Enhanced Stability and Selectivity in Pt@MFI Catalysts for n-Butane Dehydrogenation: The Crucial Role of Sn Promoter
Abstract
:1. Introduction
2. Results and Discussion
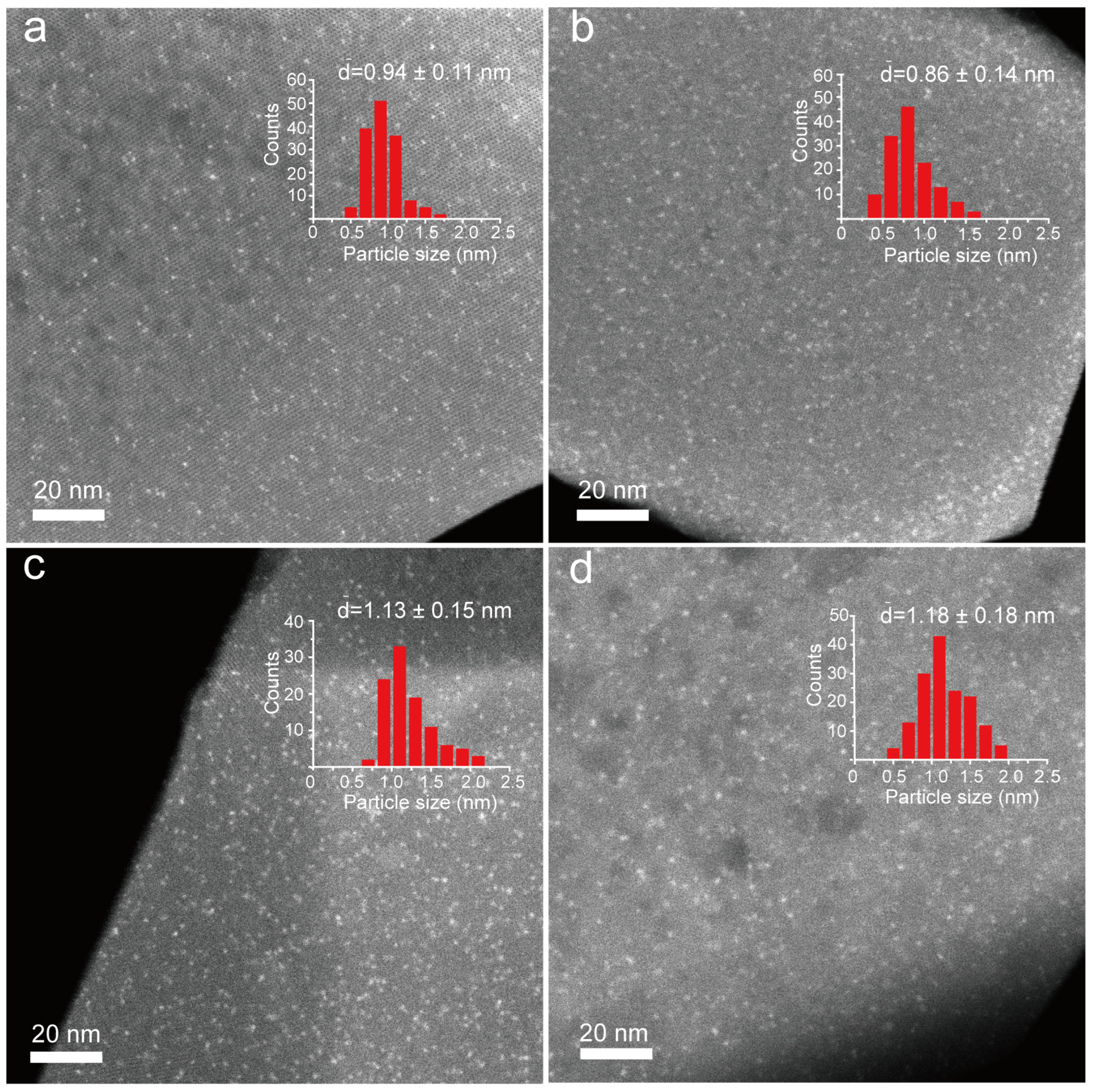
2.1. Characterization of Pt@MFI with Various Promoters
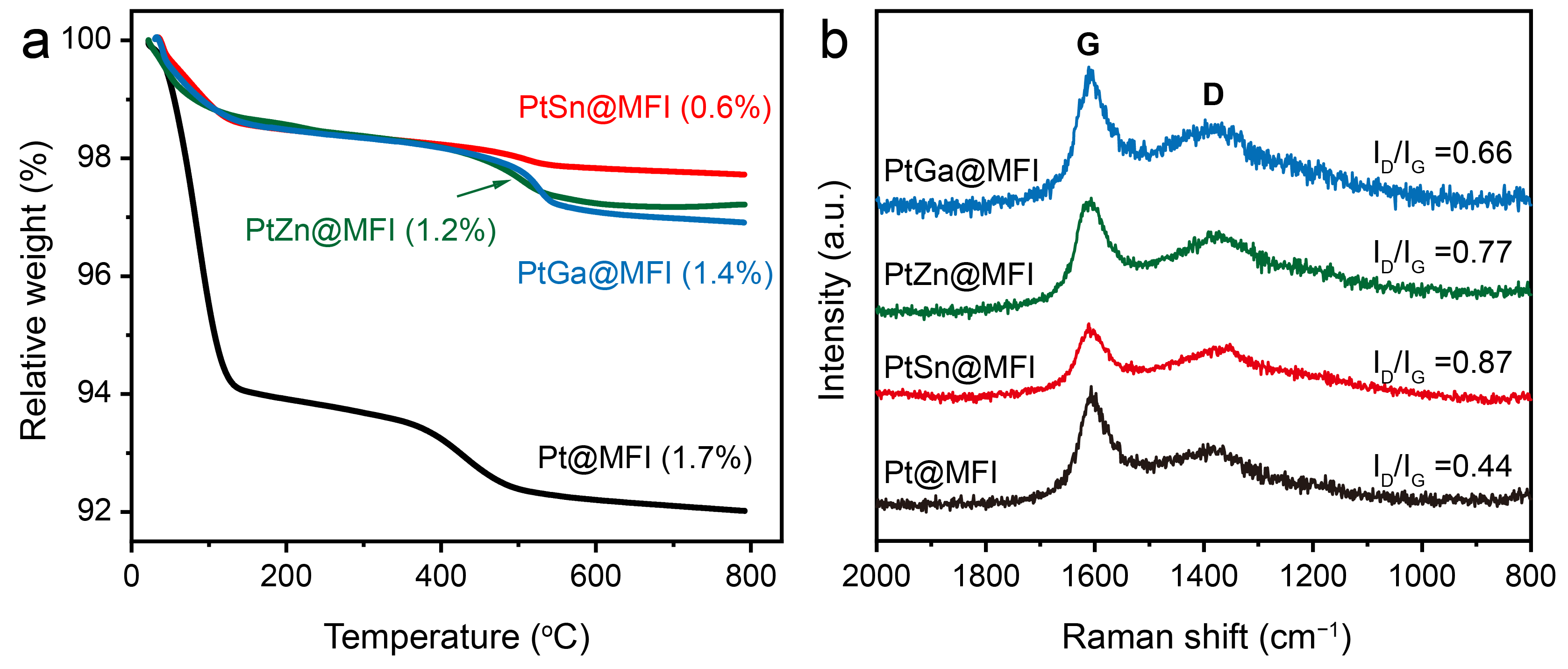
2.2. Effect of Promoter on the Structure of Pt-M@MFI
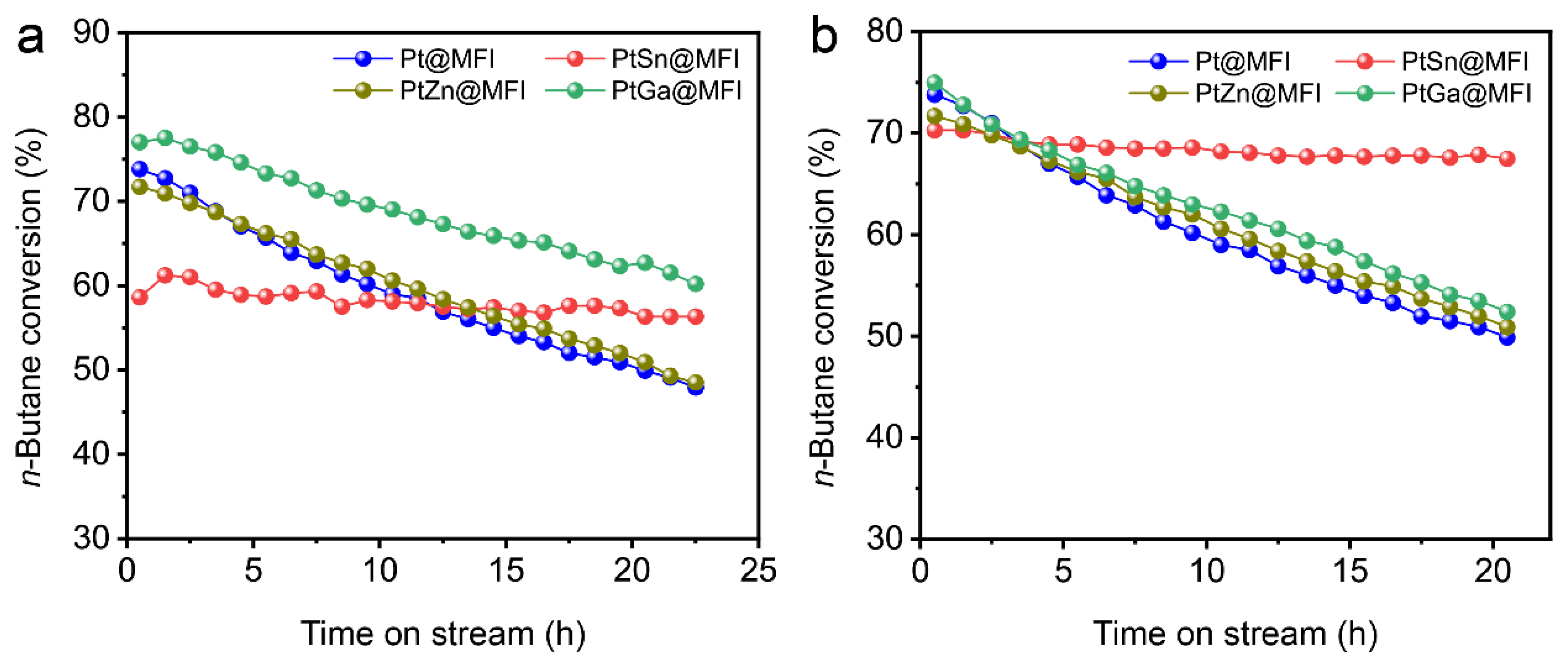
2.3. Catalytic Performance in n-Butane Dehydrogenation
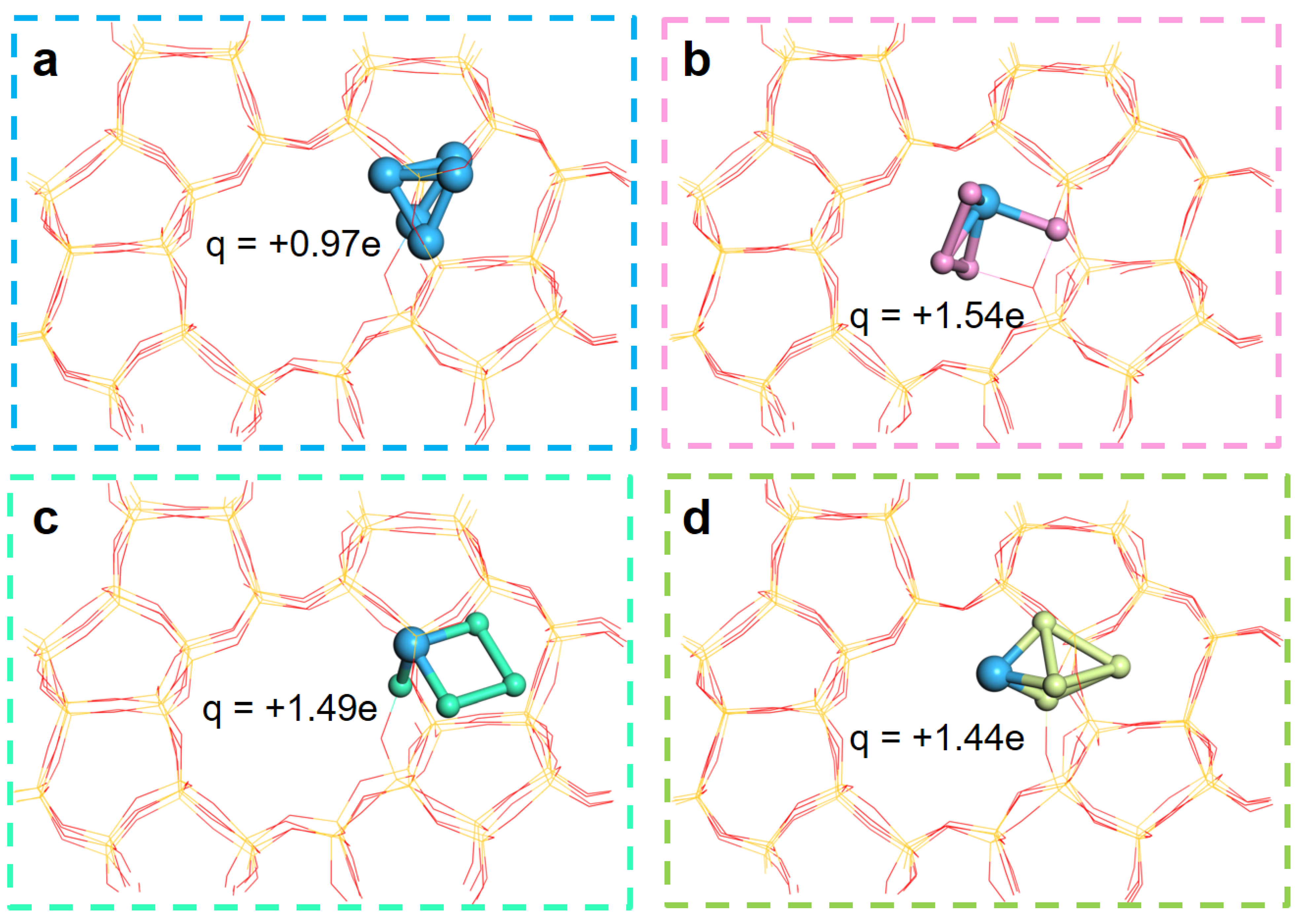
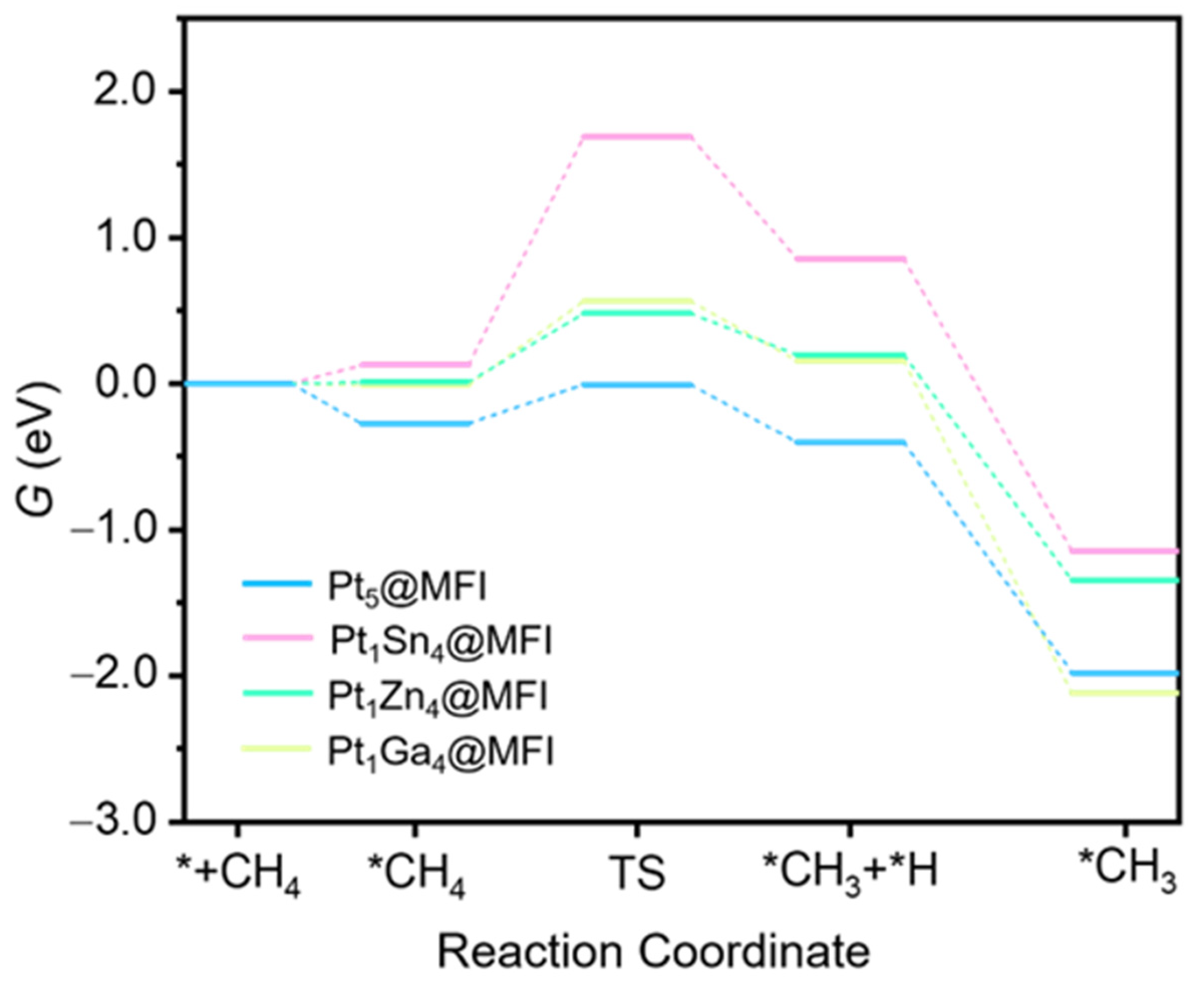
2.4. Theoretical Study on the Effect of Promoter
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Xu, Y.; Chang, X.; Pan, Y.; Sun, G.; Wang, X.; Fu, D.; Pei, C.; Zhao, Z.-J.; Su, D. Defective TiOx overlayers catalyze propane dehydrogenation promoted by base metals. Science 2024, 385, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Yan, B.; Lu, W.-D.; Qiu, B.; Gao, X.-Q.; Wang, D.; Lu, A.-H. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts. Chem. Soc. Rev. 2021, 50, 1438–1468. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Gong, N.; Liu, L. Solid catalysts for the dehydrogenation of long-chain alkanes: Lessons from the dehydrogenation of light alkanes and homogeneous molecular catalysis. Sci. Chi. Chem. 2022, 65, 2163–2176. [Google Scholar] [CrossRef]
- Jackson, S.D.; Rugmini, S. Dehydrogenation of n-butane over vanadia catalysts supported on θ-alumina. J. Catal. 2007, 251, 59–68. [Google Scholar] [CrossRef]
- Rodríguez, L.; Romero, D.; Rodríguez, D.; Sanchez, J.; Domínguez, F.; Arteaga, G. Dehydrogenation of n-butane over Pd–Ga/Al2O3 catalysts. Appl. Catal. A-Gen. 2010, 373, 66–70. [Google Scholar] [CrossRef]
- de Ballesteros, O.R.; Auriemma, F.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; De Rosa, C. Mechanical properties of isotactic 1-butene-ethylene copolymers from Ziegler-Natta catalyst. Polymer 2021, 216, 123408. [Google Scholar] [CrossRef]
