Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction
Abstract
1. Introduction
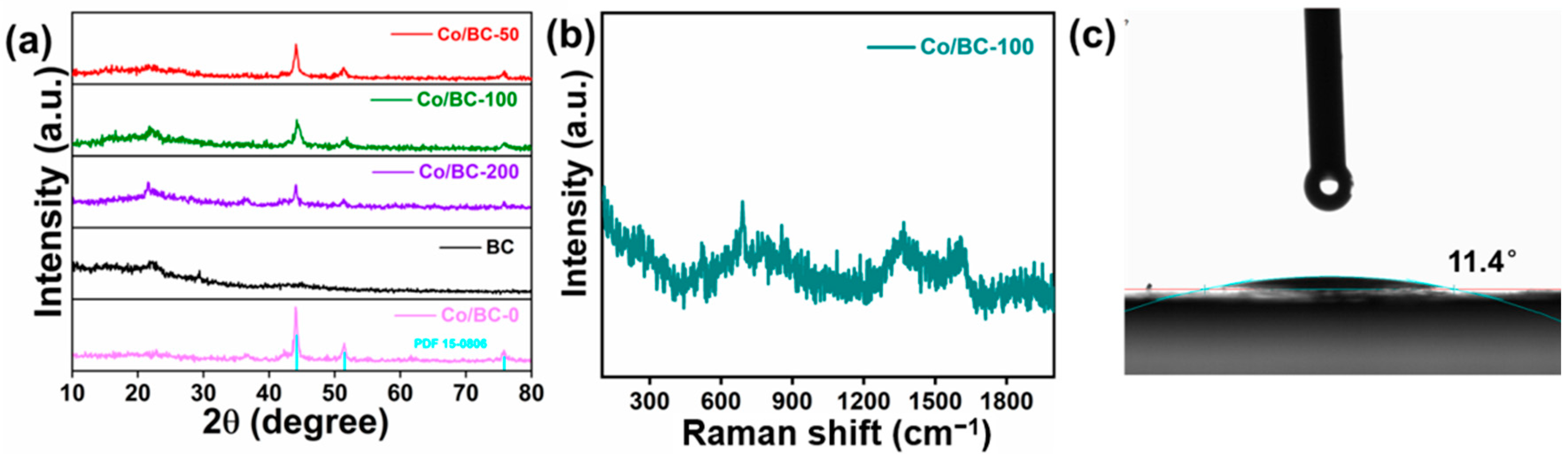
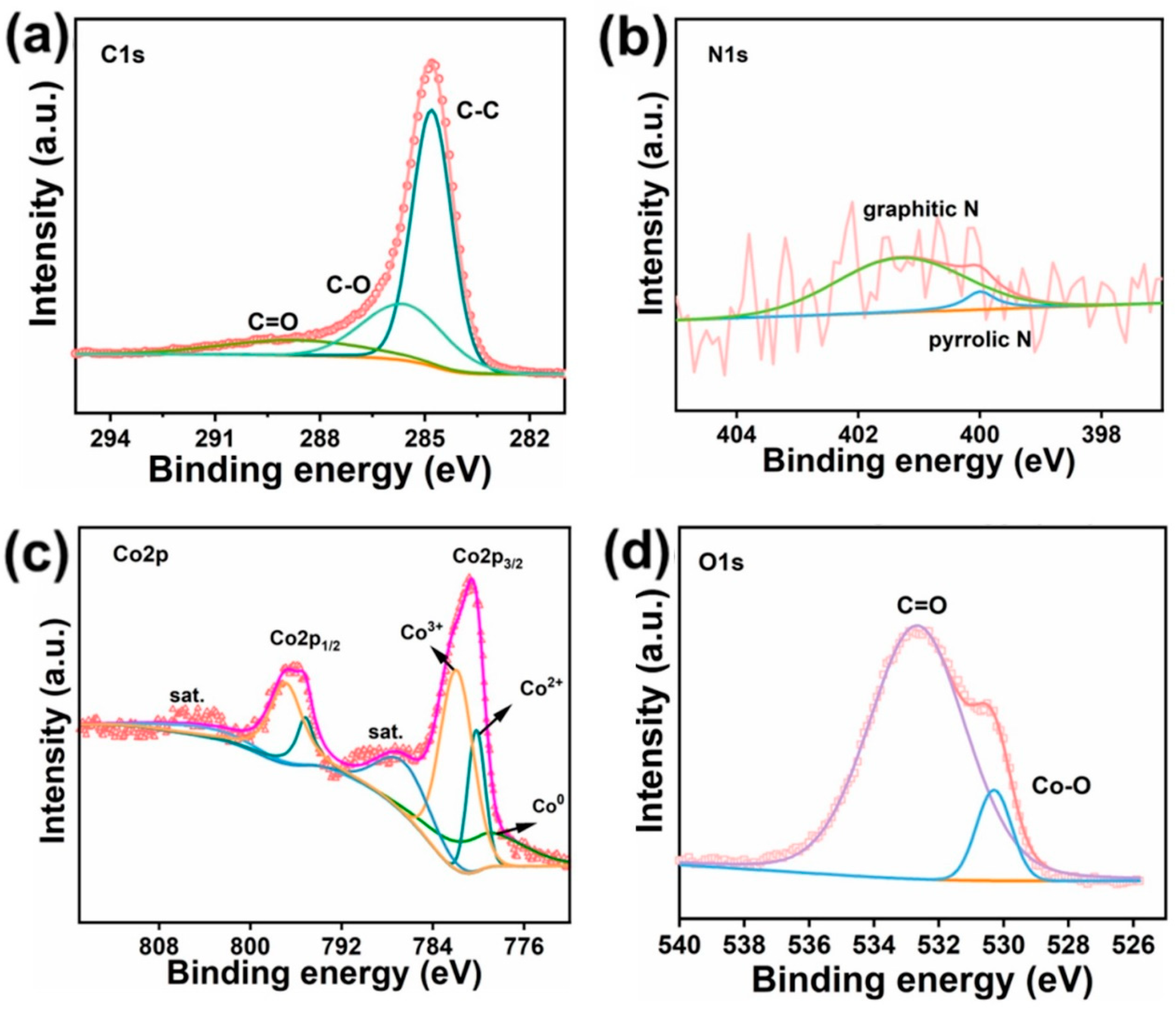
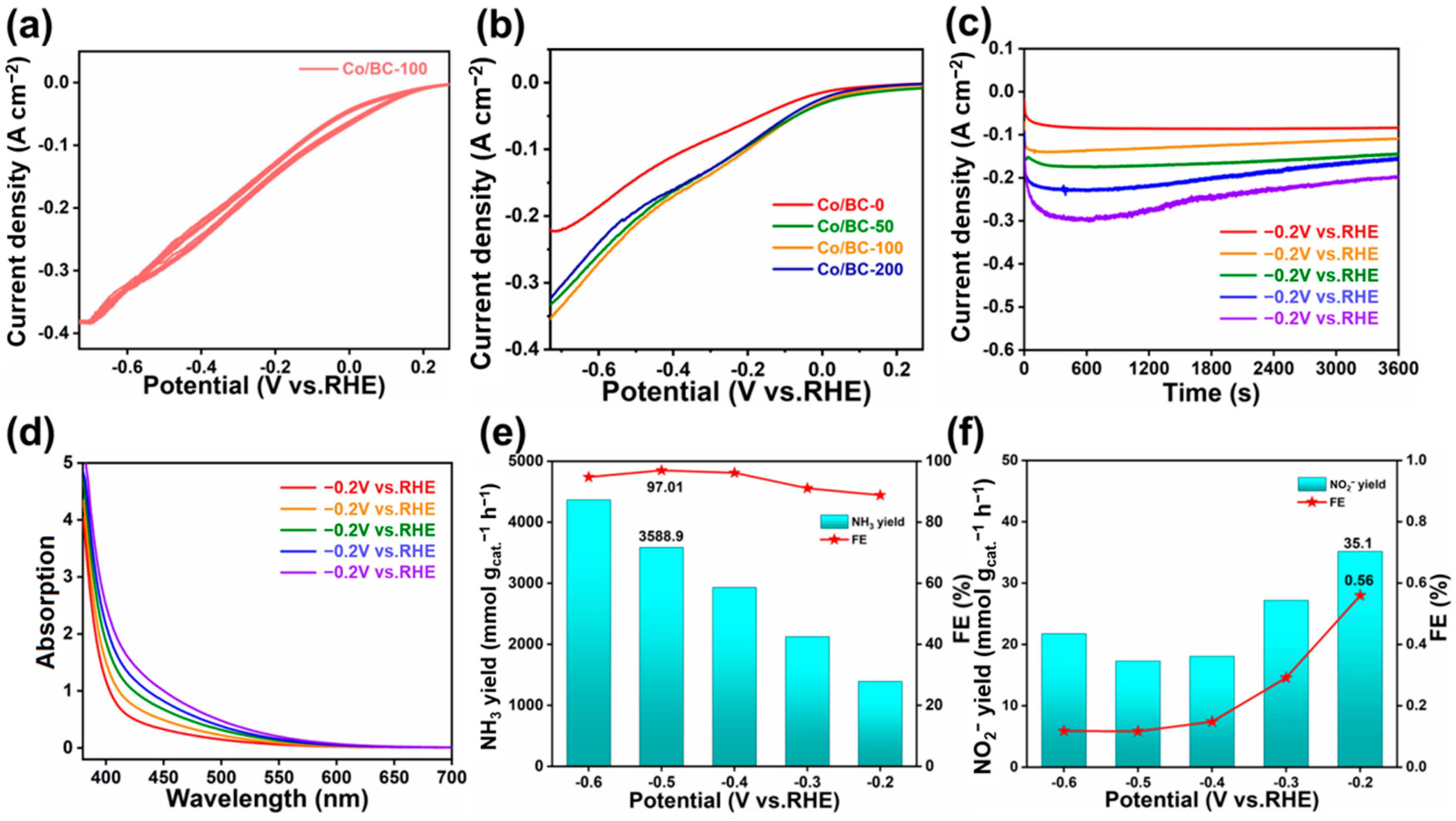
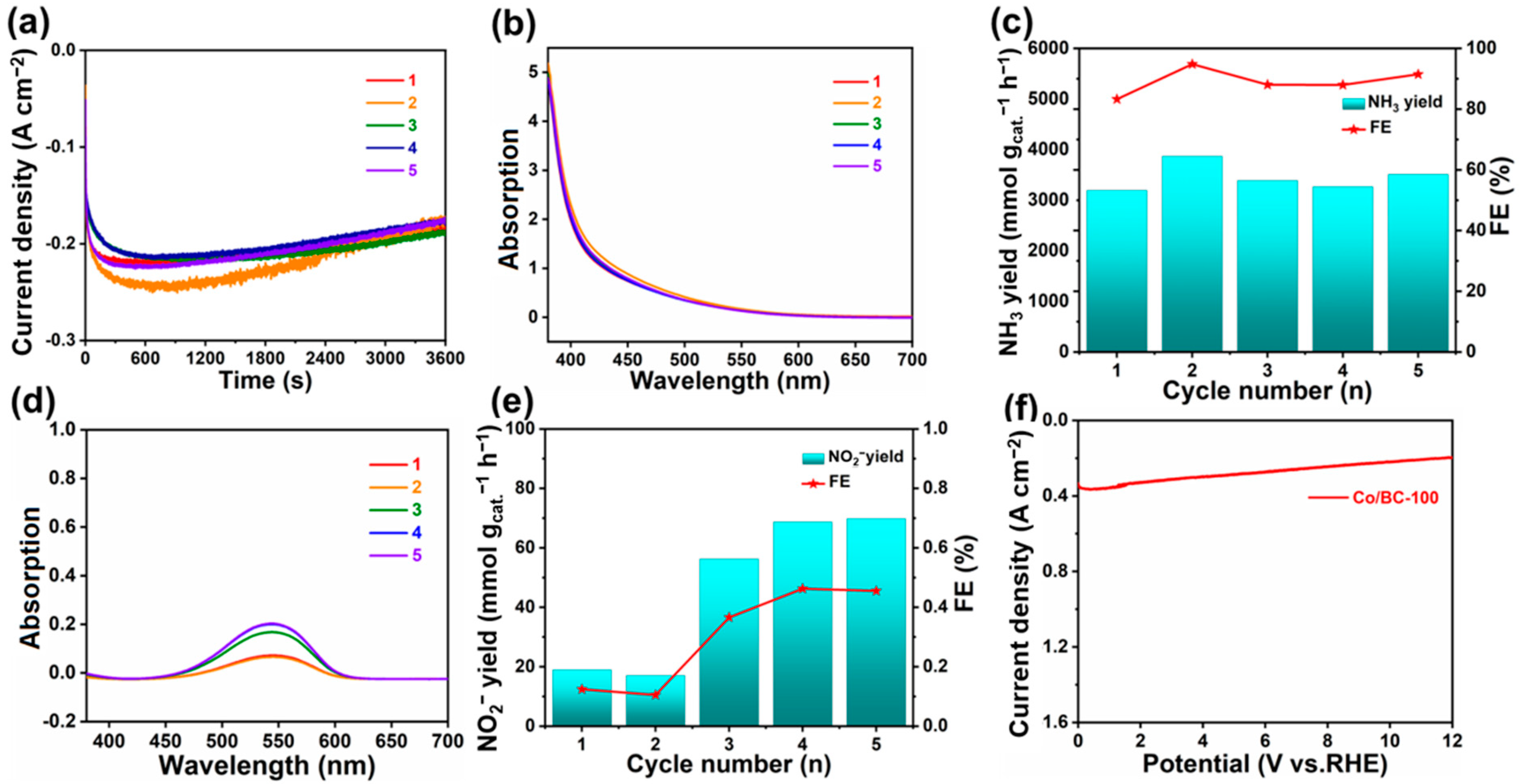
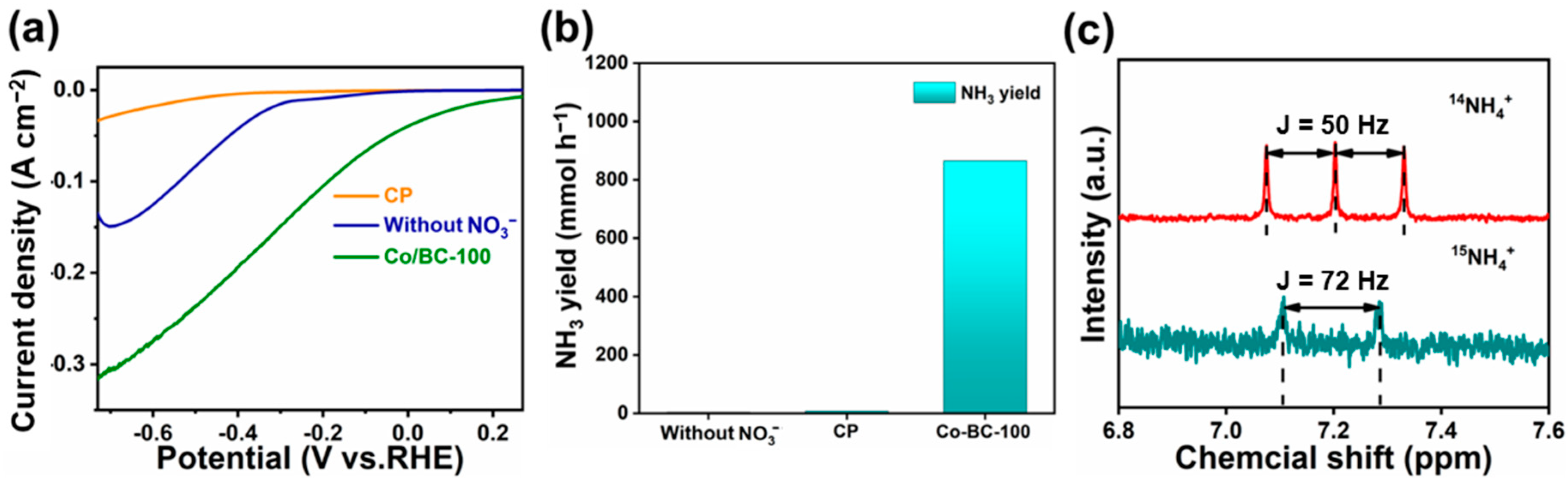
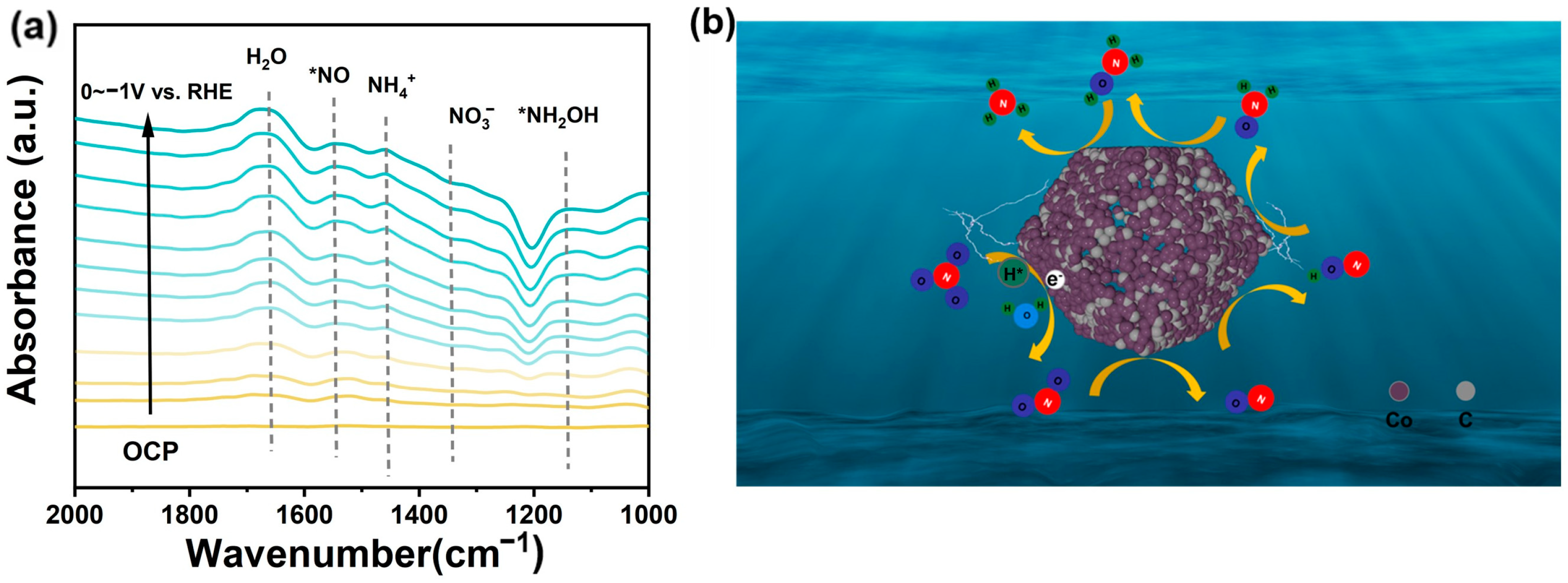
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Characterizations
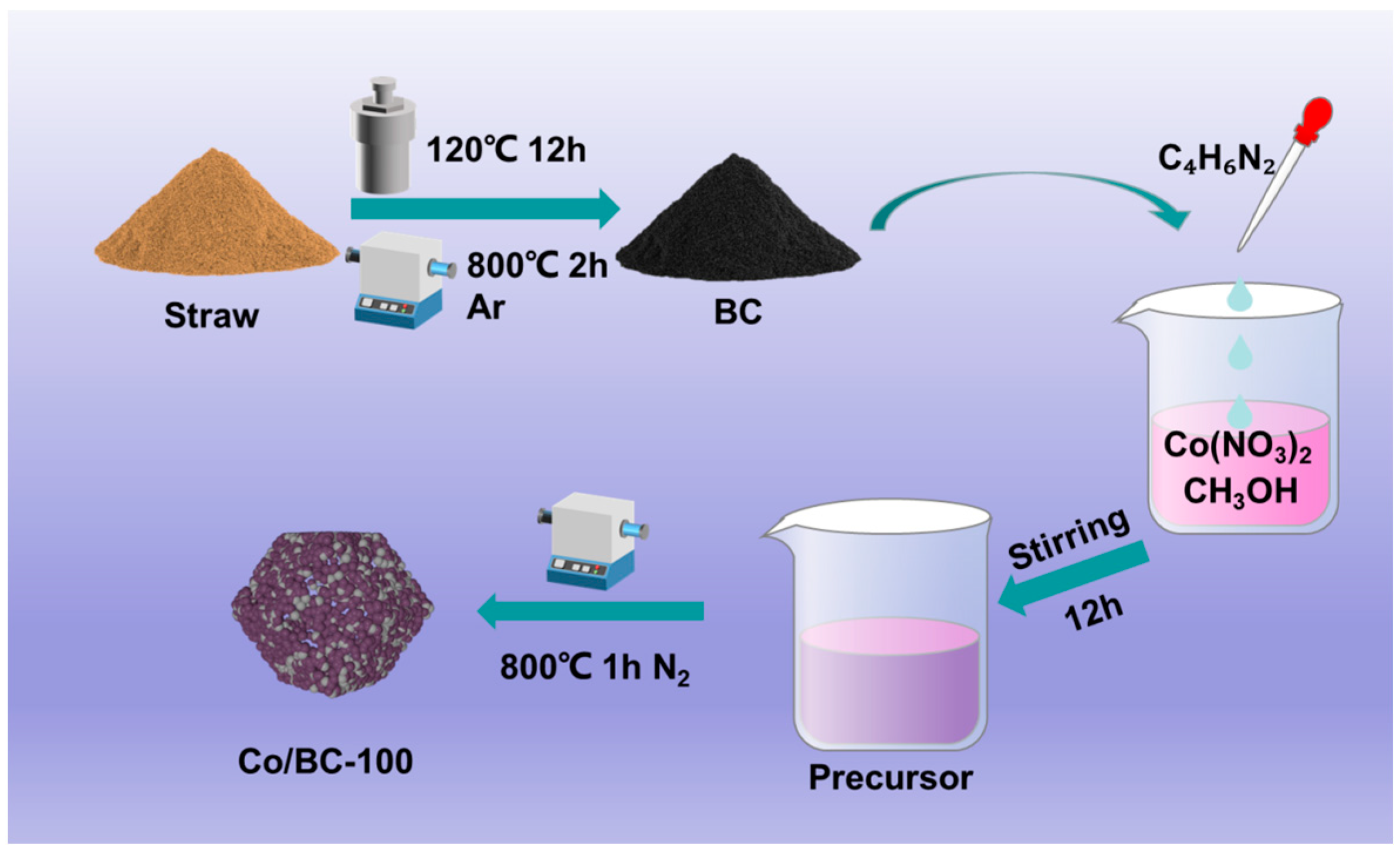
3.2. Synthesis of BC
3.3. Synthesis of Co/BC
3.4. In Situ FT-IR Testing
3.5. Electrochemical Measurement
3.6. Isotope Labeling Experiments
3.7. Determination of NH3
3.8. Determination of NO2−
3.9. Contact Angle Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, W.; Hu, Z.; Zheng, Y.; Su, P.; Zhang, Q.; Jiao, Y.; Zhou, M. Tuning mobility of intermediate and electron transfer to enhance electrochemical reduction of nitrate to ammonia on Cu2O/Cu interface. Chem. Eng. J. 2022, 433, 133680. [Google Scholar] [CrossRef]
- Fan, K.; Xie, W.; Li, J.; Sun, Y.; Xu, P.; Tang, Y.; Li, Z.; Shao, M. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, X.; Liu, Y.; Cheng, Y.; Zhang, P.; Deng, P.; Deng, J.; Kang, Z.; Li, H. Fe-doped SnO2 nanosheet for ambient electrocatalytic nitrogen reduction reaction. Nano Res. 2022, 15, 6026–6035. [Google Scholar] [CrossRef]
- Deng, P.; Liu, Y.; Liu, Y.; Li, Y.; Wu, R.; Meng, L.; Liang, K.; Gan, Y.; Qiao, F.; Liu, N.; et al. Microwave Regenerable Nickel, Zinc Co-doped Nitrogen-Coordinated Porous Carbon Catalyst for Nitrogen Fixation. ACS Appl. Mater. Interfaces 2023, 15, 44809–44819. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Yin, D.; Li, W.; Bu, X.; Quan, Q.; Lai, Z.; Wang, W.; Meng, Y.; Liu, C.; et al. Tailored p-Orbital Delocalization by Diatomic Pt-Ce Induced Interlayer Spacing Engineering for Highly-Efficient Ammonia Electrosynthesis. Adv. Energy Mater. 2023, 13, 2203201. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, T.; Hao, J.; Zhuang, Z.; Gao, G.; Lai, F.; Lu, S.; Wang, X.; Kang, Q.; Wu, G.; et al. A Coherent Pd–Pd16B3 Core–Shell Electrocatalyst for Controlled Hydrogenation in Nitrogen Reduction Reaction. Adv. Funct. Mater. 2024, 34, 2400849. [Google Scholar] [CrossRef]
