Abstract
In the current environmental scenario, the proposal of alternatives for petroleum-based products has considerably increased, with the aim of looking for bioproducts with interesting properties such as biodegradability, sustainability and efficiency, among others. In this sense, the role of biolubricants is promising, offering a wide range of possibilities through different methods and operating conditions. Specifically, double transesterification could be a suitable process in a biorefinery context. The aim of this work was to produce a biolubricant through double transesterification with methanol and neopentyl glycol (NPG) under different reaction conditions by using homogeneous catalysis (sodium methoxide). Different catalyst concentrations, among other changes in reaction conditions (temperature ranging between 100 and 140 °C and NPG/FAME ratios between 0.5 and 2), were used, obtaining high conversion values (96%) and a final product with a high viscosity (20.7 cSt), which allows for its use as engine oil (SAE 5W). In conclusion, biodiesel and biolubricant production was feasible through homogeneous catalysis, proving the feasibility of this process at the laboratory scale. Further studies, including the use of different heterogeneous catalysts, as well as the implementation of this process at a semi-industrial scale, are recommended.
1. Introduction
1.1. Global Environmental Scenario
There are different current environmental challenges that are a real concern from different points of view and at different levels (from local to international, from individuals to institutions) that provoke an urgent energy and environmental transition, with the subsequent challenges mainly related to economics and geopolitics. Indeed, different scenarios have been proposed depending on different factors, showing the challenges related to the ideal goals and the corresponding realistic steps [1,2]. Consequently, the global energy transition is mainly dependent on the economic development of countries as well as their access to energy resources [3].
In any case, a green transition is necessary, and the implementation of new green technologies is necessary to support these green policies, which are normally related to a lower energy dependency, avoiding geopolitical factors, among other advantages [4]. In this context, the role of biorefineries can be promising, as many of the products obtained could be perfect replacements for other more polluting ones, such as petroleum products, with a subsequent positive impact on the environment. For this purpose, different feedstocks can be used, like first-generation (edible crops like soybean [5]), second-generation (residues such as pulp and paper sludge or food waste [6,7]), third-generation (for instance, algae [8,9,10]) or fourth generation (non-edible) feedstocks [11].
