Significant Effects of Adding Mode on Low-Temperature De-NOx Performance and SO2 Resistance of a MnCeTiOx Catalyst Prepared by the Co-Precipitation Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural and Structural Properties
2.1.1. XRD
2.1.2. N2 Adsorption–Desorption
2.1.3. SEM/EDX
2.2. Catalytic Activity
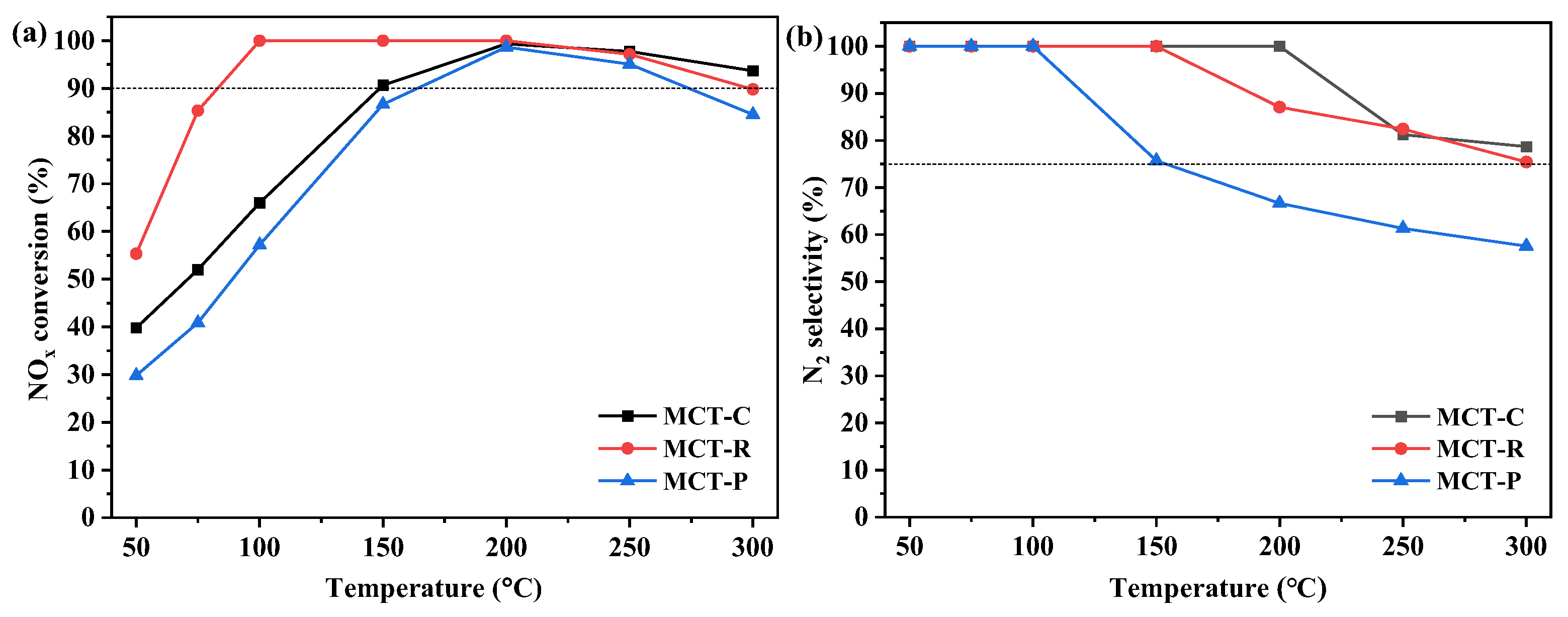
2.2.1. NH3-SCR Activity
2.2.2. H2O and SO2 Resistance
2.3. Separate Oxidation of NO and NH3
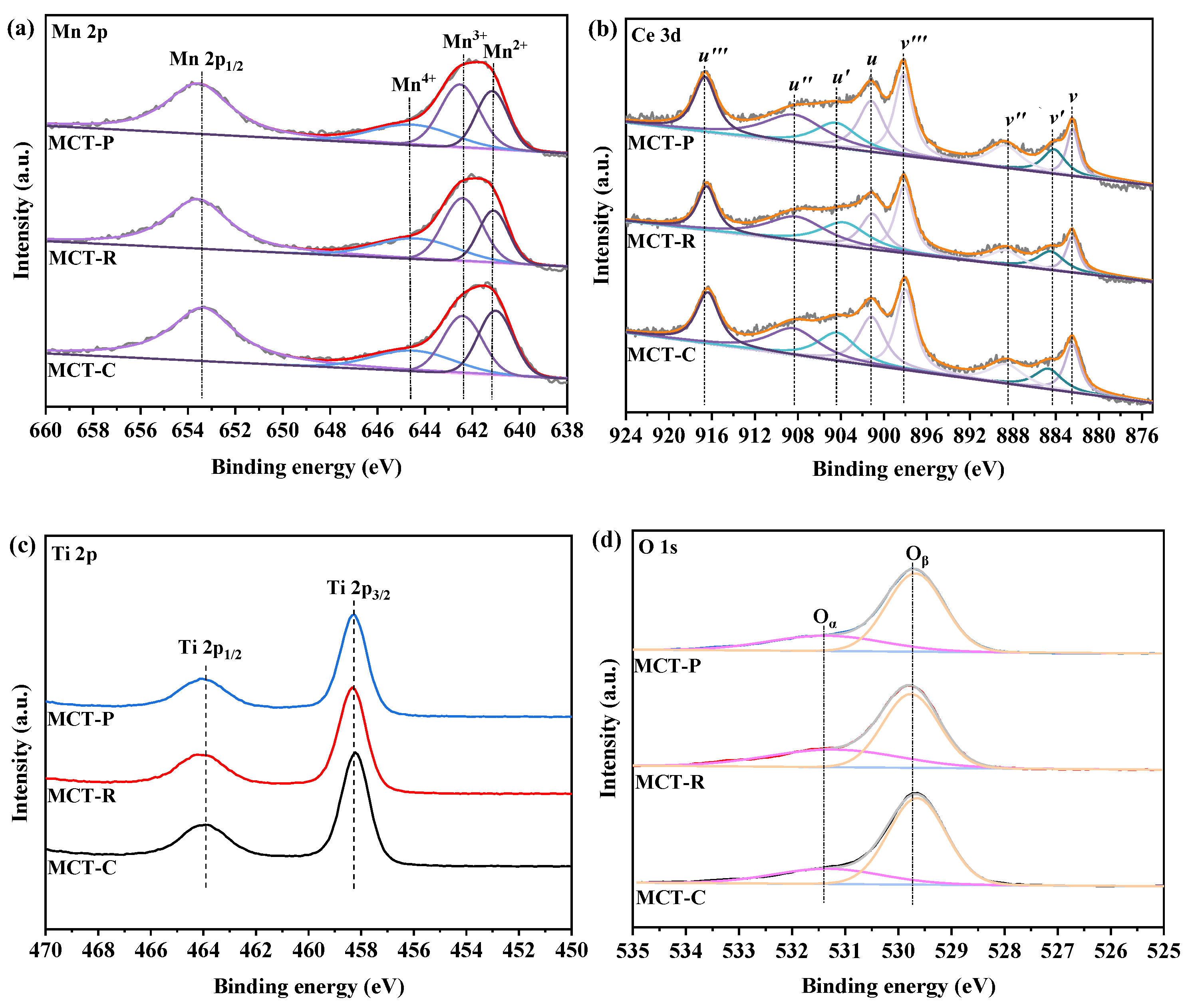
2.4. XPS Analysis
2.5. H2-TPR
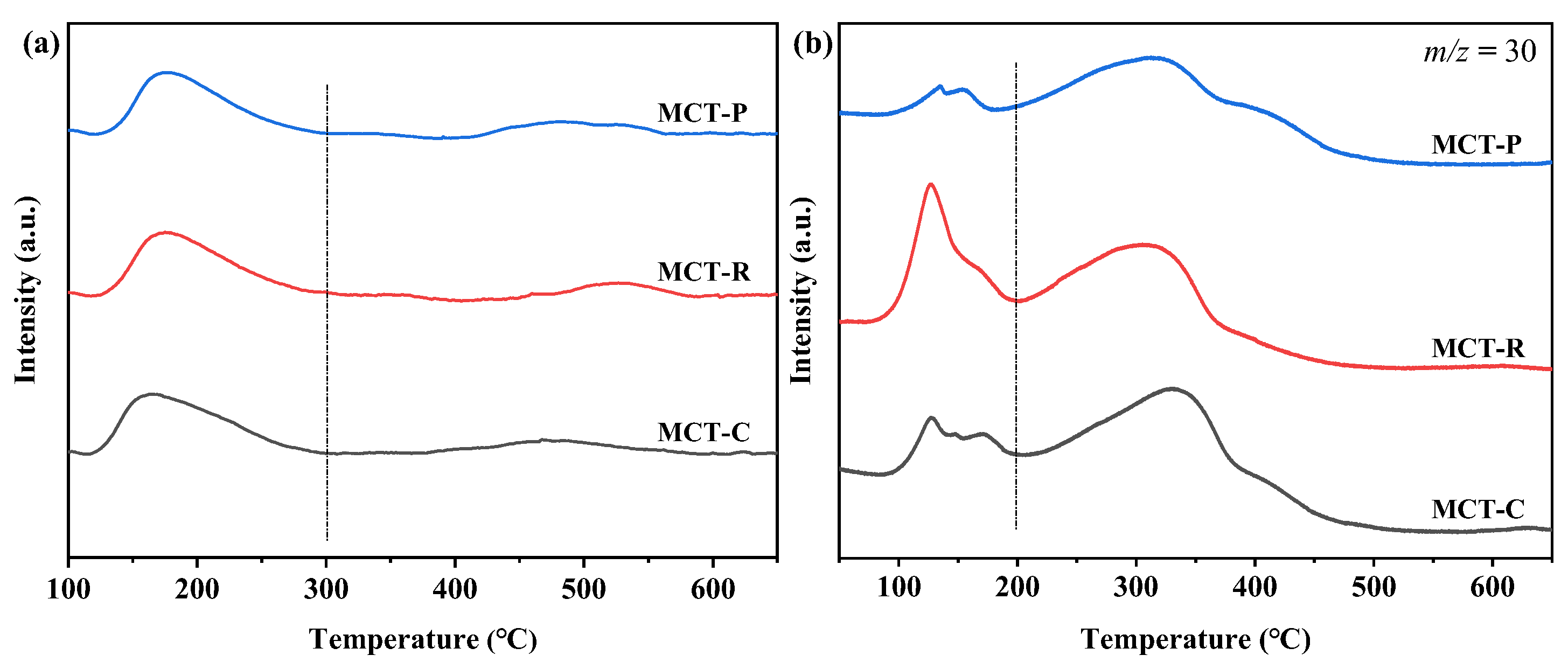
2.6. TPD Analyses for NH3 and NO
2.7. In Situ DRIFTs Characterization
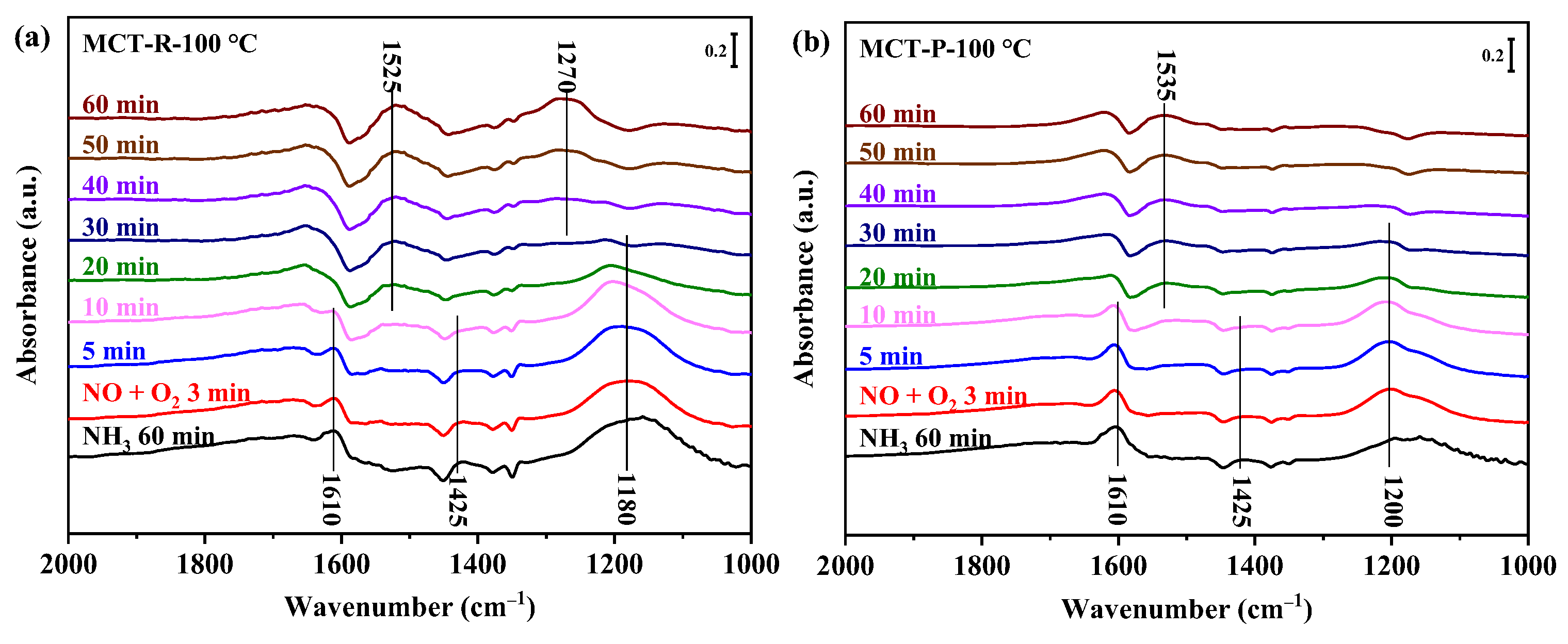
2.7.1. Adsorption of NH3 Species
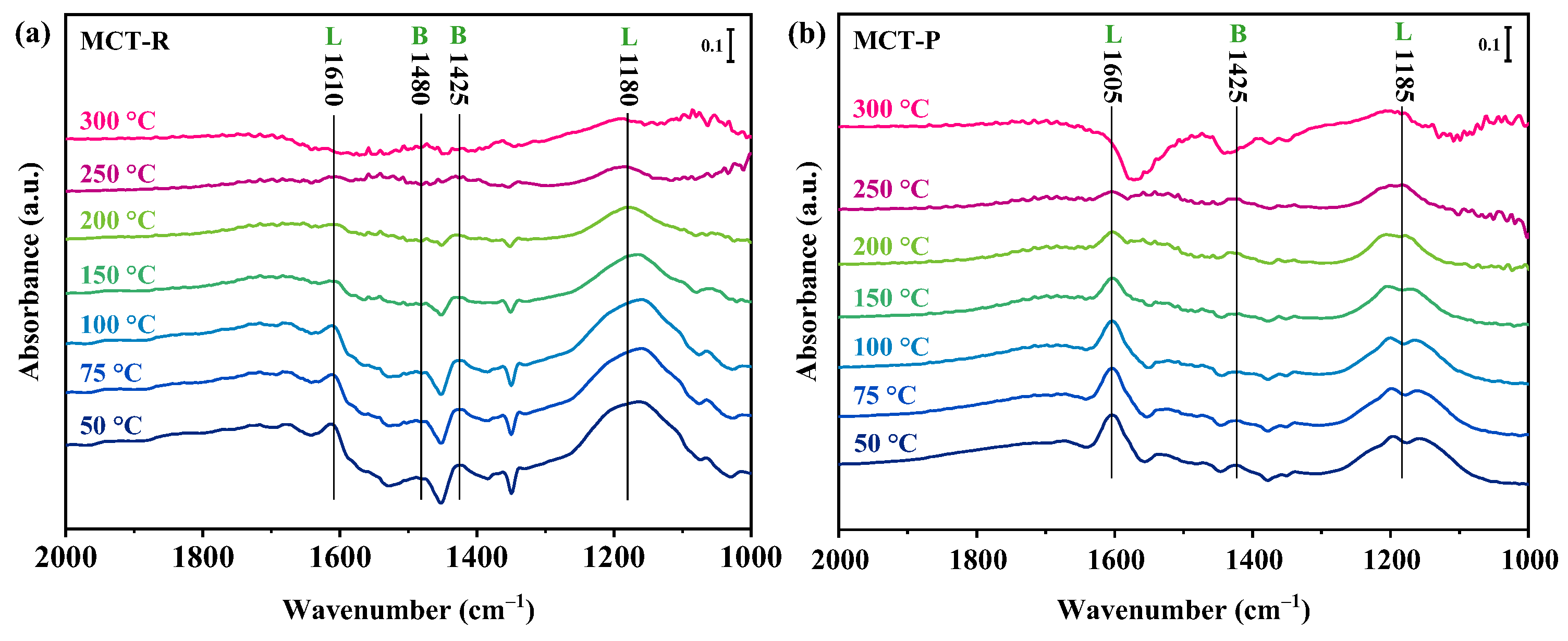
2.7.2. Adsorption of NOx Species
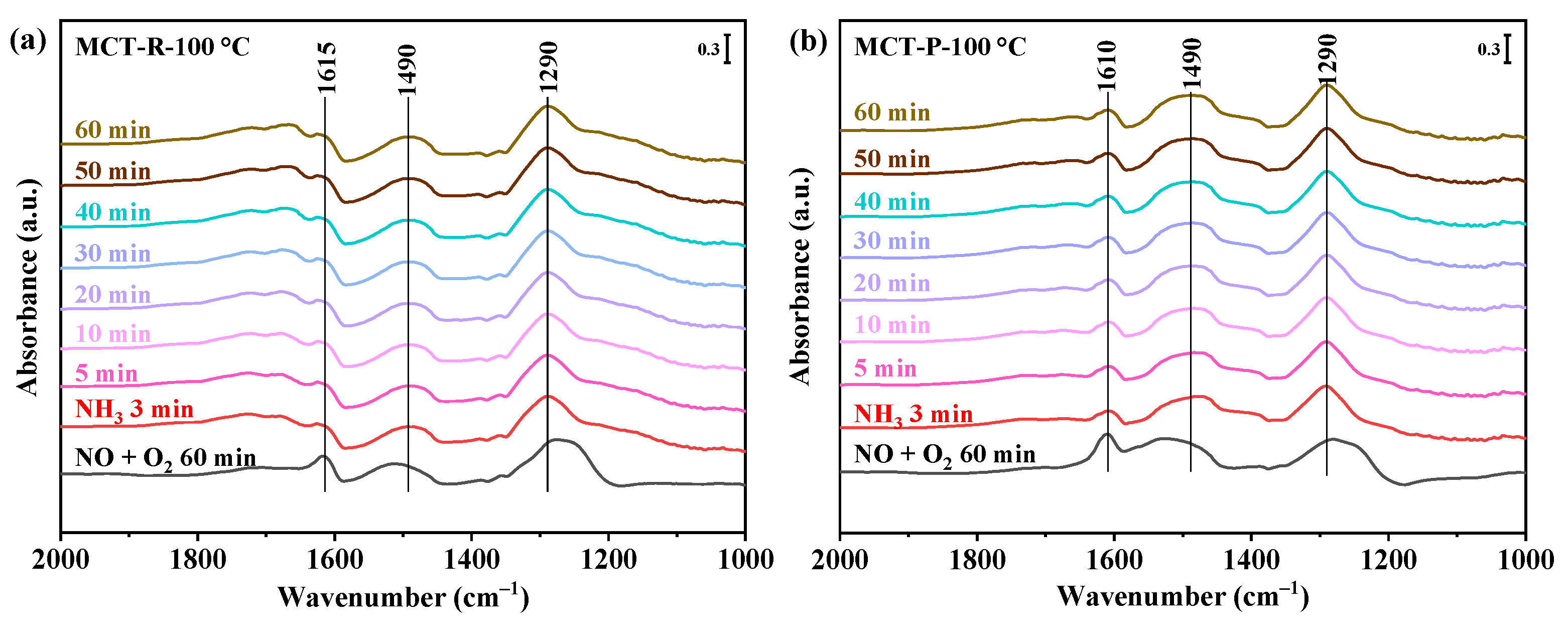
2.7.3. Reaction between NOx and Pre-Adsorbed NH3 Species
2.7.4. Reaction between NH3 and Pre-Adsorbed NOx Species
2.8. Characterization of SO2-Poisoned Samples
2.8.1. N2 Adsorption–Desorption
2.8.2. XPS Analysis
2.9. Promotional Effect of Reverse Co-Precipitation on the Catalytic Performance of MCT
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Li, X.; Li, W.; Zhong, L.; Zhang, C.; Fang, Q.Y.; Chen, G. Effect of preferential exposure of anatase TiO2 {0 0 1} facets on the performance of Mn-Ce/TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2019, 369, 26–34. [Google Scholar] [CrossRef]
- Wang, F.M.; Shen, B.X.; Zhu, S.W.; Wang, Z. Promotion of Fe and Co doped Mn-Ce/TiO2 catalysts for low temperature NH3-SCR with SO2 tolerance. Fuel 2019, 249, 54–60. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.Y.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Leng, X.S.; Zhang, Z.P.; Li, Y.S.; Zhang, T.R.; Ma, S.B.; Yuan, F.L.; Niu, X.Y.; Zhu, Y.J. Excellent low temperature NH3-SCR activity over MnaCe0.3TiOx (a = 0.1–0.3) oxides: Influence of Mn addition. Fuel Process. Technol. 2018, 181, 33–43. [Google Scholar] [CrossRef]