- Fang, J.; Zheng, Q.; Lou, Y.Y.; Zhao, K.; Hu, S.; Li, G.; Akdim, O.; Huang, X.; Sun, S. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, Z.; Lv, F.; Zhu, H.; Zhou, L.; Luo, M.; Zhu, J.; Lang, Z.; Feng, S.; Chen, W.; et al. The Marriage of the FeN4 Moiety and MXene Boosts Oxygen Reduction Catalysis: Fe 3d Electron Delocalization Matters. Adv. Mater. 2018, 30, 1803220. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Y.; Yu, R.; Zheng, M.; Hu, R.; Wu, J.; Xia, Y.; Zhuang, Z.; Wang, D. Tuning Mass Transport in Electrocatalysis Down to Sub-5 nm through Nanoscale Grade Separation. Angew. Chem. Int. Ed. 2023, 62, e202212653. [Google Scholar] [CrossRef]
- Du, H.; Guo, H.; Wang, K.; Du, X.; Beshiwork, B.A.; Sun, S.; Luo, Y.; Liu, Q.; Li, T.; Sun, X. Durable electrocatalytic reduction of nitrate to ammonia over defective pseudobrookite Fe2TiO5 nanofibers with abundant oxygen vacancies. Angew. Chem. Int. Ed. 2023, 62, e202215782. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Chu, C.; Liu, Y.; Li, Y.; Wu, R.; Wu, J.; Zhuang, C.; Kang, Z.; Li, H. Carbon armour with embedded carbon dots for building better supercapacitor electrodes. Nano Res. 2023, 16, 6815–6824. [Google Scholar] [CrossRef]
- Cheng, Y.; Jabeen, S.; Lei, S.; Liu, N.; Liu, Y.; Liu, Y.; Li, Y.; Wu, X.; Tong, Z.; Yu, J.; et al. N-doped carbon dots-modulated interfacial charge transfer and surface structure in FeNbO4 photocatalysts for enhanced CO2 conversion selectivity to CH4. Chem. Eng. J. 2024, 498, 155576. [Google Scholar] [CrossRef]
- Xia, J.; Xu, J.; Yu, B.; Liang, X.; Qiu, Z.; Li, H.; Feng, H.; Li, Y.; Cai, Y.; Wei, H.; et al. A Metal–Sulfur–Carbon Catalyst Mimicking the Two-Component Architecture of Nitrogenase. Angew. Chem. Int. Ed. 2024, 136, e202412740. [Google Scholar] [CrossRef]
- Zhuang, C.; Li, W.; Chang, Y.; Li, S.; Zhang, Y.; Li, Y.; Gao, J.; Chen, G.; Kang, Z. Coordination environment dominated catalytic selectivity of photocatalytic hydrogen and oxygen reduction over switchable gallium and nitrogen active sites. J. Mater. Chem. A 2024, 12, 5711–5718. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Y.; Xu, S.; Lu, Y.; Sun, S.; Jiang, D. Interfacing Co3Mo with CoMoOx for synergistically boosting electrocatalytic hydrogen and oxygen evolution reactions. Chem. Eng. J. 2022, 431, 133240. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, W.; Li, L.; Wang, S.; Wang, L.; Qi, Z.; Yang, Y.; Chen, Z.; Zhuang, J.; Hao, J.; et al. Discharge Induced-Activation of Phosphorus-Doped Nickel Oxyhydroxide for Oxygen Evolution Reaction. Chem. Eng. J. 2022, 435, 135049. [Google Scholar]