- Metzger, E.D.; Comito, R.J.; Hendon, C.H.; Dincă, M. Mechanism of single-site molecule-like catalytic ethylene dimerization in Ni-MFU-4 l. J. Am. Chem. Soc. 2017, 139, 757–762. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Chen, X.; Peng, M.; Cai, X.; Chen, Y.; Jia, Z.; Deng, Y.; Mei, B.; Jiang, Z.; Xiao, D.; Wen, X. Regulating coordination number in atomically dispersed Pt species on defect-rich graphene for n-butane dehydrogenation reaction. Nat. Commun. 2021, 12, 2664. [Google Scholar]
- Wang, H.; Fan, S.; Guo, S.; Wang, S.; Qin, Z.; Dong, M.; Zhu, H.; Fan, W.; Wang, J. Selective conversion of CO2 to isobutane-enriched C4 alkanes over InZrO x-Beta composite catalyst. Nat. Commun. 2023, 14, 2627. [Google Scholar]
- Zhang, Y.; Qi, L.; Leonhardt, B.; Bell, A.T. Mechanism and kinetics of n-butane dehydrogenation to 1, 3-butadiene catalyzed by isolated Pt sites grafted onto≡ SiOZn–OH nests in dealuminated zeolite Beta. ACS Catal. 2022, 12, 3333–3345. [Google Scholar] [CrossRef]
- Li, C.; Wang, G. Dehydrogenation of light alkanes to mono-olefins. Chem. Soc. Rev. 2021, 50, 4359–4381. [Google Scholar] [CrossRef] [PubMed]
- Rodemerck, U.; Sokolov, S.; Stoyanova, M.; Bentrup, U.; Linke, D.; Kondratenko, E.V. Influence of support and kind of VOx species on isobutene selectivity and coke deposition in non-oxidative dehydrogenation of isobutane. J. Catal. 2016, 338, 174–183. [Google Scholar] [CrossRef]
- Shao, M.; Song, Y.; Hu, C.; Xu, X.; Li, C. Design of PtM (M= Ru, Au, or Sn) bimetallic particles supported on TS-1 for the direct dehydrogenation of n-butane. Fuel 2023, 341, 127630. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Sun, L.-L.; Sui, Z.-J.; Zhu, Y.-A.; Ye, G.-H.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. Coke formation on Pt–Sn/Al2O3 catalyst for propane dehydrogenation. Ind. Eng. Chem. Res. 2018, 57, 8647–8654. [Google Scholar] [CrossRef]
