Studies on Electronic Structure and Optical Properties of MoS2/X (X = WSe2, MoSe2, AlN, and ZnO) Heterojunction by First Principles
Abstract
1. Introduction
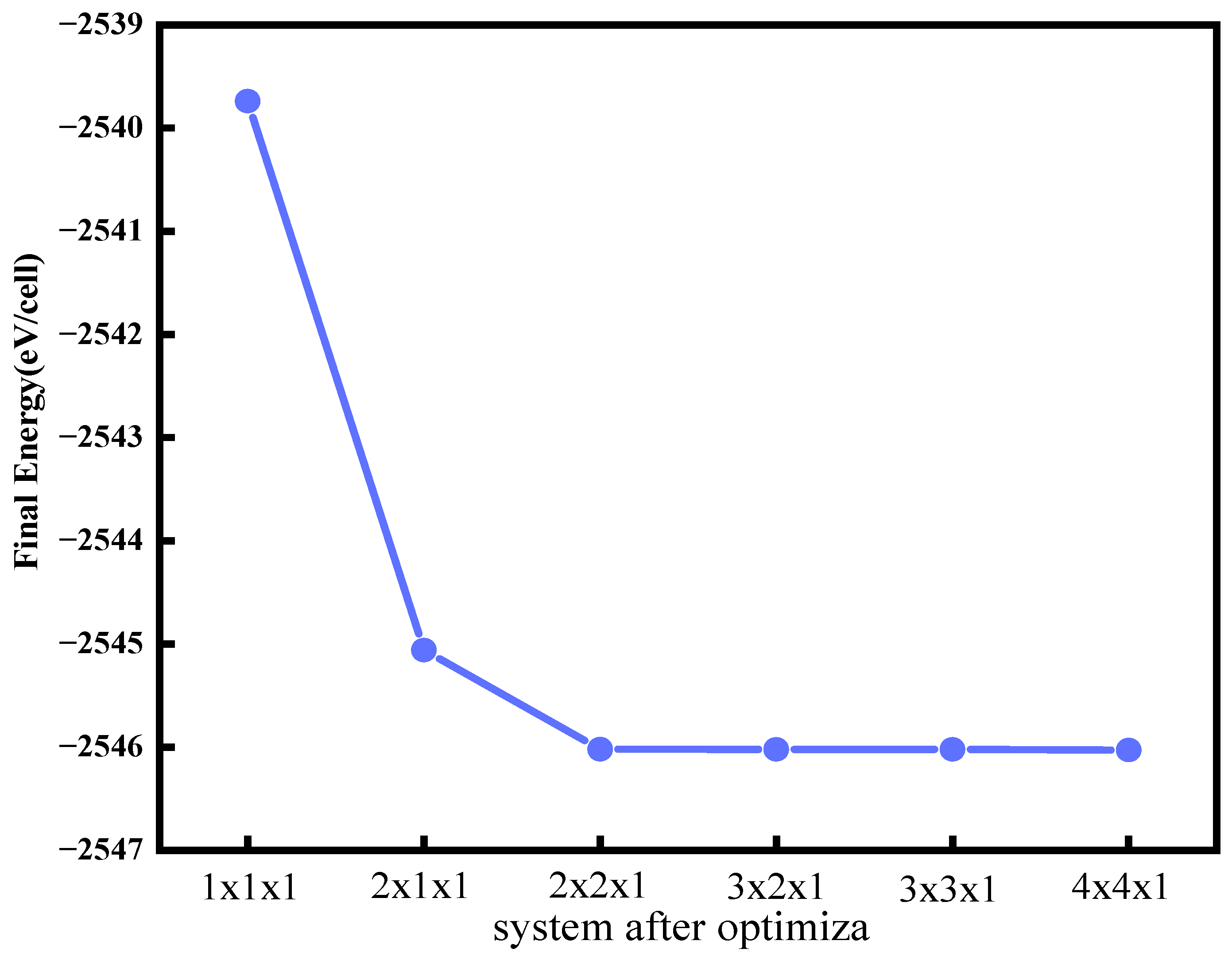
2. Computational Methods and Theoretical Model
3. Geometric Parameters and System Stability
3.1. Geometric Parameters
3.2. Stability
4. Electronic Structure
4.1. Energy Band Structure
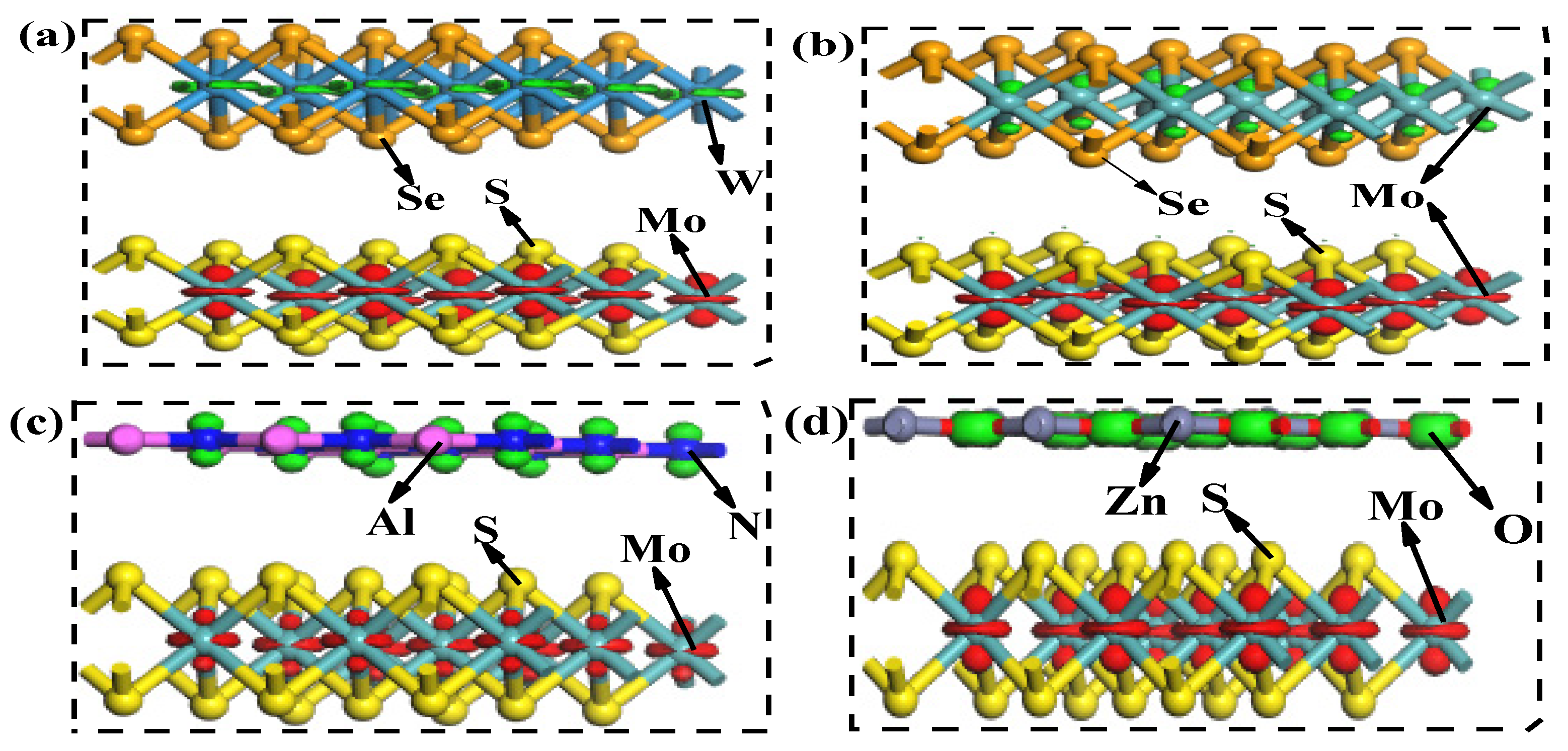
4.2. Density of States
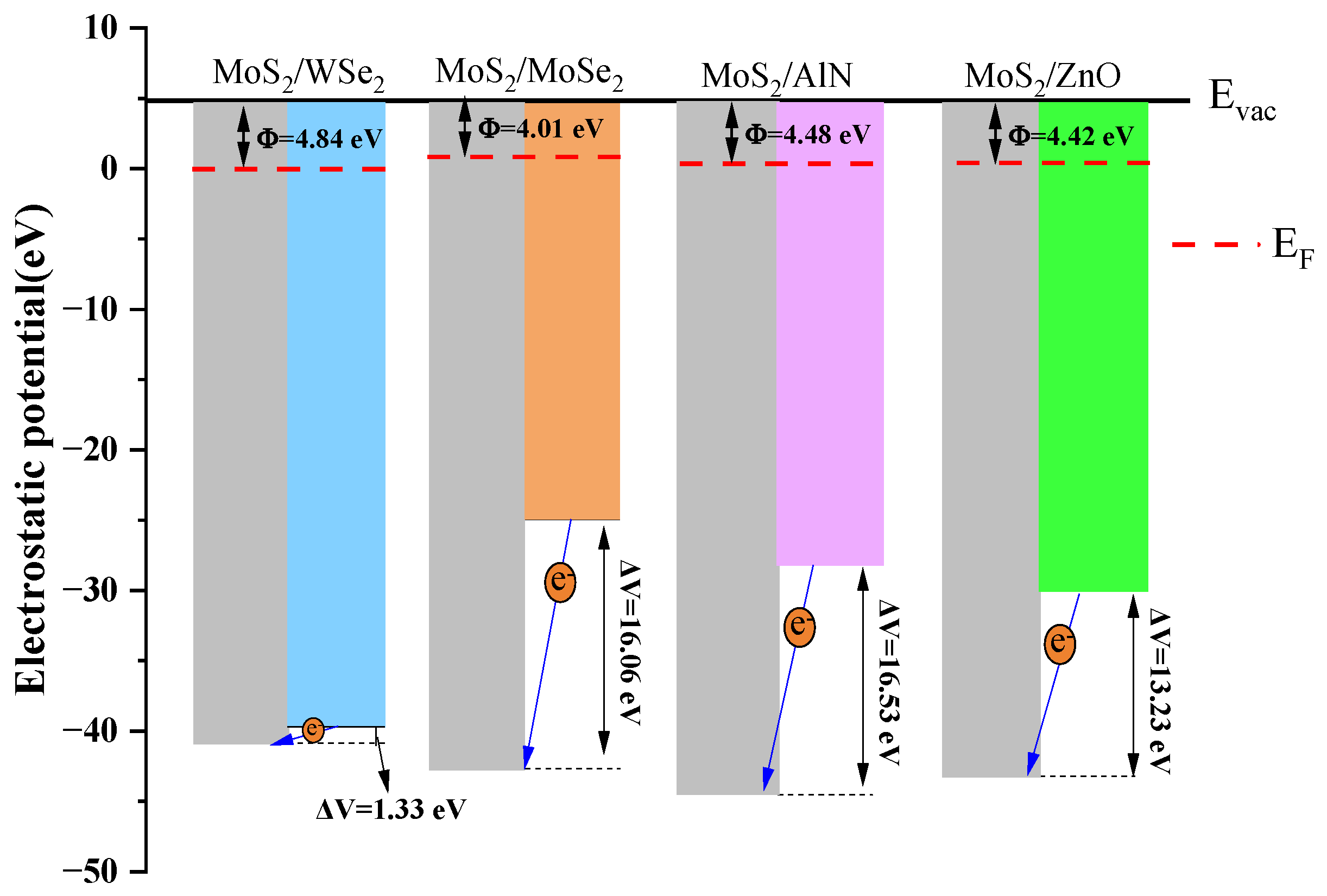
4.3. Work Function
5. Optical Property
5.1. Alignment with Edges
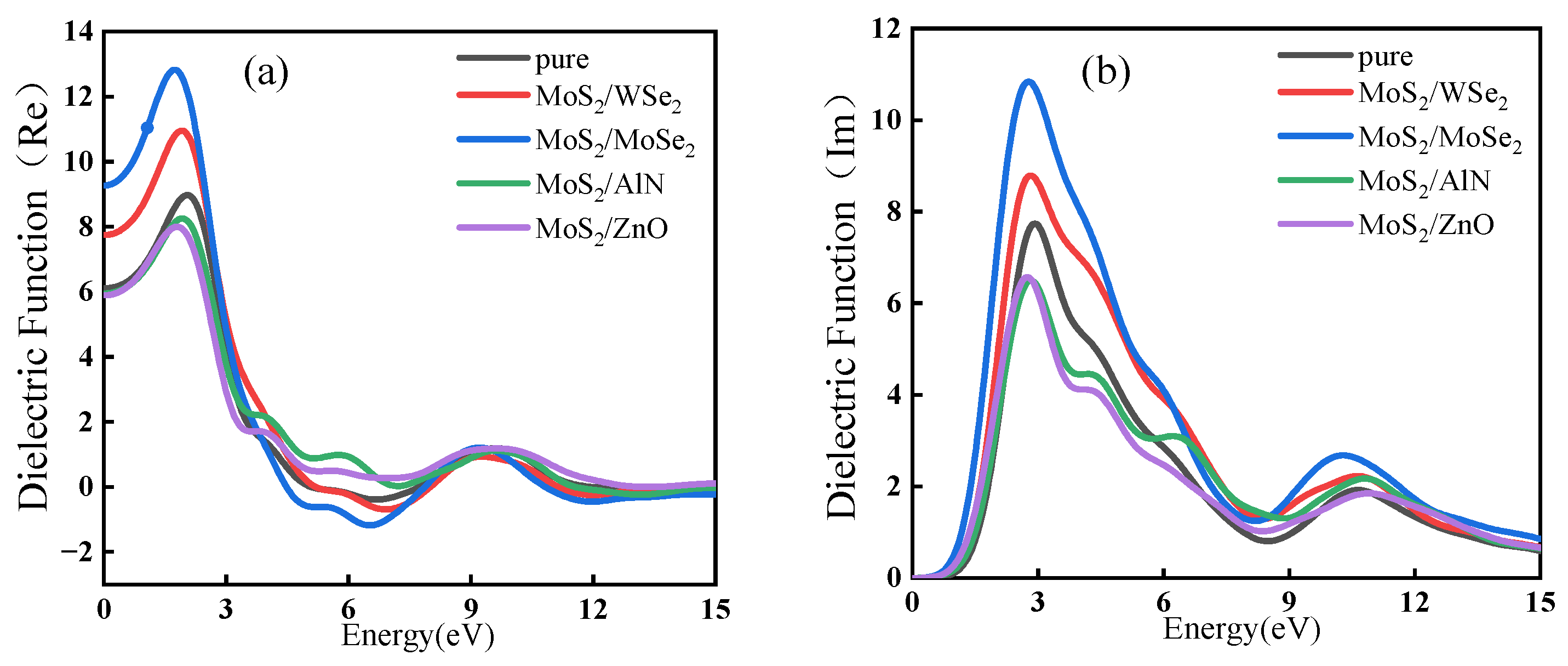
5.2. Complex Dielectric Function
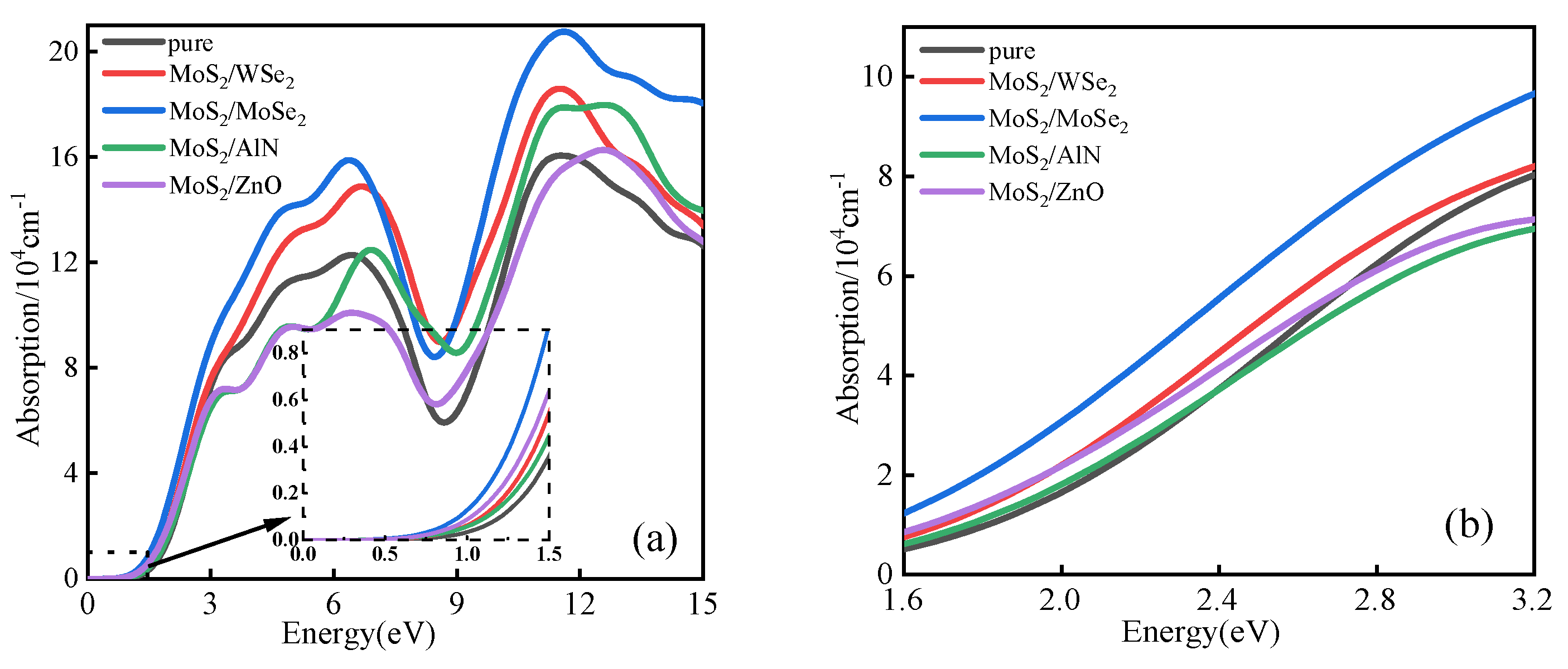
5.3. Absorption Spectrum
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- García-Dalí, S.; Paredes, J.I.; Villar-Rodil, S.; Martínez-Jódar, A.; Martínez-Alonso, A.; Tascón, J.M.D. Molecular Functionalization of 2H-Phase MoS2 Nanosheets via an Electrolytic Route for Enhanced Catalytic Performance. ACS Appl. Mater. Int. 2021, 13, 33157–33171. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, C.; Zhao, Y.F. Tunable redox potential of nonmetal doped monolayer MoS2: First principle calculations. Appl. Surf. Sci. 2016, 384, 360–367. [Google Scholar] [CrossRef]
- Lin, S.Y.; Li, W.T.; Dong, Y.Y.; Wang, C.; Chen, Q.; Zhang, H. Two-dimensional metallic MoS2: A DFT study. Comput. Mater. Sci. 2016, 124, 49–53. [Google Scholar] [CrossRef]
- Shirazi, M.; Kessels, W.M.M.; Bol, A.A. Initial stage of atomic layer deposition of 2D-MoS2 on a SiO2 surface: A DFT study. Phys. Chem. Chem. Phys. 2018, 20, 16861. [Google Scholar] [CrossRef]
- Yoon, Y.; Ganapathi, K.; Salahuddin, S. How Good Can Monolayer MoS2 Transistors Be? Nano Lett. 2011, 11, 3768–3773. [Google Scholar] [CrossRef]
- Mak, K.F.; He, K.; Shan, J.; Heinz, T.F. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nanotechnol. 2012, 7, 494–498. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Computational Search for Single-Layer Transition-Metal Dichalcogenide Photocatalysts. J. Phys. Chem. C 2013, 117, 20440–20445. [Google Scholar] [CrossRef]
