Influence of Current Collector Design and Combination on the Performance of Passive Direct Methanol Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. MEA Production
2.2. Pretreatment of Carbon Paper
2.3. Pretreatment of Nafion Membrane
2.4. Single-Cell Fixture
2.5. Electrochemical Characterization
2.6. Current Collectors Design
3. Results
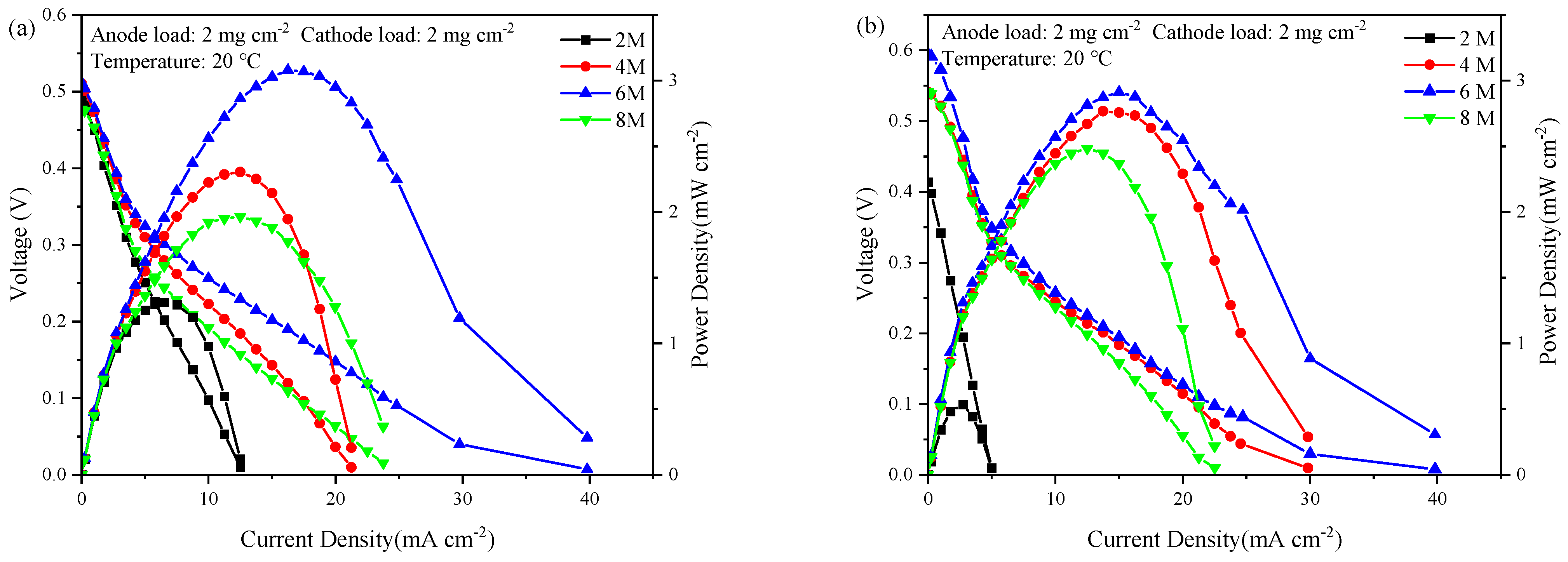
3.1. Cell Performance with Anode SF-Type Current Collector
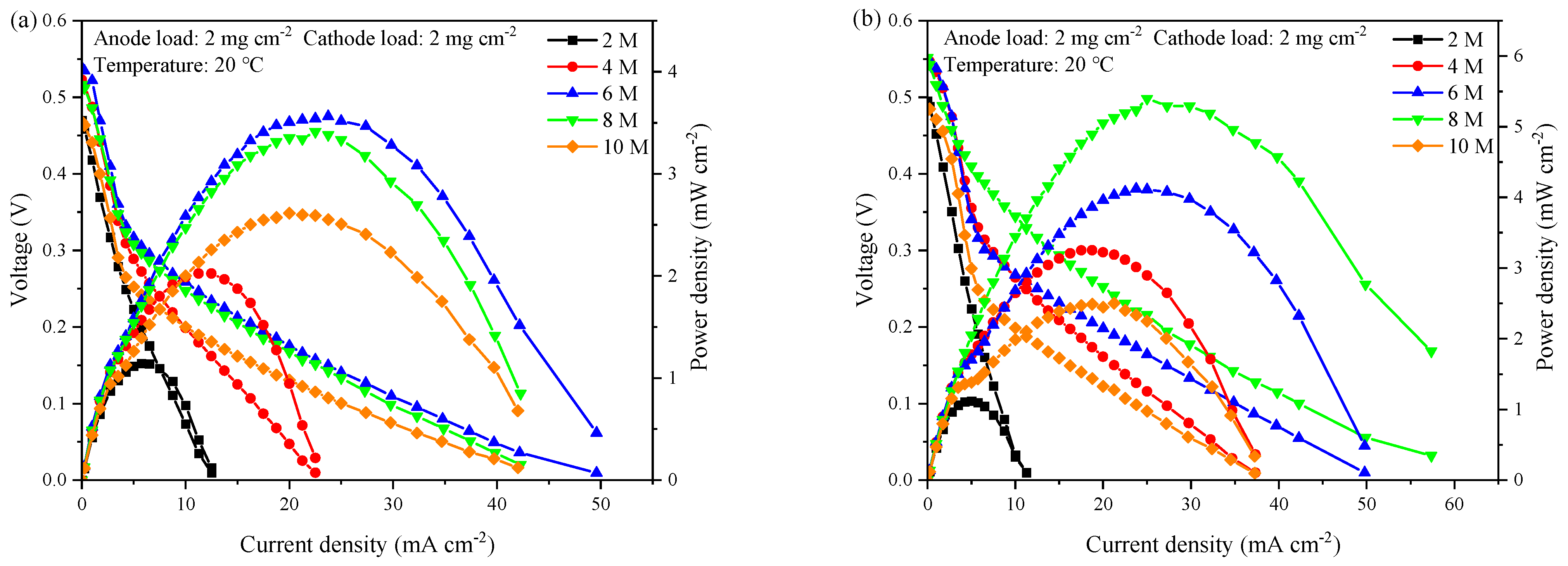
3.2. Cell Performance with Cathode X-Type Current Collector
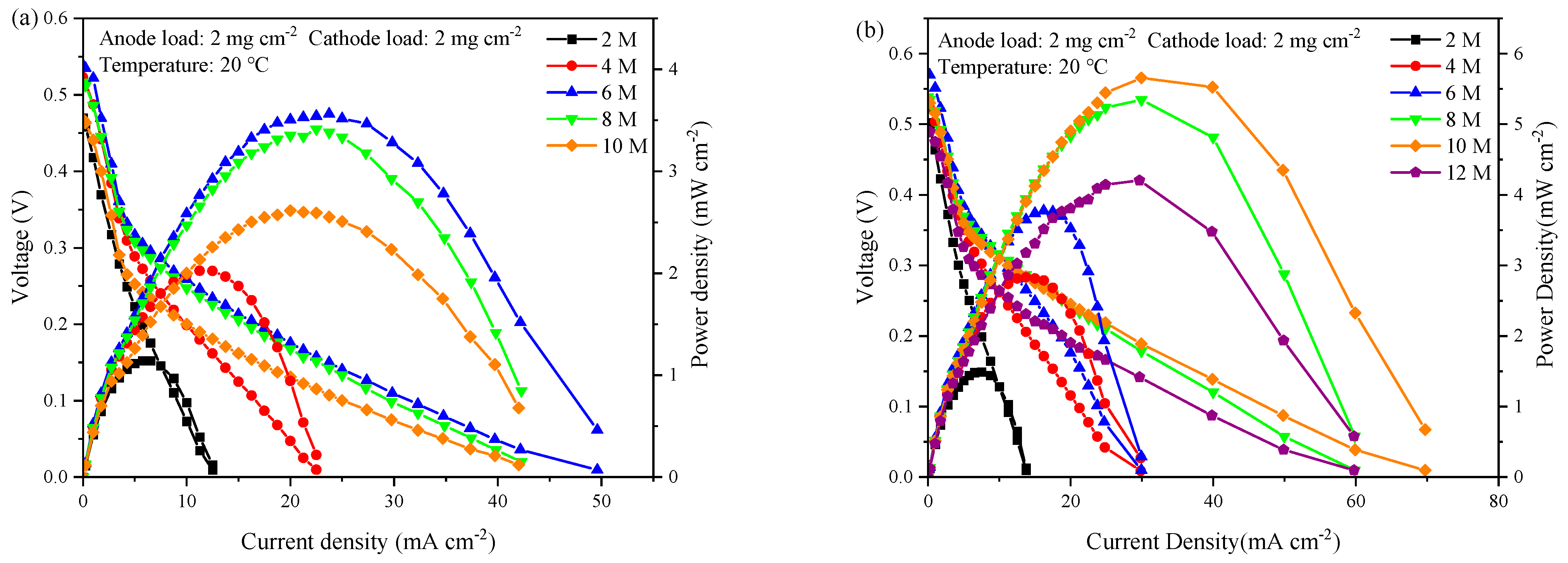
3.3. Polarization Curve of SF-X Current Collector Combination
3.4. Constant Current Discharge Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Zhang, F.H.; Xu, L.; Leung, P.; Yang, C.Z.; Li, H.M. The applications and prospect of fuel cells in medical field: A review. Renew. Sustain. Energy Rev. 2017, 67, 574–580. [Google Scholar] [CrossRef]
- Wu, Q.X.; Zhao, T.S.; Chen, R.; An, L. A sandwich structured membrane for direct methanol fuel cells operating with neat methanol. Appl. Energy 2013, 106, 301–306. [Google Scholar] [CrossRef]
- Kim, Y.S.; Peck, D.H.; Kim, S.K.; Jung, D.H.; Lim, S.; Kim, S.H. Effects of the microstructure and powder compositions of a micro-porous layer for the anode on the performance of high concentration methanol fuel cell. Int. J. Hydrogen Energy 2013, 38, 7159–7168. [Google Scholar] [CrossRef]
- Shin, J.; Nho, Y.C.; seon Hwang, I.; Fei, G.; Kim, A.R.; Nahm, K.S. Irradiated PVdF-HFP–tin oxide composite membranes for the applications of direct methanol fuel cells. J. Membr. Sci. 2010, 350, 92–100. [Google Scholar] [CrossRef]