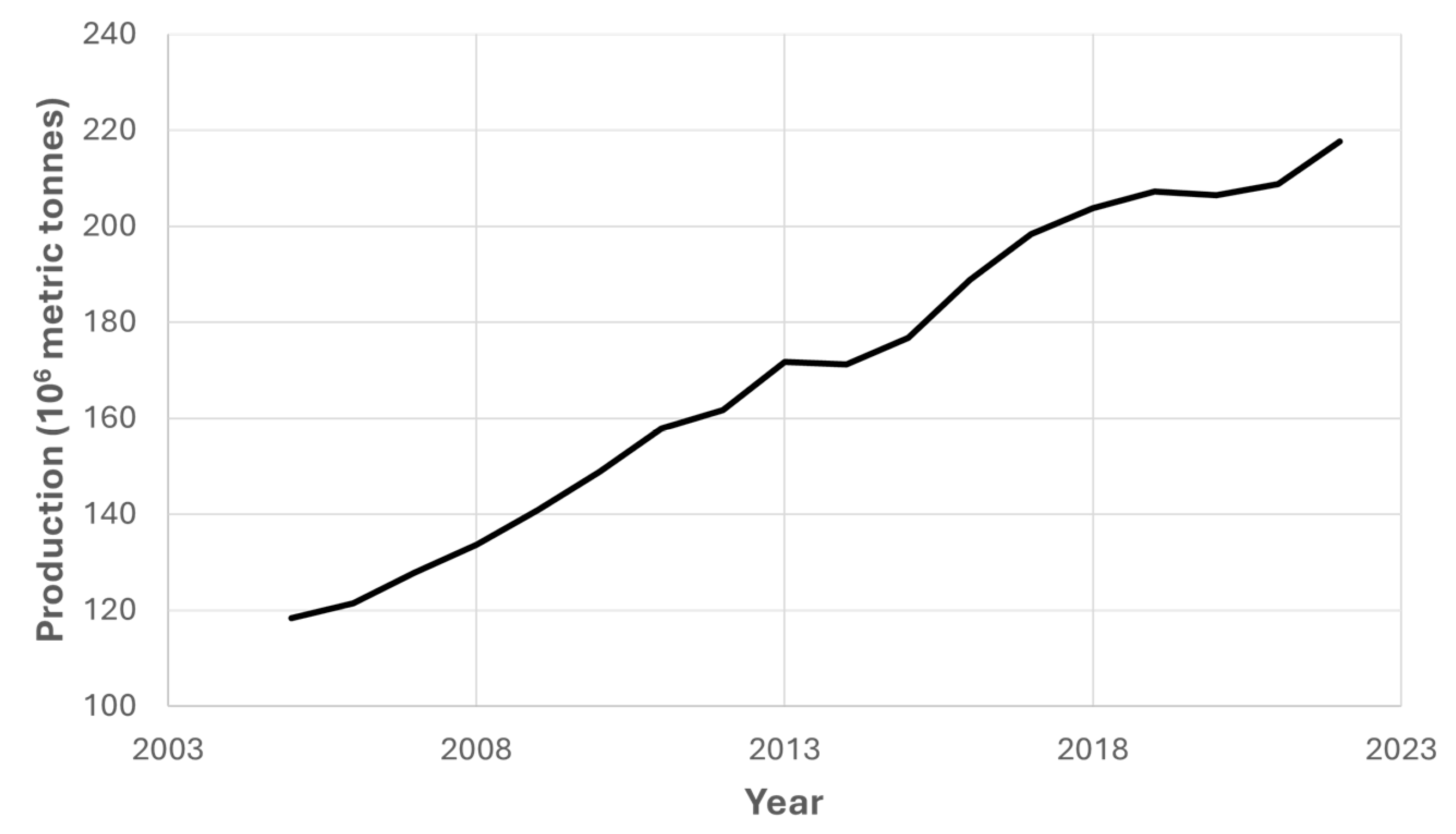
First-generation biorefineries have been widely covered in the literature [12,13], using crops such as rapeseed or canola, which could be an interesting alternative as a crop rotation due to the long taproot of this crop, which allows for the recovery of superficial soil for other agricultural practices [14,15]. Indeed, the potential of these kinds of biorefineries is still positive, as vegetable oil production has been continuously increasing according to recent data (as shown in Figure 1).
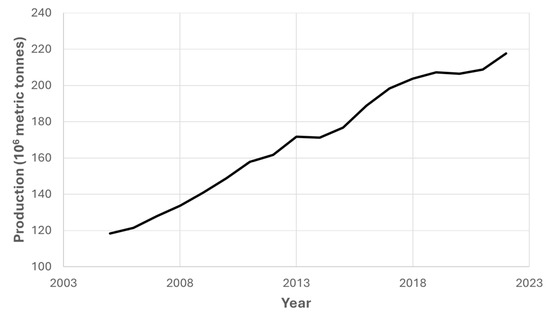
Figure 1.
Worldwide vegetable oil production in the last two decades. Source: US Department of Agriculture [16].
In this sense, different processes can be linked to a biorefinery, including biodiesel and biolubricant production, as explained in the following subsection.
1.2. Biolubricant Production: A Biorefinery Approach
Considering vegetable oils as the starting point for a biorefinery approach, there are various chemical routes to obtain biolubricants, such as epoxidation or hydrolysis and esterification, with advantages and challenges mainly related to the properties of the vegetable oil (acidity, for instance). In this sense, the process of hydrolysis followed by esterification is relatively similar to double transesterification, especially in the second step, where polyols are used with fatty acids or fatty acid methyl esters to obtain fatty acid esters. In this context, hydrolysis and esterification are advisable for samples with high acidity, thus recommending the use of acid catalysts [17,18]. Nevertheless, the use of double transesterification to produce multiple products with low emissions to the environment is interesting due to the possible implementation of this process in a biorefinery context. Thus, first regarding transesterification, vegetable oils (mainly triglycerides) react with methanol (also, ethanol can be used for this purpose) to produce fatty acid methyl esters (FAMEs) and glycerol. The former can be used (once purified) as biodiesel [19], whereas the latter has a wide range of industrial and pharmaceutical uses depending on different factors such as purity [20,21]. It should be noted that other innovative sources can be used, as in the case of microbial oil obtained from oleaginous microorganisms through fermentation of biomass-derived sugars [22,23].