- Schmidt, H.; Wang, S.; Chu, L. Transport Properties of Monolayer MoS2 Grown by Chemical Vapor Deposition. Nano Lett. 2014, 14, 1909–1913. [Google Scholar] [CrossRef]
- Li, S.S.; Sun, J.R.; Guan, J.Q. Strategies to improve electrocatalytic and photocatalytic performance of two-dimensional materials for hydrogen Strategies to improve electrocatalytic and photocatalytic performance of two-dimensional materials for hydrogen evolution reaction. Chin. J. Catal. 2021, 42, 511–556. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Lv, H.; Li, S.; Zhang, H. Construction of a 2D nanoplate-like MoS2/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activities. Mater. Lett. 2017, 188, 99–102. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, J.; Lei, H.; Zhai, X.F.; Jiang, F.H.; Sun, C.J. Novel Z-scheme MoS2/Bi2WO6 heterojunction with highly enhanced photocatalytic activity under visible light irradiation. J. Alloys Compd. 2021, 8, 157224. [Google Scholar]
- Liao, J.; Zhong, Y.; He, Z.; Ding, H.; Chen, K.; Chen, D.M. One-step synthesis of MoS2/Bi2S3 heterojunction with enhanced photocatalytic activity and high electrochemical performance. J. Mater. Sci. Mater. Electron. 2020, 32, 385–394. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Wang, F.; Lei, W. Cu2O/MoS2 composites: A novel photocatalyst for photocatalytic degradation of organic dyes under visible light. Ionics 2020, 31, 6359–6369. [Google Scholar] [CrossRef]
- Yao, H.; Guo, X.; Bao, A.; Mao, H.Y.; Ma, Y.C.; Li, X.C. Graphene-based heterojunction for enhanced photodetectors. Chin. Phys. B 2022, 31, 38501. [Google Scholar] [CrossRef]
- Deng, X.Z.; Zhang, J.R.; Zhao, Y.Q.; Yu, Z.L.; Yang, J.L.; Cai, M.Q. The energy band engineering for the high-performance infrared photodetectors constructed by CdTe/MoS2 heterojunction. J. Phys. Condens. Matter 2020, 32, 65004. [Google Scholar] [CrossRef]