- Xue, H.Y.; Guo, X.M.; Mao, D.S.; Meng, T.; Yu, J.; Ma, Z. Unveiling the temperature-dependent effect of Zn on phosphotungstic acid-modified MnOx catalyst for selective catalytic reduction of NOx: A poison at <180 °C or a promoter at >180 °C. Chem. Eng. J. 2023, 470, 144170. [Google Scholar]
- Li, S.J.; Wang, X.X.; Tan, S.; Shi, Y.; Li, W. CrO3 supported on sargassum-based activated carbon as low temperature catalysts for the selective catalytic reduction of NO with NH3. Fuel 2017, 191, 511–517. [Google Scholar] [CrossRef]
- Xie, S.Z.; Li, L.L.; Jin, L.J.; Wu, Y.H.; Liu, H.; Qin, Q.J.; Wei, X.L.; Liu, J.X.; Dong, L.H.; Li, B. Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl. Surf. Sci. 2020, 515, 146014. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.X.; Li, H.R.; Huang, L.; Shi, L.Y.; Zhang, D.S. Mechanistic aspects of deNOx processing over TiO2 supported Co-Mn oxide catalysts: Structure-activity relationships and in situ DRIFTs analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Jin, L.Y.; Xu, X.T.; Wang, Y.H.; Li, J.Y.; Fan, K.H.; Hu, B.; Shen, Y.; Liu, X.S. Study on H2O2, Ce, W modified MnO/TiO2 based NH3-SCR Catalyst: The status and effect of doped promoters and synergies. Mater. Res. Bull. 2023, 168, 112494. [Google Scholar] [CrossRef]
- Feng, J.W.; Wang, Y.; Gao, D.W.; Kang, B.T.; Li, S.; Li, C.S.; Chen, G.Z. Ce-Mn coordination polymer derived hierarchical/porous structured CeO2-MnOx for enhanced catalytic properties. Nanoscale 2020, 12, 16381–16388. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ren, S.; Xing, X.D.; Li, X.D.; Chen, L.; Wang, M.M. Unveiling the inductive strategy of different precipitants on MnFeO catalyst for low-temperature NH3-SCR reaction. Fuel 2023, 335, 126986. [Google Scholar] [CrossRef]
- Yao, X.J.; Ma, K.L.; Zou, W.X.; He, S.G.; An, J.B.; Yang, F.M.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnO-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Chao, M.X.; Mao, D.S.; Li, G.H.; Li, G.; Yu, J.; Guo, X.M. Low-temperature selective catalytic reduction of NO with NH3 over Mn-Ce-Ox/TiO2: A comparison between catalyst preparation methods. J. Sol-Gel Sci. Technol. 2020, 95, 332–343. [Google Scholar] [CrossRef]
