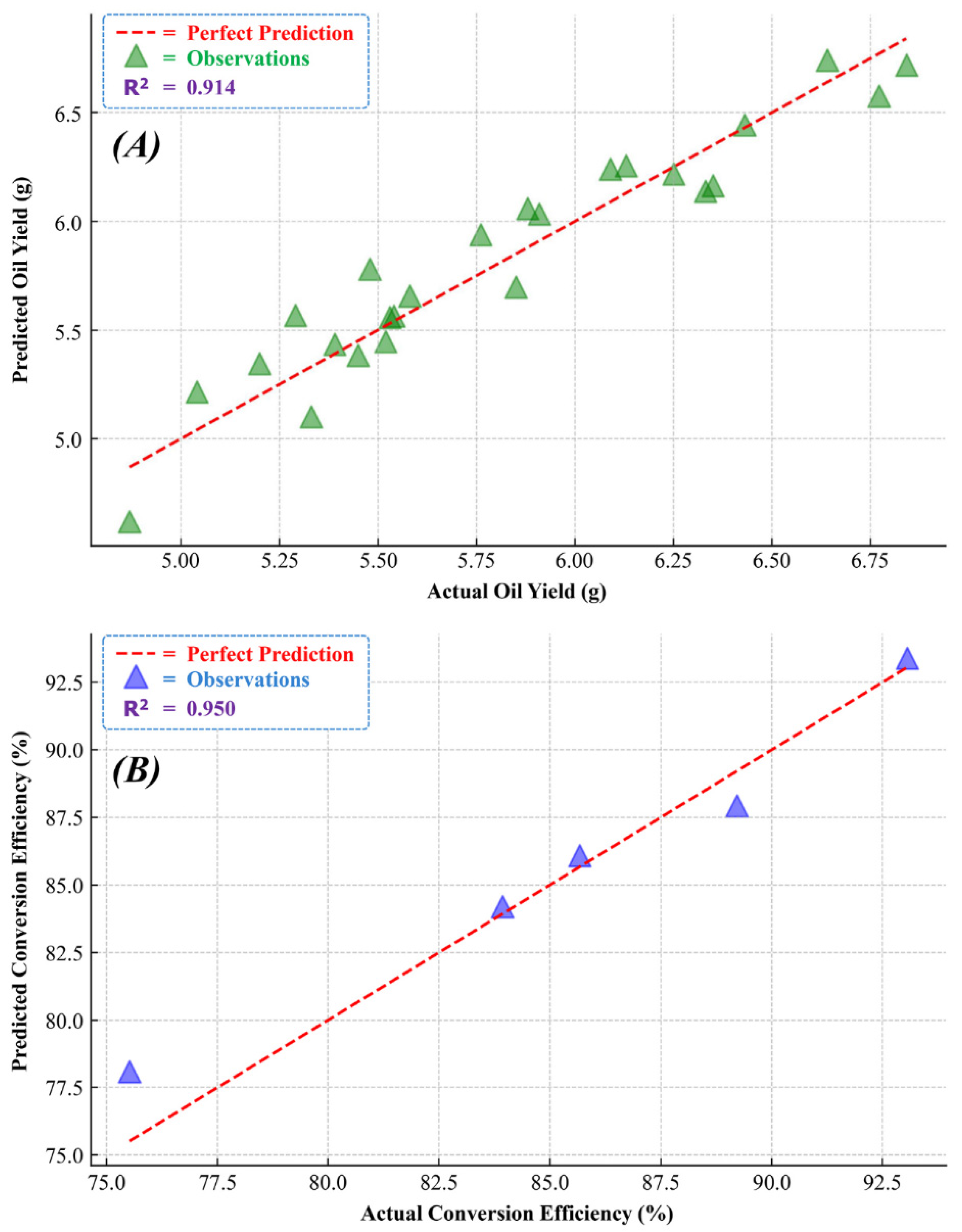
The predictive model developed using RSM for AOY is characterized by its ability to intricately map the nonlinear relationships and interaction effects among key parameters: E-CT, APS, and BSR. Exhibiting a high R2 value of 0.914, the model demonstrates exceptional predictive accuracy. This is further corroborated by the low root mean square errors (RMSEs) of 0.143 for the training set and 0.159 for the testing set. Figure 3A distinctly illustrates this accuracy, depicting a close alignment of the predicted AOY with the actual values. This model represents a significant enhancement in the predictive understanding of AOY, and its detailed consideration of both individual and combined variable impacts mark a notable advancement in biofuel research, particularly in optimizing extraction processes. The model’s equation can be expressed as Equation (1):
where Y: AOY (g). α: Intercept (5.3064). β: E-CT (h). X: APS (μm). γ: BSR. a: Coefficient for β (0.2652). b: Coefficient for X (−0.0005). c: Coefficient for γ (0.1009). d, e, f: Coefficients for squared terms of β, X and γ, respectively, indicating quadratic effects. g, h, i: Coefficients for interaction terms among β, X, and γ, showing combined effects.
