Abstract
China is one of the largest sugarcane industrial countries in the world, and the annual output of bagasse waste is abundant. Classical incineration, landfill, and other treatment methods are inefficient and seriously harmful to the environment, so it is urgent to develop a new comprehensive utilization of agricultural waste. In this work, the sugarcane waste residue is converted to biological activated carbon (BAC) through a simple pre-carbonization and KOH activation process, which is then mixed with perovskite oxide BaCo0.5Fe0.5O3−δ (BCF) to form BAC/BCF composite air electrode. BAC/BCF assembled rechargeable zinc–air battery (ZAB) exhibits a relatively good output maximum power density of 96 mW·cm−2 and considerable long-term charge–discharge cycle stability over 250 h operation. These results indicate that the BAC derived from sugarcane waste is a promising potential carbon material candidate for ZAB application, which can realize the high-value utilization of agricultural waste in the field of efficient and durable energy storage and conversion devices.
1. Introduction
As a major bioenergy crop, sugarcane has a relatively large carbon footprint. Sugarcane waste is the main by-product of the sugarcane industry. As the third largest sugarcane producer in the world, China produces countless sugarcane residues every year [1,2]. These discarded bagasse will cause secondary pollution to the environment, and most of them will be disposed of in the landfills of sugar mills [3]. Traditional methods such as incineration and landfilling will not only waste rich biomass resources but also cause environmental damage to water, air, and land, which is not sustainable for the concept of circular economy [4]. It is urgent to develop a novel way of comprehensive utilization of agricultural and industrial wastes such as bagasse [5]. So far, research on the high-value use of bagasse has attracted much attention due to its low cost and attractive technical properties, and researchers have explored a range of innovative strategies to recycle sugarcane waste [6,7,8,9,10,11,12]. For example, Andrade et al. added malted bagasse to ceramic materials as a partial substitute for clay, thus applying it in the field of civil construction [13]. Meanwhile, Ferreira et al. mixed cassava starch with agro-industrial wastes such as sugarcane bagasse and successfully applied them to biodegradable composites [14]. Rattanachueskul et al. used sugarcane bagasse ash as a precursor of alkali-activated material to prepare magnetic Fenton catalyst and applied it to biochemical fields such as tetracycline removal [15]. These studies indicate that bagasse biomass resources have broad application prospects in many fields. However, almost no literature has reported the application of sugarcane waste residue to new energy fields such as metal–air batteries (MABs) so far.
Traditional fossil fuel reserves are gradually decreasing due to the continuous expansion of consumption, and the use of such non-renewable energy will increase carbon dioxide emissions and seriously harm the ecological environment [16,17,18,19]. With the depletion of resources and the deterioration of the environment, society’s demand for safe and environmentally friendly renewable energy is increasing. In order to meet the growing demand for renewable energy, researchers have studied a series of advanced energy storage and conversion technologies [20,21,22]. As an economical and environmentally friendly energy storage technology, MABs have been widely studied and applied [23,24]. Rechargeable zinc–air battery (ZAB), one promising kind of MABs, has attracted wide attention because of their high specific capacity, high safety, and low pollution [25,26]. However, it still faces some key remaining challenges, such as electrolyte stability, parasitic processes, electrode corrosion and stability, etc. Nowadays, many strategies, including the development of new electrolytes, CO2 and H2O managements, and surface wettability of the gas diffusion layer and catalyst layer, have been proposed to address these issues and alleviate the surface side reactions and electrode corrosion, thereby increasing the service lifetime [23,24,25,26]. Recently, ZAB often uses commercial activated carbon (CAC) with a relatively single preparation source and relatively high cost as the cathode conductive material, which greatly restricts its sustainable development and application [27,28]. Biomass is regarded as a highly efficient carbon source because of its economic and environmental protection characteristics. Biomass-derived carbon shows good pore size distribution and physicochemical stability through a high-temperature activation process, which is convenient for synthesis [29,30,31]. In addition, biomass-activated carbon often contains a large number of oxygen-containing functional groups, such as C-O, C=O, and carboxyl groups, making it have more excellent properties than traditional carbon materials [32]. Researchers have converted all kinds of waste biomass resources into activated carbon for use in new energy fields, such as supercapacitors and batteries [33,34,35,36,37]. For example, Li et al. used olive leaf biomass as raw material to prepare an oxygen-rich layered porous carbon by high-temperature activation method as a cathode conductive material for zinc ion hybrid capacitors. The layered porous structure can enrich the active site and improve the ion transfer rate. At the same time, oxygen-rich functional groups improve the wettability and electrical conductivity of porous carbon and achieve excellent electrochemical properties [38]. In addition, Dubey et al. converted pineapple peel into activated carbon through a high-temperature activation process of potassium hydroxide, and applied it to an asymmetric supercapacitor, and retained 90% of its initial specific capacitance after 10,000 charge and discharge cycles, showing good cycle stability [39]. These studies show that biomass is a promising source for electrode materials, and activated carbon prepared from biomass has excellent physical and chemical properties and electrochemical conductivity, showing great prospects in energy storage devices such as batteries and supercapacitors. It can be seen that it is feasible to convert sugarcane waste residue into active carbon as a conductive material for the cathode of ZAB.
Perovskite oxide is a promising bifunctional oxygen electrocatalyst due to its low price, high catalytic activity, and flexible composition [40,41]. Our previous study demonstrated that the perovskite oxide BaCo0.5Fe0.5O3−δ (BCF) can provide sufficient oxygen vacancies and electrocatalytic oxidation of metal ions toward oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) [42]. Therefore, the electrocatalytic performance of ZAB can be effectively improved by mixing BCF with biological activated carbon (BAC) prepared from sugarcane waste residue to achieve the BAC/BCF composite air electrode.
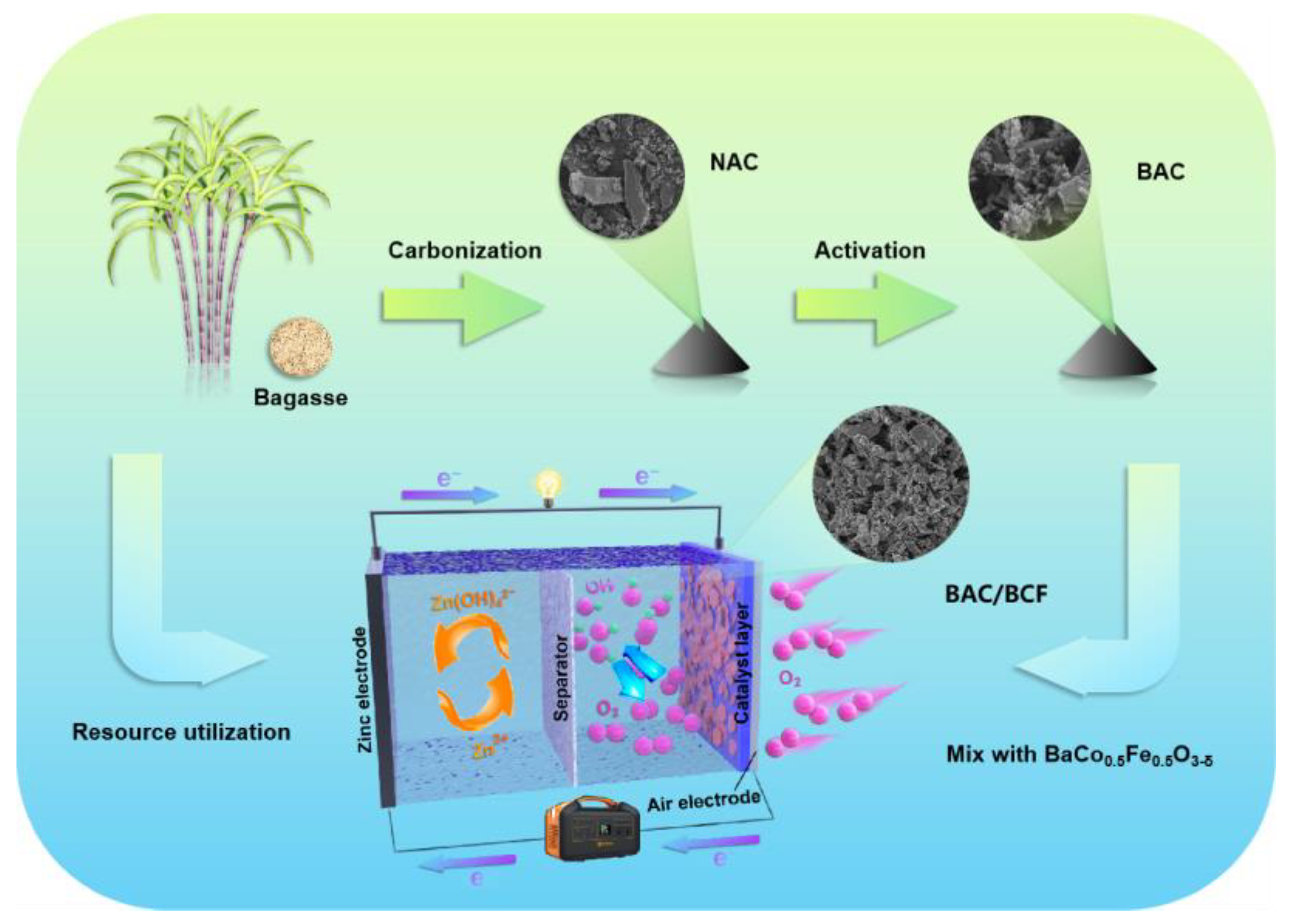
In this study, low-cost sugarcane waste residue is converted into oxygen-rich biological activated carbon (BAC) through pre-carbonization and activation processes, which is then mixed with BCF to form the BAC/BCF composite air electrode (BAC/BCF), as shown in Figure 1. In this paper, the effects of activators on the carbon properties of biomass are investigated, and the effects of different recombination ratios on the electrochemical properties of ZAB are systematically studied.