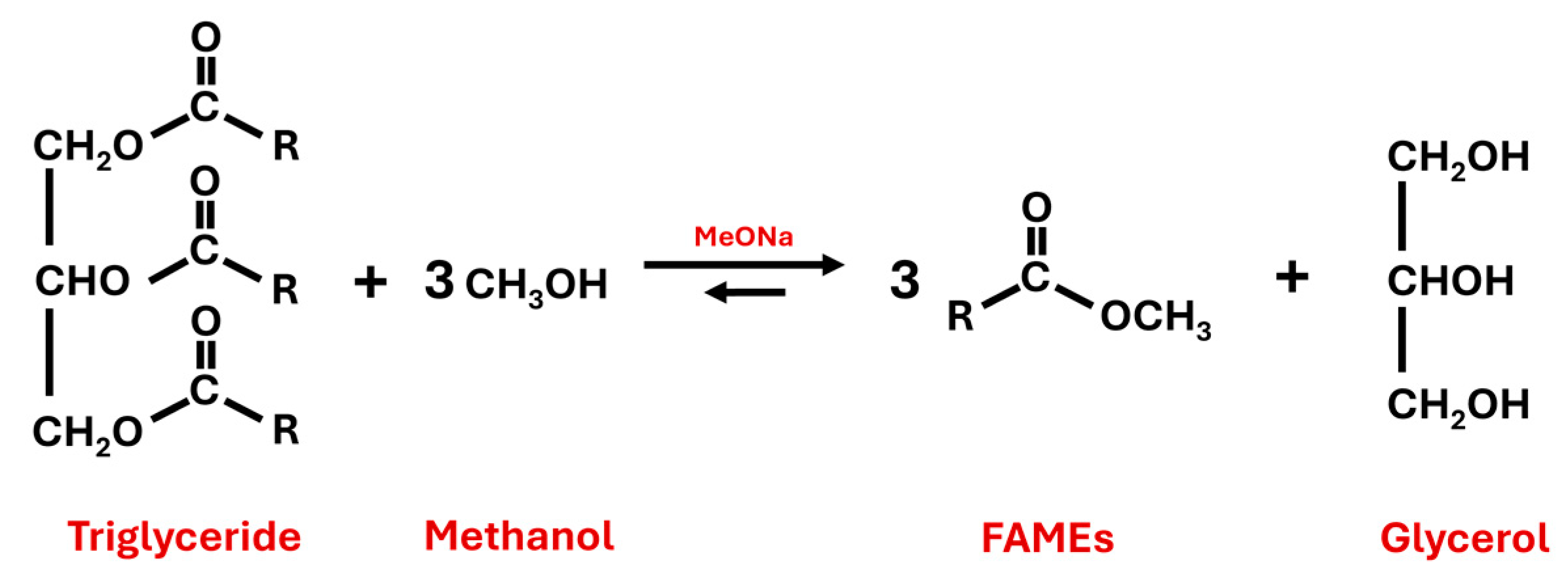
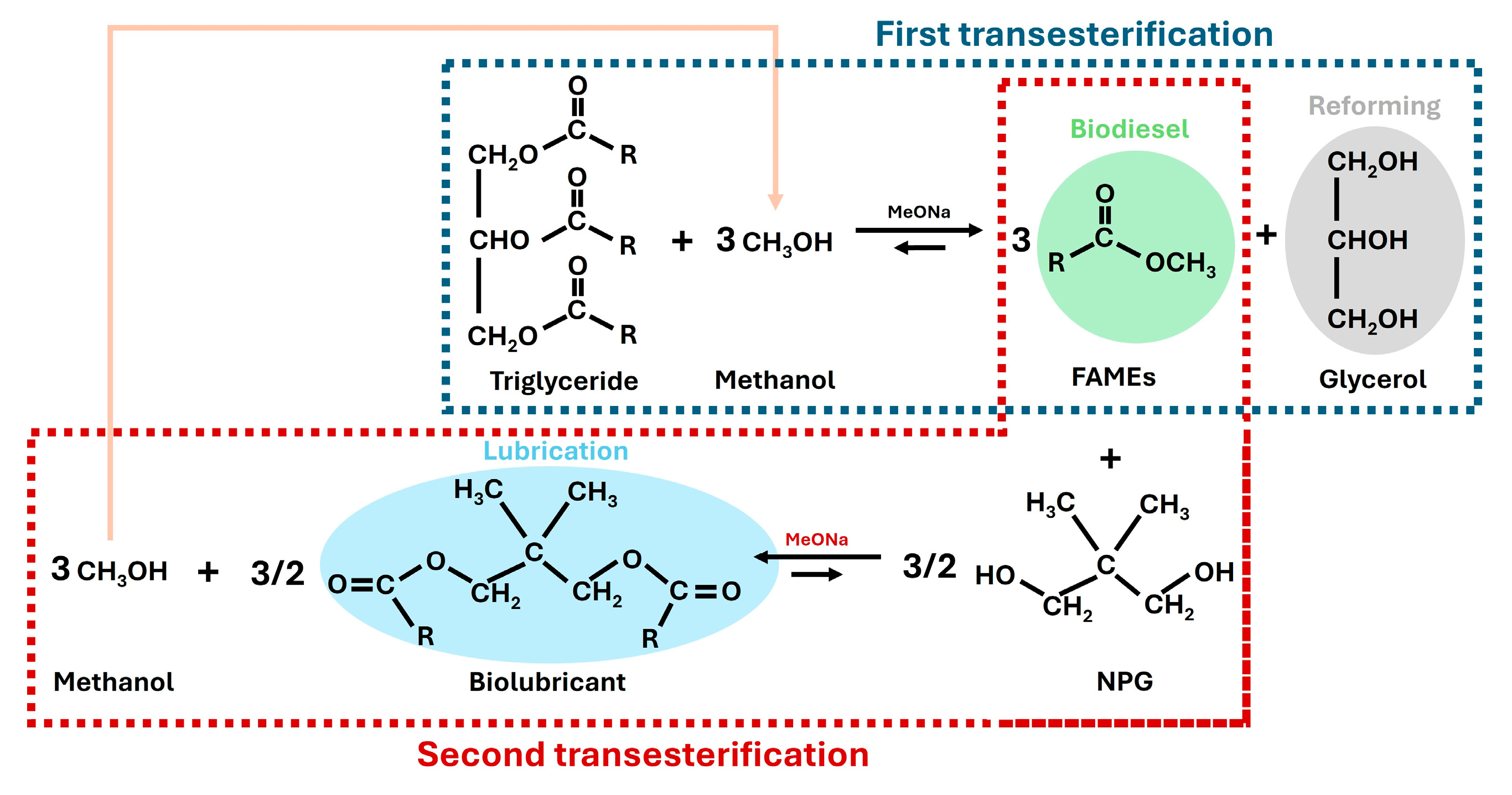
Concerning second transesterification, the FAMEs obtained in the previous steps can react with different superior alcohols (pentaerythritol, neopentylglycol or trimethylolpropane, among others) to produce the corresponding biolubricant, thereby evolving methanol that can be reused in the first transesterification (see the following section for specific chemical reactions in Figure 2 and Figure 3).
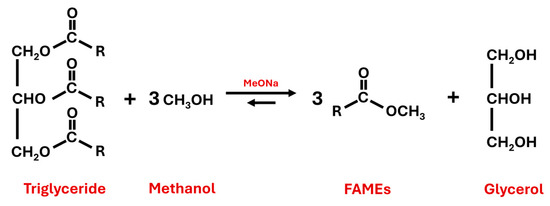
Figure 2.
First transesterification reaction of triglycerides and methanol to produce fatty acid methyl esters (FAMEs) and glycerol by using sodium methoxide (MeONa) as a catalyst.
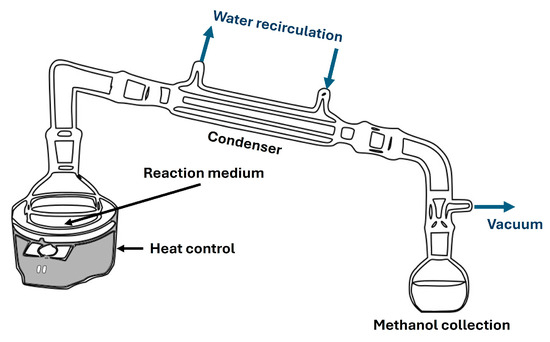
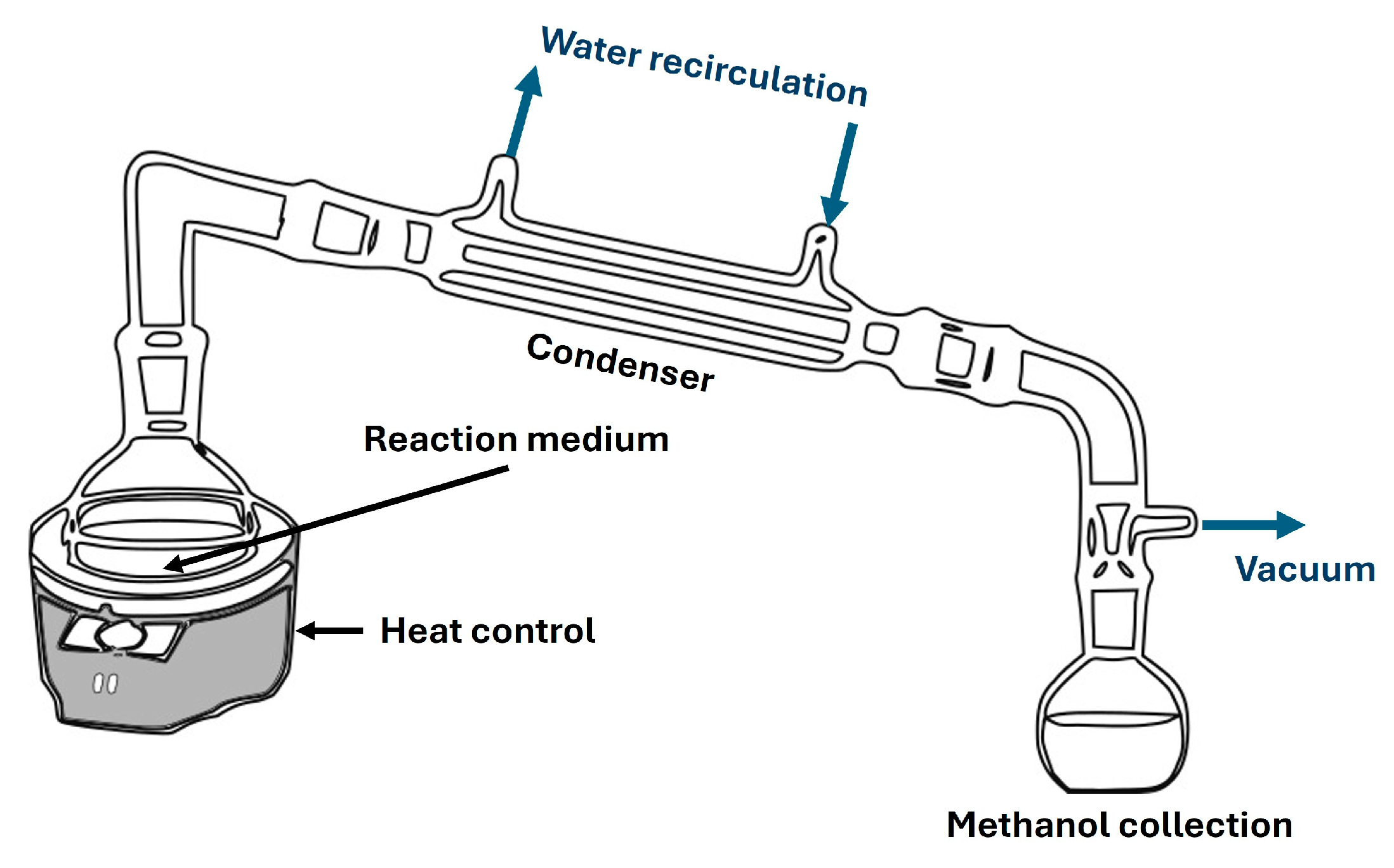
Figure 3.
Typical distillation system for biolubricant production.
As a result, this second transesterification offers a wide range of opportunities and possibilities, as different vegetable oils (for instance, edible, non-edible and