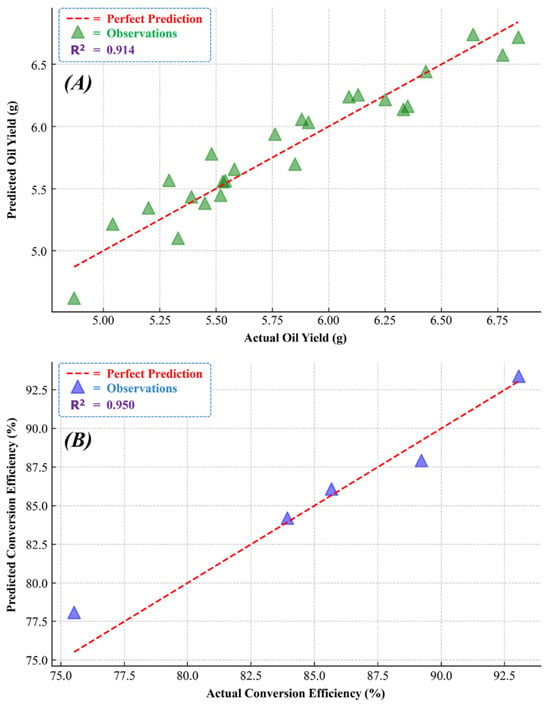
Figure 3.
RSM-based predictive models showing actual vs. predicted values: (A) AOY; (B) BCE.
2.5.2. Predictive Model for Biodiesel Conversion Efficiency
The predictive model for BCE, developed using RSM, effectively captures the complex interactions between the MOR and T-CT. This model stands out for its high predictive accuracy, as indicated by an R2 value of 0.950, suggesting it accounts for approximately 95% of the variance in the BCE. A key highlight of this model is its precision, reflected in the RMSE values of 1.16 for the training set and 1.32 for the testing set. These RMSE values validate the model’s predictive reliability and accuracy. Figure 3B visually demonstrates this precision, showing a high correlation between the predicted and actual conversion efficiencies. This RSM-based model marks an advancement over previous models by incorporating both linear and non-linear relationships, including quadratic and interaction terms. The detailed equation of this model, including critical parameters and their interdependencies, is presented as Equation (2).
Y: BCE (%). α: Intercept (72.4226). β: MOR. X: T-CT. a: Coefficient for β (0.6604). b: Coefficient for X (2.8332). c, d: Coefficients for squared terms of β and X, respectively, indicating quadratic effects. e: Coefficient for interaction terms between β and X, showing combined effects.
2.6. Analysis of Produced Biodiesel
The evaluation of the produced biodiesel synthesized via transesterification of algal oil revealed its comprehensive adherence to the international standards including ASTM D6751 and EN 14214. Notably, its density and kinematic viscosity at 40 °C comfortably fall within the prescribed ranges. The flash point surpasses the minimum threshold, while the cloud point and pour point comply with the respective upper limits. Moreover, the acid number and saponification value convincingly adhere to the stipulated maxima. While the iodine value marginally falls below the prescribed ceiling, it remains within the acceptable range. These findings, further summarized in Table 1, suggest the potential of algae-based biodiesel as a promising candidate for renewable energy applications, warranting further investigation to optimize its properties and enhance its overall competitiveness against conventional fossil fuels.