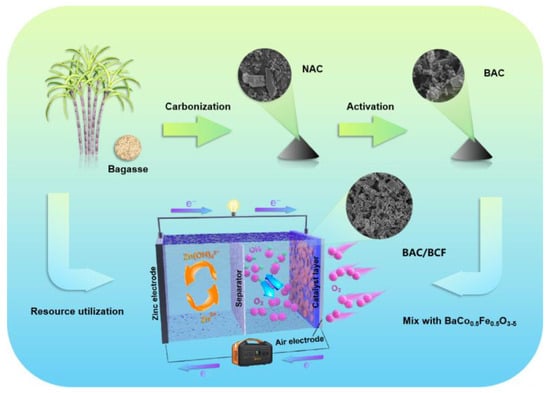
Figure 1.
Schematic illustration of the preparation of BAC/BCF composite air electrode.
2. Results and Discussion
2.1. Physicochemical Properties
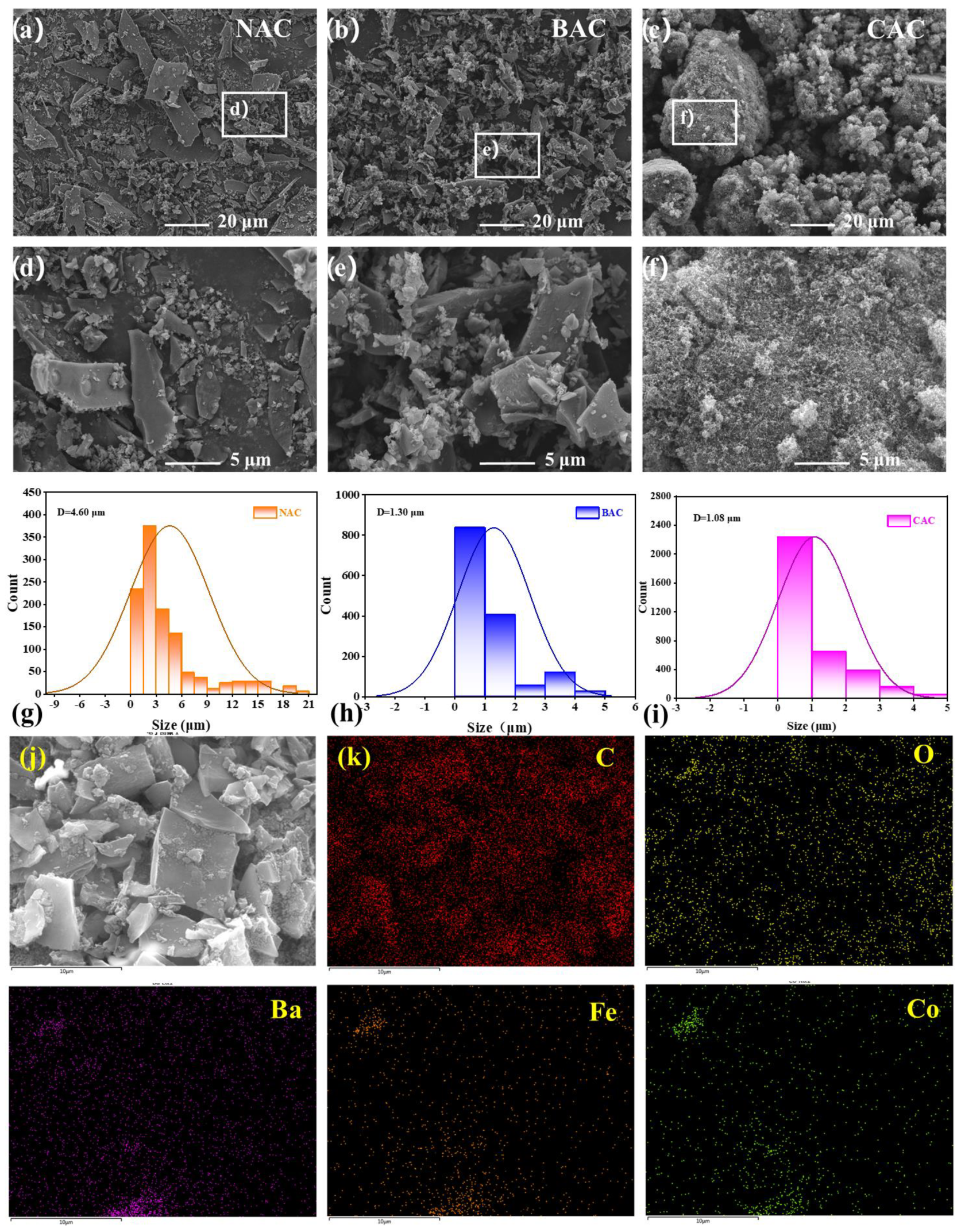
The morphology of non-activated carbon (NAC), BAC, and CAC is observed and presented in Figure 2. As shown in Figure 2a–d, the particle size of the carbon material is greatly decreased after KOH activation, and the activated BAC material with a three-dimensional (3D) structure shows more uniform particle size distribution and more porosity than the inactivated NAC, reasonably resulting in a high specific surface area, and thereby holding a great promise to enhance the electrochemical performance of the carbon material. The formation of the multilayer fragmented structure in the BAC sample is primarily due to the high-temperature KOH activation at 900 °C [39].
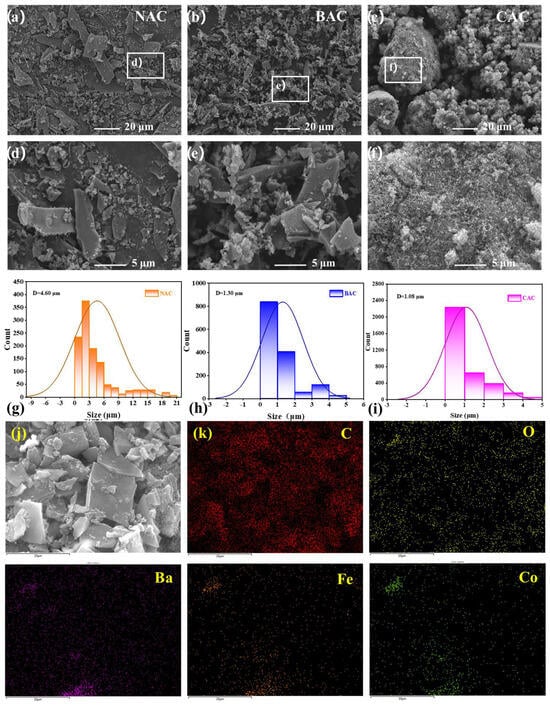
Figure 2.
(a–f) Scanning electron microscope (SEM) images at different magnifications for NAC, BAC, and CAC, (g–i) the particle size and particle size distribution of NAC, BAC, and CAC, (j,k) the EDS element mapping images for the as-prepared BAC/BCF-2 composite electrode.
Furthermore, no obvious difference in particle size and particle size distribution between BAC and CAC has been observed in Figure 2c–f. The detailed particle size and particle size distribution of NAC, BAC, and CAC are shown in Figure 2g–i. According to the calculated results, the average particle size of NAC is estimated to be about 4.60 μm, and the particles below 1 μm only account for 9.3%. In contrast, the calculated average particle size is significantly reduced to about 1.30 μm for the BAC sample after activation treatment, and 58% of the particles are smaller than 1 μm. Note that the particle size of BAC is greatly close to that of CAC (1.08 μm), implying that the as-prepared BAC material is a promising carbon material candidate for ZAB application.
Additionally, the energy dispersive spectrometer (EDS) element distribution mapping images are collected and shown in Figure 2j,k. It is clearly shown that all the elements in the BAC/BCF-2 composite catalyst, including C, Ba, Co, Fe, and O elements, are evenly distributed, and the carbon element is the predominant element. It should be pointed out that the typical signals for Ba, Co, and Fe elements belonging to the BCF phase are separately collected, indicating the successful synthesis of the BAC/BCF-2 composite catalyst.