- Um, D.; Lee, Y.; Lim, S.; Park, S.Y.; Lee, C.; Ko, H. High-Performance MoS2/CuO Nanosheet-on-One-Dimensional Heterojunction Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 33955–33962. [Google Scholar] [CrossRef]
- Abderrahmane, A.; Woo, C.; Ko, P.J. Black Phosphorus/Molybdenum Diselenide Heterojunction-Based Photodetector. J. Electron. Mater. 2021, 50, 5713–5720. [Google Scholar] [CrossRef]
- Meng, F.X.; Xu, Z.Q.; Zeng, Y.H.; Wang, C.H.; Zou, Y.N.; Wageh, S.; Al-Ghamdi, A.; Guo, Z.N.; Zhang, H. Photodetectors Based on MoS2/MAPbBr3 van der Waals Heterojunction. IEEE Electron Device Lett. 2022, 43, 414–417. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R.P. Constructing MoS2/g-C3N4 heterojunction with enhanced oxygen evolution reaction activity: A theoretical insight. Appl. Surf. Sci. 2019, 5, 254–268. [Google Scholar] [CrossRef]
- Sundaram, R.S.; Engel, M.; Lombardo, A.; Krupke, R.; Ferrari, A.C.; Avouris, P.; Steiner, M. Electroluminescence in Single Layer MoS2. Nano Lett. 2013, 13, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, P.; Luo, W.J.; Gong, F.; Liao, L.; Liu, T.D.; Tong, L.; Zang, J.F.; Xu, J.B.; Hu, W.D. Highly polarization sensitive infrared photodetector based on black phosphorus-on-WSe2 photogate vertical heterostructure. Nano Energy 2017, 37, 53–60. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Kim, H.J.; Wang, J.; Xu, B.R.; Pan, R.B.; Chang, Y.J.; Di, Z.F.; Zhou, P.; Mei, Y.F. Rolling up MoSe2 Nanomembranes as a Sensitive Tubular Photodetector. Small 2019, 15, 1902528. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Tosun, M.; Hettick, M.; Ahn, G.H.; Hu, C.M.; Javey, A. 2D-2D tunneling field-effect transistors using WSe2/SnSe2 heterostructures. Appl. Phys. Lett. 2016, 108, 83111. [Google Scholar] [CrossRef]
- Dybaa, F.; Polak, M.P.; Kopaczek, J.; Scharoch, P.; Wu, K.; Tongay, S.; Kudrawiec, R. crystals and semiconductor to metal transitions. Sci. Rep. 2016, 6, 26663. [Google Scholar]