Table 1.
The properties of produced biodiesel compared with international standards.
3. Materials and Methods
3.1. Sample Collection and Preparation
Freshwater Spirogyra algae samples were manually collected from diverse locations within Tandojam, including ponds and irrigation canals. To remove extraneous vegetation, samples underwent meticulous cleaning. Dehydration was achieved through a two-step process: initial sun-drying for 48 h followed by oven drying at 60 °C for 24 h, resulting in thoroughly dried algae samples. These samples were then finely ground using a mortar and pestle followed by sieving through ASTM Standard-E11-17 mesh sizes 60, 40, 20, 14, and 10, yielding particle sizes of 250 µm, 420 µm, 841 µm, 1410 µm, and 2000 µm, respectively. Figure 4i visually depicts the sample collection and preparation process. The resulting powder was appropriately labeled and stored in airtight containers for subsequent experimentation.

Figure 4.
(i) Algae sample preparation: (A) Harvesting Spirogyra from irrigation canal, (B) Collection and sun-drying, (C) Oven-dried samples, (D) Grinding, (E) Obtaining powdered samples. (ii) Algal oil extraction and purification: (A) Initial sample preparation, (B) Soxhlet extraction, (C) Extract obtained, (D) Rotary evaporation for n-Hexane removal, (E) Refined algal oil obtained. (iii) Biodiesel production and impurities in biodiesel post-transesterification reaction: (A) Algal oil, (B) Methanol + catalyst solution, (C) Biodiesel layering, (D) Pigments as impurities, (E) Final produced biodiesel.
3.2. Chemical Extraction of Algal Oil
The soxhlet apparatus facilitated the continuous extraction of algal oil from the powdered biomass by employing solvent reflux cycles until equilibrium was attained [46]. n-Hexane (C6H14), a non-polar aliphatic hydrocarbon, was chosen for its potent solvency and minimal toxicity, aligning with established literature [47,48]. Given the reported oil content of Spirogyra algae at approximately 30% [49], a series of experiments were conducted to optimize the algal oil yield (AOY) by varying the biomass–solvent ratio (BSR) including 1:1, 1:3, 1:5, 1:7, and 1:9 algae particle size (APS) including 250 µm, 420 µm, 841 µm, 1410 µm, and 2000 µm, and extraction-contact time (E-CT) including 1, 2, 3, 4, and 5 h. Subsequent removal of n-Hexane via rotary evaporation at atmospheric pressure, 70 °C, and 30 RPM was crucial for isolating pure algal oil from the extract [50]. This step also ensures efficient transesterification reactions by minimizing the presence of impurities that could potentially affect biodiesel conversion efficiency (BCE) and biodiesel purity [51]. The purified algal oil was then stored in sealed bottles for further investigation. The sequential steps in algal oil extraction and purification can be seen in Figure 4ii.
3.3. Transesterification for Biodiesel Production
Transesterification reaction was utilized to transform extracted algal oil into biodiesel employing methanol (CH3OH) and sodium hydroxide (NaOH) as a catalyst, as depicted in Figure 4iii. This reaction involves the interaction of triglycerides with an alcohol, yielding fatty acid esters and glycerol as byproduct [52]. A 1% w/v solution of NaOH in methanol was prepared and subsequently added to the algal oil in varying methanol–oil ratios (MORs) including 4:1, 6:1, 8:1, 10:1, and 12:1 to assess the impact of MOR on the BCE of the transesterification process. The resultant mixture was subjected to magnetic stirring on a hot plate at 60 °C and 300 RPM for varying transesterification-contact time (T-CT) including 1, 2, 3, 4, and 5 h to investigate the influence of T-CT on BCE. To isolate the biodiesel from residual sediments and impurities, a 24-hour settling process was implemented. The stratified layers were subsequently separated using a separatory funnel, and the individual weights of the biodiesel and glycerol layers were determined using an electronic balance. Although this initial separation step yields biodiesel, residual impurities, including excess methanol and trace amounts of catalyst, remain [53]. Due to the inherent water solubility of these impurities [54], the obtained biodiesel was purified through washing with distilled water at a 5% water-to-biodiesel ratio (w/w). The washed biodiesel was then dried under a running fan for 6 h to obtain the final product.