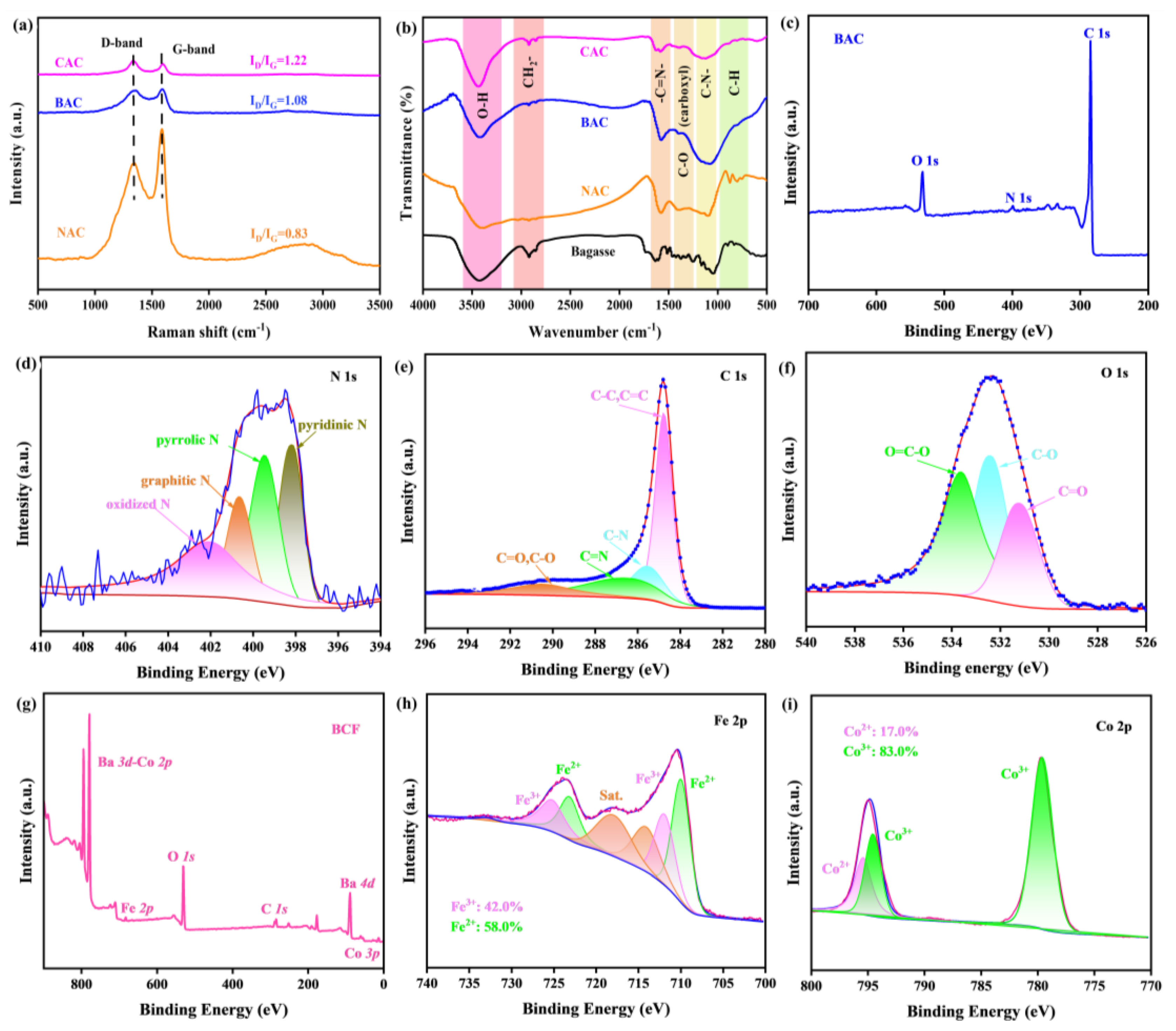
Raman spectra are highly sensitive to the crystallinity and defects in carbon materials and are usually used to analyze sample defects. The full Raman spectrum shows two typical peaks at the center of 1340 and 1590 cm−1, which corresponds to the D band and G band for carbon material, respectively. G peak represents sp2 bonded graphitic carbon, while D peak represents sp3 defects. Thus, the intensity ratio of ID/IG can be used to evaluate the defect degree in the as-prepared carbon materials [32,38]. As shown in Figure 3a, the calculated ID/IG ratios for NAC, BAC, and CAC samples are 0.83, 1.08, and 1.22, respectively, indicating that defect content in the KOH-activated BAC sample is greatly enhanced after KOH activation when compared with the inactivated NAC sample. This enhancement facilitates electron conduction, leading to improved electrode performance and energy efficiency. Notably, the ID/IG value for BAC is lower than the CAC sample, but their difference has been largely decreased, further suggesting the possibility of BAC in ZAB. At the same time, the structure of these carbon material samples is further characterized by Fourier transform infrared spectroscopy (FTIR), and the FTIR results are summarized in Figure 3b. A significant structural difference between the precursor bagasse and as-prepared is clearly obtained, again confirming the successful synthesis of BAC. The results indicate that BAC material contains plenty of hydrophilic groups, such as hydroxyl groups, while the aliphatic groups near 800 cm−1 in the precursor bagasse are almost removed. This structural modification ensures that BAC is capable of stable and uniform dispersion in the air electrode, promoting efficient and stable charge transfer [30,43].
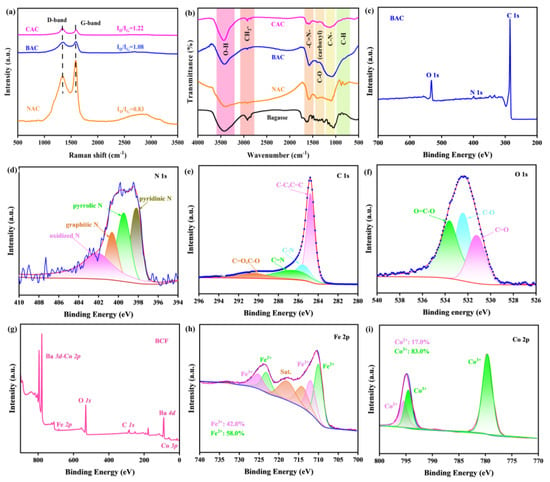
Figure 3.
(a) Raman spectra of NAC, BAC, and CAC. (b) FTIR spectra of bagasse, NAC, BAC, and CAC. (c) Survey XPS spectrum of BAC and high-resolution spectra of (d) N 1s, (e) C1s, and (f) O 1s. (g) Survey XPS spectrum of BCF and high-resolution spectra of (h) Fe 2p and (i) Co 2p.
In order to better understand the thermal stability of these carbon materials, the thermogravimetric analysis (TGA) is conducted in the air, and the data are shown in (Supplementary Materials, Figure S1). Only slight weight loss is observed for these three samples at the temperature below 200 °C, which is due to the desorption of the adsorbed H2O and gaseous species. The onset thermal decomposition temperatures for NAC, BAC, and CAC are measured to be 338, 519, and 598 °C, respectively, and the sudden drop in mass occurs as the temperature over the decomposition point, which is likely ascribed to the carbon oxidation reaction [39]. These results reveal that KOH activation improves the thermal stability of BAC, making its stability close to that of the commercial one.
To explore the surface valance states of BAC material, we collect its X-ray photoelectron spectroscopy (XPS) spectrum in the binding energy (BE) range of 200–700 eV (Figure 3c–f), and the characteristic XPS peaks for the C1s, N1s, and O1s orbits are clearly detected. The presence of N1s orbits in the BE range of 396–408 eV indicates the successful retention of the heteroatom N during the high-temperature carbonization and KOH activation (Figure 3d) [30]. The C1s spectrum of BAC (Figure 3e) shows four typical fitting peaks centered at 284.8, 285.5, 286.4, and 290.5 eV, corresponding to C=C/C-C, C-N, C=N, and C=O/C-O groups, respectively, confirming the abundant oxygen-containing polar groups on the BAC surface, which is highly consistent with the FTIR results (Figure 3b). The high-resolution N1s spectrum (Figure 3d) also shows four typical peaks, including oxidized N at 402.3 eV, graphitic N at 400.6 eV, pyrrolic N at 399.5 eV, and pyridinic N at 398.1 eV. Generally, the introduction of N atoms can effectively adjust the electronic structure of adjacent C atoms, resulting in charge redistribution and increased catalytic activity. Specifically, pyridinic N can increase the onset potential, while graphitic N determines the diffusion-limiting current of the ORR [5]. These fitting peaks for all the elements are quantified by the Rietveld method by using the XPSPeak4.1 software, and the relative atomic percentages of oxidized N, graphitic N, pyrrolic N, and pyridinic N in BAC are determined to be 31.7%, 26.8%, 25.6%, and 15.9%, respectively, indicating their important roles in oxygen electrocatalysis. In the high-resolution O1s spectrum (Figure 3f), the fitting peaks centered at 533.6, 532.4, and 531.2 eV correspond to the O=C-O group, O-C group, and O=C group, respectively. The nitrogen and oxygen-containing functional groups can greatly improve its hydrophilicity, thus ensuring its stability in the ZAB. Additionally, these functional groups can enhance the electrical conductivity of the carbon materials, thereby improving their electrochemical performance [30,44].