waste cooking oil, with different properties) and alcohols can be used to synthetize biolubricants with variable properties, which are easily adaptable to specific industrial requirements [24]. Also, the different operating conditions, which are dependent on many factors such as the kind of catalyst or alcohol used, play an important role in the biolubricant characteristics, thus recommending mild reaction conditions [25] or the incorporation of additives to improve their performance [26,27,28], as observed in the literature.
The advantages of double transesterification should be noted, as follows:
- It is a process that can be easily implemented on an industrial scale (the first and second transesterification steps are like typical biodiesel production in industries).
- A wide range of products can be obtained, like biodiesel, glycerol or biolubricants.
- Some intermediate products can be reused, like methanol or biodiesel (as the starting point for biolubricant production).
- As a consequence, low amounts of pollutants are emitted to the environment, implying a high atom economy.
In both processes (first and second transesterification), the role of catalysts is essential, as high conversions are required to compete with petroleum-based refineries. This way, high conversions avoid the presence of unreacted reagents (that would require further purification steps), thus reducing the environmental impact and increasing the atom economy. In the following subsection, more details about catalytic biodiesel and, especially, biolubricant production are included.
1.3. Catalytic Biolubricant Production Through Double Transesterification
Catalytic conversion of FAMEs to produce biolubricants is essential to make this process efficient and competitive and in order for it to be a feasible alternative for petroleum-based lubricant production. In this sense, there has been an increasing interest in the application of different catalysts to obtain biolubricants with industrial uses, with high conversion rates in most cases.