- Zhu, J.; Osuga, R.; Ishikawa, R.; Shibata, N.; Ikuhara, Y.; Kondo, J.N.; Ogura, M.; Yu, J.; Wakihara, T.; Liu, Z. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: Catalytic application for propane dehydrogenation. Angew. Chem. Int. Ed. 2020, 59, 19669–19674. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, Q. Advances in zeolite-supported metal catalysts for propane dehydrogenation. Inorg. Chem. Front. 2022, 9, 3095–3115. [Google Scholar] [CrossRef]
- Zhang, K.; Dou, X.; Zhou, Z.; Wang, Y.; Hou, H.; Meira, D.; Liu, L.; He, P. Tuning the size and spatial distribution of Pt in bifunctional Pt-zeolite catalysts for direct coupling of ethane and benzene. Chem. Eng. J. 2024, 497, 154874. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Liu, L.; Diaz, U.; Arenal, R.; Agostini, G.; Concepcion, P.; Corma, A. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 2017, 16, 132–138. [Google Scholar] [CrossRef]
- Liu, L.; Lopez-Haro, M.; Lopes, C.W.; Li, C.; Concepcion, P.; Simonelli, L.; Calvino, J.J.; Corma, A. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 2019, 18, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lopez-Haro, M.; Lopes, C.W.; Rojas-Buzo, S.; Concepcion, P.; Manzorro, R.; Simonelli, L.; Sattler, A.; Serna, P.; Calvino, J.J. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 2020, 3, 628–638. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, X.; Doronkin, D.E.; Han, S.; Kondratenko, V.A.; Grunwaldt, J.-D.; Perechodjuk, A.; Vuong, T.H.; Rabeah, J.; Eckelt, R. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Babucci, M.; Zhang, Y.; Lund, A.; Liu, L.; Li, J.; Chen, Y.; Hoffman, A.S.; Bare, S.R.; Han, Y. Propane dehydrogenation catalyzed by isolated Pt atoms in≡ SiOZn–OH nests in dealuminated zeolite Beta. J. Am. Chem. Soc. 2021, 143, 21364–21378. