Degradation of Tetracycline (TC) by ZrO2-3DG/PMS System: Revealing the Role of Defects in the Conditions of Light Irradiation and Sulfate Accumulation
Abstract
1. Introduction
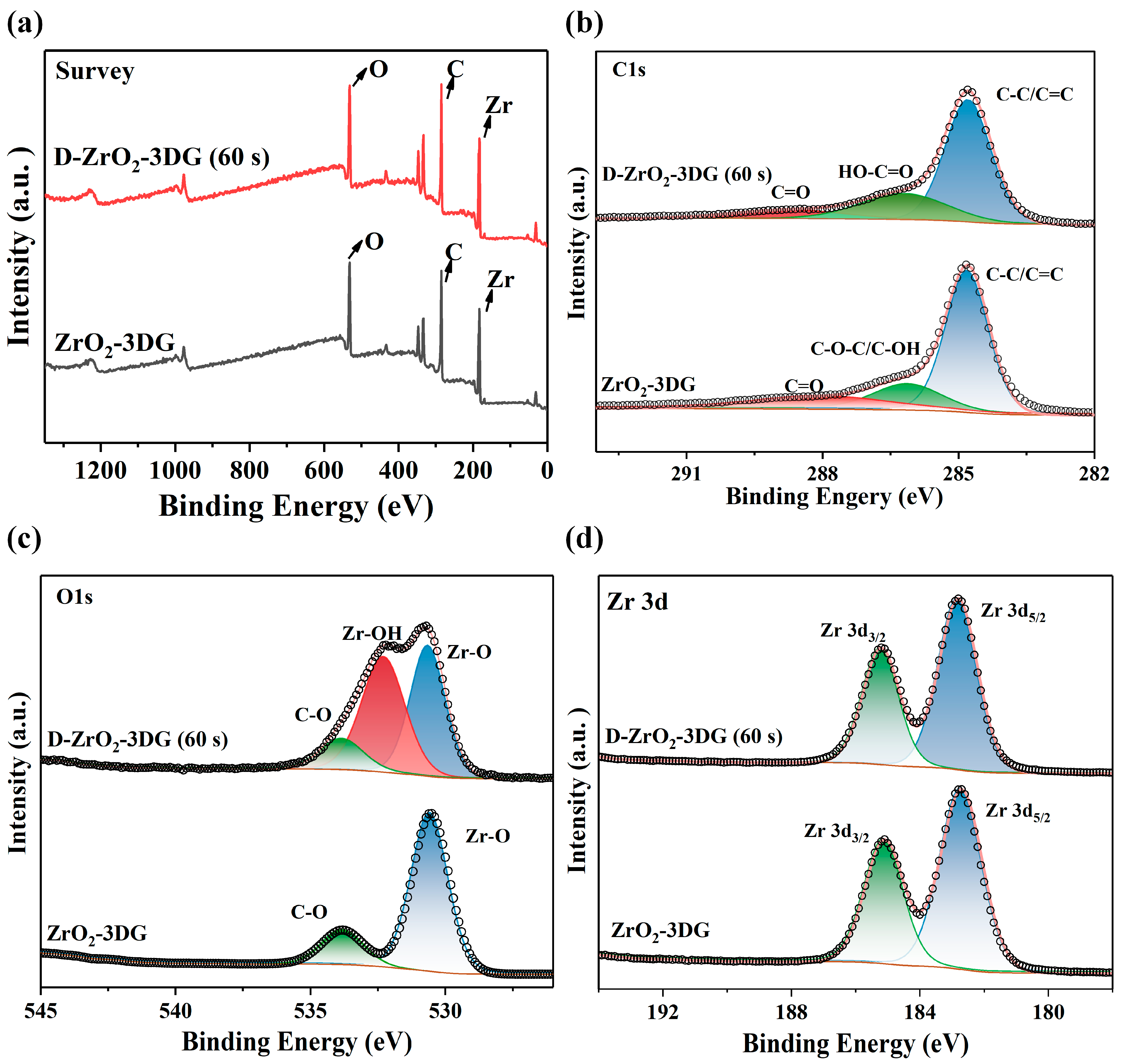
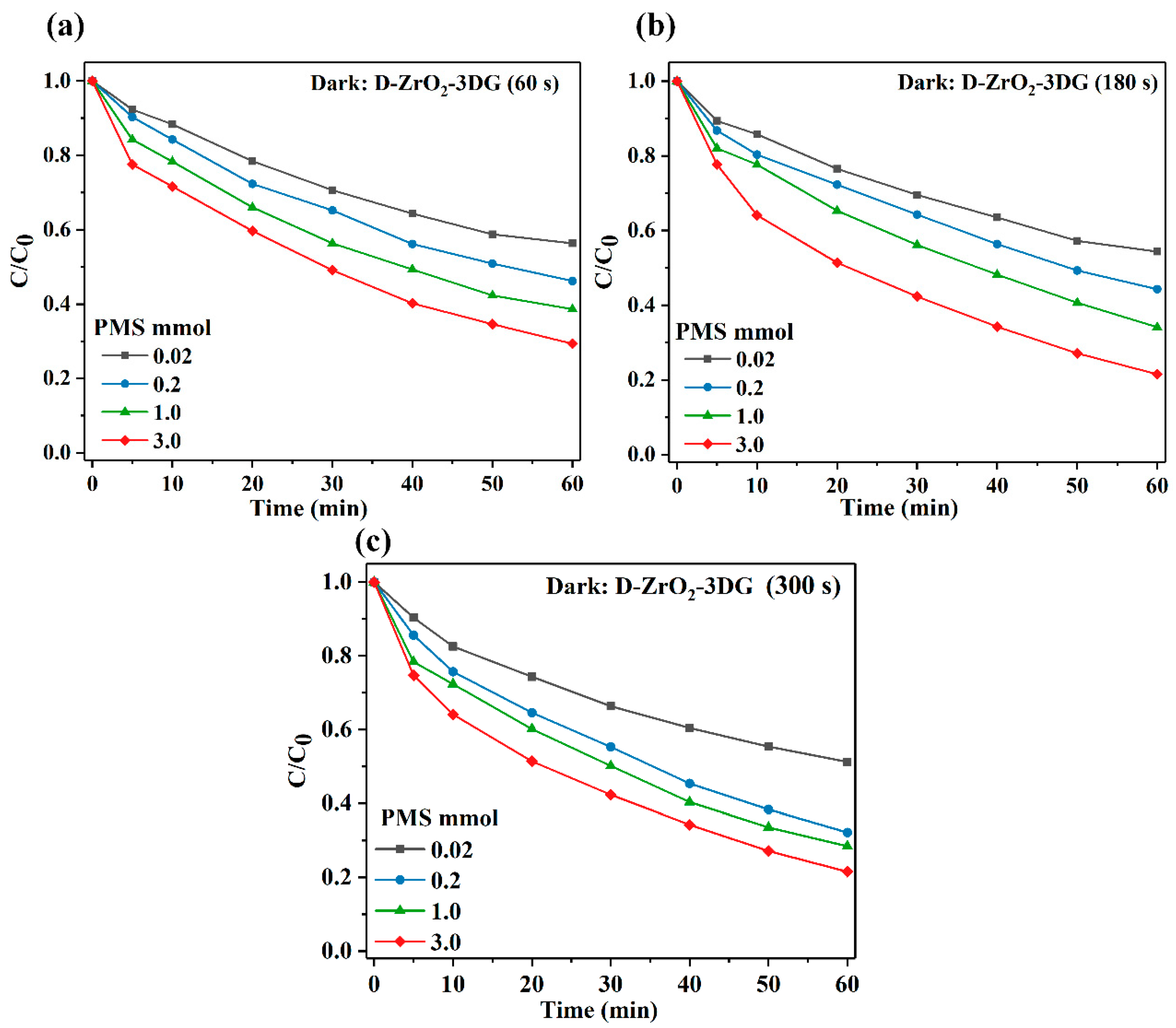
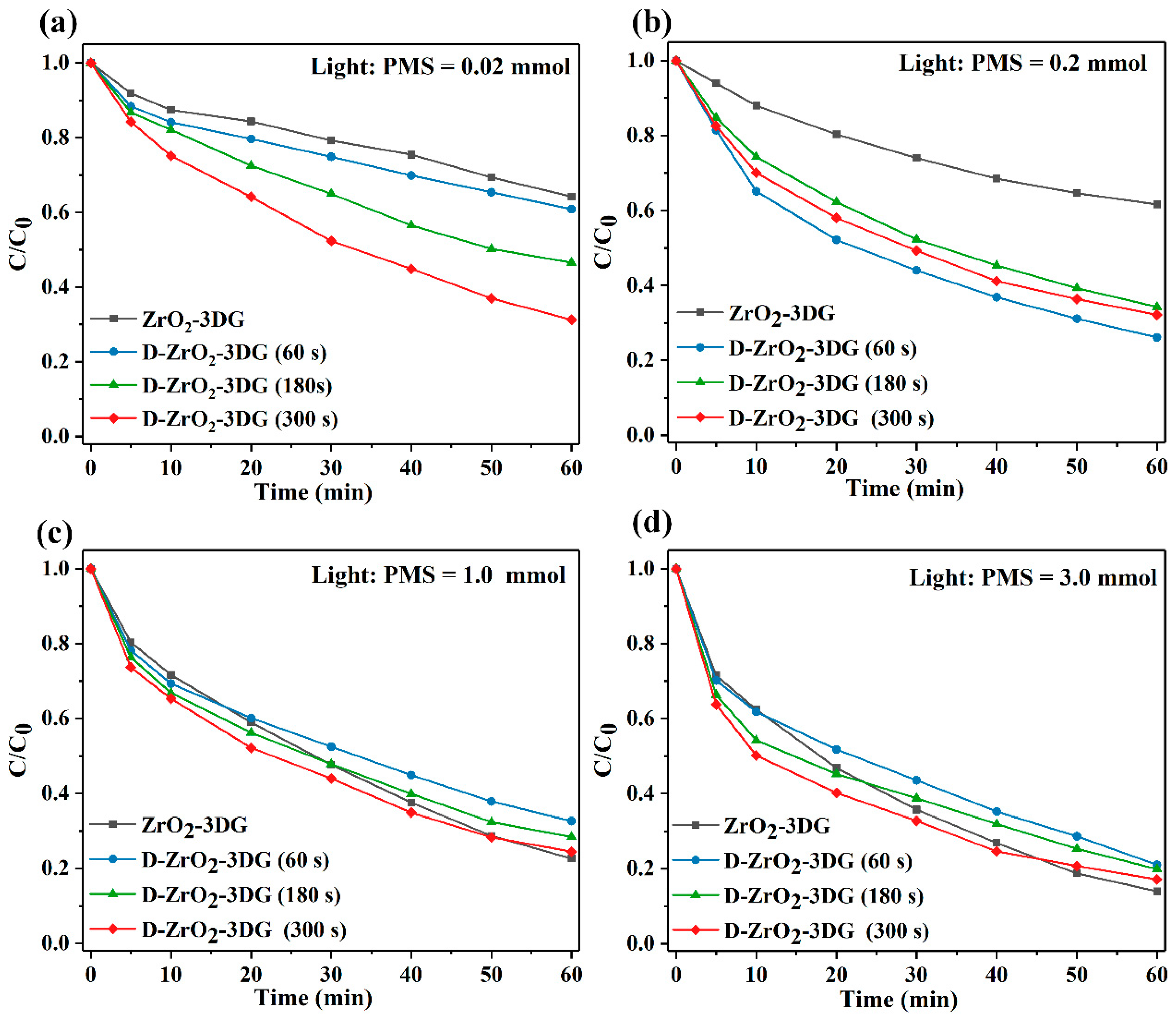
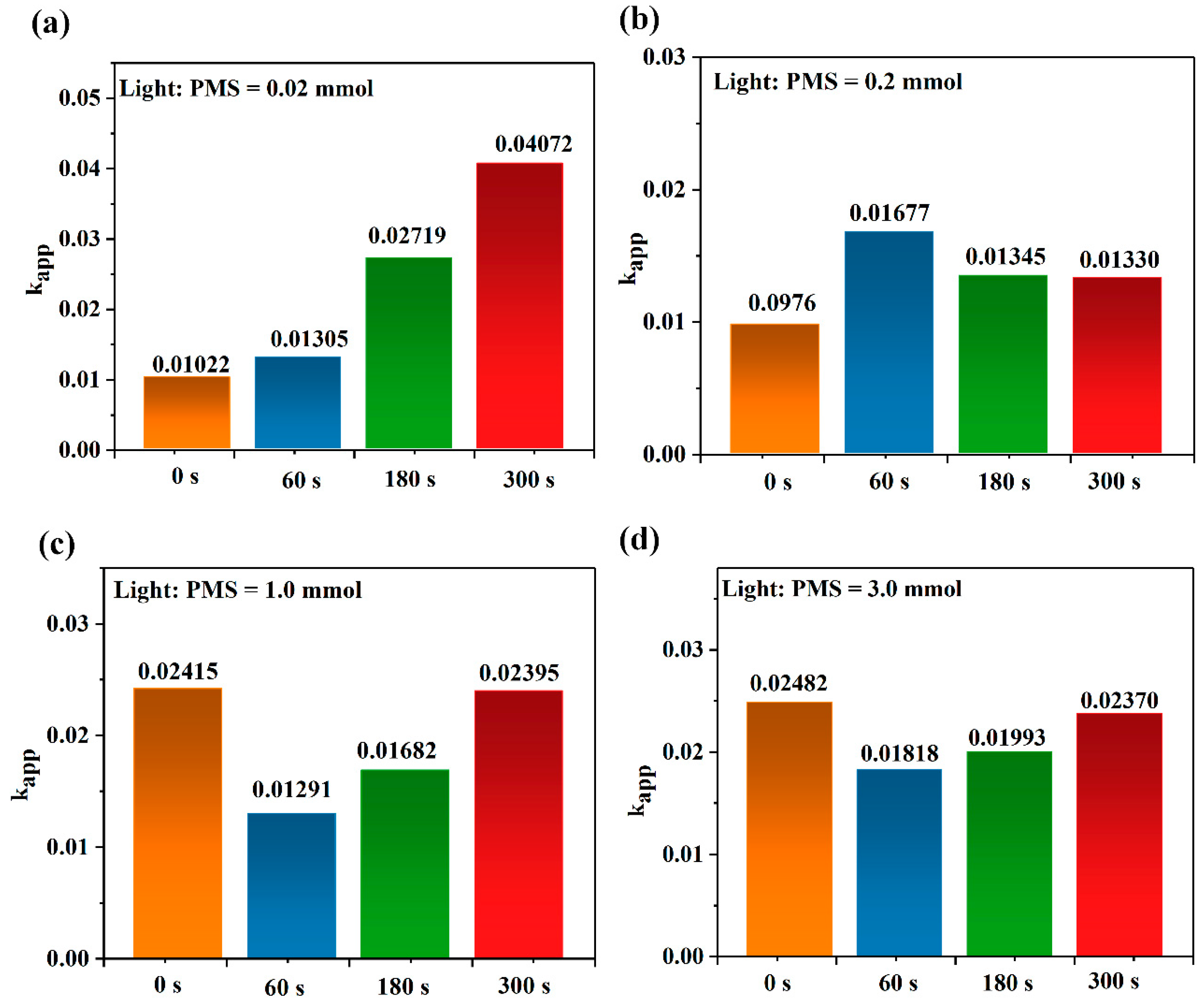
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation of ZrO2-3DG
3.3. Preparation of D-ZrO2-3DG
3.4. Characterization
3.5. The Degradation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yin, H.; Varis, O. Impacts of industrial transition on water use intensity and energy-related carbon intensity in China: A spatio-temporal analysis during 2003–2012. Appl. Energy 2016, 183, 1112–1122. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdacs, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.-S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, Q.; Nan, T.; Zhou, M.; Xia, Y.; Hu, G.; Zheng, Q.; Wu, Y.; Bian, T.; Wei, T.; et al. Cobalt-based catalysts for heterogeneous peroxymonosulfate (PMS) activation in degradation of organic contaminants: Recent advances and perspectives. J. Alloys Compd. 2023, 958, 170370. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Chen, K.-Y.; Wang, S.-X.; Zhao, S.-Y.; Yu, L.-Q.; Huang, B.-C.; Jin, R.-C. Synergizing electron transfer with singlet oxygen to expedite refractory contaminant mineralization in peroxymonosulfate based heterogeneous oxidation system. Appl. Catal. B-Environ. 2024, 341, 123324. [Google Scholar] [CrossRef]
- Xiao, Z.-J.; Feng, X.-C.; Shi, H.-T.; Zhou, B.-Q.; Wang, W.-Q.; Ren, N.-Q. Why the cooperation of radical and non-radical pathways in PMS system leads to a higher efficiency than a single pathway in tetracycline degradation. J. Hazard. Mater. 2022, 424, 127247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, X.; Lu, W.; Wang, H.; Liu, J.; Zhang, R.; Wang, H.; Wang, J. Multi-dimensional induction and analysis of singlet oxygen oxidation in persulfate-based catalytic oxidation systems. Sep. Purif. Technol. 2024, 332, 125811. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- Loffredo, C.M.; Dennehy, M.; Alvarez, M. Fe-Mn/ZrO2 catalysts: Sulfate-based-advanced oxidation process for the degradation of olive oil industry model pollutants. Catal. Commun. 2023, 174, 106578. [Google Scholar] [CrossRef]