- Wu, Y.X.; Liang, H.L.; Chen, X.; Tang, J.; Chen, Y.F.; Zhao, C.L. Influences of preparation parameters of catalyst support on the SCR denitration activity of Mn-Ce/TiO2. Key Eng. Mater. 2016, 697, 279–283. [Google Scholar] [CrossRef]
- Sheng, Z.Y.; Hu, Y.F.; Xue, J.M.; Wang, X.M.; Liao, W.P. A novel co-precipitation method for preparation of Mn-Ce/TiO2 composites for NOx reduction with NH3 at low temperature. Environ. Technol. 2012, 33, 2421–2428. [Google Scholar] [CrossRef]
- Rahmani, M.; Mirzaee, O.; Tajally, M.; Loghman-Estarki, M.R. A comparative study of synthesis and spark plasma sintering of YAG nano powders by different co-precipitation methods. Ceram. Int. 2018, 44, 10035–10046. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, Y.H.; Hou, X.Y.; Chu, B.X.; Nan, B.; Qin, Q.J.; Fan, M.G.; Sun, C.Z.; Li, B.; Dong, L.H.; et al. Investigation of two-phase intergrowth and coexistence in Mn-Ce-Ti-O catalysts for the selective catalytic reduction of NO with NH3: Structure-activity relationship and reaction mechanism. Ind. Eng. Chem. Res. 2018, 58, 849–862. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhao, Y.C.; Yang, J.P.; Zhang, J.Y.; Zheng, C.G. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas. Chem. Eng. J. 2018, 348, 618–629. [Google Scholar] [CrossRef]
- Chen, J.Y.; Fu, P.; Lv, D.F.; Chen, Y.; Fan, M.L.; Wu, J.L.; Meshram, A.; Mu, B.; Li, X.; Xia, Q.B. Unusual positive effect of SO2 on Mn-Ce mixed-oxide catalyst for the SCR reaction of NOx with NH3. Chem. Eng. J. 2021, 407, 127071. [Google Scholar] [CrossRef]
- Wang, H.J.; Huang, B.C.; Yu, C.L.; Lu, M.J.; Huang, H.; Zhou, Y.L. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, C.; Li, Q.; Gao, T.; Yu, S.H.; Tan, P.; Fang, Q.Y.; Chen, G. Promoting mechanism of SO2 resistance performance by anatase TiO2 {0 0 1} facets on Mn-Ce/TiO2 catalysts during NH3-SCR reaction. Chem. Eng. Sci. 2022, 251, 117438. [Google Scholar] [CrossRef]