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, E.; Head-Gordon, M.; Bell, A.T. Computational modeling of the nature and role of Ga species for light alkane dehydrogenation catalyzed by Ga/H-MFI. ACS Catal. 2018, 8, 6146–6162. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and kinetics of propane dehydrogenation and cracking over Ga/H-MFI prepared via vapor-phase exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2018, 141, 1614–1627. [Google Scholar] [CrossRef]
- Phadke, N.M.; Van der Mynsbrugge, J.; Mansoor, E.; Getsoian, A.B.; Head-Gordon, M.; Bell, A.T. Characterization of isolated Ga3+ cations in Ga/H-MFI prepared by vapor-phase exchange of H-MFI zeolite with GaCl3. ACS Catal. 2018, 8, 6106–6126. [Google Scholar] [CrossRef]
- Dou, X.; Li, W.; Zhang, K.; Hou, H.; He, Z.; Zhu, C.; Meira, D.M.; Lopez-Haro, M.; Xia, Z.; He, P. Size-dependent structural features of subnanometer PtSn catalysts encapsulated in zeolite for alkane dehydrogenation. ACS Catal. 2024, 14, 2859–2871. [Google Scholar] [CrossRef]
- Wan, H.; Qian, L.; Gong, N.; Hou, H.; Dou, X.; Zheng, L.; Zhang, L.; Liu, L. Size-dependent structures and catalytic properties of supported bimetallic PtSn catalysts for propane dehydrogenation reaction. ACS Catal. 2023, 13, 7383–7394. [Google Scholar] [CrossRef]
- Schallmoser, S.; Ikuno, T.; Wagenhofer, M.; Kolvenbach, R.; Haller, G.; Sanchez-Sanchez, M.; Lercher, J. Impact of the local environment of Brønsted acid sites in ZSM-5 on the catalytic activity in n-pentane cracking. J. Catal. 2014, 316, 93–102. [Google Scholar] [CrossRef]
- Wu, Y.; Xi, S.; Chen, C.; Hu, Q.; Xiong, Z.; Wang, J.; Dai, Y.; Han, Y.; Jiang, S.; Wang, J. Synergistic roles of platinum nanoparticles and sodium ions within beta zeolites in N-alkylation of amines with aromatic alcohols. Sci. Chi. Chem. 2023, 66, 2690–2699. [Google Scholar] [CrossRef]