From the perspective of ORR and OER catalysis, the contents of Co and Fe are the main components of the active reactive sites. Therefore, we collect the XPS spectrum of BCF at 0–900 eV, as shown in Figure 3g. The Co3p, Co2p, Ba4d, Ba3d, Fe2p, and O1s signals, belonging to BCF material, can be clearly observed in the entire XPS spectrum, which indicates the successful synthesis of BCF material. Figure 3h shows that Fe has a mixed valence state. The peaks centered at 712.2 and 725.5 eV belong to Fe3+, while those located at 710.1 and 723.3 eV correspond to Fe2+. The content of Fe3+ and Fe2+ in BCF material is 42.0% and 58.0%, respectively. Previous studies have shown that the presence of Fe2+ is a negative factor affecting OER activity [45,46]. Therefore, BCF material has good catalytic activity. Figure 3i illustrates the XPS spectrum of the Co2p orbit, represented by the observed peaks of Co3+ (779.7 and 795.5 eV) and Co2+ (794.4 eV). The previous literature reported that higher Co3+/Co2+ ratio and Co3+ content were conducive to improving the catalytic activity of bifunctional oxygen catalysts [42,47]. The Co3+ content and Co3+/Co2+ ratio of BCF material reach the expected high values of 83.0% and 4.88, respectively, and these high values are conducive to achieving an improved eg occupancy rate, thereby demonstrating that BCF material has a good ability toward OER and ORR.
2.2. Electrochemical Performance
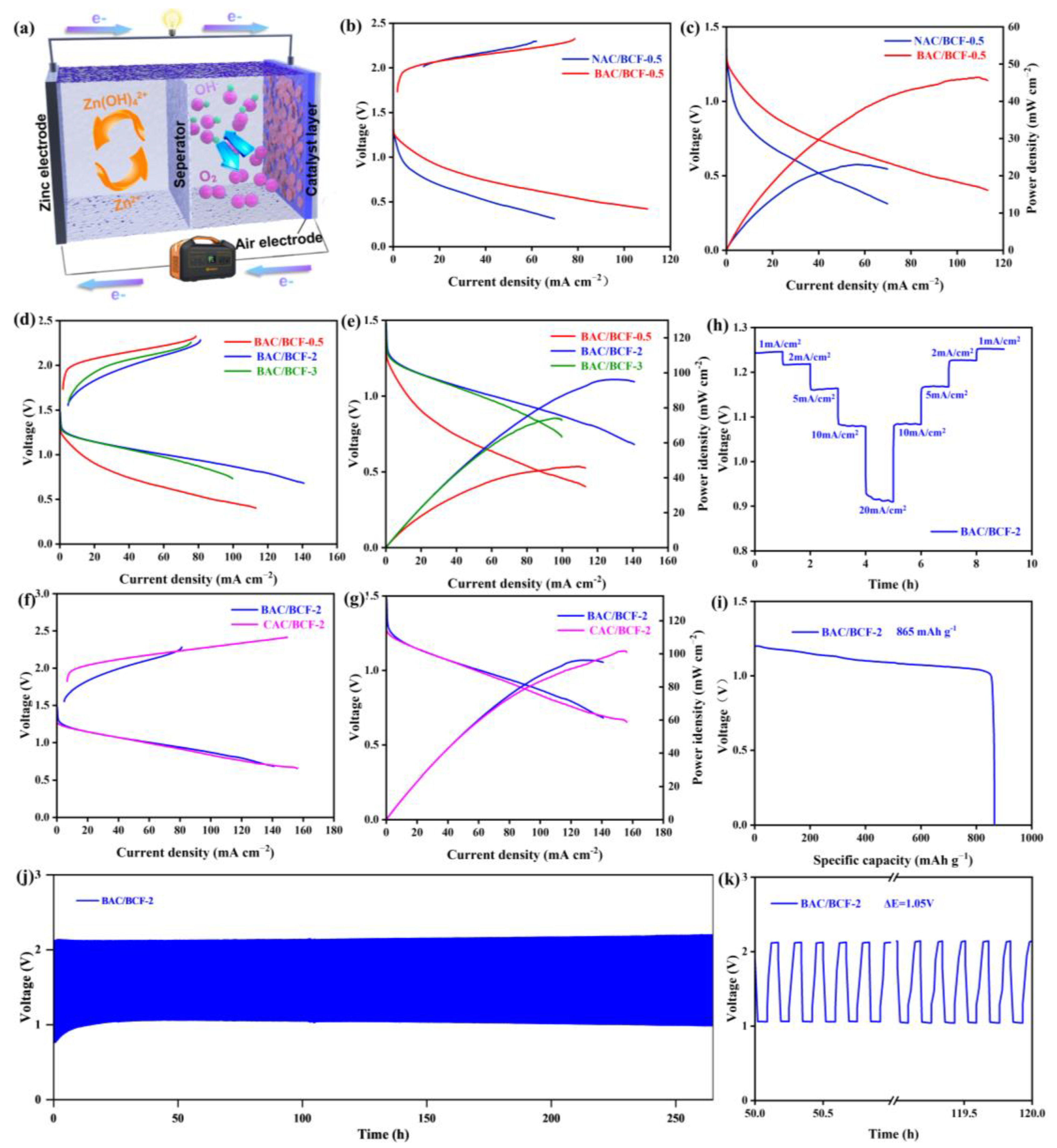
To obtain the optimal BAC to BCF ratio, five typical ZABs with NAC/BCF-0.5 air electrode, BAC/BCF-0.5 air electrode, BAC/BCF-2 air electrode, BAC/BCF-3 air electrode, and CAC/BCF-2 air electrode, are assembled and tested, and their electrochemical performance results are summarized in Figure 4. The BAC/BCF cathode-based ZAB not only can maintain a stable open circuit voltage (OCV) of 1.354 V during operation (Supplementary Materials, Figure S2) but also can successfully light up a digital LED screen with “Zn-Air” letters (Supplementary Materials, Figure S3).
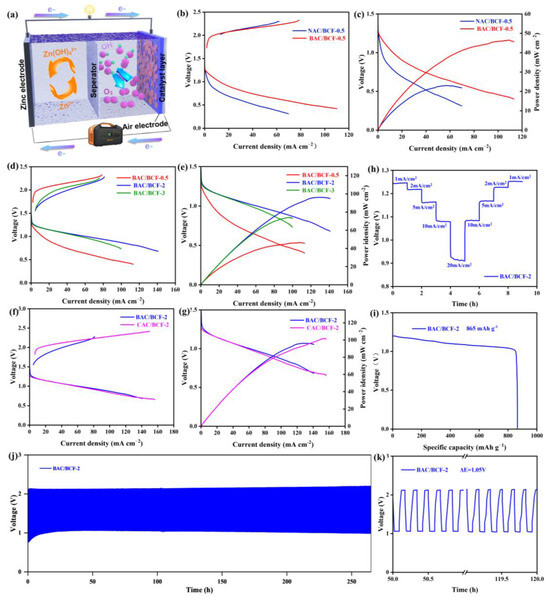
Figure 4.
(a) ZAB schematic diagram, (b) linear sweep voltammetry (LSV) curves at 0–2.5 V, and (c) current density–voltage–power density (i-V-p) curves of ZABs based on NAC/BCF-0.5 cathode and BAC/BCF-0.5 cathode, (d) LSV curves at 0–2.5 V, and (e) i-V-p curves of ZABs based on BAC/BCF-0.5 cathode, BAC/BCF-2 cathode and BAC/BCF-0.5 cathode, (f) LSV curves at 0–2.5 V, and (g) i-V-p curves of ZABs based on BAC/BCF-2 and CAC/BCF-2, (h) rate performance of BAC/BCF-2-based ZAB at 1, 5, 10, and 20 mA cm−2, (i) specific capacity, (j) charge–discharge cycle curve, and (k) the magnified (j) for the BAC/BCF-2-based ZAB.