- Patel, A.; Arkatkar, A.; Singh, S.; Rabbani, A.; Medina, J.D.S.; Ong, E.S.; Habashy, M.M.; Jadhav, D.A.; Rene, E.R.; Mungray, A.A.; et al. Physico-chemical and biological treatment strategies for converting municipal wastewater and its residue to resources. Chemosphere 2021, 282, 130881. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Hao, Z.; Huang, Y.; Wang, Y.; Meng, X.; Wang, X.; Liu, X. Enhanced degradation and mineralization of estriol over ZrO2/OMS-2 nanocomposite: Kinetics, pathway and mechanism. Chemosphere 2022, 308, 136521. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Fan, Y.; Sun, Z.; Hu, X. ZrO2 supported perovskite activation of peroxymonosulfate for sulfamethoxazole removal from aqueous solution. Chemosphere 2022, 298. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Rasool, R.T.; Ul Rasool, R.; Saleem, M.F.; Ali, J.; Ghernaout, D.; Hassan, M.; Aljuwayid, A.M.; Habila, M.A.; Guo, H. Photocatalytic stimulation of peroxymonosulfate by novel MoO3@ZrO2 with Z-scheme heterojunction for diclofenac sodium degradation. J. Water Process Eng. 2023, 51, 103435. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Cleaning and anti-reflective (AR) hydrophobic coating of glass surface: A review from materials science perspective. J. Sol-Gel Sci. Technol. 