- He, P.; Jarvis, J.S.; Meng, S.; Li, Q.; Bernard, G.M.; Liu, L.; Mao, X.; Jiang, Z.; Zeng, H.; Michaelis, V.K. Co-aromatization of methane with propane over Zn/HZSM-5: The methane reaction pathway and the effect of Zn distribution. Appl. Catal. B-Environ. 2019, 250, 99–111. [Google Scholar] [CrossRef]
- Liu, B.; Wang, F.; Dou, X.; Li, P.; Xiang, H.; Yang, Y.; He, P. Co-aromatization of methane and hexane over Pt encapsulated in ZSM-5 zeolite and the electronic effect of K promoters. Sci. Chi. Chem. 2024, 67, 1017–1027. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.; Beato, P. Structure–deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Danilova, I.G.; Arzumanov, S.S.; Toktarev, A.V.; Freude, D.; Stepanov, A.G. Strong acidity of silanol groups of zeolite beta: Evidence from the studies by IR spectroscopy of adsorbed CO and 1H MAS NMR. Micropor. Mesopor. Mat. 2010, 131, 210–216. [Google Scholar] [CrossRef]
- Grand, J.; Talapaneni, S.N.; Vicente, A.; Fernandez, C.; Dib, E.; Aleksandrov, H.A.; Vayssilov, G.N.; Retoux, R.; Boullay, P.; Gilson, J.-P. One-pot synthesis of silanol-free nanosized MFI zeolite. Nat. Mater. 2017, 16, 1010–1015. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2014; Volume 57, pp. 99–318. [Google Scholar]
- Burton, A.; Terefenko, E.; Wang, H.; Paccagnini, M.; Sattler, A. Structure-property relationships that influence platinum stability in all-silica or highly siliceous zeolites. Micropor. Mesopor. Mat. 2023, 358, 112655. [Google Scholar] [CrossRef]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effects of alkali additives on the physicochemical characteristics and chemisorptive properties of Pt/TiO2 catalysts. J. Catal. 2008, 260, 141–149. [Google Scholar] [CrossRef]