3.4. Estimation of Biodiesel Yield after Transesterification Reaction
Since algal oil has a molecular weight of 850.55 g/mol, the moles of algal oil utilized were calculated using Equation (3).
Equation (4) was used to calculate molar yield. The actual molecular weight of glycerol is 92.1 g/mol.
The average molecular mass of methyl ester was calculated using known molecular weight (MW) of algal oil, methanol, and glycerol and using Equation (5):
The biodiesel conversion efficiency (BCE) was calculated using Equation (6)
The percent yield on a weight basis was calculated using Equation (7)
where W1 = Initial weight of algal oil, W2 = Final weight of produced biodiesel.
3.5. Analysis of Produced Biodiesel
The density of the biodiesel was measured using the graduated cylinder method (ASTM D4052), while the viscosity of the biodiesel was measured at 40 °C, utilizing the viscometer. The calculation of viscosity was carried out using Equation (8) incorporating two constants specific to viscometer. The values of these constants were A = 0.26 and B = 179 when time taken was less than 100s, or A = 0.24 and B = 50 when time taken exceeded 100s (ASTM D445).
Flash point was determined by heating the biodiesel until the first visible flashes of fire appeared (ASTM D93), the cloud point by observing the temperature at which the first haze or cloudiness appeared (ASTM D2500), and the pour point by measuring the lowest temperature at which the biodiesel could still flow when tilted (ASTM D97).
Acid number of biodiesel was estimated using titration method. A conical flask was used to hold 0.1–0.5 mL of biodiesel. An amount of 100 mL ethanol was added to flask and mixed with biodiesel. Solvent–oil mixture was titrated with 0.1 M NaOH using 1% phenolphthalein indicator. Calculations were made using Equation (9) (ASTM D664).
where MWNaOH = Molecular weight of sodium hydroxide, VNaOH = Volume of sodium hydroxide used, N = Normality of NaOH solution, W = Weight of the sample taken.
The saponification value was determined by mixing the oil sample with KOH and leaving it in a water bath for an hour. After cooling, it was titrated with HCl using phenolphthalein as an indicator and was calculated using Equation (10) (ASTM D1693).
where V1 = Volume of HCl for blank titration, V2 = Volume of HCl for sample titration, NHCl = Normality of HCl, W = Weight of the oil.
Iodine value of an oil sample was determined by mixing it with chloroform, Hannus solution, and potassium iodide, and then titrating it against sodium thiosulphate until the blue color disappeared, and was calculated using Equation (11) (ASTM D6584).
where B = Volume of sodium thiosulphate for blank titration, S = Volume of sodium thiosulphate for sample titration, N = Normality of sodium thiosulphate, W = Oil sample mass in grams.
3.6. Statistical Analysis
For the optimization of algal oil extraction and transesterification parameters, a combination of Response Surface Methodology (RSM), Principal Component Analysis (PCA), and Multivariate Regression Analysis (MRA) was employed. The experimental data were analyzed using RSM, which involved the generation of response surface plots and contour plots to visualize the relationships between the input variables and the responses (AOY and BCE). Predictive RSM models were developed for both algal oil extraction and transesterification parameters and validated by comparing the predicted and actual responses. PCA was employed to identify the principal components that accounted for the majority of the variance in the data and to explore the relationships between the different variables. MRA was performed to establish the relationships between the process parameters and the responses. Statistical analyses were performed using Python libraries (such as NumPy, SciPy, Matplotlib, and Scikit-learn) and R libraries (such as “rsm” and “ggplot2”). The significance of the models and the individual factors were assessed using appropriate statistical tests, such as analysis of variance (ANOVA), correlation analysis, and Tukey’s HSD test, with a significance level of p < 0.05.