- Kopaczek, J.; Polak, M.P.; Scharoch, P.; Wu, K.; Chen, B.; Tongay, S.; Kudrawiec, R. Direct optical transitions at K- and H-point of Brillouin zone in bulk MoS2, MoSe2, WS2, and WSe2. J. Appl. Phys. 2016, 119, 1244. [Google Scholar] [CrossRef]
- Long, L.; Guo, Y.P.; He, C.Z.; Tao, H.L.; Huang, J.T.; Yu, W.Y.; Chen, R.X.; Lou, M.S.; Yan, L.B. First-principles study of magnetism of 3d transition metals and nitrogen co-doped monolayer MoS2. Chin. Phys. B 2020, 29, 517–524. [Google Scholar]
- Weinhardt, L.; Blum, B.; Bär, M.; Heske, C.; Cole, B.; Marsen, B.; Miller, E.L. Electronic Surface Level Positions of WO3 Thin Films for Photoelectrochemical Hydrogen Production. J. Phys. Chem. C 2008, 112, 3078–3082. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 69–72. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2010, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, S.; Zhao, H.; Liu, K.Y. First Principles Study on Properties of Monolayer MoS2 Under Different Strains. Braz. J. Phys. 2021, 51, 1230–1236. [Google Scholar] [CrossRef]
- Gustafsson, M.V.; Yankowitz, M.; Forsythe, C.; Rhodes, D.; Watanabe, K.; Taniguchi, T.; Hone, J.; Zhu, X.Y.; Dean, C.R. Ambipolar Landau levels and strong band-selective carrier interactions in monolayer WSe2. Nat. Mater. 2018, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Saki, N.; Lu, Z.; Goto, H.; Eguchi, R.; Ota, H.; Kambe, T.; Terashima, K.; Yokoya, K.; Huyen, T.L.; et al. Emergence of superconductivity in (NH3)yMxMoSe2 (M: Li, Na and K). Sci. Rep. 2016, 6, 29292. [Google Scholar]
- Wu, H.; Qin, Z.; Yan, Z.; Sun, Z.H.; Li, B.K.; Zheng, R.S. First Principles Study on Li-doped and Li, O-codoped AlN. Medžiagotyra 2019, 25, 17–20. [Google Scholar]
- Badreddine, K.; Kazah, I.; Rekaby, M.; Awad, R. Structural, Morphological, Optical, and Room Temperature Magnetic Characterization on Pure and Sm-Doped ZnO Nanoparticles. J. Nanomater. 2018, 7096195. [Google Scholar] [CrossRef]