- Wang, S.; Zhao, K.; Chen, Z.; Wang, L.; Qi, Z.; Hao, J.; Shi, W. New insights into cations effect in oxygen evolution reaction. Chem. Eng. J. 2022, 433, 133518. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Liu, Y.; Li, Y.; Wu, R.; Du, Y.; Askari, N.; Liu, N.; Qiao, F.; Sun, C.; et al. A core-satellite structured type II heterojunction photocatalyst with enhanced CO2 reduction under visible light. Nano Res. 2022, 15, 8880–8889. [Google Scholar] [CrossRef]
- Cheng, X.-F.; He, J.-H.; Ji, H.-Q.; Zhang, H.-Y.; Cao, Q.; Sun, W.-J.; Yan, C.-L.; Lu, J.-M. Coordination Symmetry Breaking of Single-Atom Catalysts for Robust and Efficient Nitrate Electroreduction to Ammonia. Adv. Mater. 2022, 34, 2205767. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Li, Z.; Lv, F.; Lang, Z.; Zhao, K.; Zhou, L.; Moskaleva, L.; Guo, S.; Mai, L. MoB/g-C3N4 Interface Materials as a Schottky Catalyst to Boost Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 496–500. [Google Scholar] [CrossRef]
- Zhuang, Z.; Xia, L.; Huang, J.; Zhu, P.; Li, Y.; Ye, C.; Xia, M.; Yu, R.; Lang, Z.; Zhu, J.; et al. Continuous Modulation of Electrocatalytic Oxygen Reduction Activities of Single-Atom Catalysts through p-n Junction Rectification. Angew. Chem. Int. Ed. 2023, 62, e202212335. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tong, Z.; Liu, Y.; Li, Y.; Cheng, Y.; Yu, J.; Cao, P.; Zhuang, C.; Shi, Q.; Liu, N.; et al. Modification of the CuO electronic structure for enhanced selective electrochemical CO2 reduction to ethylene. Nano Res. 2024, 17, 7194–7202. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, Y.; Wu, X.; Cheng, Y.; Yu, J.; Zhang, X.; Liu, N.; Liu, X.; Li, H. Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol. Nanomaterials 2024, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.; Hao, J.; Zhuang, Z.; Zhang, S.; Wang, T.; Kang, Q.; Lu, S.; Wang, X.; Lai, F.; et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis. Energy Environ. Sci. 2023, 16, 619–628. [Google Scholar] [CrossRef]
- Wang, S.; Zhuang, Z.; Xu, J.; Fu, C.; Qiu, Z.; Feng, H.; Xiang, H.; Chen, Z.; Li, H.; Zhang, L.; et al. Non-rigid metal–oxygen bonding empowered nitrate reduction on ruthenium catalysts. Nano Energy 2024, 130, 110088. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Yu, R.; Xia, L.; Yang, J.; Lang, Z.; Zhu, J.; Huang, J.; Wang, J.; Wang, Y.; et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat. Catal. 2022, 5, 300–310. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Li, Y.; Huang, J.; Wei, B.; Sun, R.; Ren, Y.; Ding, J.; Zhu, J.; Lang, Z.; et al. Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides. Energy Environ. Sci. 2021, 14, 1016–1028. [Google Scholar] [CrossRef]
