High-Performance Methanol Oxidation via Ni12-Metal8/CNF Catalyst for Fuel Cell Applications
Abstract
:1. Introduction
2. Result and Discussion
2.1. XRD Analysis
2.2. Morphological Study
2.3. Elemental Analysis
2.4. Electrochemistry Analysis
2.4.1. Effect of Electrolyte Concentration
2.4.2. Effect of Scan Rate
2.4.3. Linear Sweep Voltammetry
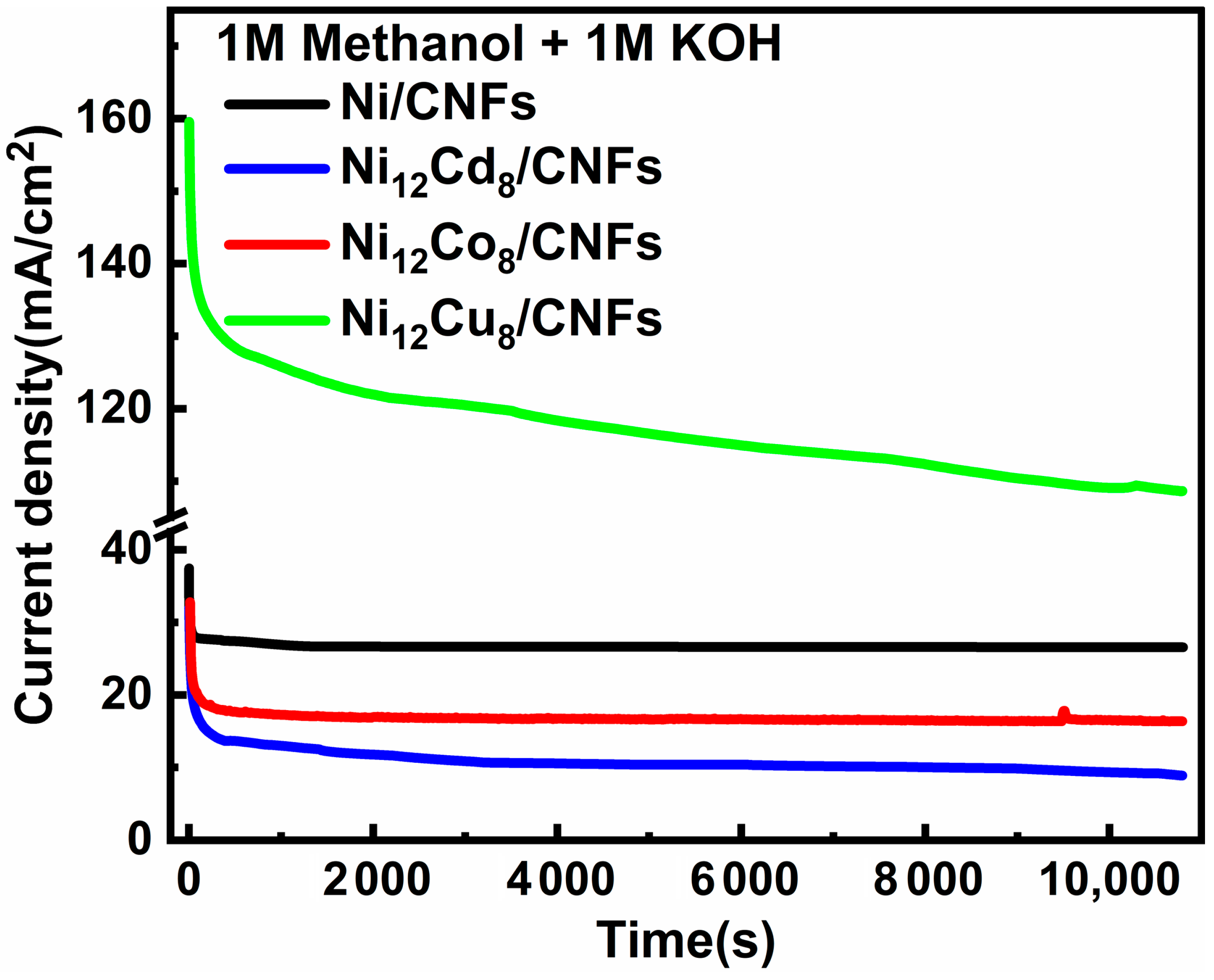
2.4.4. Chronoamperometry
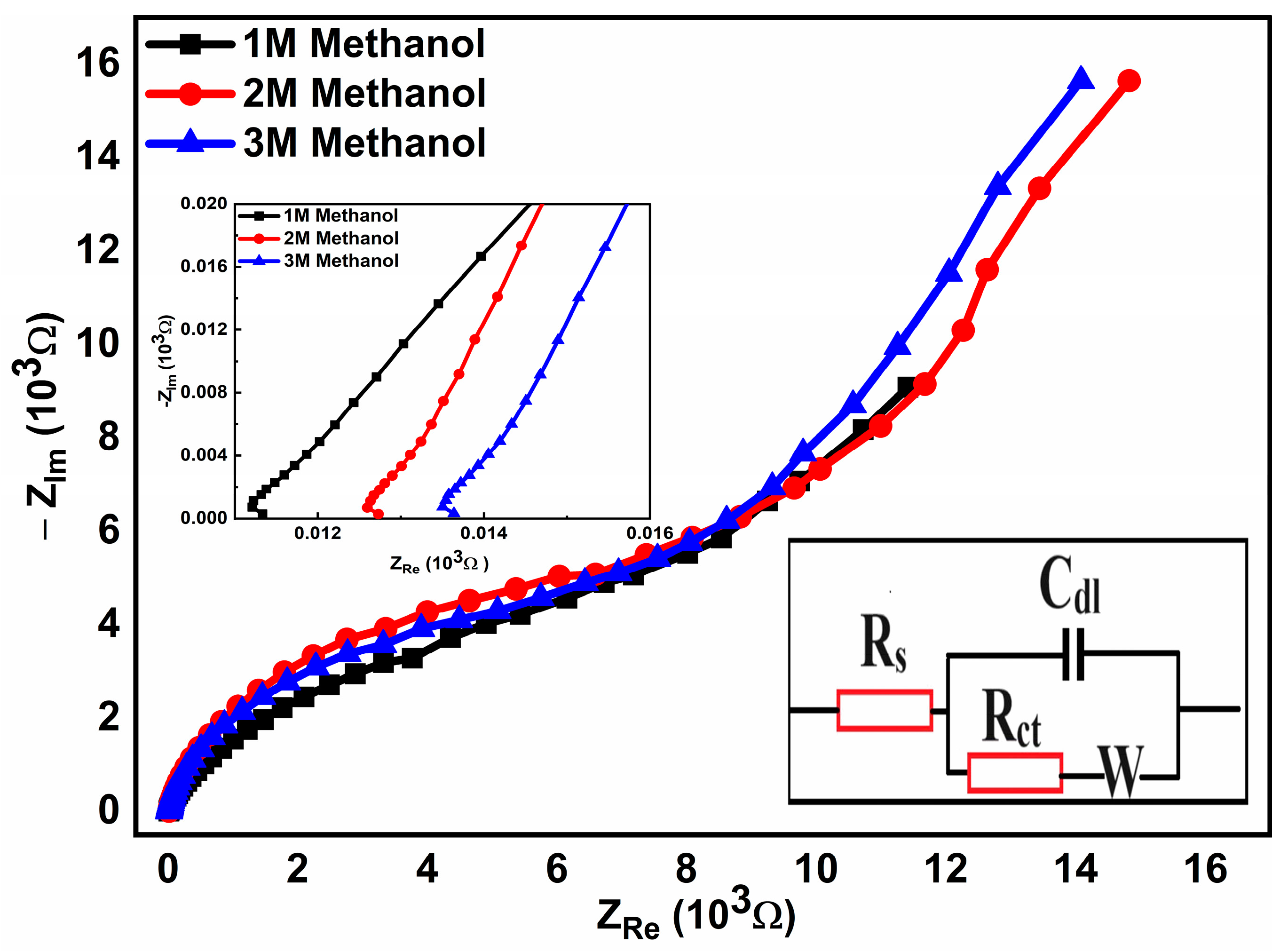
2.4.5. Electrochemical Impedance Spectroscopy (EIS)
3. Experimental Details
3.1. Samples Preparation
3.2. Synthesis of Metal/CNFs
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dincer, I.; Zamfirescu, C. Sustainable Energy Systems and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Güler, S.; Yavaş, A.; Mustafov, S.D.; Şen, F. The material development and characterization of direct alcohol fuel cells. In Nanomaterials for Direct Alcohol Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–73. [Google Scholar]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Suter, T.A.; Smith, K.; Hack, J.; Rasha, L.; Rana, Z.; Angel, G.M.A.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Engineering catalyst layers for next-generation polymer electrolyte fuel cells: A review of design, materials, and methods. Adv. Energy Mater. 2021, 11, 2101025. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO stripping on nanosized Pt surfaces in acid media: A review on the issue of peak multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on carbon-alternative materials as Pt catalyst supports for a durable oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Mohamed, H.O.; Obaid, M.; Rezk, H.; Chae, K.-J. Nonprecious anodic catalysts for low-molecular-hydrocarbon fuel cells: Theoretical consideration and current progress. Prog. Energy Combust. Sci. 2020, 77, 100805. [Google Scholar] [CrossRef]
- Lee, J.D.; Jishkariani, D.; Zhao, Y.; Najmr, S.; Rosen, D.; Kikkawa, J.M.; Stach, E.A.; Murray, C.B. Tuning the electrocatalytic oxygen reduction reaction activity of Pt–Co nanocrystals by cobalt concentration with atomic-scale understanding. ACS Appl. Mater. Interfaces 2019, 11, 26789–26797. [Google Scholar] [CrossRef]
- Eshghi, A.; Sabzehmeidani, M.M. Platinum–Iron nanoparticles supported on reduced graphene oxide as an improved catalyst for methanol electro oxidation. Int. J. Hydrogen Energy 2018, 43, 6107–6116. [Google Scholar] [CrossRef]
- Colmati, F.; Magalhães, M.M.; Sousa, R.; Ciapina, E.G.; Gonzalez, E.R. Direct Ethanol Fuel Cells: The influence of structural and electronic effects on Pt–Sn/C electrocatalysts. Int. J. Hydrogen Energy 2019, 44, 28812–28820. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, S.; Omasta, T.J.; Roller, J.M.; Mustain, W.E. Activity and durability of Pt-Ni nanocage electocatalysts in proton exchange membrane fuel cells. Appl. Catal. B Environ. 2017, 203, 927–935. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-based core–shell electrocatalysts for fuel cell cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Leonard, B.M.; Zhou, Q.; Wu, D.; DiSalvo, F.J. Facile synthesis of PtNi intermetallic nanoparticles: Influence of reducing agent and precursors on electrocatalytic activity. Chem. Mater. 2011, 23, 1136–1146. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Chen, L.; Shi, J.; He, M. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 2019, 10, 5335. [Google Scholar] [CrossRef]