The electrochemical performance of ZABs based on various composite electrodes is evaluated by collecting LSV curves at 0–2.5 V. Figure 4b shows the charge and discharge voltage difference (∆Egap) for ZABs with NAC/BCF-0.5 and BAC/BCF-0.5 composite air electrodes. In the entire current density range, the ∆Egap value for the BAC/BCF-0.5 cathode is smaller than that of the NAC/BCF-0.5 cathode, indicating its superior electrocatalytic performance. Additionally, the maximum output power (pmax) of the ZAB with BAC/BCF-0.5 composite electrode is 46 mWcm−2 and is also significantly higher than 23 mW cm−2 for the latter one, reflecting an enhancement rate of 100% (Figure 4c). This further confirms the superiority of the charge–discharge performance of the BAC/BCF-0.5 air electrode, which can be explained by the extended specific surface area and uniform 3D structure induced by the KOH activation [28,30]. Furthermore, the ∆Egap value for the BAC/BCF-2 cathode is smaller than other batteries, demonstrating the best electrochemical performance. As shown in Figure 4e, the pmax of the ZAB with BAC/BCF-2 composite electrode can reach 96 mWcm−2, which is higher than the 74 mWcm−2 of BAC/BCF-3 and the 46 mW cm−2 of BAC/BCF-0.5, indicating the optimal BAC/BCF ratio is 2. Figure 4f compares the electrochemical performance of BAC/BCF-2 and CAC/BCF-2 cathodes. At the low current density range, BAC/BCF-2 ZAB exhibits better charging performance than that of the CAC/BCF-2 ZAB, but comparative discharge performance and similar pmax values are achieved for the ZABs with BAC/BCF-2 cathode (96 mW cm−2) and CAC/BCF-2 cathode (101 mW cm−2), which are comparative with the results reported previously [42,48,49,50,51,52,53,54,55,56], and the comparison of the electrochemical performance has been summarized in Table S2 in the Supplementary Materials file. These results highly agree with the differences in physicochemical properties between BAC and CAC, demonstrating that the BAC/BCF-2 cathode has comparative electrochemical with the commercial CAC/BCF-2 cathode, making it suitable for practical application.
In addition, the charge–discharge cycling durability and specific capacity of the BAC/BCF-2 ZAB, which has the optimal BAC/BCF recombination ratio, are systematically investigated. Figure 4h shows that the ZAB loaded with BAC/BCF-2 cathode maintains stability at the current densities of 1, 2, 5, 10, and 20 mA cm−2. The discharge potential can return to its initial value after applying a high current density of 20 mA cm−2, indicating its good short-term stability under high current densities. More importantly, this ZAB has a high specific capacity of 865 mAh g−1 at 10 mA cm−2 (Figure 4i), slightly exceeding the theoretical value, and this discrepancy is possible due to the overestimation of zinc consumption because of the accumulation of ZnO on the Zn surface. Additionally, its charge–discharge cycling durability at 10 mA cm−2 with a charge–discharge cycle period of 10 min is presented in Figure 4j,k. The BAC/BCF battery exhibits excellent charge–discharge cycling stability and maintains its function at a lower charge–discharge voltage difference of 1.05 V after 1590 charge–discharge cycles over 265 h operation. Overall, these results, including the output power density, the charge–discharge stability, and specific capacity, confirm that BAC is a promising carbon material candidate.
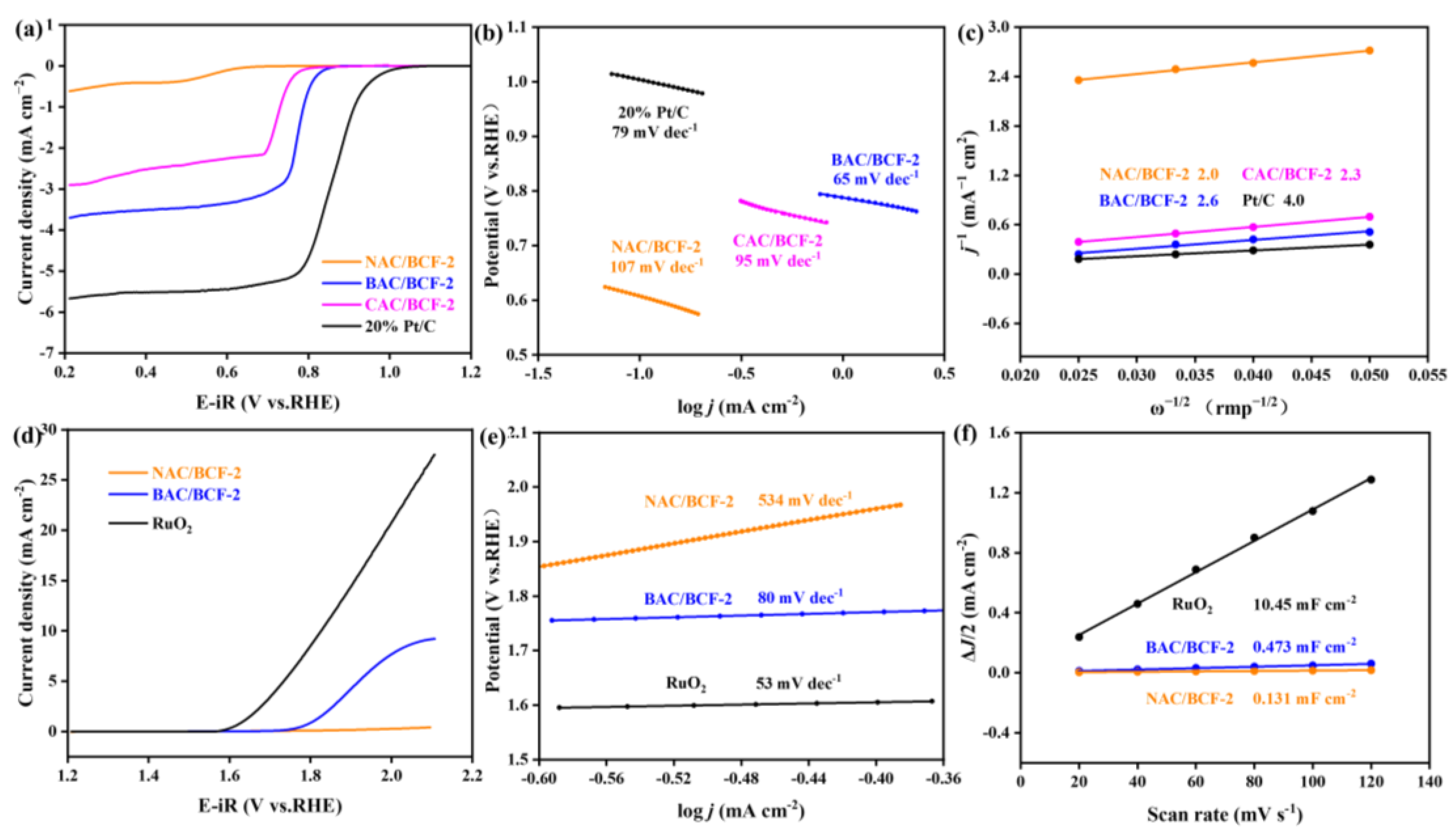
To understand the effect of KOH activation on the ZAB performance, their ORR and OER performance are performed via the rotating disk electrode (RDE) measurements and summarized in Figure 5. The measured half-wave potential (E₁/₂) for the BAC/BCF-2 catalyst is 0.776 V, which is 204 mV higher than the inactivated NBC/BCF catalyst and also 52 mV better than the commercial CAC/BCF-2 (Figure 5a). Figure 5b shows that the Tafel slope value for the BAC/BCF-2 catalyst is 65 mV dec−1, lower than 95 mV dec−1 for the CAC/BCF-2 catalyst and 107 mV dec−1 for the NAC/BCF-2 catalyst. A higher E₁/₂ and lower Tafel slope value indicate better ORR catalytic performance for BAC/BCF-2. Figure S4 in the Supplementary Materials file presents the current densities of BAC/BCF-2 at different scanning rates. The current density increases with raising the rotating rate, indicating that the ORR activity of BAC/BCF-2 is also controlled by the mass diffusion process. According to the Koutecky–Levich (K-L) equation, the calculated electron transfer numbers (n) are 2.6, 2.0, and 2.3 for the BAC/BCF-2, NAC/BCF-2, and CAC/BCF-2 catalysts, respectively (Figure 5c), suggesting that the addition of KOH activation enhances the electron transfer pathway of the ORR process [53,54,55,56,57].
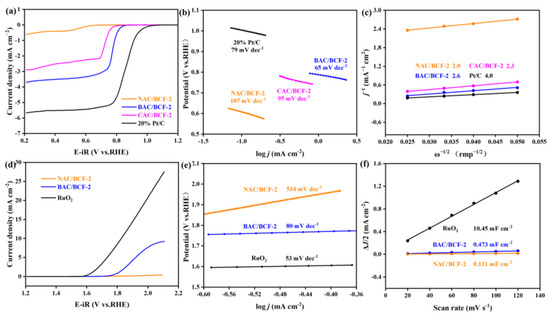
Figure 5.
The electrocatalytic properties for the NAC/BCF, BAC/BCF, and CAC/BCF catalysts, (a–c) ORR performances. (a) LSV curves at 1600 rpm, (b) Tafel plots, (c) K-L curves. (d–f) OER performances. (d) LSV curves at 1600 rpm, (e) Tafel plots, (f) Cdl curves.