4. Conclusions
This study provides a comprehensive analysis of Spirogyra algae-based biodiesel production, optimizing both oil extraction and transesterification processes. The research reveals complex, non-linear relationships between process parameters and outcomes, with optimal conditions identified for BSR (1:7), APS (400 μm), and E-CT (3–4 h). Transesterification parameters were similarly optimized including MOR (12:1) and T-CT (4 h). Advanced statistical methods, including RSM, PCA, and MRA, were employed to develop highly accurate predictive models for algal oil yield (R² = 0.916) and biodiesel conversion efficiency (R² = 0.950). The produced biodiesel largely conformed to ASTM D6751 and EN 14214 standards, demonstrating its potential as a viable renewable fuel source. This research contributes significant insights into the optimization of algae-based biodiesel production, offering a robust framework for enhancing process efficiency and sustainability in biofuel manufacturing.
Author Contributions
Conceptualization, A.R.O. and M.L.; methodology, A.R.O., M.L. and S.A.B.; software, F.A.C. and S.A.O.; validation, A.R.J., J.D. and J.A.C.; formal analysis, M.L., S.A.O., S.A.B. and F.A.C.; investigation, L.H., I.A.M., J.D. and J.A.C.; data curation, A.R.O., I.A.M. and A.R.J.; writing—original draft preparation, A.R.O., L.T. and A.R.J.; writing—review and editing, A.R.J. and L.H.; visualization, A.R.J., S.A.O. and F.A.C.; supervision, M.L. and S.A.B.; funding acquisition, L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52109105, the Jiangsu Association for Science and Technology Young Science and Tech-neology Talent Support Project, grant number JSTJ-2023-XH029, and the National Key Research and Development Program of China, grant number 2023YFD1900704-02.
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Naveenkumar, R.; Baskar, G. Optimization and Techno-Economic Analysis of Biodiesel Production from Calophyllum Inophyllum Oil Using Heterogeneous Nanocatalyst. Bioresour. Technol. 2020, 315, 123852. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Renganathan, S.; Baskar, G. Production of Biodiesel from Waste Cooking Oil Using MgMoO4 -Supported TiO2 as a Heterogeneous Catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 2053–2059. [Google Scholar] [CrossRef]
- Chakraborty, R.; Chatterjee, S.; Mukhopadhyay, P.; Barman, S. Progresses in Waste Biomass Derived Catalyst for Production of Biodiesel and Bioethanol: A Review. Procedia Environ. Sci. 2016, 35, 546–554. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Ganachari, S.V.; Pengadeth, D.; Mohanakrishna, G.; Aminabhavi, T.M. Biobased Heterogeneous Renewable Catalysts: Production Technologies, Innovations, Biodiesel Applications and Circular Bioeconomy. Environ. Res. 2024, 261, 119745. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-S.; Chew, L.-L. Valuable Compounds Produced by Microalgae. In Handbook of Biorefinery Research and Technology: Production of Biofuels and Biochemicals; Springer Nature Singapore: Singapore, 2024; pp. 731–749. [Google Scholar]
- Feyzi, M.; Shahbazi, E. Catalytic Performance and Characterization of Cs–Ca/SiO2–TiO2 Nanocatalysts for Biodiesel Production. J. Mol. Catal. A Chem. 2015, 404–405, 131–138. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the Time Finally Come for Green Oleochemicals and Biodiesel Production Using Large-Scale Enzyme Technologies? Current Status and New Developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable Fuels from Algae: An Answer to Debatable Land Based Fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ali Ijaz Malik, M.; Zeeshan, S.; Khubaib, M.; Ikram, A.; Hussain, F.; Yassin, H.; Qazi, A. A Review of Major Trends, Opportunities, and Technical Challenges in Biodiesel Production from Waste Sources. Energy Convers. Manag. X 2024, 23, 100675. [Google Scholar] [CrossRef]