- Yousefi, S.; Zohoor, M. Conceptual design and statistical overview on the design of a passive DMFC single cell. Int. J. Hydrogen Energy 2014, 39, 5972–5980. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Wu, Q.X.; Zhao, T.S.; Chen, R.; Yang, W.W. Enhancement of water retention in the membrane electrode assembly for direct methanol fuel cells operating with neat methanol. Int. J. Hydrogen Energy 2010, 35, 10547–10555. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Daud, W.R.W.; Ho, S.L.; Hasran, U.A. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 2007, 163, 743–754. [Google Scholar] [CrossRef]
- Yang, W.; Chou, S.; Shu, C. Effect of current-collector structure on performance of passive micro direct methanol fuel cell. J. Power Sources 2007, 164, 549–554. [Google Scholar] [CrossRef]
- de Sa, M.H.; Pinto, A.M.F.R.; Oliveira, V.B. Passive direct methanol fuel cells as a sustainable alternative to batteries in hearing aid devices—An overview. Int. J. Hydrogen Energy 2022, 37, 47. [Google Scholar] [CrossRef]
- Carneiro, L.P.; Ferreira, N.S.; Tavares, A.P.; Pinto, A.M.; Mendes, A.; Sales, M.G.F. A passive direct methanol fuel cell as transducer of an electrochemical sensor applied to the detection of carcinoembryonic antigen. Biosens. Bioelectron. 2021, 175, 112877. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Carneiro, L.P.; Viezzer, C.; Almeida, M.J.; Marques, A.C.; Pinto, A.M.; Fortunato, E.; Sales, M.G.F. Passive direct methanol fuel cells acting as fully autonomous electrochemical biosensors: Application to sarcosine detection. J. Electroanal. Chem. 2022, 922, 116710. [Google Scholar] [CrossRef]
- Carneiro, L.P.; Pinto, A.M.; Mendes, A.; Sales, M.G.F. An all-in-one approach for self-powered sensing: A methanol fuel cell modified with a molecularly imprinted polymer for cancer biomarker detection. J. Electroanal. Chem. 2022, 906, 116009. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Bresciani, F.; Rabissi, C.; Zago, M.; Gazdzicki, P.; Schulze, M.; Guétaz, L.; Escribano, S.; Bonde, J.L.; Marchesi, R.; Casalegno, A. A combined in-situ and post-mortem investigation on local permanent degradation in a direct methanol fuel cell. J. Power Sources 2016, 306, 49–61. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Recent progress in development of efficient electrocatalyst for methanol oxidation reaction in direct methanol fuel cell. Int. J. Energy Res. 2021, 45, 6550–6583. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Structural mechanism investigation on methanol crossover and stability of a passive direct methanol fuel cell performance via modified micro-porous layer. Int. J. Energy Res. 2021, 45, 12928–12943. [Google Scholar] [CrossRef]
- Shrivastava, N.K.; Thombre, S.B.; Motghare, R.V. Wire mesh current collectors for passive direct methanol fuel cells. J. Power Sources 2014, 272, 629–638. [Google Scholar] [CrossRef]
- Gholami, O.; Imen, S.J.; Shakeri, M. Effect of anode and cathode flow field geometry on passive direct methanol fuel cell performance. Electrochim. Acta 2015, 158, 410–417. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B.; Shrivastava, N.K. A critical review of the current collector for passive direct methanol fuel cells. J. Power Sources 2015, 285, 510–529. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A.M.F.R. Effect of the current collector design on the performance of a passive direct methanol fuel cell. Electrochim. Acta 2019, 300, 306–315. [Google Scholar] [CrossRef]
- Chan, Y.H.; Zhao, T.S.; Chen, R.; Xu, C. A small mono-polar direct methanol fuel cell stack with passive operation. J. Power Sources 2008, 178, 118–124. [Google Scholar] [CrossRef]