Specifically, different kinds of catalysts can be used in this context, which are mainly classified as homogeneous and heterogenenous. Regarding the former, they are widely used due to their price and efficiency, reducing the production process to a few hours (normally from 2 to 5, depending on other factors such as the molecular complexity of the alcohol used for the second transesterification). However, their purification and removal from the final product can be a challenge, as some traces can imply a worsening of the characteristics of the biolubricants, especially their oxidation stability.
Alternatively, heterogeneous catalysts (normally in a solid state) can contribute to a green approach during the second transesterification step, as they are easily separated from the final biolubricant and can be reused. In contrast, the conversion values are low, and the reaction times are normally longer (compared to the use of homogeneous catalysts), whereas the reusability of these heterogeneous catalysts is still reduced (less than 10 cycles in many cases).
With their advantages and challenges, the catalytic conversion of vegetable oils is an interesting research field at the industrial and laboratory levels. For instance, there have been a few attempts to obtain patents by using the technology or procedures covered in this work. In these cases, different heterogeneous catalysts, mainly immobilized lipases, were used. Particularly, some patents have covered this subject, as in the case of patent number KR102564510B1 [29], where a method for producing neopentyl glycol diester as a biolubricant using an enzymatic reaction was presented. The use of immobilized lipase derived from Thermomyces lanuginosus, along with the correct temperature, time (8–10 h) and vacuum conditions, obtained high conversions (>95%). On the other hand, patent number JP2015059176A [30] provided a method for manufacturing a biolubricant by stirring castor oil methyl biodiesel and/or jatropha oil methyl biodiesel (a mixture of methyl oleate and methyl linoleate), trimethylolpropane (TMP) as a poly hydroxylated alcohol, neopentyl glycol or pentaerythritol, water and an enzymatic catalyst under specific stirring and vacuum conditions.
These kinds of catalysts offer advantages and challenges, like the possibility of reusing several cycles with the subsequent positive environmental impact in this regard. However, the reaction times and conversions are not still optimized, normally offering less efficient results compared to traditional homogeneous catalysts so far [31,32]. On the other hand, there has been an increasing scientific interest in biolubricant synthesis through the use of alcohols like neopentylgycol (NPG), as observed in Table 1, where a selection of the main works included in the literature are shown. As observed, high reaction times are usually required, exceeding 10 h in some cases, and a wide range of conversion values are obtained (from 80 to 97%).

Table 1.
Main research works focused on biolubricant synthesis with neopentylglycol.
It should be noted that these works are focused on the alcohol used in this research work. If other alcohols are considered, it could increase the interest in this field up to date, as observed in the literature.
1.4. Aim of This Work
Considering the above, the aim of this work was to produce a specific biolubricant based on canola oil through double transesterification with methanol and neopentylglycol (NPG), offering a product with unique properties for industrial purposes. For this purpose, homogeneous catalysis (sodium methoxide) was selected for both biodiesel and biolubricant production, assessing conversion depending on different factors like the catalyst concentration or temperature, among others. Finally, a characterization of the final product, including parameters such as its viscosity and oxidative stability, was carried out to classify this biolubricant according to its industrial use.
To the best of our knowledge, the specific study of biolubricant production from canola oil by using double transesterification with methanol and neopentylglycol has not been widely studied in the literature, showing an innovative approach that could expand the knowledge about the feasible use of vegetable oils as raw materials for this purpose, including a preliminary catalytic study.
2. Materials and Methods
As previously explained, canola (a genetically modified sample of rapeseed crop) was selected for the production of biolubricant through double transesterification. The following subsections give more details about the process, from raw material pre-treatment to biolubricant characterization. The procedures were similar to those explained in previous works, included in the literature [39,40,41], with slight modifications, as explained in following subsections. Nevertheless, the use of canola oil, along with NPG for the second transesterification step (see Section 2.3), provides a different product with unique properties (especially concerning the viscosity and oxidation stability, determining factors for the specific use of a certain biolubricant and its stability during storage, respectively).
2.1. Raw Material
In this work, canola oil was provided by CICYTEX (Technological and Scientific Research Center of Extremadura, Guadajira, Badajoz, Spain), and the coordinates of the crop were as follows: 38°51′10.0″ N 6°40′15.1″ W. The harvesting took place in the spring of 2022, and the corresponding oil was obtained through mechanical extraction. Once the oil was properly filtered, the acid number was determined (see Section 2.4 for more details), obtaining low levels (<1%), which justified the use of basic homogeneous catalysts during the first and second transesterification steps, as explained in following subsections.