- Hao, J.; Wang, T.; Yu, R.; Cai, J.; Gao, G.; Zhuang, Z.; Kang, Q.; Lu, S.; Liu, Z.; Wu, J.; et al. Integrating few-atom layer metal on high-entropy alloys to catalyze nitrate reduction in tandem. Nat. Commun. 2024, 15, 9020. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13, 2662. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, S.; Wang, H.; Li, H.; Shao, M. A Spectroscopic Study of Electrochemical Nitrogen and Nitrate Reduction on Rhodium Surfaces. Angew. Chem. Int. Ed. 2020, 59, 10479–10483. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.; Liu, Y.; Li, Y.; Cheng, Y.; Li, H.T. Modulation of photogenerated holes for enhanced photoelectrocatalytic performance. Microstructures 2023, 3, 2023001. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Guo, X.; Kong, X.; Ke, J.; Chi, M.; Li, Q.; Geng, Z.; Zeng, J. A Highly Efficient Metal-Free Electrocatalyst of F-Doped Porous Carbon toward N2 Electroreduction. Adv. Mater. 2020, 32, 1907690. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Chang, Y.; Li, W.; Li, S.; Xu, P.; Zhang, H.; Zhang, Y.; Zhang, C.; Gao, J.; Chen, G.; et al. Light-Induced Variation of Lithium Coordination Environment in g-C3N4 Nanosheet for Highly Efficient Oxygen Reduction Reactions. ACS Nano 2024, 18, 5206–5217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, R.; Liu, Y.; Deng, P.; Li, Y.; Cheng, Y.; Du, Y.; Li, Z.; Yan, X.; Liu, N.; et al. Interface engineering of CoS/MoS2 heterostructure for the electrocatalytic reduction of N2 to NH3. Inorg. Chem. Front. 2023, 10, 5700–5709. [Google Scholar] [CrossRef]
- Ling, C.; Zhang, Y.; Li, Q.; Bai, X.; Shi, L.; Wang, J. New Mechanism for N2 Reduction: The Essential Role of Surface Hydrogenation. J. Am. Chem. Soc. 2019, 141, 18264–18270. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Jabeen, S.; Liu, N.; Liu, Y.; Cheng, Y.; Li, Y.; Yu, J.; Wu, X.; Yan, N.; et al. Mechanochemical route to fabricate an efficient nitrate reduction electrocatalyst. Nano Res. 2024, 17, 4889–4897. [Google Scholar] [CrossRef]