- Ponnuswamy, I.; Madhavan, S.; Shabudeen, S. Isolation and Characterization of Green Microalgae for Carbon Sequestration, Waste Water Treatment and Bio-Fuel Production. Int. J. Bio-Sci. Bio-Technol. 2013, 5, 17–26. [Google Scholar]
- Nguyen, L.N.; Vu, M.T.; Vu, H.P.; Johir, M.A.H.; Labeeuw, L.; Ralph, P.J.; Mahlia, T.M.I.; Pandey, A.; Sirohi, R.; Nghiem, L.D. Microalgae-Based Carbon Capture and Utilization: A Critical Review on Current System Developments and Biomass Utilization. Crit. Rev. Environ. Sci. Technol. 2023, 53, 216–238. [Google Scholar] [CrossRef]
- Dębowski, M.; Świca, I.; Kazimierowicz, J.; Zieliński, M. Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies 2022, 16, 81. [Google Scholar] [CrossRef]
- Pate, R.C. Resource Requirements for the Large-Scale Production of Algal Biofuels. Biofuels 2013, 4, 409–435. [Google Scholar] [CrossRef]
- Mehrotra, R.; Hasan Khanc, Z. Lipase Catalysed Transesterfication. Enzym. Eng. 2016, 5, e113. [Google Scholar] [CrossRef]
- Jamaluddin, M.’A.; Ismail, K.; Mohd Ishak, M.A.; Ab Ghani, Z.; Abdullah, M.F.; Safian, M.T.; Idris, S.S.; Tahiruddin, S.; Mohammed Yunus, M.F.; Mohd Hakimi, N.I.N. Microwave-Assisted Pyrolysis of Palm Kernel Shell: Optimization Using Response Surface Methodology (RSM). Renew. Energy 2013, 55, 357–365. [Google Scholar] [CrossRef]
- Outili, N.; Kerras, H.; Nekkab, C.; Merouani, R.; Meniai, A.H. Biodiesel Production Optimization from Waste Cooking Oil Using Green Chemistry Metrics. Renew. Energy 2020, 145, 2575–2586. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Liang, K.-Y.; Zeger, S.L.; Qaqish, B. Multivariate Regression Analyses for Categorical Data. J. R. Stat. Soc. Ser. B Stat. (Methodol.) 1992, 54, 3–24. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil from Spent Coffee Grounds for Biofuel Production. Waste Biomass Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef]
- Singh, S.; Meena, P.; Bhoi, R.; Saharan, V.K.; George, S. Optimization of Bio-Oil Extraction from Chlorella Biomass via a Green Approach to Obtain Algal-Based Di-Ethyl Phthalate. Environ. Sci. Pollut. Res. 2023, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Evaristo, R.; de Macedo, J.; Ghesti, G. Extraction and Characterization of Pequi Seed Oil for Biodiesel Production: A Green Management of Waste to Biofuel Using Ethanol and Heterogeneous Catalysis. J. Braz. Chem. Soc. 2022, 33, 327–339. [Google Scholar] [CrossRef]
- Larida, A.Q.; Bañaga, J.B. Extraction and Characterization of Algal Oil from Lake Sebu, South Cotabato. J. Sci. Sci. Educ. 2021, 5, 12–25. [Google Scholar] [CrossRef]
- Ntalikwa, J.W. Solvent Extraction of Jatropha Oil for Biodiesel Production: Effects of Solvent-to-Solid Ratio, Particle Size, Type of Solvent, Extraction Time, and Temperature on Oil Yield. J. Renew. Energy 2021, 2021, 9221168. [Google Scholar] [CrossRef]
- Haris Mulyadi, A.; Setianingsih, E.; Hasanah, Y.R. Effect of Extraction Parameters (Raw Material Particle Size, Volume of Solvent, and Time) on the Process Yield of Rice Bran Oil. Res. Chem. Eng. (RiCE) 2022, 1, 1–6. [Google Scholar] [CrossRef]