- Monnerie, N.; Gan, P.G.; Roeb, M.; Sattler, C. Methanol production using hydrogen from concentrated solar energy. Int. J. Hydrogen Energy 2020, 45, 26117–26125. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Li, J.; Wei, N.; Gary, D.; et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl. Catal. B Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Screening of Ni-Cu bimetallic catalysts for hydrogen and carbon nanofilaments production via catalytic decomposition of methane. Appl. Catal. A Gen. 2018, 559, 10–19. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Halim, E.M.; Chemchoub, S.; El Attar, A.; Salih, F.E.; Oularbi, L.; EL Rhazi, M. Recent advances in anode metallic catalysts supported on conducting polymer-based materials for direct alcohol fuel cells. Front. Energy Res. 2022, 10, 843736. [Google Scholar] [CrossRef]
- Roy, A.; Talarposhti, M.R.; Normile, S.J.; Zenyuk, I.V.; De Andrade, V.; Artyushkova, K.; Serov, A.; Atanassov, P. Nickel–copper supported on a carbon black hydrogen oxidation catalyst integrated into an anion-exchange membrane fuel cell. Sustain. Energy Fuels 2018, 2, 2268–2275. [Google Scholar] [CrossRef]
- Pieta, I.S.; Rathi, A.; Pieta, P.; Nowakowski, R.; Holdynski, M.; Pisarek, M.; Kaminska, A.; Gawande, M.B.; Zboril, R.; Pieta, I.S.; et al. Electrocatalytic methanol oxidation over Cu, Ni and bimetallic Cu-Ni nanoparticles supported on graphitic carbon nitride. Appl. Catal. B Environ. 2019, 244, 272–283. [Google Scholar] [CrossRef]
- Hu, B.; Xie, Y.; Yang, Y.; Meng, J.; Cai, J.; Chen, C.; Yu, D.; Zhou, X. Lattice strain controlled Ni@ NiCu efficient anode catalysts for direct borohydride fuel cells. Dalton Trans. 2023, 52, 12002–12009. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Shaban, M.; Abdel-Hamed, M.O.; Gamal, A.; Yehia, H.; Ahmed, A.M. Synthesis and characterization of NiCoPt/CNFs nanoparticles as an effective electrocatalyst for energy applications. Nanomaterials 2022, 12, 492. [Google Scholar] [CrossRef]
- Utkan, G.; Yumusak, G.; Tunali, B.C.; Ozturk, T.; Turk, M. Production of reduced graphene oxide by using three different microorganisms and investigation of their cell interactions. ACS Omega 2023, 8, 31188–31200. [Google Scholar] [CrossRef]
- Mohammed, S.; Aburabie, J.; Nassrullah, H.; Hashaikeh, R. Porous rGO/networked cellulose composite membranes: Towards enhanced nanofiltration performance of rGO-based membranes. Mater. Today Sustain. 2024, 25, 100682. [Google Scholar] [CrossRef]
- Aragaw, B.A. Reduced graphene oxide-intercalated graphene oxide nano-hybrid for enhanced photoelectrochemical water reduction. J. Nanostruct. Chem. 2020, 10, 9–18. [Google Scholar] [CrossRef]
- Guan, J.; Liu, Y.; Fang, Y.; Du, X.; Fu, Y.; Wang, L.; Zhang, M. Co-Ni alloy nanoparticles supported by carbon nanofibers for hydrogen evolution reaction. J. Alloys Compd. 2021, 868, 159172. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni–Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, P.; Peng, Q.; Chen, J.; Huang, J.; Wang, F.; Noor, N. Layer-by-layer assembly of polyelectrolyte multilayer onto PET fabric for highly tunable dyeing with water soluble dyestuffs. Polymers 2017, 9, 735. [Google Scholar] [CrossRef]
- Han, H.; Dai, R.; Wang, Z. Fabrication of high-performance thin-film composite nanofiltration membrane by dynamic calcium-carboxyl intra-bridging during post-treatment. Membranes 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Li, W.; Liu, L. Vertically aligned porous nickel (II) hydroxide nanosheets supported on carbon paper with long-term oxygen evolution performance. Chem. Asian J. 2017, 12, 543–551. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Zhang, W.; Yu, M.; Cui, L.; Cao, X.; Liu, J. Super-light Cu@ Ni nanowires/graphene oxide composites for significantly enhanced microwave absorption performance. Sci. Rep. 2017, 7, 1584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bhatt, V.; Nayal, O.S.; Sharma, S.; Kumar, V.; Thakur, M.S.; Kumar, N.; Bal, R.; Singh, B.; Sharma, U. CuI nanoparticles as recyclable heterogeneous catalysts for C–N bond formation reactions. Catal. Sci. Technol. 2017, 7, 2857–2864. [Google Scholar] [CrossRef]