- Liu, N.; Wu, R.; Liu, Y.; Liu, Y.; Deng, P.; Li, Y.; Du, Y.; Cheng, Y.; Zhuang, Z.; Kang, Z.; et al. Oxygen Vacancy Engineering of Fe-Doped NiMoO4 for Electrocatalytic N2 Fixation to NH3. Inorg. Chem. 2023, 62, 11990–12000. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, Z.; Liu, Y.; Liu, N.; Li, Y.; Cheng, Y.; Yu, J.; Yu, R.; Li, H.; Wang, D. Shear-strained Pd Single-atom Electrocatalysts for Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202411396. [Google Scholar]
- Bhuvanendran, N.; Ravichandran, S.; Jayaseelan, S.S.; Xu, Q.; Khotseng, L.; Su, H. Improved bi-functional oxygen electrocatalytic performance of Pt–Ir alloy nanoparticles embedded on MWCNT with Pt-enriched surfaces. Energy 2020, 211, 118695. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Bi, X. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies. Chem. Eng. J. 2020, 382, 123034. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Niu, Z.; Fan, S.; Li, X.; Liu, Z.; Wang, J.; Duan, J.; Tadé, M.O.; Liu, S. Facile tailoring of the electronic structure and the d-band center of copper-doped cobaltate for efficient nitrate electrochemical hydrogenation. ACS Appl. Mater. Interfaces 2022, 14, 35477–35484. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; An, N.; Zhang, S.; Song, Q.; Yang, Y.; Li, J.; Liu, X. Boosted ammonium production by single cobalt atom catalysts with high Faradic efficiencies. Proc. Natl. Acad. Sci. USA 2022, 119, e2123450119. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.; Lebedeva, O.; Kultin, D.; Mashkin, M.; Kalmykov, K.; Kustov, L. Enhancing Efficiency of Nitrate Reduction to Ammonia by Fe and Co Nanoparticle-Based Bimetallic Electrocatalyst. Int. J. Mol. Sci. 2024, 25, 7089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, J.; Liu, B.; Yan, S. Electrocatalytic nitrate reduction to ammonia by sea-urchin-like CoNiO2 under mild conditions. CRPS 2024, 5, 101994. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Lu, D.; Wang, H. The synergistic tandem effect of Cu2+1O and Co3O4 enhances the activity and selectivity of nitrate reduction to ammonia in neutral solution. Appl. Catal. A 2024, 677, 119695. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, H.; Mao, B.; Zhang, Z.; Liu, Y.; Liu, Y.; Li, F.; Zhang, D.; Zhang, D.; Shi, W. Efficient MOF- derived V–Ni3S2 nanosheet arrays for electrocatalytic overall water splitting in alkali. Int. J. Hydrogen Energy 2021, 46, 10773–10782. [Google Scholar] [CrossRef]