- Guo, L.J.; Hu, J.S.; Ma, X.G.; Xiang, J. Interfacial interaction and Schottky contact of two-dimensional WS2/graphene heterostructure. Acta Phys. Sin.-Chin. Ed. 2019, 68, 97101. [Google Scholar] [CrossRef]
- Ma, H.H.; Zhang, X.B.; Wei, X.Y.; Cao, J.M. Theoretical study on Schottky regulation of WSe2/graphene heterostructure doped with nonmetallic elements. Acta Phys. Sin.-Chin. Ed. 2020, 69, 117101. [Google Scholar] [CrossRef]
- Hu, J.; Ji, G.; Ma, X.; He, H.; Huang, C.Y. Probing interfacial electronic properties of graphene/CH3NH3PbI3 heterojunctions: A theoretical study. Appl. Surf. Sci. 2018, 440, 35–41. [Google Scholar] [CrossRef]
- Zhang, J.R.; Yu, Q.; Chen, L.; Yin, S.F.; Cai, M.Q. Density functional theory calculation on facet-dependent photocatalytic activity of MoS2/CdS heterostructures. Appl. Surf. Sci. 2019, 469, 27–33. [Google Scholar] [CrossRef]
- Sun, M.; Nelson, A.E.; Adjaye, J. Ab initio DFT study of hydrogen dissociation on MoS2, NiMoS, and CoMoS: Mechanism, kinetics, and vibrational frequencies. J. Catal. 2005, 233, 411–421. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 24, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Bucher, D.; Pierce, L.C.T.; McCammon, J.A.; Markwick, P.R.L. On the Use of Accelerated Molecular Dynamics to Enhance Configurational Sampling in Ab Initio Simulations. J. Chem. Theory Comput. 2011, 7, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.K.; Parkinson, B.A. Detailed photocurrent spectroscopy of the semiconducting group VIB transition metal dichalcogenides. J. Phys. Chem. 1982, 86, 463–467. [Google Scholar] [CrossRef]
- Liu, J.; Hua, E. Photocatalytic properties and energy band offset of a tungsten disulfide/graphitic carbon nitride van der Waals heterojunction. RSC Adv. 2020, 10, 5260–5267. [Google Scholar] [CrossRef]