- Swadźba-Kwaśny, M.; Chancelier, L.; Ng, S.; Manyar, H.G.; Hardacre, C.; Nockemann, P. Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles. Dalton Trans. 2012, 41, 219–227. [Google Scholar] [CrossRef]
- Chen, G.; Pan, Y.; Lu, T.; Wang, N.; Li, X. Highly catalytical performance of nanoporous copper for electro-oxidation of methanol in alkaline media. Mater. Chem. Phys. 2018, 218, 108–115. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jalali, F. Zr-MOF@ Polyaniline as an efficient platform for nickel deposition: Application to methanol electro-oxidation. Fuel 2021, 296, 120677. [Google Scholar] [CrossRef]
- Li, F.; Chang, X.; Wang, S.; Guo, Y.; Li, H.; Wu, K. Excellent electrocatalytic performance toward methanol oxidation of hierarchical porous NiCu obtained by electrochemical dealloying. J. Alloys Compd. 2023, 934, 167811. [Google Scholar] [CrossRef]
- Mahmoud, I.; Farghali, A.A.; El-Rouby, W.M.A.; Abdelwahab, A. Nickel and cobalt-based tungstate nanocomposites as promising electrocatalysts in alkaline direct methanol fuel cells. Nanoscale Adv. 2024, 6, 2059–2074. [Google Scholar] [CrossRef]
- Liu, C.; Yang, F.; Schechter, A.; Feng, L. Recent progress of Ni-based catalysts for methanol electrooxidation reaction in alkaline media. Adv. Sens. Energy Mater. 2023, 2, 100055. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Y.; Huang, L.; Luo, Y.; Chen, S.; Xu, M.; Chen, W. Recent Advances in Electrochemical Sensors for Formaldehyde. Molecules 2024, 29, 327. [Google Scholar] [CrossRef]
- Awad, S.; Al-Sheqefi, F.U.Y.; Al-Ahmadi, A.N.; Ibrahim, M.; Abdel-Hady, E.E. Valuation of bimetallic Pd/Ni nanoparticles catalyst for the applications in direct methanol fuel cells. Polym. Adv. Technol. 2023, 34, 3137–3153. [Google Scholar] [CrossRef]
- Neghmouche, N.S.; Lanez, T. Calculation of electrochemical parameters starting from the polarization curves of ferrocene at glassy carbon electrode. Int. Lett. Chem. Phys. Astron. 2013, 4, 37–45. [Google Scholar] [CrossRef]
- Parsa, A.; Amanzadeh-Salout, S. Electrocatalytic activity and electrochemical impedance spectroscopy of poly (aniline-co-ortho-phenylenediamine) modified electrode on ascorbic acid. Orient. J. Chem 2016, 32, 2051–2058. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhang, X.-H.; Zhao, D.-M.; Wang, S.-F. Electrochemical studies of bovine serum albumin immobilization onto the poly-o-phenylenediamine and carbon-coated nickel composite film and its interaction with papaverine. Sens. Actuators B Chem. 2011, 152, 88–93. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Pandian, M.S.; Pandikumar, A.; Ramasamy, P. Electrochemical interfacial charge transfer dynamics and photovoltaic performances of nanofibrous vanadium derivatives based platinum free counter electrodes in dye sensitized solar cells. Mater. Sci. Eng. B 2017, 222, 7–17. [Google Scholar] [CrossRef]
- Chen, M.; Du, C.; Yin, G.; Shi, P.; Zhao, T. Numerical analysis of the electrochemical impedance spectra of the cathode of direct methanol fuel cells. Int. J. Hydrogen Energy 2009, 34, 1522–1530. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Huang, W.-C.; Weng, J.-H.; Chen, L.-C.; Chou, C.-F.; Wei, P.-K. Impedimetric aptasensing using a symmetric Randles circuit model. Electrochim. Acta 2020, 337, 135750. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, M.; Wang, Z.; Wang, A.; Huang, Z.; Yue, S.; Hang, C. Mof Derived Carbon Encapsulation Coni Nanocrystal In-Situ Grafting of N-Doped Carbon Nanotubes for Synergistically Enhancing Electrocatalytic Active for Methanol Oxidation. Available at SSRN 4081666. 2022. Available online: https://ssrn.com/abstract=4081666 (accessed on 12 April 2022).