The OER curve in Figure 5d shows that BAC/BCF-2 has a lower overpotential and better OER performance compared to NAC/BCF-2, which is further confirmed by the largely decreased Tafel slope value from 534 mV dec−1 for NAC/BCF-2 to 80 mV dec−1 for BAC/BCF-2 (Figure 5e). Additionally, their corresponding electrochemical surface area (ECSA) and double-layer capacitance (Cdl) are calculated using the cyclic voltammetry (CV) measurement (Supplementary Materials, Figure S5) and provided in Figure 5f. The Cdl value is greatly increased from 0.131 mF cm−2 for NAC/BCF-2 to 0.473 mF cm−2 for BAC/BCF-2, indicating the enlarged active sites and specific surface areas for the bifunctional oxygen catalyst after KOH activation. To date, the ORR and OER processes have been dominated by three representative oxygen reaction mechanisms, including the adsorbate evolution mechanism (AEM), lattice oxygen-mediated mechanism (LOM), and the labile oxygen participant adsorbate evolving mechanism (LAM) [58,59,60,61,62]. However, the oxygen reaction mechanism for the cathode studied in this work cannot be clearly explained due to the lack of density function theory calculation results, which needs to be further studied in the future to better understand the feasibility of the carbon materials prepared in this work.
It should be noted that the ORR/OER performance of the BAC/BCF catalyst is still lower than the conventional 20% Pt/C-RuO2 catalyst. However, it is expected that the performance of the composite catalyst can be improved by adjusting the activation temperature or other ways in future studies, for example, the preparation of the hybrid catalysts with the synergistic effect by combining the metals and perovskites [63].
Overall, the BAC/BCF composite cathode based on activated BAC exhibits superior ORR, OER, and ZAB performance. The ORR performance of the BAC/BCF-2 composite cathode surpasses that of the commercial carbon material, further confirming that the BAC/BCF-2 composite electrode can achieve excellent electrocatalytic performance for ZABs.
3. Materials and Methods
3.1. Materials Synthesis
After washing and drying, the waste bagasse is put into a tube furnace, which is then heated at 600 °C in a N2 atmosphere with a dwell time of 2 h. After natural cooling, inactivated sugarcane carbon (NAC) is obtained. Followingly, the NAC is activated by KOH at 900 °C in a N2 atmosphere for 1 h to achieve the BAC. Meanwhile, the perovskite-type BCF is synthesized by the combined solution combustion method and rapid cooling treatment, which is described in our previous work [42]. In this work, the as-prepared NAC and BAC materials are mixed with BCF to prepare the NAC/BCF composite air electrode and BAC/BCF composite air electrode by ultrasonically mixing, respectively. NAC/BCF and BAC/BCF represent composite electrodes composed of inactivated sugarcane carbon and biological activated carbon and BCF, respectively.
3.2. Characterizations
The morphologies of NAC and BAC powders, as well as the commercial activated carbon purchased from Kelude Ketjen black EC-300J, are observed by SEM (MIRA3 LMA, Tescan, Brno, Czech), while the element distribution of BAC/BCF composite material is collected by the equipped EDS (Maxx 20, Oxford Aztec Energy, Oxford Instruments, Abingdon, UK). In addition, the defect degree and structure of all these carbon materials are studied by Raman Spectroscope (Raman, XploRA Plus, HORIBA Jobin Yvon, Paris, France) and FTIR (Nicolet iS50, Thermo Fisher Scientific Instruments, Carlsbad, CA, USA), respectively. Moreover, the valance states of the BAC and BCF material are determined by XPS (ESCALAB250Xi, Thermo Fisher Scientific, Carlsbad, CA, USA) with an Al source.
3.3. Electrochemical Measurements
To investigate the effects of KOH activation and BAC to BCF weight ratio on the electrochemical performance, we prepare five typical air electrode inks, simplified as NAC/BCF-0.5, BAC/BCF-0.5, BAC/BCF-2, BAC/BCF-3, and CAC/BCF-2. Note that Table S1 in the Supplementary Materials file summarizes the detailed parameters of these five air electrode inks, while the descriptions of the ink preparation process are also provided in the Supplementary Materials file.
ZABs are assembled by using a carbon paper loaded with 2 mg cm−2 catalysts mentioned above, a 0.3 mm thick zinc plate anode, and 6 M KOH/0.2 M Zn(CH3COO)2 electrolyte solution, and the cathode and anode are separated by a thin polymer film Celgard 2340. To compare the electrochemical performance of the five ZABs prepared, their LSV curves in the voltage range of 0–2.5 V are recorded by using an electrochemical workstation (Admiral Squidstat Plus, Tempe, AZ, USA), while the charge–discharge cycling stability for the optimal ZABs is evaluated at 10 mA cm−2 with a cycle period of 10 min (CT2001A, LAND Instruments, Wuhan, China).
ORR and OER performance are evaluated in O2-saturated and N2-saturated 0.1 M KOH by a rotating disk electrode (RED, AF01MV10, Pine Instrument, Grove City, OH, USA) connected to the above-mentioned electrochemical workstation in the voltage range of 1.2–0.2 V and 1.2–2.0 V vs. RHE, respectively. Meanwhile, the K-L equation is used to determine the n values,
where J, JK, and JL are measured current density, dynamic current density, and diffusion limit, respectively. F is Faraday’s constant (96, 485 C mol−1), and DO2 is the diffusion coefficient of oxygen with a value of 1.9 × 10−5 cm2 s−1. Meanwhile, k is the rate constant of ORR, v is the kinetic viscosity of the solution (1.0 × 10−2 cm2 s−1), C0 is the saturation concentration of oxygen (1.2 × 10−3 mol L−1 in 0.1 m KOH solution), and ω is the rotational speed (rpm).
To determine selectivity, RDE measurement is performed, and n can be calculated by using the following Equation (2):
where the collection efficiency N is 0.37, IR is the Faraday current at the disk electrode, and ID is the Faraday current at the disk.
4. Conclusions
In summary, the activated carbon derived from sugarcane waste residue can support the sustainable development of efficient and durable ZAB electrode materials and holds great application potential. This work demonstrates that a simple activation process can produce multilayered, oxygen-rich BAC from sugarcane waste. In addition, the combined BAC/BCF cathode significantly enhances the electrochemical and electrocatalytic properties. The pmax of the BAC-based cathode increased by 100% when compared with the inactivated NBC sample, highlighting the substantial impact of the activator on electrochemical performance. The BAC/BCF-2 composite air electrode-based ZAB performs 1590 charge–discharge cycles over 265 h, showing excellent electrocatalytic stability. Additionally, the BAC/BCF-2 catalyst exhibits better ORR performance compared to the CAC/BCF-2 composite electrode. In conclusion, our work could expand the sources of high-efficiency carbon materials for ZABs, propose a new circular economy approach to reduce the environmental impact and promote sustainable ZAB development, and thus provide new insights into surface engineering strategies for advanced ZAB electrode materials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal14100740/s1, Figure S1: TGA curves of the NAC, BAC and CAC in air; Figure S2: The measured OCV for the ZAB based on BAC/BCF-2 air electrode; Figure S3: Photograph of a light-emitting diode (LED) with the “Zn air” logo, powered by the ZAB based on BAC/BCF-2 air electrode; Figure S4: ORR performance: LSV curves of BAC/BCF-2 at different rotating rates; Figure S5: CV curves of (a) NAC/BCF-2, and (b) BAC/BCF-2; Table S1: the detailed parameters of NAC/BCF-0.5, BAC/BCF-0.5, BAC/BCF-2, BAC/BCF-3, and CAC/BCF-2; Table S2: Comparison of electrochemical performance of zinc-air batteries with different cathodes. The references [42,48—55] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, T.L., X.H., and Y.W.; methodology, L.D. and C.W.; validation, L.D., C.W., A.X., and F.Z.; formal analysis, L.D., C.W., A.X., and F.Z.; investigation, L.D. and C.W.; writing—original draft preparation, L.D., C.W., and T.L.; writing—review and editing, T.L., X.H., and Y.W.; supervision, T.L., X.H., and Y.W.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Hubei Province of China (grant number 2024AFB754), the start-up research funds from Wuhan Institute of Technology (grant number K202201), and Innovation and Entrepreneurship Training Program Funded by Wuhan Institute of Technology (grant number 202410490005), Science and Technology Project of State Grid Hunan Electric Power Co., Ltd. (grant number 5216A5240007).
Data Availability Statement
All the data will be available upon request.