- Chai, H.; Gao, L.; Wang, P.; Li, F.; Hu, G.W.; Jin, J. In2S3/F-Fe2O3 type-II heterojunction bonded by interfacial S-O for enhanced charge separation and transport in photoelectrochemical water oxidation. Appl. Catal. B Environ. 2022, 305, 121011. [Google Scholar] [CrossRef]
- Zhou, F.; Ren, X.H.; Liu, J.; Liu, P. Development of Photocatalytic Degradation of Organic Pollutants in Water. J. Mater. Eng. 2018, 46, 9–19. [Google Scholar]
- Liu, L.W.; Liu, C.S.; Huang, X.H.; Zeng, S.F.; Tang, Z.W.; Zhang, D.W.; Zhou, P. Tunable Current Regulative Diode Based on Van der Waals Stacked MoS2/WSe2 Heterojunction–Channel Field-Effect Transistor. Adv. Electron. Mater. 2022, 8, 2100869. [Google Scholar] [CrossRef]
- Shimada, T.; Ohuchi, F.S.; Parkinson, B.A. Work function and photo threshold of layered metal dichalcogenides. Jpn. J. Appl. Phys. 1994, 33, 2696–2698. [Google Scholar] [CrossRef]
- Sun, H.; Torres, C.G.; Liu, K.; Li, K.H.; Guo, W.Z.; Lin, R.H.; Liu, X.W.; Li, J.T.; Li, X.H. Valence and conduction band offsets of β-Ga2O3/AlN heterojunction. Appl. Phys. Lett. 2017, 111, 162105. [Google Scholar] [CrossRef]
- Ozawa, K.; Edamoto, K. Photoelectron spectroscopy study of K adsorption on ZnO(1 0 -1 0). Surf. Sci. 2003, 524, 78–88. [Google Scholar] [CrossRef]
- Wei, D.; Li, Y.; Feng, Z.; Guo, G.F.; Ma, Y.Q.; Yu, H.; Luo, Q.Q.; Tang, Y.N.; Dai, X.Q. Electronic and optical properties of 3N-doped graphdiyne/MoS2 heterostructures tuned by biaxial strain and external electric field. Chin. Phys. B 2021, 30, 438–445. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Tian, W.; Zhang, Y.; Shan, P.Y.; Chen, Y.J.; Wei, W.H.; Yuan, H.K.; Cui, H. Mechanism for hydrogen evolution from water splitting based on a MoS2/WSe2 heterojunction photocatalyst: A first-principle study. RSC Adv. 2020, 10, 41127–41136. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zang, W.; Liu, X.; Pennycook, S.J.; Kou, Z.K.; Yang, C.H.; Guan, G.; Wang, J. Heterojunction engineering of MoSe2/MoS2 with electronic modulation towards synergetic hydrogen evolution reaction and supercapacitance performance. Chem. Eng. J. 2019, 359, 1419–1426. [Google Scholar] [CrossRef]