2.2. First Transesterification
For the first transesterification reaction, optimum reaction conditions were selected to obtain a high FAME yield according to previous works (already included in the literature) [41,42]. Thus, biodiesel was obtained by a methanol transesterification technique in a three-neck flask coupled with a water-cooled condenser. The reaction is explained in Figure 2, where the main products are shown.
The process was carried out under the following conditions (for each experiment, 150 g of canola oil was used): 1.5% catalyst (sodium methylate, 30% in methanol, Panreac, Castellar del Vallès, Barcelona, Spain); temperature, 60 °C; methanol/oil molar ratio, 6:1; stirring rate, 700 rpm; and reaction time, 180 min. Methanol (Panreac, Castellar del Vallès, Barcelona, Spain) was added when the canola oil had been heated to 50 °C. Afterwards, catalyst addition was carried out when the reaction medium reached 60 °C, and this point was considered as the beginning of the first transesterification step (t = 0 min).
Once the reaction was finished, the glycerol was removed through decantation in a separation funnel, followed by several washing steps with distilled water to withdraw the catalyst and salts. The final product was dehydrated by heating to 110 °C for 30 min and was stored in amber glass bottles.
Under these operating conditions, high-purity biodiesel with a high FAME content (exceeding 97%) was expected to be obtained in order to use this product as an intermediate reagent for the second transesterification process to obtain the biolubricant.
2.3. Second Transesterification
Once the canola FAMEs were purified in the previous step, the second transesterification step with neopentylglycol was carried out. This alcohol, along with pentaerythritol and trimethylolpropane, is widely used in the literature for biolubricant production, each offering specific properties to the final product, especially regarding viscosity [24]. Following a scheme similar to that shown in Figure 3, a round flask containing biodiesel was heated in a thermostatic oil bath under a 400 rpm stirring rate. Methanol was condensed in a flask.
First of all, a fixed quantity of canola biodiesel (150 g) was added to the reactor, heating it to a suitable temperature (depending on the experiment). When the set temperature was reached, the necessary amount of NPG (99%, Sigma-Aldrich, Darmstadt, Germany) was added, depending on the molar ratio required for each experiment. The catalyst was added (titanium isopropoxide, p-toluenesulfonic acid or sodium methoxide, depending on the experiment) dropwise carefully, avoiding any overflow reaction. These homogeneous catalysts (all of them purchased in Sigma-Aldrich, Darmstadt, Germany) were selected due to their high efficiency according to the literature [24,43,44]. This moment was set as the starting point of the chemical reaction. Samples were taken at 5, 15, 30, 90 and 210 min for chromatography analysis. Also, due to the highly intense reaction produced, a vacuum was only applied after 90 min.
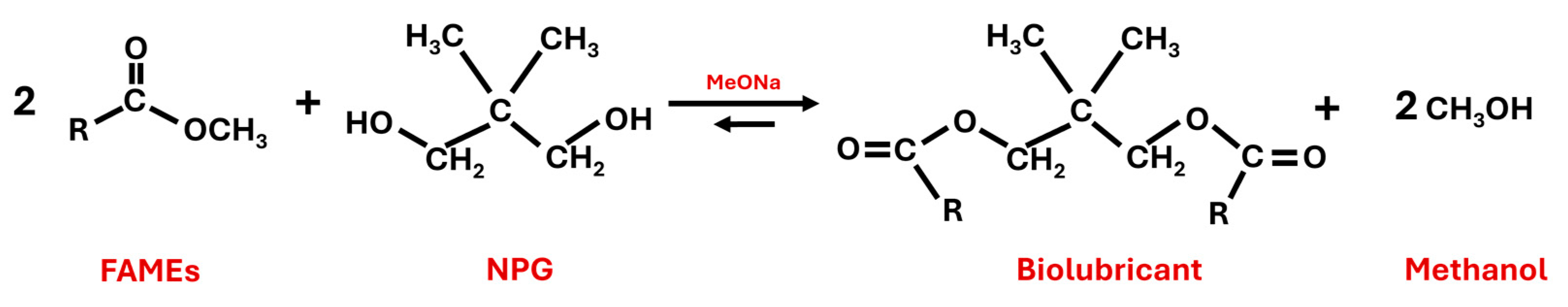
The reaction took place by substituting the methoxide group for the NPG molecule. Evaporation of methanol forced the equilibrium towards the formation of biolubricant (see Figure 4).