- Kim, W.-g.; So, J.; Choi, S.-W.; Liu, Y.; Dixit, R.S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W. Hierarchical Ga-MFI catalysts for propane dehydrogenation. Chem. Mater. 2017, 29, 7213–7222. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, W.-G.; So, J.-S.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G.; Sievers, C.; Sholl, D.S.; Nair, S. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Yuan, Y.; Brady, C.; Lobo, R.F.; Xu, B. Understanding the correlation between Ga speciation and propane dehydrogenation activity on Ga/H-ZSM-5 catalysts. ACS Catal. 2021, 11, 10647–10659. [Google Scholar] [CrossRef]
- He, S.; Sun, C.; Yang, X.; Wang, B.; Dai, X.; Bai, Z. Characterization of coke deposited on spent catalysts for long-chain-paraffin dehydrogenation. Chem. Eng. J. 2010, 163, 389–394. [Google Scholar] [CrossRef]
- Filez, M.; Walke, P.; Le-The, H.; Toyouchi, S.; Peeters, W.; Tomkins, P.; Eijkel, J.C.; De Feyter, S.; Detavernier, C.; De Vos, D.E. Nanoscale Chemical Diversity of Coke Deposits on Nanoprinted Metal Catalysts Visualized by Tip-Enhanced Raman Spectroscopy. Adv. Mater. 2024, 36, 2305984. [Google Scholar] [CrossRef]
- Lin, L.; Lin, W.; Zhu, Y.X.; Zhao, B.Y.; Xie, Y.C.; Jia, G.Q.; Li, C. Uniformly Carbon-Covered Alumina and Its Surface Characteristics. Langmuir 2005, 21, 5040–5046. [Google Scholar] [CrossRef]
- Li, J.; Xiong, G.; Feng, Z.; Liu, Z.; Xin, Q.; Li, C. Coke formation during the methanol conversion to olefins in zeolites studied by UV Raman spectroscopy. Micropor. Mesopor. Mat. 2000, 39, 275–280. [Google Scholar] [CrossRef]
- Negri, F.; Castiglioni, C.; Tommasini, M.; Zerbi, G. A Computational Study of the Raman Spectra of Large Polycyclic Aromatic Hydrocarbons: Toward Molecularly Defined Subunits of Graphite. J. Phys. Chem. A 2002, 106, 3306–3317. [Google Scholar] [CrossRef]