- Zou, H.; Peng, M.; Zhou, W.; Pan, J.L.; Ouyang, F.P. Type II GaS/AlN van der Waals heterostructure: Vertical strain, in-plane biaxial strain and electric field effect. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114481. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Xiong, Z.; Cui, Y.Y.; Luo, H.J.; Gao, Y.F. Electronic and electron emission properties of X/ZnO monolayer (X = Li, Na, K, Rb, or Cs): A first-principles study. J. Phys. Chem. Solids 2020, 147, 109618. [Google Scholar] [CrossRef]
- Li, B.; Nengzi, L.C.; Guo, R.N.; Cui, Y.Q.; Zhang, Y.X.; Cheng, X.W. Novel synthesis of Z-scheme α-Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance: Influence of calcination temperature. Chin. Chem. Lett. 2020, 31, 2705–2711. [Google Scholar] [CrossRef]
- Tong, T.; He, B.; Zhu, B.H.; Cheng, B.; Zhang, L.Y. First-principle investigation on charge carrier transfer in transition-metal single atoms loaded g-C3N4. Appl. Surf. Sci. 2018, 459, 385–392. [Google Scholar] [CrossRef]
- Wang, J.N.; Huang, Y.H.; Guo, J.X.; Zhang, J.M.; Wei, X.M.; Ma, F. Optoelectronic response and interfacial properties of BiOI/BiOX (X = F, Cl, Br) heterostructures based on DFT investigation. Solid State Chem. 2020, 284, 121181. [Google Scholar] [CrossRef]
- Bartels, C.; Schultze, J.W.; Stimming, U.; Habib, M.A. The electrochemical behaviors of hafnium. Electrochim. Acta 1982, 27, 129–140. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xia, T.; Liu, G.A. Electronic and optical properties of n-pr co-doped anatase TiO2 from first principles. Acta Phys. Sin. 2019, 68, 245–253. [Google Scholar]
- Mahmood, Q.; Hassan, M.; Faridi, M.A. Study of magnetic and optical properties of Zn_(1−x)TM_xTe(TM = Mn,Fe,Co,Ni) diluted magnetic semiconductors: First principle approach. Chin. Phys. B 2017, 26, 456–464. [Google Scholar] [CrossRef]
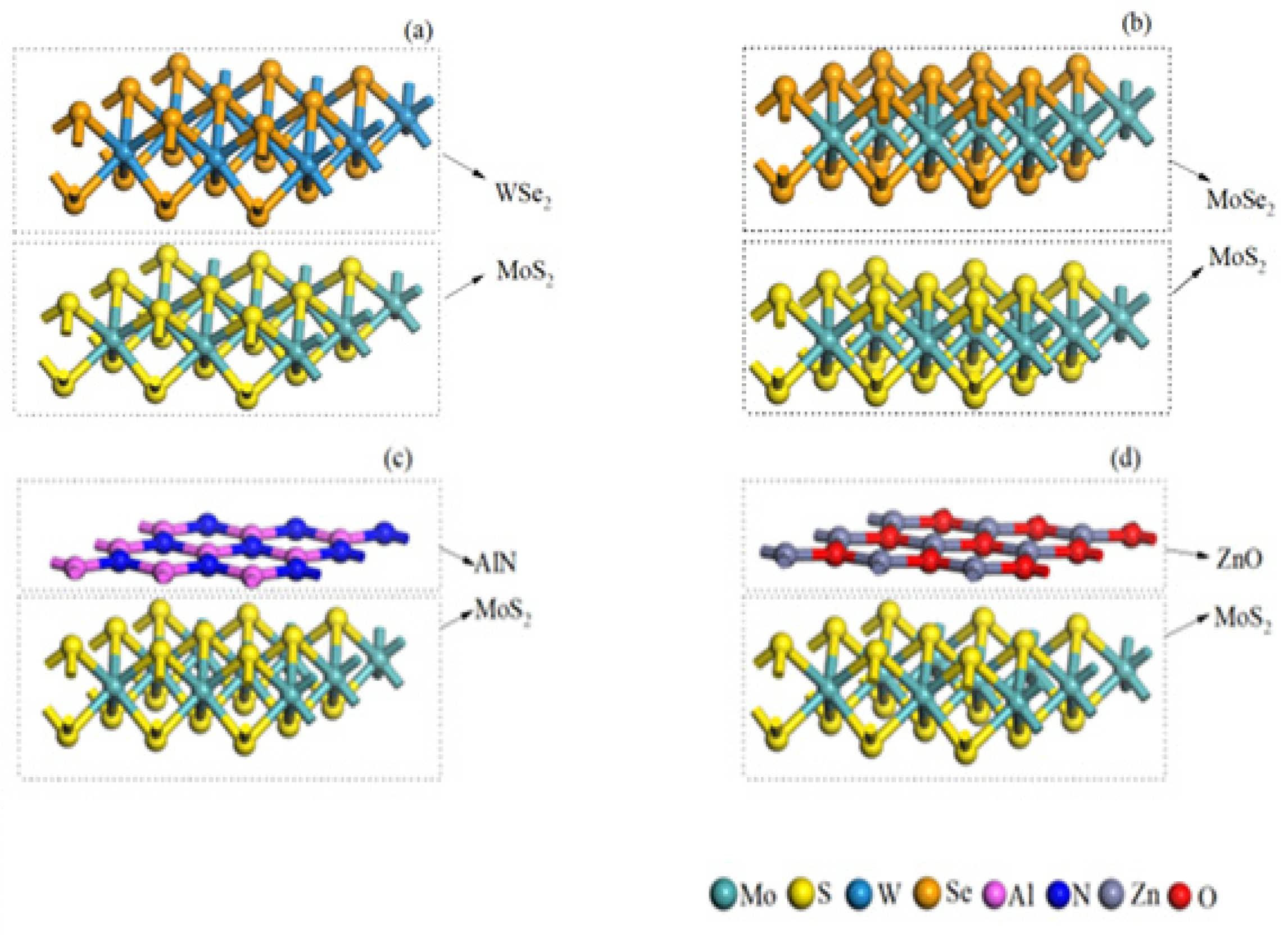