- Sjoerd Kijlstra, W.; Biervliet, M.; Poels, E.K.; Bliek, A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 1998, 16, 327–337. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Metal sulfate poisoning effects over MnFe/TiO2 for selective catalytic reduction of NO by NH3 at low temperature. Ind. Eng. Chem. Res. 2018, 57, 4848–4858. [Google Scholar] [CrossRef]
- Shi, W.; Liu, J.J.; Zhu, Y.; Zhao, L.; Wang, Y.G.; Cheng, Z.H.; Peng, X.P.; Shi, X.Y.; Yu, Y.B.; He, H. Extruded monolith MnO-CeO2-TiO2 catalyst for NH3-SCR of low temperature flue gas from an industry boiler: Deactivation and recovery. J. Rare Earths 2023, 41, 1336–1343. [Google Scholar] [CrossRef]
- Zhang, B.; Li, D.; Wang, X.Y. Catalytic performance of La-Ce-O mixed oxide for combustion of methane. Catal. Today 2010, 158, 348–353. [Google Scholar] [CrossRef]
- Meng, D.M.; Zhan, W.C.; Guo, Y.; Guo, Y.L.; Wang, L.; Lu, G.Z. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, H.N.; Chen, L.; Zhang, Y.Y.; Mi, Y.Y.; Liao, M.Y.; Liu, W.M.; Wu, D.S.; Li, Z.G.; Peng, H.G. Ternary MnCoVOx catalysts with remarkable deNOx performance: Dual acid-redox sites control strategy. Appl. Catal. B Environ. 2022, 318, 121779. [Google Scholar] [CrossRef]
- Chen, R.Y.; Fang, X.Y.; Li, J.H.; Zhang, Y.; Liu, Z.M. Mechanistic investigation of the enhanced SO2 resistance of Co-modified MnOx catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2023, 452, 139207. [Google Scholar] [CrossRef]
- Niu, C.H.; Wang, B.R.; Xing, Y.; Su, W.; He, C.; Xiao, L.; Xu, Y.R.; Zhao, S.Q.; Cheng, Y.H.; Shi, J.W. Thulium modified MnOx/TiO2 catalyst for the low-temperature selective catalytic reduction of NO with ammonia. J. Clean. Prod. 2021, 290, 125858. [Google Scholar] [CrossRef]
- Mao, D.; Yang, W.; Xia, J.; Zhang, B.; Song, Q.Y.; Chen, Q.L. Highly effective hybrid catalyst for the direct synthesis of dimethyl ether from syngas with magnesium oxide-modified HZSM-5 as a dehydration component. J. Catal. 2005, 230, 140–149. [Google Scholar] [CrossRef]