- Yu, Z.; Si, C.; Lagrow, A.P.; Tai, Z.; Caliebe, W.A.; Tayal, A.; Sampaio, M.J.; Sousa, J.P.S.; Amorim, I.; Araujo, A.; et al. Iridium–Iron Diatomic Active Sites for Efficient Bifunctional Oxygen Electrocatalysis. ACS Catal. 2022, 12, 9397–9409. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, G.; Diaconescu, V.M.; Simonelli, L.; Lagrow, A.P.; Tai, Z.; Xiang, X.; Xiong, D.; Liu, L. Atomically dispersed dinuclear iridium active sites for efficient and stable electrocatalytic chlorine evolution reaction. Chem. Sci. 2024, 15, 9216–9223. [Google Scholar] [CrossRef]
- Li, C.; Zhao, D.-H.; Long, H.-L.; Li, M. Recent advances in carbonized non-noble metal–organic frameworks for electrochemical catalyst of oxygen reduction reaction. Rare Met. 2021, 40, 2657–2689. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, L.; Chen, Y.; Zhou, P.; Feng, J.; Leong, C.C.; Ng, K.W.; Peng, S.; Wang, S.; Ip, W.F.; et al. Electrocatalytic reduction of nitrate to ammonia on low-cost manganese-incorporated Co3O4 nanotubes. Appl. Catal. B 2023, 324, 122293. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Hu, Y.; Li, L.; Yan, T.; Yang, G.; Ji, D.; Srinivasan, M.; Pan, Z.; Ramakrishna, S. Cobalt nanoparticles encapsulated in carbon nanotube-grafted nitrogen and sulfur co-doped multichannel carbon fibers as efficient bifunctional oxygen electrocatalysts. J. Mater. Chem. A 2017, 5, 4949–4961. [Google Scholar] [CrossRef]
- Wu, Y.-j.; Wu, X.-h.; Tu, T.-x.; Zhang, P.-f.; Li, J.-t.; Zhou, Y.; Huang, L.; Sun, S.-g. Controlled synthesis of FeNx-CoNx dual active sites interfaced with metallic Co nanoparticles as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Appl. Catal. B 2020, 278, 119259. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cai, C.; Liu, Y.; Wu, D.; Wang, M.; Li, M.; Wei, X.; Shao, M.; Gu, M. Cu-doped Iron oxide for the efficient electrocatalytic nitrate reduction reaction. Nano Lett. 2023, 23, 1897–1903. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhang, X.Y.; Yao, F.; Huang, R.P.; Chen, Y.; Chen, C.L.; Fei, J.W.; Chen, Y.Q.; Wang, Z.C.; Liang, H.F. A Hydrazine-Nitrate Flow Battery Catalyzed by a Bimetallic RuCo Precatalyst for Wastewater Purification along with Simultaneous Generation of Ammonia and Electricity. Angew. Chem. Int. Ed. 2023, 62, e202300390. [Google Scholar] [CrossRef]
- Zhu, W.J.; Yao, F.; Wu, Q.F.; Jiang, Q.; Wang, J.X.; Wang, Z.C.; Liang, H.F. Weakened d–p orbital hybridization in in situ reconstructed Ru/β-Co(OH)2 heterointerfaces for accelerated ammonia electrosynthesis from nitrates. Energy Environ. Sci. 2023, 16, 2483–2493. [Google Scholar] [CrossRef]