- Mahapatra, S.S.; Datta, J. Characterization of Pt-Pd/C electrocatalyst for methanol oxidation in alkaline medium. Int. J. Electrochem. 2011, 2011, 563495. [Google Scholar] [CrossRef]
- Mohamed, A.; Shaban, M.; Kordy, M.G.M.; Al-Senani, G.M.; Eissa, M.F.; Hamdy, H. Fabrication and characterization of NiCu/GO and NiCu/rGO nanocomposites for fuel cell application. RSC Adv. 2024, 14, 6776–6792. [Google Scholar] [CrossRef]
- Kotp, A.A.; Abdelwahab, A.; Farghali, A.A.; El Rouby, W.M.; Allah, A.E. Ornated hydrangea-like M-MO/graphitic porous carbon derived via direct carbonization of MOFs for electrooxidation of methanol in alkaline DMFC. Diam. Relat. Mater. 2024, 145, 111119. [Google Scholar] [CrossRef]
- Sarwar, E.; Noor, T.; Iqbal, N.; Mehmood, Y.; Ahmed, S.; Mehek, R. Effect of Co-Ni ratio in graphene based bimetallic electro-catalyst for methanol oxidation. Fuel Cells 2018, 18, 189–194. [Google Scholar] [CrossRef]
- Patil, K.; Babar, P.; Lee, D.M.; Karade, V.; Jo, E.; Korade, S.; Kim, J.H. Bifunctional catalytic activity of Ni–Co layered double hydroxide for the electro-oxidation of water and methanol. Sustain. Energy Fuels 2020, 4, 5254–5263. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; E Abdel-Hady, E.; Hmamm, M.F.M.; Ibrahim, M.; Ahmed, H.; Mondy, M.; Yehia, H. A promising fuel cell catalyst using non-precious metal oxide. IOP Conf. Ser. Mater. Sci. Eng. 2018, 464, 012002. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Ahmed, J.; Al-Hartomy, O.A. Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 2014, 4, 491–498. [Google Scholar] [CrossRef]