- Wu, Q.X.; Zhao, T.S.; Chen, R.; Yang, W.W. A microfluidic-structured flow field for passive direct methanol fuel cells operating with highly concentrated fuels. J. Micromech. Microeng. 2010, 20, 045014. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yuan, Z.Y.; Li, Y.L.; Jia, Q.; Chen, S.; Liu, X.W. Design and fabrication of a silicon-based direct methanol fuel cell with a new cathode spoke structure. J. Power Sources 2011, 196, 3015–3025. [Google Scholar] [CrossRef]
- Gholami, O.; Imen, S.J.; Shakeri, M. Effect of non-uniform parallel channel on performance of passive direct methanol fuel cell. Int. J. Hydrogen Energy 2013, 38, 3395–3400. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B. Performance of passive DMFC with expanded metal mesh current collectors. Electrochim. Acta 2017, 243, 299–309. [Google Scholar] [CrossRef]
- Raghavaiah, N.V.; Srinivasulu, G.N. Analysis and experimental investigation on passive direct methanol fuel cell current collectors with taper cylindrical openings. Fuel Cells 2022, 22, 169–185. [Google Scholar] [CrossRef]
- Dong, H.; Su, H.M.; Chen, Z.; Yu, H.; Yu, H.B. Fabrication of Electrochemically Reduced Graphene Oxide Modified Gas Diffusion Electrode for In-situ Electrochemical Advanced Oxidation Process under Mild Conditions. Electrochim. Acta 2016, 222, 1501–1509. [Google Scholar] [CrossRef]
- Guan, L.; Yu, W.; Asghar, M.R.; Zhang, W.; Su, H.; Li, C.; Xing, L.; Xu, Q. Effect of graphene aerogel as a catalyst layer additive on performance of direct methanol fuel cell. Fuel 2024, 360, 130503. [Google Scholar] [CrossRef]
- Kang, K.; Lee, G.; Gwak, G.; Choi, Y.; Ju, H. Development of an advanced MEA to use high-concentration methanol fuel in a direct methanol fuel cell system. Int. J. Hydrogen Energy 2012, 37, 6285–6291. [Google Scholar] [CrossRef]
- Braz, B.A.; Oliveira, V.B.; Pinto, A.M.F.R. Optimization of a passive direct methanol fuel cell with different current collector materials. Energy 2020, 208, 118394. [Google Scholar] [CrossRef]