- Jang, W.; Oh, D.; Lee, J.; Kim, J.; Matthews, J.E.; Kim, H.; Lee, S.W.; Lee, S.; Xu, Y.; Yu, J.M.; et al. Homogeneously Mixed Cu–Co Bimetallic Catalyst Derived from Hydroxy Double Salt for Industrial-Level High-Rate Nitrate-to-Ammonia Electrosynthesis. J. Am. Chem. Soc. 2024, 146, 27417–27428. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, T.; Fu, H.; Chen, Z.; Qu, X.; Zheng, S. Construction and identification of highly active single-atom Fe1-NC catalytic site for electrocatalytic nitrate reduction. Appl. Catal. B 2023, 323, 122181. [Google Scholar] [CrossRef]
- Zhang, J.; He, W.; Quast, T.; Junqueira, J.R.C.; Saddeler, S.; Schulz, S.; Schuhmann, W. Single-entity Electrochemistry Unveils Dynamic Transformation during Tandem Catalysis of Cu2O and Co3O4 for Converting NO3− to NH3. Angew. Chem. Int. Ed. 2023, 62, e202214830. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, S.; Wang, Y.; Wei, P.; Shao, J.; Liu, T.; Wang, G.; Bao, X. Enhancing Electrochemical Nitrate Reduction to Ammonia over Cu Nanosheets via Facet Tandem Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202303327. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Bu, X.; Zhang, R.; Quan, Q.; Lai, Z.; Wang, W.; Meng, Y.; Yin, D.; Yip, S.; et al. Synergistic modulation of local environment for electrochemical nitrate reduction via asymmetric vacancies and adjacent ion clusters. Nano Energy 2022, 98, 107338. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Zhong, Y.; Guo, Q.; Zeng, J.; Geng, Z. Efficient Electroreduction of Nitrate into Ammonia at Ultralow Concentrations Via an Enrichment Effect. Adv. Mater. 2022, 34, 2204306. [Google Scholar] [CrossRef]
- Gao, W.; Xie, K.; Xie, J.; Wang, X.; Zhang, H.; Chen, S.; Wang, H.; Li, Z.; Li, C. Alloying of Cu with Ru Enabling the Relay Catalysis for Reduction of Nitrate to Ammonia. Adv. Mater. 2023, 35, 2202952. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. [Google Scholar] [CrossRef]
- Fang, Z.; Jin, Z.; Tang, S.; Li, P.; Wu, P.; Yu, G. Porous Two-dimensional Iron-Cyano Nanosheets for High-rate Electrochemical Nitrate Reduction. ACS Nano 2022, 16, 1072–1081. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Ren, T.; Wang, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Ultralow-content Pd in-situ incorporation mediated hierarchical defects in corner-etched Cu2O octahedra for enhanced electrocatalytic nitrate reduction to ammonia. Appl. Catal. B 2022, 306, 121094. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Ren, T.; Wang, M.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Cooperativity of Cu and Pd active sites in CuPd aerogels enhances nitrate electroreduction to ammonia. Chem. Commun. 2021, 57, 7525–7528. [Google Scholar] [CrossRef]