- Li, G.H.; Xue, H.Y.; Yu, J.; Mao, D.S. Insights into the simultaneously enhanced activity, selectivity, and H2O resistance of cobalt modified MnCeOx/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Fuel 2023, 354, 129416. [Google Scholar] [CrossRef]
- Chen, L.Q.; Yuan, F.L.; Li, Z.B.; Niu, X.Y.; Zhu, Y.J. Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NOx removal over the Co0.2CexMn0.8−xTi10 (x = 0–0.40) oxides catalysts. Chem. Eng. J. 2018, 354, 393–406. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, W.C.; Luan, T.; Li, H.; Zhang, W.K.; Feng, W.C.; Jiang, H.L. High-efficiency catalytic conversion of NOx by the synergy of nanocatalyst and plasma: Effect of Mn-based bimetallic active species. Catalysts 2019, 9, 103. [Google Scholar] [CrossRef]
- Liu, F.D.; He, H.; Ding, Y.; Zhang, C.B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2009, 93, 194–204. [Google Scholar] [CrossRef]
- Ali, S.; Chen, L.Q.; Yuan, F.L.; Li, R.; Zhang, T.R.; Bakhtiar, S.u.H.; Leng, X.S.; Niu, X.Y.; Zhu, Y.J. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5−x-Zr0.5 (x = 0.1–0.5) oxides catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B Environ. 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Cheng, J.; Song, L.Y.; Wu, R.; Li, S.N.; Sun, Y.M.; Zhu, H.T.; Qiu, W.G.; He, H. Promoting effect of microwave irradiation on CeO2-TiO2 catalyst for selective catalytic reduction of NO by NH3. J. Rare Earths 2020, 38, 59–69. [Google Scholar] [CrossRef]
- Chen, W.S.; Li, Z.; Hu, F.L.; Qin, L.B.; Han, J.; Wu, G.M. In situ DRIFTS investigation on the selective catalytic reduction of NO with NH3 over the sintered ore catalyst. Appl. Surf. Sci. 2018, 439, 75–81. [Google Scholar] [CrossRef]
- Mu, J.C.; Li, X.Y.; Sun, W.B.; Fan, S.Y.; Wang, X.Y.; Wang, L.; Qin, M.C.; Gan, G.Q.; Yin, Z.F.; Zhang, D.K. Enhancement of low-temperature catalytic activity over a highly dispersed Fe-Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Qiu, L.; Pang, D.D.; Zhang, C.L.; Meng, J.J.; Zhu, R.S.; Ouyang, F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2015, 357, 189–196. [Google Scholar] [CrossRef]