Conflicts of Interest
Author Fanglin Zha was employed by the company Electric Power Research Institute of State Grid Hunan Electric Power Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yang, L.S.; Zhou, Y.F.; Meng, B.; Li, H.J.; Zhan, J.; Xiong, H.Y.; Zhao, H.Y.; Cong, W.F.; Wang, X.Z.; Zhang, W.S.; et al. Reconciling productivity, profitability and sustainability of small-holder sugarcane farms: A combined life cycle and data envelopment analysis. Agric. Syst. 2022, 199, 103392. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Hamawand, I.; da Silva, W.; Seneweera, S.; Bundschuh, J. Value Proposition of Different Methods for Utilisation of Sugarcane Wastes. Energies 2021, 14, 5483. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Biomass fly ash geopolymer monoliths for effective methylene blue removal from wastewaters. J. Clean. Prod. 2018, 171, 783–794. [Google Scholar] [CrossRef]
- Sousa, L.N.; Figueiredo, P.F.; França, S.; de Moura Solar Silva, M.V.; Borges, P.H.R.; Bezerra, A.C.d.S. Effect of Non-Calcined Sugarcane Bagasse Ash as an Alternative Precursor on the Properties of Alkali-Activated Pastes. Molecules 2022, 27, 1185. [Google Scholar] [CrossRef]
- Farias, D.; Maugeri-Filho, F. Sequential fed batch extractive fermentation for enhanced bioethanol production using recycled Spathaspora passalidarum and mixed sugar composition. Fuel 2021, 288, 119673. [Google Scholar] [CrossRef]
- Biswas, S.; Mohapatra, S.S.; Kumari, U.; Meikap, B.C.; Sen, T.K. Batch and continuous closed circuit semi-fluidized bed operation: Removal of MB dye using sugarcane bagasse biochar and alginate composite adsorbents. J. Environ. Chem. Eng. 2020, 8, 103637. [Google Scholar] [CrossRef]
- Yin, W.W.; Fu, Z.W. The Potential of Na-Air Batteries. ChemCatChem 2017, 9, 1545–1553. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, A.; Bhatti, H.N.; Zahid, M.; Alissa, S.A.; El-Badry, Y.A.; Hussein, E.E.; Iqbal, M. Composite of polypyrrole with sugarcane bagasse cellulosic biomass and adsorption efficiency for 2,4-dichlorophenoxyacetic acid in column mode. J. Mater. Res. Technol. 2021, 15, 2016–2025. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, A.; Ostrowski, K.A.; Aslam, F.; Joyklad, P.; Zajdel, P. Sustainable approach of using sugarcane bagasse ash in cement-based composites: A systematic review. Case Stud. Constr. Mater. 2021, 15, e00698. [Google Scholar] [CrossRef]
- Madhoushi, M.; Malakani, A.; Ebrahimi, G.; Rashidi, A. Influence of spherical-shaped carbon nanoparticles on the mechanical properties of a foamed sugarcane bagasse/polypropylene composite. Ind. Crops Prod. 2021, 172, 114041. [Google Scholar] [CrossRef]
- Kumari, N.; Sarangi, S.K. Bagasse reinforced epoxy-based green composite for orthotic callipers: A tribological study. Mater. Today Proc. 2022, 56, 1156–1159. [Google Scholar] [CrossRef]
- Andrade, J.P.d.S.C.; Cecchin, D.; Vieira, C.M.F.; Delaqua, G.C.G.; Silva, F.C.d.; Hamacher, L.d.S.; da Silva, T.R.; Amran, M.; Paes, J.L.; Moll Hüther, C. Agro-Industrial Waste of Malt Bagasse: Perspectives on the Development of Eco-Friendly Ceramic Material. Sustainability 2023, 15, 9120. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Molina, G.; Pelissari, F.M. Biodegradable trays based on cassava starch blended with agroindustrial residues. Compos. Part B Eng. 2020, 183, 107682. [Google Scholar] [CrossRef]
- Rattanachueskul, N.; Dokkathin, O.; Dechtrirat, D.; Panpranot, J.; Watcharin, W.; Kaowphong, S.; Chuenchom, L. Sugarcane Bagasse Ash as a Catalyst Support for Facile and Highly Scalable Preparation of Magnetic Fenton Catalysts for Ultra-Highly Efficient Removal of Tetracycline. Catalysts 2022, 12, 446. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Borelli, D.; Devia, F.; Schenone, C.; Silenzi, F.; Sollai, F.; Tagliafico, L.A. Assessing environmental benefits of the transition from standard fossil fuels to liquefied natural gas: The Sardinia Region case study. Energy Sustain. Dev. 2023, 73, 205–217. [Google Scholar] [CrossRef]
- Rashedi, A.; Khanam, T.; Jonkman, M. On Reduced Consumption of Fossil Fuels in 2020 and Its Consequences in Global Environment and Exergy Demand. Energies 2020, 13, 6048. [Google Scholar] [CrossRef]
- Shelare, S.D.; Belkhode, P.N.; Nikam, K.C.; Jathar, L.D.; Shahapurkar, K.; Soudagar, M.E.M.; Veza, I.; Khan, T.M.Y.; Kalam, M.A.; Nizami, A.-S.; et al. Biofuels for a sustainable future: Examining the role of nano-additives, economics, policy, internet of things, artificial intelligence and machine learning technology in biodiesel production. Energy 2023, 282, 128874. [Google Scholar] [CrossRef]
- Wang, M.; Su, K.; Zhang, M.; Du, X.; Li, Z. Advanced trifunctional electrocatalysis with Cu-, N-, S-doped defect-rich porous carbon for zinc-air batteries. ACS Sustain. Chem. Eng. 2021, 9, 13324–13336. [Google Scholar] [CrossRef]
- Lin, H.; Xie, J.; Zhang, Z.; Wang, S.; Chen, D. Perovskite nanoparticles@N-doped carbon nanofibers as robust and efficient oxygen electrocatalysts for Zn-air batteries. J. Colloid Interface Sci. 2021, 581 Pt. A, 374–384. [Google Scholar] [CrossRef]
- Ma, R.; Gao, F.; Breaz, E.; Huangfu, Y.; Briois, P. Multidimensional Reversible Solid Oxide Fuel Cell Modeling for Embedded Applications. IEEE Trans. Energy Convers. 2018, 33, 692–701. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Martins, M.; Sequeira, C.A.C.; Šljukić, B. Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries 2023, 9, 394. [Google Scholar] [CrossRef]
- Wang, H.F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, H.; Wang, Y.; Guo, J.H.; Sun, X.W.; Sun, W.Y.; Zhao, H.T.; Bai, J.; Li, C.P. Tailoring the d-band electronic structure of deficient LaMn0.3Co0.7O3-δ perovskite nanofibers for boosting oxygen electrocatalysis in Zn-Air batteries. J. Colloid Interface Sci. 2023, 650, 951–960. [Google Scholar] [CrossRef]
- Ran, J.Q.; Wu, J.F.; Hu, Y.F.; Shakouri, M.; Xia, B.; Gao, D.Q. Atomic-level coupled spinel@perovskite dual-phase oxides toward enhanced performance in Zn–air batteries. J. Mater. Chem. A 2022, 10, 1506–1513. [Google Scholar] [CrossRef]
- Bu, Y.F.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Ajay, K.M.S.; Dinesh, M.N.; Yashaswini, M.; Gopalakrishna, B.; Kathyayini, N.; Sundarayya, Y.; Vijeth, H. Natural Biomass Derived Microporous Activated Carbon Electrodes for Highly Efficient Supercapacitor Applications. ChemistrySelect 2022, 7, e202201301. [Google Scholar] [CrossRef]
- Liu, R.M.; Liu, H.N.; Yang, Q.Y.; Ma, Y.J.; Dong, D.Q.; Wang, J. Longan-Derived Biomass Carbon-Induced Cubic-Type Ferric Oxide Nanoparticles for Efficient Lithium-Ion Battery Anodes. Energy Fuels 2023, 37, 16979–16987. [Google Scholar] [CrossRef]
- Yang, S.; Qu, K.Q.; Huang, Z.H. Optimizing Hierarchical Porous Carbon from Biomass Waste for High-Performance Supercapacitors. ES Food Agrofor. 2022, 10, 39–50. [Google Scholar] [CrossRef]
- Xu, X.J.; Huang, Y.; Wei, W.R.; Fan, Y.; Jiang, Z.D.; Li, R.H.; Yuan, X.X. Long-Cycling and High-Rate Potassium Storage Enabled by Sulfur-Doped Carbon Derived from Disposable Chopsticks. Energy Fuels 2023, 37, 14375–14382. [Google Scholar] [CrossRef]
- Wu, J.Q.; Ma, Z.H.; Wang, G.N.; Chen, T.T. Biomass Porous Carbon Derived from Celery Leaves with High Capacitance for Supercapacitor. ChemistrySelect 2023, 8, e202204616. [Google Scholar] [CrossRef]
- Yu, J.; Jia, X.F.; Peng, J.X.; Meng, B.; Wei, Y.B.; Hou, X.Y.; Zhao, J.K.; Yang, N.X.; Xie, K.Y.; Chu, D.W.; et al. Synergistic Effect of Nitrogen–Sulfur Codoping on Honeycomb-like Carbon-Based High-Energy-Density Zinc-Ion Hybrid Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 2728–2738. [Google Scholar] [CrossRef]