- Baig, R.U.; Malik, A.; Ali, K.; Arif, S.; Hussain, S.; Mehmood, M.; Sami, K.; Mengal, A.N.; Khan, M.N. Extraction of Oil from Algae for Biodiesel Production, from Quetta, Pakistan. IOP Conf. Ser. Mater. Sci. Eng. 2018, 414, 012022. [Google Scholar] [CrossRef]
- Andersson, V.; Heyne, S.; Harvey, S.; Berntsson, T. Integration of Algae-based Biofuel Production with an Oil Refinery: Energy and Carbon Footprint Assessment. Int. J. Energy Res. 2020, 44, 10860–10877. [Google Scholar] [CrossRef]
- Devi, T.; Pravin, R.; Baskar, G. Chlorella Biomass as a Potential Source of Algal Oil: Investigations on Optimization of Ultrasonic Assisted Extraction, Kinetics and Characterization of Algal Oil. Indian J. Chem. Technol. 2023, 30, 430–434. [Google Scholar] [CrossRef]
- Rani, D.S.; Watanabe, M.; Demura, M.; Yoshida, M.; Ahamed, T.; Noguchi, R. A Novel Polyculture Growth Model of Native Microalgal Communities to Estimate Biomass Productivity for Biofuel Production. Biotechnol. Prog. 2021, 37, e3156. [Google Scholar] [CrossRef]
- Hassan Najim, Y.; Al-Abdraba, M.S.W.; Hassan Ahmad, A. Effects of Temperature, Alkaline Catalysts and Molar Ratio of Alcohol to Oil on the Efficiency of Production Biodiesel from Castor Oil. Kirkuk Univ. J. Sci. Stud. 2016, 11, 56–69. [Google Scholar] [CrossRef]
- Andami, P.; Zinatizadeh, A.A.; Feyzi, M.; Zangeneh, H.; Azizi, S.; Norouzi, L.; Maaza, M. Optimization of Biodiesel Production from Sunflower Oil Transesterification Using Ca-K/Al2O3 Nanocatalysts. Int. J. Eng. 2022, 35, 351–359. [Google Scholar] [CrossRef]
- Jain, S. An Assessment of the Operation and Emission Characteristics of a Diesel Engine Powered by a New Biofuel Prepared Using In Situ Transesterification of a Dry Spirogyra Algae–Jatropha Powder Mixture. Energies 2023, 16, 1470. [Google Scholar] [CrossRef]
- Hundie, K.B. Optimization of Biodiesel Production Parameters from Cucurbita Maxima Waste Oil Using Microwave Assisted via Box-Behnken Design Approach. J. Chem. 2022, 2022, 8516163. [Google Scholar] [CrossRef]
- Li, H.; Niu, S.; Lu, C.; Cheng, S. Comparative Evaluation of Thermal Degradation for Biodiesels Derived from Various Feedstocks through Transesterification. Energy Convers. Manag. 2015, 98, 81–88. [Google Scholar] [CrossRef]
- Ayu, D.; Aulyana, R.; Astuti, E.W.; Kusmiyati, K.; Hidayati, N. Catalytic Transesterification of Used Cooking Oil to Biodiesel: Effect of Oil-Methanol Molar Ratio and Reaction Time. Automot. Exp. 2019, 2, 73–77. [Google Scholar] [CrossRef]
- Riayatsyah, T.M.I.; Thaib, R.; Silitonga, A.S.; Milano, J.; Shamsuddin, A.H.; Sebayang, A.H.; Rahmawaty; Sutrisno, J.; Mahlia, T.M.I. Biodiesel Production from Reutealis Trisperma Oil Using Conventional and Ultrasonication through Esterification and Transesterification. Sustainability 2021, 13, 3350. [Google Scholar] [CrossRef]
- Pasawan, M.; Chen, S.-S.; Das, B.; Chang, H.-M.; Chang, C.-T.; Nguyen, T.X.Q.; Ku, H.-M.; Chen, Y.-F. Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles. Fuels 2022, 3, 113–131. [Google Scholar] [CrossRef]