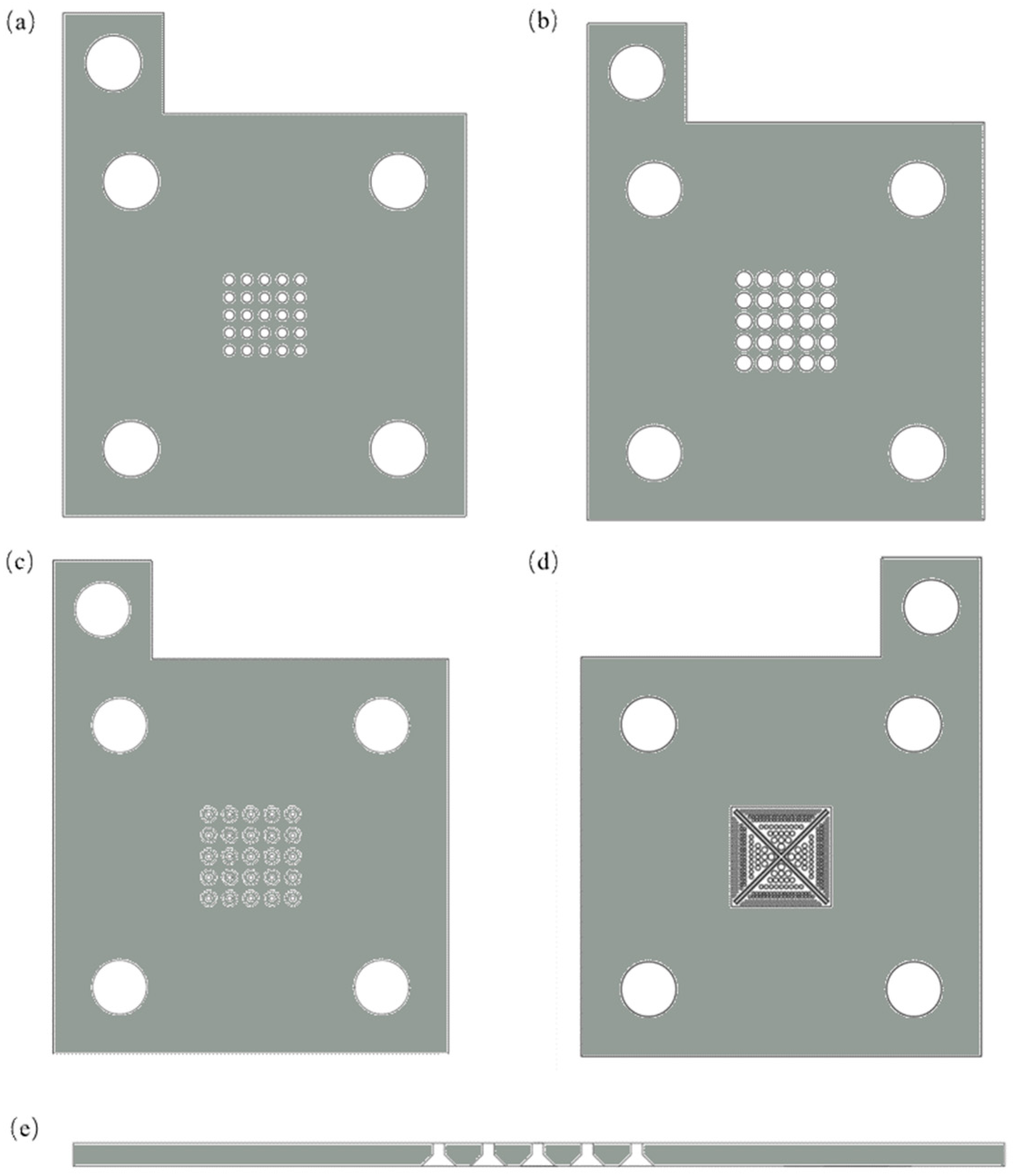


| Material | Density (g/cm3) | Electrical Conductivity (S/m) | Material Cost (USD) | Processing Cost (USD) | Total Cost (USD) |
|---|---|---|---|---|---|
| Stainless steel (316 L) | 7.99 | 1.33 × 107 | 3.09 | 2.53 | 5.62 |
| Titanium (TA2) | 4.51 | 4.30 × 107 | 3.37 | 3.66 | 7.03 |
| Graphite plate | 2.2 | 6.5 × 103 | 1.69 | 16.59 | 18.28 |
| Current Collector Design | Operating Temperature | Optimum Methanol Concentration | Anode and Cathode Catalyst Load (mg cm−2) | Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|
| SF-type | 20 °C | 8 M | 2; 2 | 5.40 | This Study |
| X-type | 20 °C | 10 M | 2; 2 | 5.65 | This Study |
| SF-X | 20 °C | 8 M | 2; 2 | 6.22 | This Study |
| perforated CC | - | 2 M | 3; 1.3 | 3.14 | [22] |
| spoke structure | 20 °C | 1 M | 4; 4 | 14.79 | [25] |
| EMCC | 25 °C | 3 M | 4; 2 | 3.04 | [27] |
| taper cylindrical | - | 3 M | 4; 2 | 7.06 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Xiao, Z.; Zhang, W.; Ma, Q.; Li, Z.; Yan, X.; Su, H.; Xing, L.; Xu, Q. Influence of Current Collector Design and Combination on the Performance of Passive Direct Methanol Fuel Cells. Catalysts 2024, 14, 632. https://doi.org/10.3390/catal14090632
Yu W, Xiao Z, Zhang W, Ma Q, Li Z, Yan X, Su H, Xing L, Xu Q. Influence of Current Collector Design and Combination on the Performance of Passive Direct Methanol Fuel Cells. Catalysts. 2024; 14(9):632. https://doi.org/10.3390/catal14090632
Chicago/Turabian StyleYu, Weibin, Zhiyuan Xiao, Weiqi Zhang, Qiang Ma, Zhuo Li, Xiaohui Yan, Huaneng Su, Lei Xing, and Qian Xu. 2024. "Influence of Current Collector Design and Combination on the Performance of Passive Direct Methanol Fuel Cells" Catalysts 14, no. 9: 632. https://doi.org/10.3390/catal14090632