- Liu, S.M.; Guo, R.T.; Sun, P.; Wang, S.X.; Pan, W.G.; Li, M.Y.; Liu, S.W.; Sun, X.; Liu, J. The enhancement of Zn resistance of Mn/TiO2 catalyst for NH3-SCR reaction by the modification with Al2(SO4)3. J. Taiwan Inst. Chem. Eng. 2017, 78, 370–377. [Google Scholar] [CrossRef]
- Li, G.; Mao, D.S.; Chao, M.X.; Li, G.H.; Yu, J.; Guo, X.M. Low-temperature NH3-SCR of NO over MnCeO/TiO2 catalyst: Enhanced activity and SO2 tolerance by modifying TiO2 with Al2O3. J. Rare Earths 2021, 39, 805–816. [Google Scholar] [CrossRef]
- He, Z.H.; Wang, Y.; Liu, Y.X.; Lian, L.Q.; Kong, D.X.; Zhao, Y.C. Recent advances in sulfur poisoning of selective catalytic reduction (SCR) denitration catalysts. Fuel 2024, 365, 131126. [Google Scholar] [CrossRef]
- Wu, H.L.; Liu, W.Z.; Cao, J.; Huang, J.B.; Liu, Q.C. Mechanistic and performance insights into low-temperature NH3-SCR based on Ce-modified Mn-Ti catalysts. J. Environ. Chem. Eng. 2023, 11, 110072. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ren, S.; Wang, M.M.; Yang, J.; Chen, L.; Liu, W.Z.; Liu, Q.C.; Su, B.X. Insights into samarium doping effects on catalytic activity and SO2 tolerance of MnFeO catalyst for low-temperature NH3-SCR reaction. Fuel 2022, 321, 124113. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Qu, P.Y.; Qiu, L.M.; Wang, J.T.; Lian, C.; Ma, C.; Jia, X.F.; Qiao, W.M.; Ling, L.C. Polymer-directed self-assembly synthesis of tin-titanium-manganese compounded oxides with enhanced activity and sulfur tolerance for NH3-SCR. Appl. Surf. Sci. 2023, 607, 154956. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.D.; Yang, J.; Li, X.D.; Wang, M.M.; Chen, Z.C.; Liu, Q.C. Poisoning mechanism of KCl, K2O and SO2 on Mn-Ce/CuX catalyst for low-temperature SCR of NO with NH3. Process Saf. Environ. 2022, 167, 609–619. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.J.; Cheng, Y.; Cen, W.L. Mechanism of Ce-modified Birnessite-MnO2 in promoting SO2 poisoning resistance for low-temperature NH3-SCR. ACS Catal. 2021, 11, 4125–4135. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.T.; Liu, S.M.; Wang, S.X.; Pan, W.G.; Li, M.Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Zhang, Y.P.; Xiao, R.; Huang, T.J.; Shen, K. Novel holmium-modified Fe-Mn/TiO2 catalysts with a broad temperature window and high sulfur dioxide tolerance for low-temperature SCR. Chem. Commun. 2017, 88, 64–67. [Google Scholar] [CrossRef]
- Chen, C.; Xie, H.D.; He, P.W.; Liu, X.; Yang, C.; Wang, N.; Ge, C.M. Comparison of low-temperature catalytic activity and H2O/SO2 resistance of the Ce-Mn/TiO2 NH3-SCR catalysts prepared by the reverse co-precipitation, co-precipitation and impregnation method. Appl. Surf. Sci. 2022, 571, 151285. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Liang, G.T.; Liu, S.J.; Gao, W.Q.; Yang, Z.D.; Wang, X.W.; Lin, R.Y.; Zhu, X.B. The poisoning effect of PbO on CeO2-MoO3/TiO2 catalyst for selective catalytic reduction of NO with NH3. Mol. Catal. 2020, 486, 110877. [Google Scholar] [CrossRef]
- Wang, J.W.; Xie, H.; Shu, D.B.; Chen, T.H.; Liu, H.B.; Zou, X.H.; Chen, D. The promotion of NH3-SCR performance and its mechanism on Sm modified birnessite. Fuel 2024, 356, 129604. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.T.; Li, S.H.; Zhang, W.; Du, X.Y.; Huang, L.; Zhu, Y.C.; Zhai, Y.B.; Zeng, G.M. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal. Chem. Eng. J. 2019, 371, 781–795. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.P.; Guo, H.X.; Ma, X.Y.; Zhang, L.J. DRIFT and DFT study of cerium addition on SO2 of Manganese-based catalysts for low temperature SCR. J. Mol. Catal. A Chem. 2016, 421, 102–108. [Google Scholar] [CrossRef]
- Xie, Q.; An, D.Q.; Zhou, L.S.; Li, T.Z.; Hu, Z.H.; Chen, M.H.; Ma, M.L.; Zhang, L.; Sun, J.F.; Dong, L. Deactivation induced by metal sulfate over MnCeO catalyst in NH3-SCR reaction at low temperature. J. Rare Earths 2024, 42, 1056–1065. [Google Scholar] [CrossRef]