- Zhao, R.; Cao, K.; Ye, R.; Tang, Y.; Du, C.; Liu, F.; Zhao, Y.; Chen, R.; Shan, B. Deciphering the stability mechanism of Pt-Ni/Al2O3 catalysts in syngas production via DRM. Chem. Eng. J. 2024, 491, 151966. [Google Scholar] [CrossRef]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nat. Chem. 2016, 8, 718–724. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Heard, C.J.; Gołąbek, K.; Ju, X.; Čejka, J.; Mazur, M. Encapsulating metal nanoparticles into a layered zeolite precursor with surface silanol nests enhances sintering resistance. Angew. Chem. Int. Ed. 2023, 62, e202213361. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L. Database of Zeolite Structures; International Zeolite Association: Napoli, Italy, 2001. [Google Scholar]
- Seo, H.; Lee, J.K.; Hong, U.G.; Park, G.; Yoo, Y.; Lee, J.; Chang, H.; Song, I.K. Direct dehydrogenation of n-butane over Pt/Sn/M/γ-Al2O3 catalysts: Effect of third metal (M) addition. Catal. Comm. 2014, 47, 22–27. [Google Scholar] [CrossRef]
- Ballarini, A.D.; Zgolicz, P.; Vilella, I.M.; de Miguel, S.R.; Castro, A.A.; Scelza, O.A. n-Butane dehydrogenation on Pt, PtSn and PtGe supported on γ-Al2O3 deposited on spheres of α-Al2O3 by washcoating. Appl. Catal. A-Gen. 2010, 381, 83–91. [Google Scholar] [CrossRef]
- Nawaz, Z.; Fei, W. Pt−Sn-Based SAPO-34 Supported Novel Catalyst for n-Butane Dehydrogenation. Ind. Eng. Chem. Res. 2009, 48, 7442–7447. [Google Scholar] [CrossRef]
- Deng, L.; Miura, H.; Ohkubo, T.; Shishido, T.; Wang, Z.; Hosokawa, S.; Teramura, K.; Tanaka, T. The importance of direct reduction in the synthesis of highly active Pt–Sn/SBA-15 for n-butane dehydrogenation. Catal. Sci. Technol. 2019, 9, 947–956. [Google Scholar] [CrossRef]
- Bocanegra, S.; Ballarini, A.; Zgolicz, P.; Scelza, O.; De Miguel, S. Highly selective and stable bimetallic catalysts supported on different materials for n-butane dehydrogenation. Catal. Today 2009, 143, 334–340. [Google Scholar] [CrossRef]
- De Miguel, S.R.; Bocanegra, S.A.; Vilella, I.J.; Guerrero-Ruiz, A.; Scelza, O.A. Characterization and catalytic performance of PtSn catalysts supported on Al2O3 and Na-doped Al2O3 in n-butane dehydrogenation. Catal. Lett. 2007, 119, 5–15. [Google Scholar] [CrossRef]