- Zheng, S.M.; Yuan, Z.H.; Dionysiou, D.D.; Zhong, L.B.; Zhao, F.; Yang, J.C.E.; Zheng, Y.M. Silkworm cocoon waste-derived nitrogen-doped hierarchical porous carbon as robust electrode materials for efficient capacitive desalination. Chem. Eng. J. 2023, 458, 141471. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.Y.; Luo, Q.T.; Li, H.J.; Li, J.; Yang, W.Q. Capillary Evaporation on High-Dense Conductive Ramie Carbon for Assisting Highly Volumetric-Performance Supercapacitors. Small 2023, 19, 2303349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.X.; Zhang, X.; Chen, H.S.; Hu, X.; Yang, P. Formation of g-C3N4 Nanotubes towards Superior Photocatalysis Performance. ChemCatChem 2019, 11, 4558–4567. [Google Scholar] [CrossRef]
- Shi, K.J.; Li, Y.; Zhang, Y.Y.; Li, X.F.; Zhu, Z.J.; Xu, H.Y.; Zheng, L.C.; Gao, J. N-doped 3D hierarchical carbon from water hyacinth for high-performance Zn-air batteries. Diam. Relat. Mater. 2023, 135, 109841. [Google Scholar] [CrossRef]
- Jaiswal, K.; Groutchik, K.; Bawari, D.; Dobrovetsky, R. An “On-Demand”, Selective Dehydrogenative Borylation or Hydroboration of Terminal Alkynes Using Zn2±based Catalyst. ChemCatChem 2022, 14, e202200004. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Jain, M.; Pant, K.K.; Maheshwari, P.H.; Sundriyal, S. Facile Synthesis of Pineapple Waste-Derived Carbon and Polyaniline Composite for High-Energy-Density Asymmetric Supercapacitors. Energy Fuels 2023, 37, 8659–8671. [Google Scholar] [CrossRef]
- Yu, J.; Ran, R.; Zhong, Y.; Zhou, W.; Ni, M.; Shao, Z. Advances in porous perovskites: Synthesis and electrocatalytic performance in fuel and metal–air batteries. Energy Environ. Mater. 2020, 3, 121–145. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-performance perovskite composite electrocatalysts enabled by controllable interface engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Q.; Zhou, J.; Liu, T.; Wang, J.; Wang, Y.; Zhang, D.; Huang, D.X.; Liu, Y.L.; Hu, X.L. Compositional engineering of perovskite oxide BaCo0.5Fe0.5O3-δ as an efficient bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Catal. Sci. Technol. 2024, 14, 589–605. [Google Scholar] [CrossRef]
- Abbas, A.; Rubab, S.; Rehman, A.; Irfan, S.; Sharif, H.M.A.; Liang, Q.; Tabish, T.A. One-step green synthesis of biomass-derived graphene quantum dots as a highly selective optical sensing probe. Mater. Today Chem. 2023, 30, 101555. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zou, Y.H.; Chen, H.J.; Zhang, K.W.; Hui, B. Bamboo-Modulated Helical Carbon Nanotubes for Rechargeable Zn-Air Battery. Small 2023, 20, 2307776. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.R.; Jin, C.; Lu, J.; Sun, J.W.; Yang, R.Z. Preparation of Perovskite Oxides/(CoFe)P2 Heterointerfaces to Improve Oxygen Evolution Activity of La0.8Sr0.2Co0.2Fe0.8O4+δ Layered Perovskite Oxide. Int. J. Hydrogen Energy 2020, 45, 22959–22964. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Chen, Y.B.; Yang, G.M.; Xu, X.M.; Tadé, M.O.; Shao, Z.P. SrCo0.9Ti0.1O3-δ As a New Electrocatalyst for the Oxygen Evolution Reaction in Alkaline Electrolyte with Stable Performance. ACS Appl. Mater. Interfaces 2015, 7, 17663–17670. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Sun, Y.F.; Zhang, Y.Q.; Yan, N.; Chen, J.; Thundat, T.; Li, J.; Luo, J.L. A Coupling for Success: Controlled Growth of Co/CoOx Nanoshoots on Perovskite Mesoporous Nanofibres as High-Performance Trifunctional Electrocatalysts in Alkaline Condition. Nano Energy 2017, 32, 247–254. [Google Scholar] [CrossRef]
- Huang, Y.L.; Liu, Y.Z.; Deng, Y.Z.; Zhang, J.; He, B.; Sun, J.; Yang, Z.; Zhou, W.; Zhao, L. Enhancing the Bifunctional Activity of CoSe2 Nanocubes by Surface Decoration of CeO2 for Advanced Zinc-Air Batteries. J. Colloid Interface Sci. 2022, 625, 839–849. [Google Scholar] [CrossRef]
- Li, P.Z.; Wei, B.; Lü, Z.; Wu, Y.Y.; Zhang, Y.H.; Huang, X.Q. La1.7Sr0.3CO0.5Ni0.5O4+δ Layered Perovskite as an Efficient Bifunctional Electrocatalyst for Rechargeable Zinc-Air Batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Gui, L.Q.; Wang, Z.B.; Zhang, K.; He, B.B.; Liu, Y.Z.; Zhou, W.; Xu, J.M.; Wang, Q.; Zhao, L. Oxygen Vacancies-Rich Ce0.9Gd0.1O2-δ Decorated Pr0.5Ba0.5CoO3-δ Bifunctional Catalyst for Efficient and Long-Lasting Rechargeable Zn-Air Batteries. Appl. Catal. B Environ. 2020, 266, 118656. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, T.; Zhang, J.; Zhao, L.; He, W.; Wang, Y. Rational Design of Ultrafine Cobalt-Free Electrospun Nanofibers as Efficient and Durable Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Battery. Sep. Purif. Technol. 2023, 304, 122316. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhang, D.; Zhong, D.; Liu, T.; Ding, M.Y. Electrospun 3D Structured Double Perovskite Oxide PrBa0.8Ca0.2Co2O5+δ Bifunctional Electrocatalyst for Zinc-Air Battery. J. Am. Ceram. Soc. 2024, 107, 3265–3276. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.Y.; Yuan, M.K.; Hao, H.R.; Lv, Z.; Xu, L.L.; Wei, B. Operando Spectroscopies Unveil Interfacial FeOOH Induced Highly Reactive β-Ni(Fe)OOH for Efficient Oxygen Evolution. Appl. Catal. B Environ. 2022, 318, 121825. [Google Scholar] [CrossRef]
- Huang, D.X.; Liu, T.; Xu, A.Q.; Zhou, J.; Wang, Y.; Hu, X.L. A Novel Layered Cobalt Oxide Ba2Co9O14 as an Efficient and Durable Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. Electrochim. Acta 2024, 494, 144450. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.R.; Cai, W.Z.; He, W.; Ni, M. In-situ Growth of Co3O4 Nanowire-Assembled Clusters on Nickel Foam for Aqueous Rechargeable Zn-Co3O4 and Zn-Air Batteries. Appl. Catal. B Environ. 2019, 241, 104–112. [Google Scholar] [CrossRef]
- Wang, Y.B.; Ge, X.L.; Lu, Q.; Bai, W.J.; Ye, C.C.; Shao, Z.P.; Bu, Y.F. Accelerated Deprotonation with a Hydroxy-Silicon Alkali Solid for Rechargeable Zinc-Air Batteries. Nat. Commun. 2023, 14, 6968. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Liu, T.; Dai, Y.W.; Wang, J.; Wang, Y.; Guo, Z.J.; Yu, J.; Bello, I.T.; Ni, M. Pt/C as a Bifunctional ORR/Iodide Oxidation Reaction (IOR) Catalyst for Zn-Air Batteries with Unprecedentedly High Energy Efficiency of 76.5%. Appl. Catal. B Environ. 2023, 320, 121992. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Liu, J.; Lang, X.; Wang, W. Labile oxygen participant adsorbate evolving mechanism to enhance oxygen reduction in SmMn 2 O 5 with double-coordinated crystal fields. J. Mater. Chem. A 2023, 9, 380–389. [Google Scholar] [CrossRef]
- Yoo, J.S.; Rong, X.; Liu, Y.; Kolpak, A.M. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites. ACS Catal. 2018, 8, 4628–4636. [Google Scholar] [CrossRef]
- Zagalskaya, A.; Alexandrov, V. Role of defects in the interplay between adsorbate evolving and lattice oxygen mechanisms of the oxygen evolution reaction in RuO2 and IrO2. ACS Catal. 2020, 10, 3650–3657. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, L.; Shi, J.; Xie, M.Y.; Nie, J.; Huang, G.F.; Li, B.; Hu, W.; Pan, A.; Huang, W.Q. Oxygen defect engineering promotes synergy between adsorbate evolution and single lattice oxygen mechanisms of OER in transition metal-based (oxy) hydroxide. Adv. Sci. 2023, 10, 2303321. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-supported perovskite as an efficient bifunctional electrocatalyst for oxygen reduction and evolution: Substrate effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).