- Heineman, W.R. Laboratory Techniques in Electroanalytical Chemistry; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ahammad, A.J.S.; Choi, Y.-H.; Koh, K.; Kim, J.-H.; Lee, J.-J.; Lee, M. Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochem. Sci. 2011, 6, 1906–1916. [Google Scholar] [CrossRef]
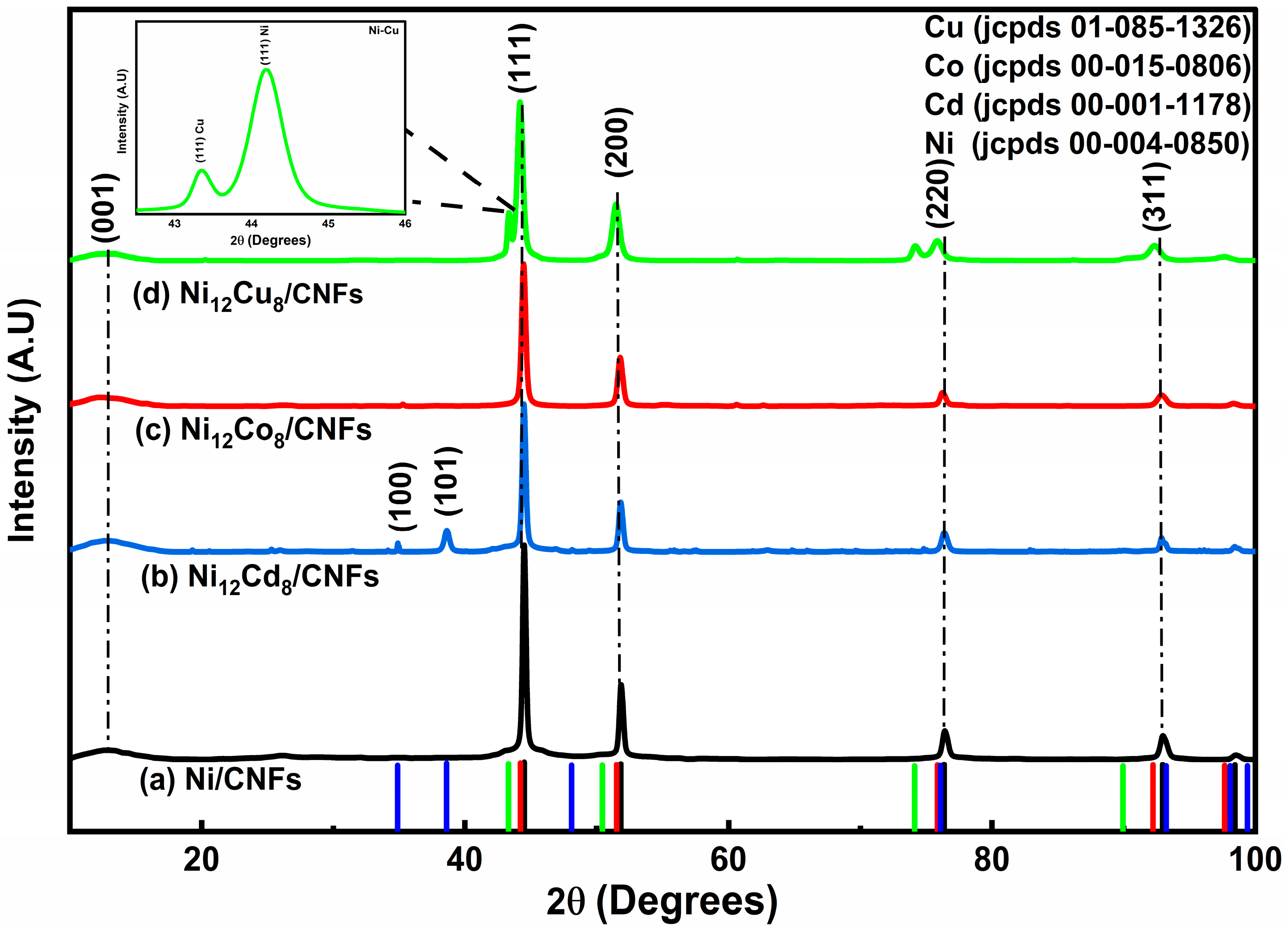
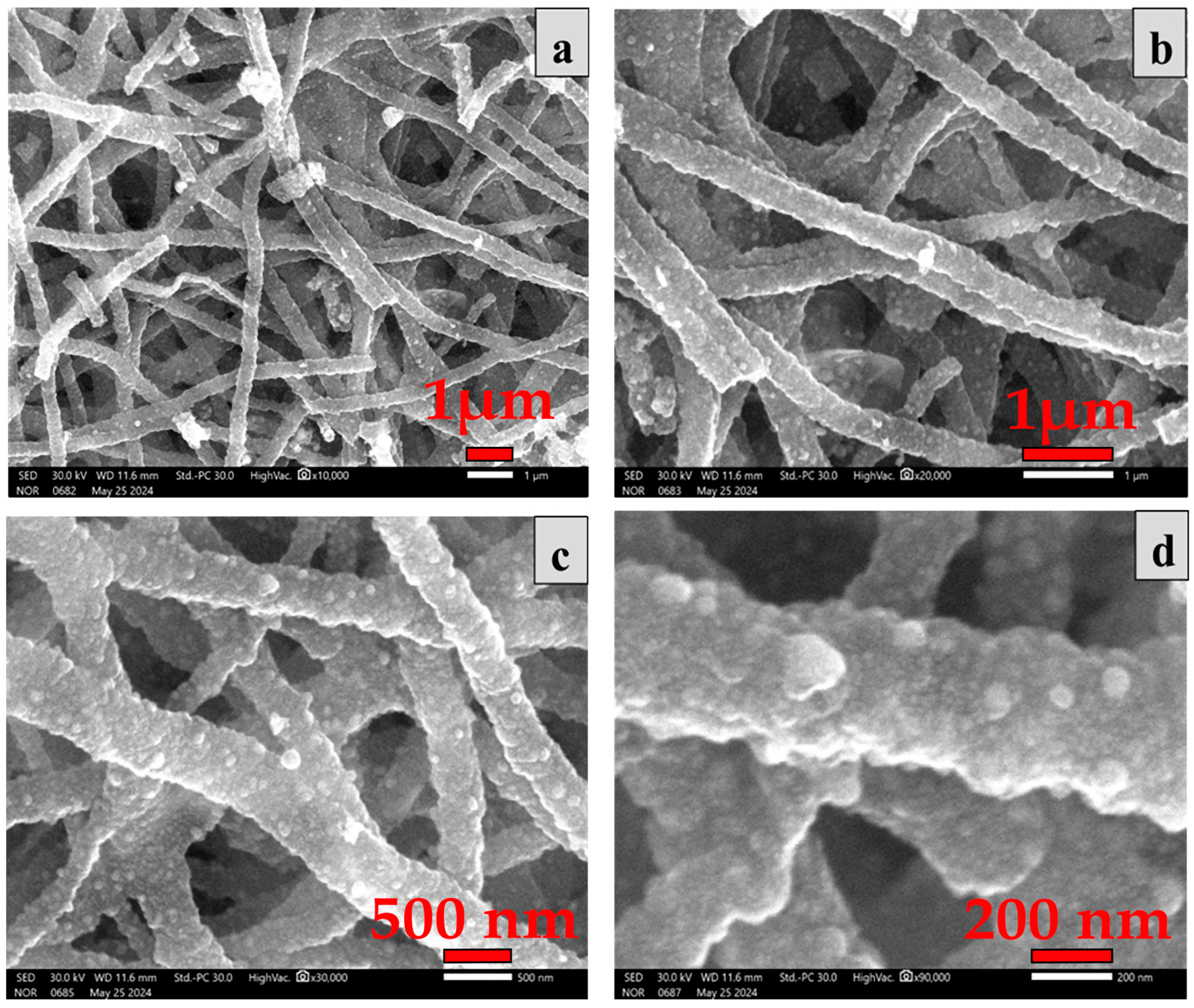
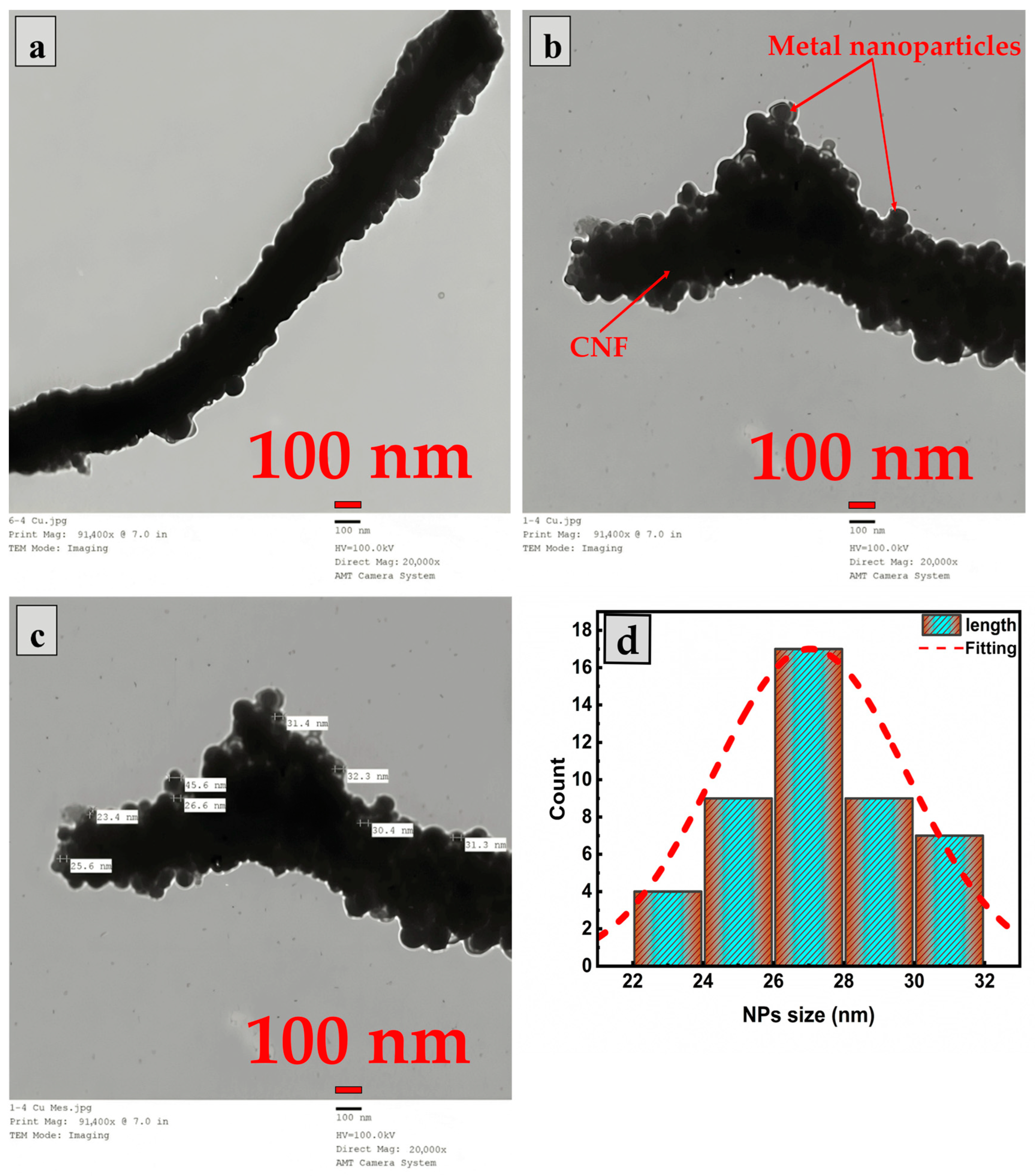
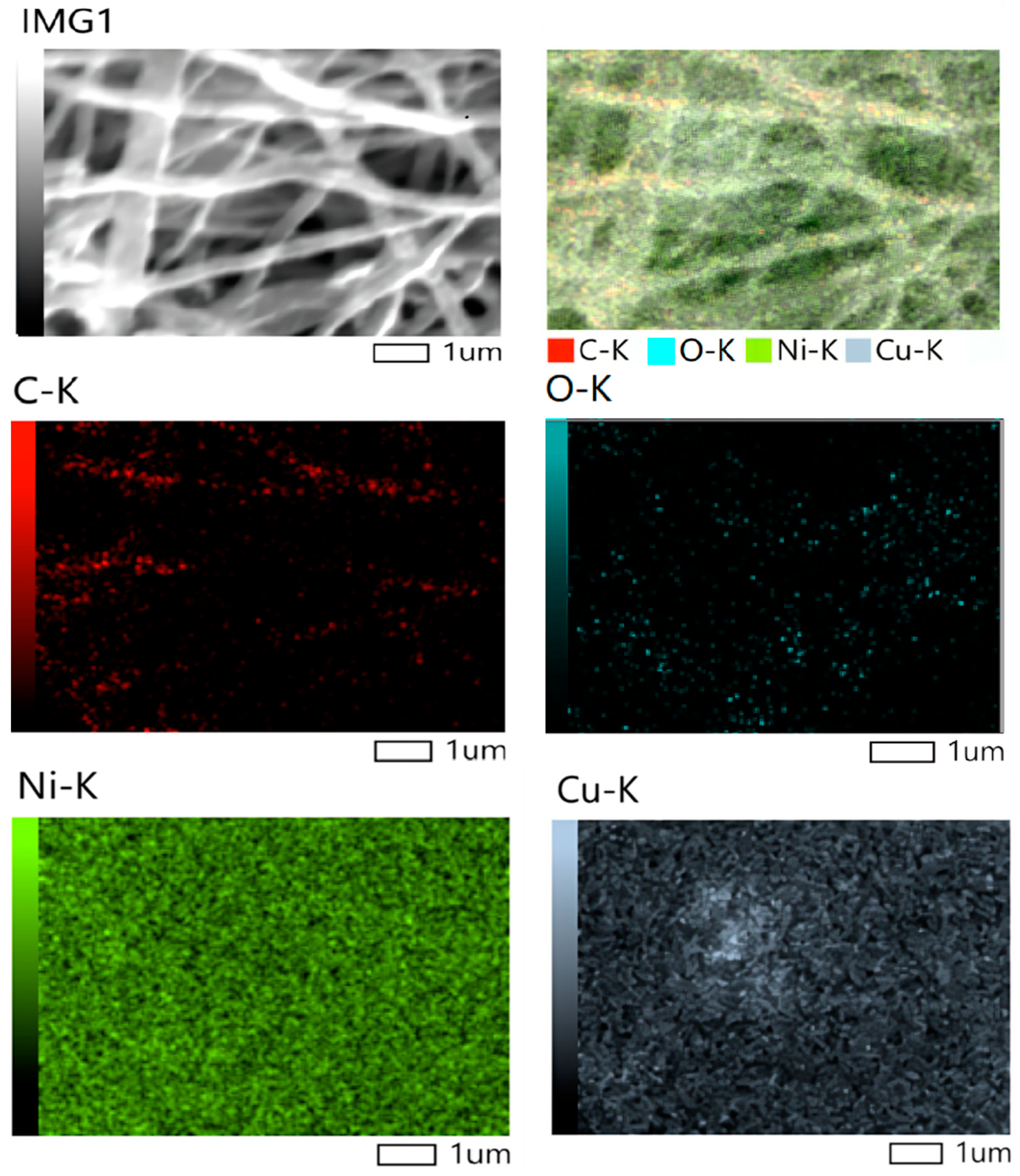
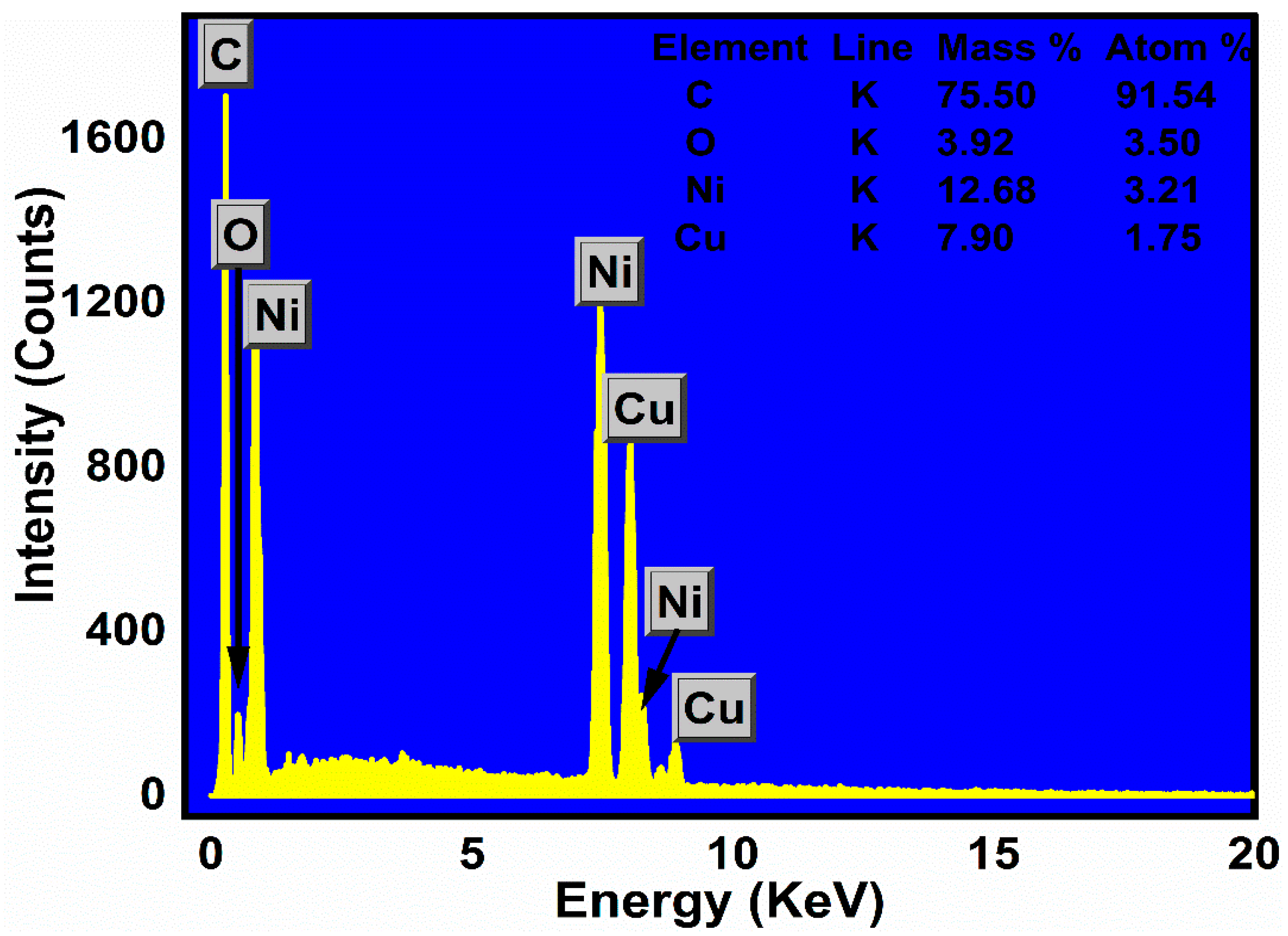
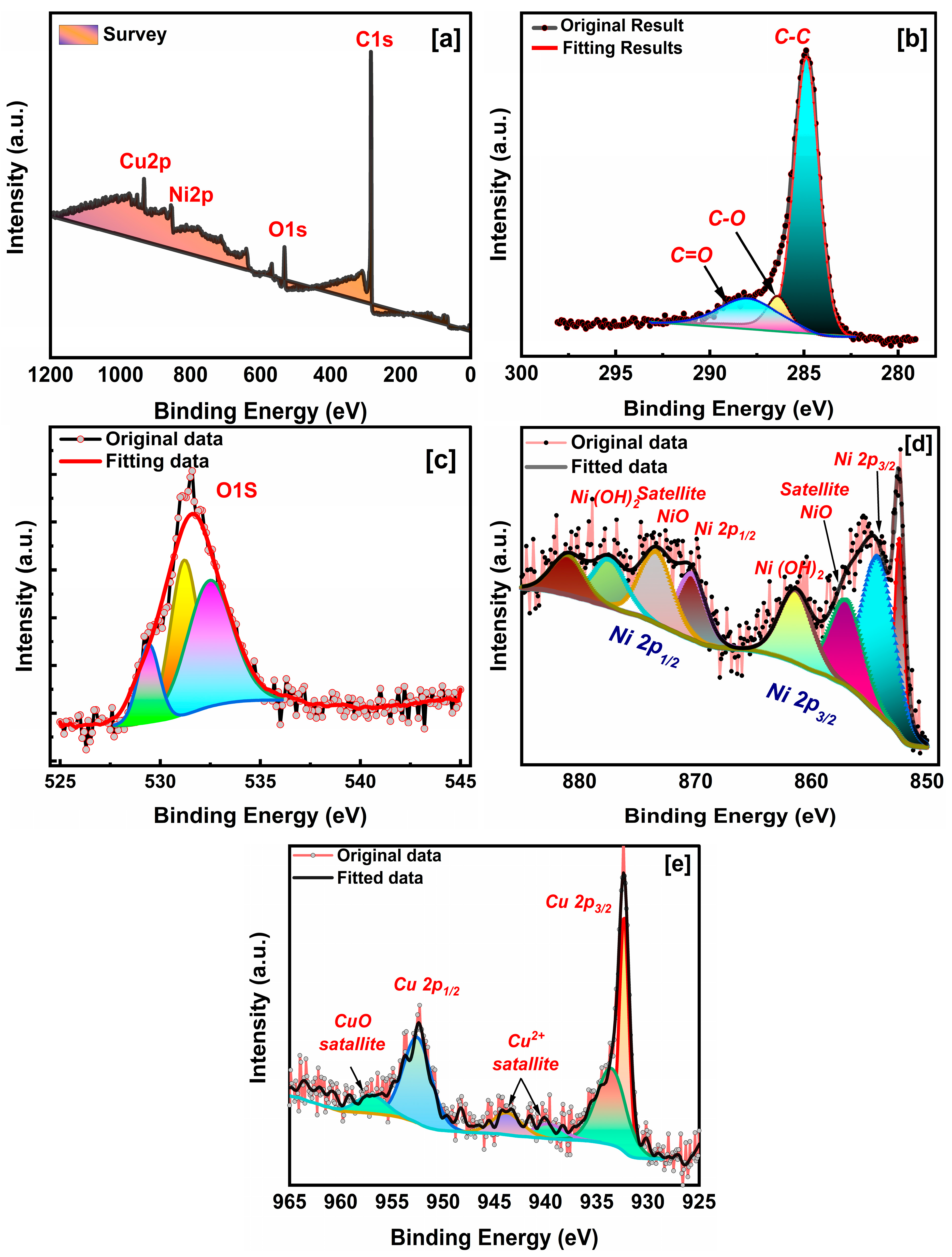
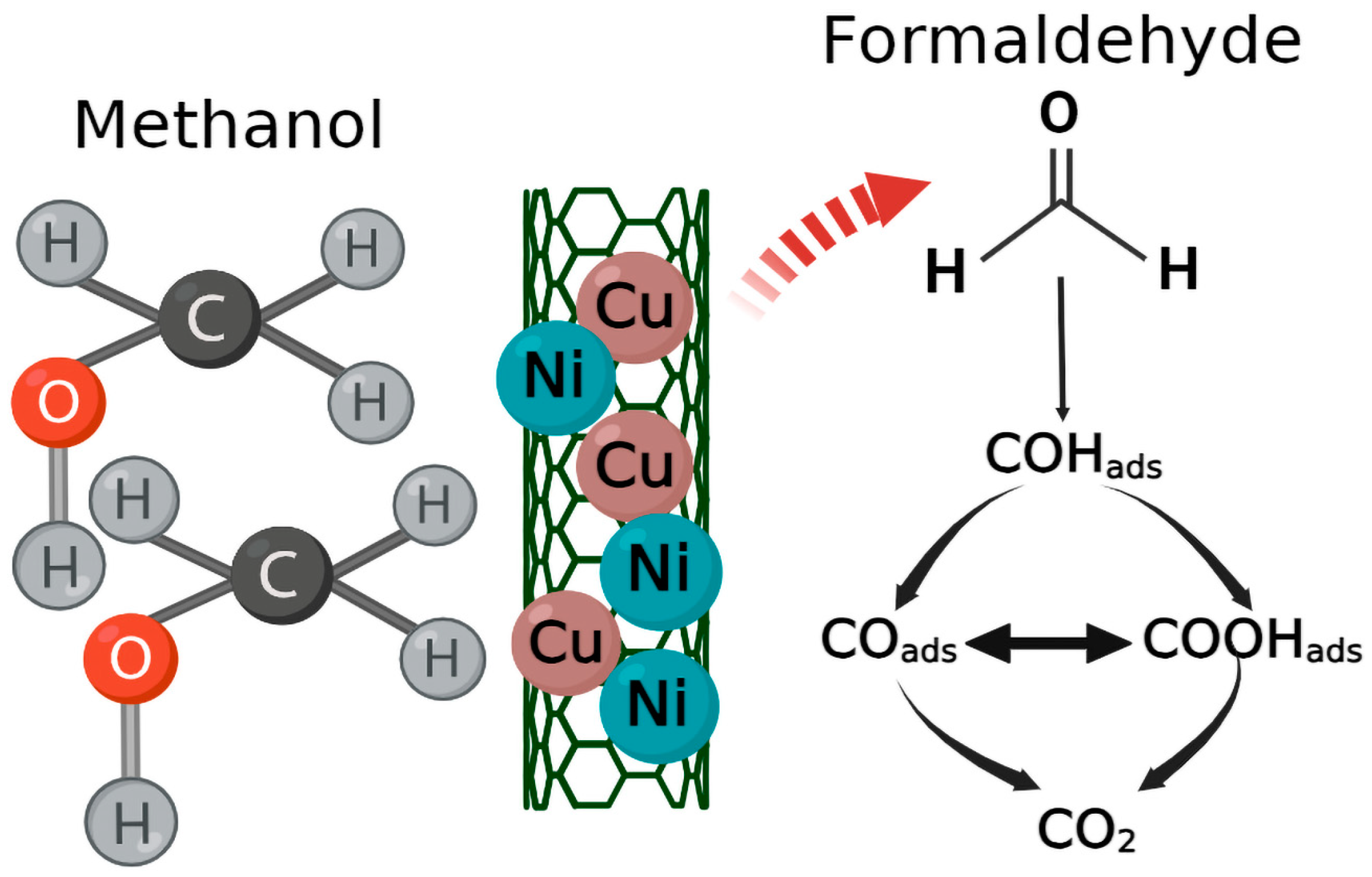


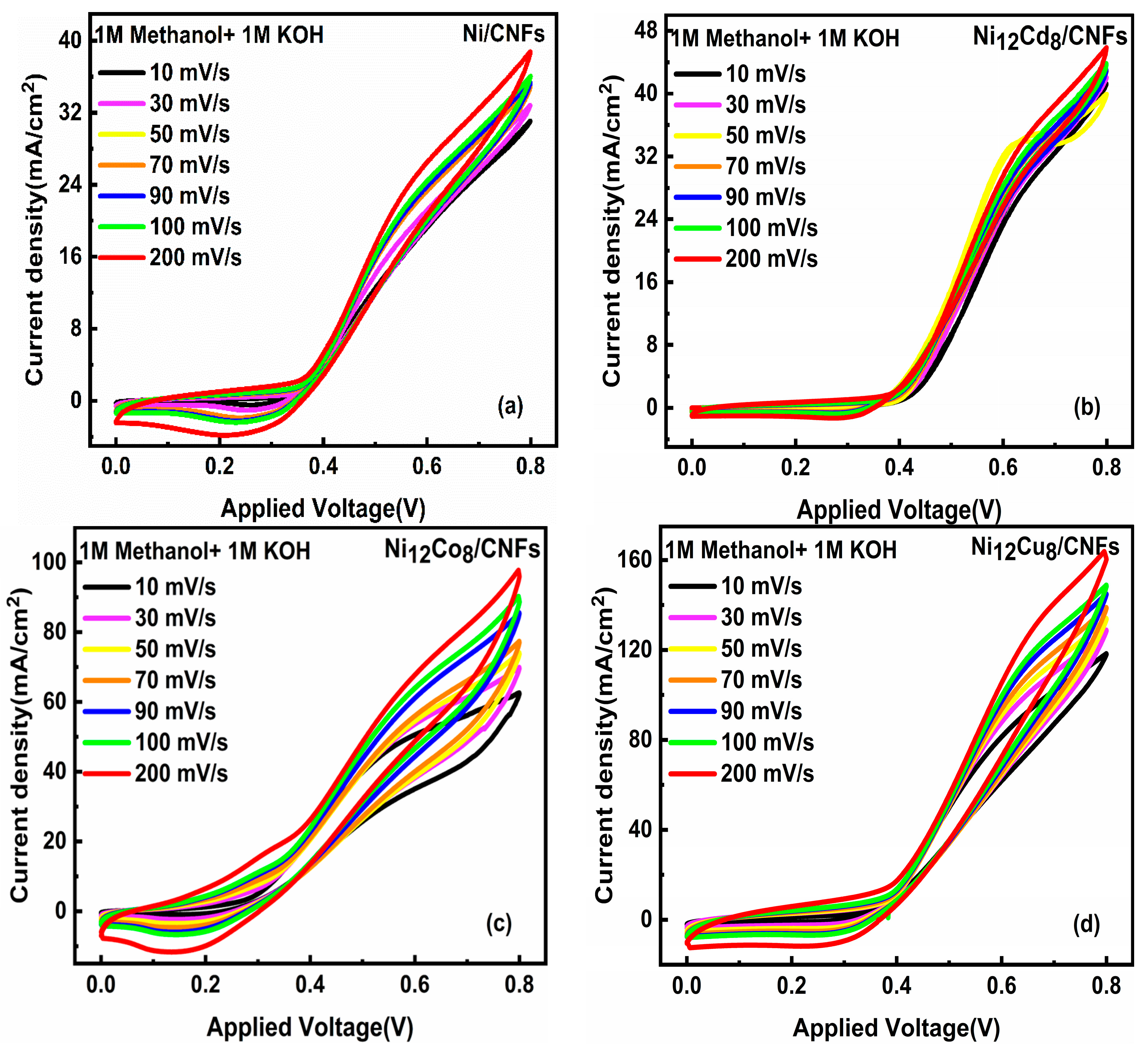



| Samples | hkl | Peak Position (Degree) | FWHM (Degree) | D (nm) (Scherrer Equation) | Surface Area (m2/g) |
|---|---|---|---|---|---|
| Ni/CNFs | (111) | 44.47 | 0.24 | 31.69 | 21.24 |
| (200) | 51.83 | 0.27 | |||
| (220) | 76.38 | 0.38 | |||
| (311) | 92.94 | 0.44 | |||