- Kang, T.H.; Youn, S.; Kim, D.H. Improved catalytic performance and resistance to SO2 over V2O5-WO3/TiO2 catalyst physically mixed with Fe2O3 for low-temperature NH3-SCR. Catal. Today 2021, 376, 95–103. [Google Scholar] [CrossRef]
- Wang, X.P.; Ye, Q.; Liu, W.Y.; Meng, F.W.; Yang, F.; Zhang, X.; Dai, H.X. Improved sulfur dioxide resistance of the Mn/Fe-pillared interlayered clay by the doping of ceria in the selective catalytic reduction of NO with NH3. React. Kinet. Mech. Catal. 2023, 136, 1263–1281. [Google Scholar] [CrossRef]





| Catalyst | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| MCT-C | 97.9 | 0.22 | 8.8 |
| MCT-R | 141.6 | 0.46 | 12.9 |
| MCT-P | 54.0 | 0.23 | 17.2 |
| MCT-C-S | 79.3 | 0.18 | 9.2 |
| MCT-R-S | 112.7 | 0.36 | 12.6 |
| MCT-P-S | 39.4 | 0.19 | 18.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xue, H.; Wang, L.; Yu, J.; Han, L.; Mao, D. Significant Effects of Adding Mode on Low-Temperature De-NOx Performance and SO2 Resistance of a MnCeTiOx Catalyst Prepared by the Co-Precipitation Method. Catalysts 2024, 14, 690. https://doi.org/10.3390/catal14100690
Yang X, Xue H, Wang L, Yu J, Han L, Mao D. Significant Effects of Adding Mode on Low-Temperature De-NOx Performance and SO2 Resistance of a MnCeTiOx Catalyst Prepared by the Co-Precipitation Method. Catalysts. 2024; 14(10):690. https://doi.org/10.3390/catal14100690
Chicago/Turabian StyleYang, Xi, Hongyan Xue, Lei Wang, Jun Yu, Lupeng Han, and Dongsen Mao. 2024. "Significant Effects of Adding Mode on Low-Temperature De-NOx Performance and SO2 Resistance of a MnCeTiOx Catalyst Prepared by the Co-Precipitation Method" Catalysts 14, no. 10: 690. https://doi.org/10.3390/catal14100690
APA StyleYang, X., Xue, H., Wang, L., Yu, J., Han, L., & Mao, D. (2024). Significant Effects of Adding Mode on Low-Temperature De-NOx Performance and SO2 Resistance of a MnCeTiOx Catalyst Prepared by the Co-Precipitation Method. Catalysts, 14(10), 690. https://doi.org/10.3390/catal14100690