| MoS2 | WSe2 | MoSe2 | AlN | ZnO | |
|---|---|---|---|---|---|
| a = b/nm (this work) | 0.317 | 0.331 | 0.330 | 0.312 | 0.329 |
| a = b/nm (experimental) | 0.316 [32] | 0.327 [33] | 0.329 [34] | 0.311 [35] | 0.325 [36] |
| Relative error | 0.3% | 1.2% | 0.3% | 0.3% | 1.2% |
| MoS2/WSe2 | MoS2/MoSe2 | MoS2/AlN | MoS2/ZnO | |
|---|---|---|---|---|
| ΔEmis/meV·Å−2 | −0.332 | −1.138 | −8.675 | −0.548 |
| Eb/eV | −0.78 | −0.79 | −1.59 | −1.05 |
| MoS2 | WSe2 | MoSe2 | AlN | ZnO | |
|---|---|---|---|---|---|
| Φ (this work) | 5.78 eV | 4.57 eV | 4.64 eV | 5.04 eV | 5.09 eV |
| Φ (experimental) | 4.80 eV [46] | 3.70 eV [45] | 4.40 eV [46] | 4.45 eV [47] | 4.85 eV [48] |
| Φ (calculation) | 5.67 eV [53] GGA + PBE | 4.36 eV [53] GGA + PBE | 4.57 eV [54] PBE + SOC | 4.92 eV [55] GGA + PBE | 5.06 eV [56] GGA + PBE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jin, Y.; Lei, B.; Zhao, X.; Huang, Y.; Zhang, L.; Zhu, Y. Studies on Electronic Structure and Optical Properties of MoS2/X (X = WSe2, MoSe2, AlN, and ZnO) Heterojunction by First Principles. Catalysts 2024, 14, 678. https://doi.org/10.3390/catal14100678
Liu J, Jin Y, Lei B, Zhao X, Huang Y, Zhang L, Zhu Y. Studies on Electronic Structure and Optical Properties of MoS2/X (X = WSe2, MoSe2, AlN, and ZnO) Heterojunction by First Principles. Catalysts. 2024; 14(10):678. https://doi.org/10.3390/catal14100678
Chicago/Turabian StyleLiu, Jibo, Yuheng Jin, Bocheng Lei, Xucai Zhao, Yineng Huang, Lili Zhang, and Youliang Zhu. 2024. "Studies on Electronic Structure and Optical Properties of MoS2/X (X = WSe2, MoSe2, AlN, and ZnO) Heterojunction by First Principles" Catalysts 14, no. 10: 678. https://doi.org/10.3390/catal14100678
APA StyleLiu, J., Jin, Y., Lei, B., Zhao, X., Huang, Y., Zhang, L., & Zhu, Y. (2024). Studies on Electronic Structure and Optical Properties of MoS2/X (X = WSe2, MoSe2, AlN, and ZnO) Heterojunction by First Principles. Catalysts, 14(10), 678. https://doi.org/10.3390/catal14100678