| Ni12Cd8/CNFs | (101) Cd | 38.60 | 0.44 | 30.86 | 21.81 |
| (111) | 44.45 | 0.27 | |||
| (200) | 51.80 | 0.30 | |||
| (220) | 76.32 | 0.44 | |||
| (311) | 92.87 | 0.26 | |||
| Ni12Co8/CNFs | (111) | 44.42 | 0.33 | 24.37 | 27.63 |
| (200) | 51.76 | 0.38 | |||
| (220) | 76.20 | 0.38 | |||
| (311) | 92.80 | 0.66 | |||
| Ni12Cu8/CNFs | (111) Cu | 43.32 | 0.22 | 23.07 | 29.18 |
| (111) | 44.15 | 0.46 | |||
| (200) | 51.44 | 0.60 | |||
| (220) | 75.78 | 0.77 | |||
| (311) | 92.27 | 0.88 |
| Samples | Tafel Slope (mV/dec.) |
|---|---|
| Ni/CNFs | 35.6 ± 0.002 |
| Ni12Cd8/CNFs | 29.9 ± 0.003 |
| Ni12Co8/CNFs | 28.6 ± 0.001 |
| Ni12Cu8/CNFs | 22.6 ± 0.001 |
| Concentration | Rs (Ω) | Rct (Ω) | Cdl (µF) |
|---|---|---|---|
| 1 M Methanol | 16.07 | 14.25 | 2.47 |
| 2 M Methanol | 12.87 | 17.46 | 2.74 |
| 3 M Methanol | 12.59 | 36.25 | 4.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomaa, M.M.; Abdel-Hamed, M.O.; Ibrahim, M.; Abdel-Hady, E.E.; Elsharkawy, Y.S. High-Performance Methanol Oxidation via Ni12-Metal8/CNF Catalyst for Fuel Cell Applications. Catalysts 2024, 14, 680. https://doi.org/10.3390/catal14100680
Gomaa MM, Abdel-Hamed MO, Ibrahim M, Abdel-Hady EE, Elsharkawy YS. High-Performance Methanol Oxidation via Ni12-Metal8/CNF Catalyst for Fuel Cell Applications. Catalysts. 2024; 14(10):680. https://doi.org/10.3390/catal14100680
Chicago/Turabian StyleGomaa, Mahmoud. M., Mohamed. O. Abdel-Hamed, Mohamed Ibrahim, Esam. E. Abdel-Hady, and Yehya S. Elsharkawy. 2024. "High-Performance Methanol Oxidation via Ni12-Metal8/CNF Catalyst for Fuel Cell Applications" Catalysts 14, no. 10: 680. https://doi.org/10.3390/catal14100680