2016, 77, 1–27. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, X.; Sun, H.; Li, Y.; Chen, J.; Yuan, X.; Zhang, P. Nonequilibrium Plasma Cleaning of Organic Contaminants on the Surface of Diffraction Gratings. Ieee Trans. Plasma Sci. 2023, 51, 3008–3017. [Google Scholar] [CrossRef]
- Wang, M.; Kang, J.; Li, S.; Zhang, J.; Tang, Y.; Liu, S.; Liu, J.; Tang, P. Electro-assisted heterogeneous activation of peroxymonosulfate by g-C3N4 under visible light irradiation for tetracycline degradation and its mechanism. Chem. Eng. J. 2022, 436, 135278. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Photo-Fenton degradation of phenol by CdS/rGO/Fe2+ at natural pH with in situ-generated H2O2. Appl. Catal. B-Environ. 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Krishnakumar, A.; Srinivasan, P.; Kulandaisamy, A.J.; Babu, K.J.; Rayappan, J.B.B. A facile microwave synthesis of rGO, ZrO2 and rGO-ZrO2 nanocomposite and their room temperature gas sensing properties. J. Mater. Sci.-Mater. Electron. 2019, 30, 17094–17105. [Google Scholar] [CrossRef]
- Mylarappa, M.; Chandruvasan, S.; Harisha, K.S.; Krishnamurthy, G. Graphene Loaded ZrO2 Nanocomposite for Antioxidant, Dye Removal, Electrochemical and Green Sensor Studies. Chemistryselect 2024, 9, e202401218. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Basahel, S.N.; Mokhtar, M.; Alsharaeh, E.H.; Mahmoud, H.A. Template Assisted Microwave Synthesis of rGO-ZrO2 Composites: Efficient Photocatalysts Under Visible Light. J. Nanosci. Nanotechnol. 2019, 19, 5177–5188. [Google Scholar] [CrossRef]
- Kallawar, G.A.; Barai, D.P.; Bhanvase, B.A. Bismuth titanate based photocatalysts for degradation of persistent organic compounds in wastewater: A comprehensive review on synthesis methods, performance as photocatalyst and challenges. J. Clean. Prod. 2021, 318, 128563. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef] [PubMed]
- Teeparthi, S.R.; Awin, E.W.; Kumar, R. Dominating role of crystal structure over defect chemistry in black and white zirconia on visible light photocatalytic activity. Sci. Rep. 2018, 8, 5541. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Thakur, S.; Mutreja, V.; Kaur, R. Synergistic integration of ZrO2-enriched reduced graphene oxide-based nanostructures for advanced photodegradation of tetracycline hydrochloride. Environ. Sci. Pollut. Res. Int. 2024, 31, 31562–31576. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Wang, X.; Ye, Z.; Chen, F. Degradation of Tetracycline (TC) by ZrO2-3DG/PMS System: Revealing the Role of Defects in the Conditions of Light Irradiation and Sulfate Accumulation. Catalysts 2024, 14, 846. https://doi.org/10.3390/catal14120846
Duan J, Wang X, Ye Z, Chen F. Degradation of Tetracycline (TC) by ZrO2-3DG/PMS System: Revealing the Role of Defects in the Conditions of Light Irradiation and Sulfate Accumulation. Catalysts. 2024; 14(12):846. https://doi.org/10.3390/catal14120846
Chicago/Turabian StyleDuan, Jixiang, Xin Wang, Zhihong Ye, and Fuming Chen. 2024. "Degradation of Tetracycline (TC) by ZrO2-3DG/PMS System: Revealing the Role of Defects in the Conditions of Light Irradiation and Sulfate Accumulation" Catalysts 14, no. 12: 846. https://doi.org/10.3390/catal14120846
APA StyleDuan, J., Wang, X., Ye, Z., & Chen, F. (2024). Degradation of Tetracycline (TC) by ZrO2-3DG/PMS System: Revealing the Role of Defects in the Conditions of Light Irradiation and Sulfate Accumulation. Catalysts, 14(12), 846. https://doi.org/10.3390/catal14120846