- Camacho-Bunquin, J.; Ferrandon, M.S.; Sohn, H.; Kropf, A.J.; Yang, C.; Wen, J.; Hackler, R.A.; Liu, C.; Celik, G.; Marshall, C.L. Atomically precise strategy to a PtZn alloy nanocluster catalyst for the deep dehydrogenation of n-butane to 1,3-butadiene. ACS Catal. 2018, 8, 10058–10063. [Google Scholar] [CrossRef]




| Samples | r1-hexene | rcyclooctene | X a | Y b |
|---|---|---|---|---|
| Pt/SiO2 | 145,000 | 6054 | 24.0 | - |
| PtSn/SiO2 | 131,957 | 3543 | 37.2 | - |
| PtZn/SiO2 | 105,028 | 4023 | 26.1 | - |
| PtGa/SiO2 | 96,011 | 5066 | 19.0 | - |
| Pt@MFI | 61,558 | 89 | 691.7 | 28.9 |
| PtSn@MFI | 37,073 | 31 | 1195.9 | 32.1 |
| PtZn@MFI | 32,105 | 48 | 668.9 | 25.6 |
| PtGa@MFI | 28,954 | 64 | 452.4 | 23.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, N.; Qin, G.; Li, P.; Zhang, X.; Chen, Y.; Yang, Y.; He, P. Enhanced Stability and Selectivity in Pt@MFI Catalysts for n-Butane Dehydrogenation: The Crucial Role of Sn Promoter. Catalysts 2024, 14, 760. https://doi.org/10.3390/catal14110760
Gong N, Qin G, Li P, Zhang X, Chen Y, Yang Y, He P. Enhanced Stability and Selectivity in Pt@MFI Catalysts for n-Butane Dehydrogenation: The Crucial Role of Sn Promoter. Catalysts. 2024; 14(11):760. https://doi.org/10.3390/catal14110760
Chicago/Turabian StyleGong, Nengfeng, Gaolei Qin, Pengfei Li, Xiangjie Zhang, Yan Chen, Yong Yang, and Peng He. 2024. "Enhanced Stability and Selectivity in Pt@MFI Catalysts for n-Butane Dehydrogenation: The Crucial Role of Sn Promoter" Catalysts 14, no. 11: 760. https://doi.org/10.3390/catal14110760
APA StyleGong, N., Qin, G., Li, P., Zhang, X., Chen, Y., Yang, Y., & He, P. (2024). Enhanced Stability and Selectivity in Pt@MFI Catalysts for n-Butane Dehydrogenation: The Crucial Role of Sn Promoter. Catalysts, 14(11), 760. https://doi.org/10.3390/catal14110760