- Shaah, M.A.; Hossain, M.S.; Allafi, F.; Ab Kadir, M.O.; Ahmad, M.I. Biodiesel Production from Candlenut Oil Using a Non-Catalytic Supercritical Methanol Transesterification Process: Optimization, Kinetics, and Thermodynamic Studies. RSC Adv. 2022, 12, 9845–9861. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.; Barik, D.; Ashok, N. Optimization of Oil Yield from the Macro Algae Spirogyra by Solvent Extraction Process Using RSM and ANN. Int. J. Photoenergy 2022, 2022, 3690635. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Ibrahim, S.K.; Nor, M.Z.M.; Hamzah, A.F.A.; Shamsudin, R.; Ali, A.H.M. Optimization of Electrochemical Pre-Treatment for Essential Oil Extraction from Lemon Myrtle (B. Citriodora) Leaves by Response Surface Methodology. J. Food Meas. Charact. 2023, 17, 3732–3744. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, J.; Hernández-Santos, B.; Herman-Lara, E.; Gómez-Aldapa, C.A.; Garcia, H.S.; Martínez-Sánchez, C.E. Effect of Some Variables on Oil Extraction Yield from Mexican Pumpkin Seeds. CyTA J. Food 2014, 12, 9–15. [Google Scholar] [CrossRef]
- Falletti, P.; Barrera Vázquez, M.F.; Cabrera, J.L.; Martini, R.E.; Comini, L.R. Valorization of Biomass Generated by Weeding of Flaveria Bidentis: Optimization of the Process of Extraction Sulfated Flavonoids Using a Doehlert Experimental Design. Waste Biomass Valorization 2023, 14, 3739–3749. [Google Scholar] [CrossRef]
- da Silva, F.C.; Guardiola, J.F.H.; Teixeira, L.P.; Maria, A.C.L.; da Souza, L.A.; Belém, A.L. Optimization of Palm Oil Biodiesel Production Using Response Surface Methodology. Rev. Bras. Ciências Ambient. 2021, 56, 274–285. [Google Scholar] [CrossRef]
- Razzaq, L.; Abbas, M.M.; Miran, S.; Asghar, S.; Nawaz, S.; Soudagar, M.E.M.; Shaukat, N.; Veza, I.; Khalil, S.; Abdelrahman, A.; et al. Response Surface Methodology and Artificial Neural Networks-Based Yield Optimization of Biodiesel Sourced from Mixture of Palm and Cotton Seed Oil. Sustainability 2022, 14, 6130. [Google Scholar] [CrossRef]
- Dharmegowda, I.Y.; Muniyappa, L.M.; Siddalingaiah, P.; Suresh, A.B.; Gowdru Chandrashekarappa, M.P.; Prakash, C. MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization. Sustainability 2022, 14, 11132. [Google Scholar] [CrossRef]
- Jain, D. Soxhlet Apparatus: Hot Continuous Extraction. In DNA Centre for Applied Sciences (DLCAS) Training Manual; DLCAS: Dehra Dun, India, 2020. [Google Scholar]
- Mckee, R.H.; Adenuga, M.D.; Carrillo, J.-C. Characterization of the Toxicological Hazards of Hydrocarbon Solvents. Crit. Rev. Toxicol. 2015, 45, 273–365. [Google Scholar] [CrossRef]
- Akaranta, O.; Anusiem, A.C.I. A Bioresource Solvent for Extraction of Castor Oil. Ind. Crops Prod. 1996, 5, 273–277. [Google Scholar] [CrossRef]
- Saeed, A.; Hanif, M.A.; Hanif, A.; Rashid, U.; Iqbal, J.; Majeed, M.I.; Moser, B.R.; Alsalme, A. Production of Biodiesel from Spirogyra Elongata, a Common Freshwater Green Algae with High Oil Content. Sustainability 2021, 13, 12737. [Google Scholar] [CrossRef]
- Aravind, S.; Barik, D.; Ragupathi, P.; Vignesh, G. Investigation on Algae Oil Extraction from Algae Spirogyra by Soxhlet Extraction Method. Mater. Today Proc. 2021, 43, 308–313. [Google Scholar] [CrossRef]
- Sánchez, A.; Maceiras, R.; Cancela, A.; Rodríguez, M. Influence of N-Hexane on In Situ Transesterification of Marine Macroalgae. Energies 2012, 5, 243–257. [Google Scholar] [CrossRef]
- Makareviciene, V.; Skorupskaite, V. Transesterification of Microalgae for Biodiesel Production. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–510. [Google Scholar]
- Thao, N.T.P.; Thanh Tin, N.; Thanh, B.X. Biodiesel Production from Microalgae by Extraction–Transesterification Method. Waste Technol. 2013, 1, 6–9. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, H. Effect of Major Impurities in Crude Glycerol on Solubility and Properties of Glycerol/Methanol/Bio-Oil Blends. Fuel 2015, 159, 118–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).