- Gao, Q.; Pillai, H.S.; Huang, Y.; Liu, S.; Mu, Q.; Han, X.; Yan, Z.; Zhou, H.; He, Q.; Xin, H.; et al. Breaking adsorption-energy scaling limitations of electrocatalytic nitrate reduction on intermetallic CuPd nanocubes by machine-learned insights. Nat. Commun. 2022, 13, 2338. [Google Scholar] [CrossRef]
- Xie, M.; Tang, S.; Li, Z.; Wang, M.; Jin, Z.; Li, P.; Zhan, X.; Zhou, H.; Yu, G. Intermetallic Single-Atom Alloy In–Pd Bimetallene for Neutral Electrosynthesis of Ammonia from Nitrate. J. Am. Chem.Soc. 2023, 145, 13957–13967. [Google Scholar] [CrossRef]
- Sun, L.; Liu, B. Mesoporous PdN Alloy Nanocubes for Efficient Electrochemical Nitrate Reduction to Ammonia. Adv. Mater. 2023, 35, 2207305. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, Z.; Li, X.; Kang, J.; Ma, D.; Chu, K. Single-Atom Bi Alloyed Pd Metallene for Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2209890. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Cai, W.; Zhao, H.; Zhang, Y.; Sun, Y.; Hu, Z.; Li, S.; Lai, J.; Wang, L. Facet-controlled palladium nanocrystalline for enhanced nitrate reduction towards ammonia. J. Colloid Interface Sci. 2021, 600, 620–628. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Du, Y.; Liu, S.; Liu, Y.; Li, Y.; Cheng, Y.; Cao, P.; Zhang, X.; Yuan, X.; Liu, N.; et al. Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction. Catalysts 2024, 14, 817. https://doi.org/10.3390/catal14110817
Yu J, Du Y, Liu S, Liu Y, Li Y, Cheng Y, Cao P, Zhang X, Yuan X, Liu N, et al. Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction. Catalysts. 2024; 14(11):817. https://doi.org/10.3390/catal14110817
Chicago/Turabian StyleYu, Jingwen, Yongchao Du, Shuaiqi Liu, Yunliang Liu, Yaxi Li, Yuanyuan Cheng, Peng Cao, Xinyue Zhang, Xinya Yuan, Naiyun Liu, and et al. 2024. "Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction" Catalysts 14, no. 11: 817. https://doi.org/10.3390/catal14110817
APA StyleYu, J., Du, Y., Liu, S., Liu, Y., Li, Y., Cheng, Y., Cao, P., Zhang, X., Yuan, X., Liu, N., Liu, Y., & Li, H. (2024). Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction. Catalysts, 14(11), 817. https://doi.org/10.3390/catal14110817