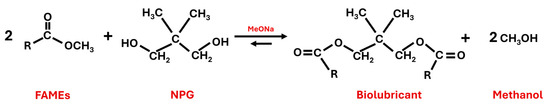
Figure 4.
Second transesterification reaction to produce biolubricants from fatty acid methyl esters (FAMEs) and neopentylglycol (NPG) by using sodium methoxide (MeONa) as a catalyst.
Once the reaction finished, the resulting biolubricant was separated from the unreacted alcohol by using an initial gravity filtration process using filter paper (Dorsan, Igualada, Barcelona, Spain). Afterwards, successive vacuum filtrations were carried out using a Büchner funnel with filter paper (Dorsan, Igualada, Barcelona, Spain) connected to a 500 mL vacuum flask, until a translucent product was obtained (normally after 3 or 4 vacuum filtrations). Finally, the canola biolubricant was stored in amber glass bottles at room temperature.
To sum up, both the first and second transesterification steps are included in Figure 5, showing the interconnections in a biorefinery context. Thus, valuable products were obtained for multiple purposes, including the reutilization of the methanol evolved in the second transesterification step for the first transesterification step (see the pink arrow). Consequently, a high atom economy was reached (if high conversions are assumed for each transesterification), as the evolved products could be reused for different energy scenarios or in the same process.
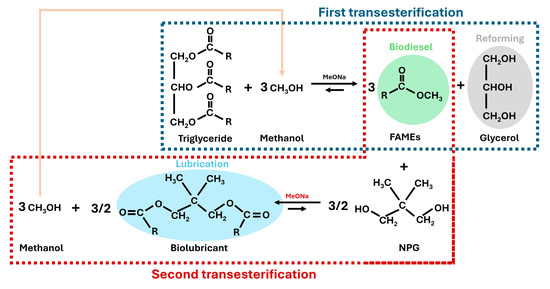
Figure 5.
First (in blue dashed line) and second transesterification (in red dashed line) steps of canola oil with methanol and neopentyl glycol, respectively, and their connections in a biorefinery context, including the applications of the main products obtained.
Finally, different variables were studied in this work, like the kind of catalyst (sodium methoxide, p-toluenesulfonic acid and titanium isopropoxide), reaction temperature (from 100 to 140 °C), molar ratio (from 0.5 to 2) and catalyst concentration (from 0.25 to 0.50% w/w).
2.4. Biolubricant Characterization
Once the canola biodiesel and biolubricant were produced and purified, the characterization of their main properties was carried out, mainly following the UNE-EN 14214 standard [45]. Different parameters were analyzed, following the corresponding standard, whose procedures were explained in previous research works. Table 2 shows the different analyses carried out.

Table 2.
Main procedures for biodiesel and biolubricant characterization.
According to this table, viscosity was measured at 40 °C by using a silicone gel and a temperature probe for temperature control. For the acid and iodine number, the corresponding analytical methods were used, using a titration with alcoholic potassium and sodium thiosulfate, respectively. For the Rancimat method, three grams of sample were heated at 110 °C, bubbling air and registering the conductivity of degradation products in distilled water. An abrupt increase in conductivity indicated the induction point or oxidation stability. Finally, the flash and fire points were determined by heating the sample at a regular heating rate, observing the temperature at which a discontinuous flame occurred for the flash point and a permanent one for the fire point.
For the FAME conversion, Equation (1) was followed, according to the decrease in the FAME content in the biolubricant obtained compared to the original FAME content in the canola biodiesel:
where %FAMEbiodiesel is the fatty acid methyl ester content of the biodiesel, whereas %FAMEbiolubricant is the fatty acid methyl ester content in the product obtained in the second transesterification step.
3. Results and Discussion
Regarding biodiesel production, high conversion values (97.8%) were obtained under the selected operating conditions. Consequently, the fatty acid methyl ester profile is included in Figure 6.
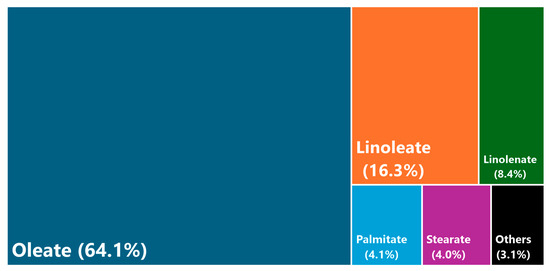
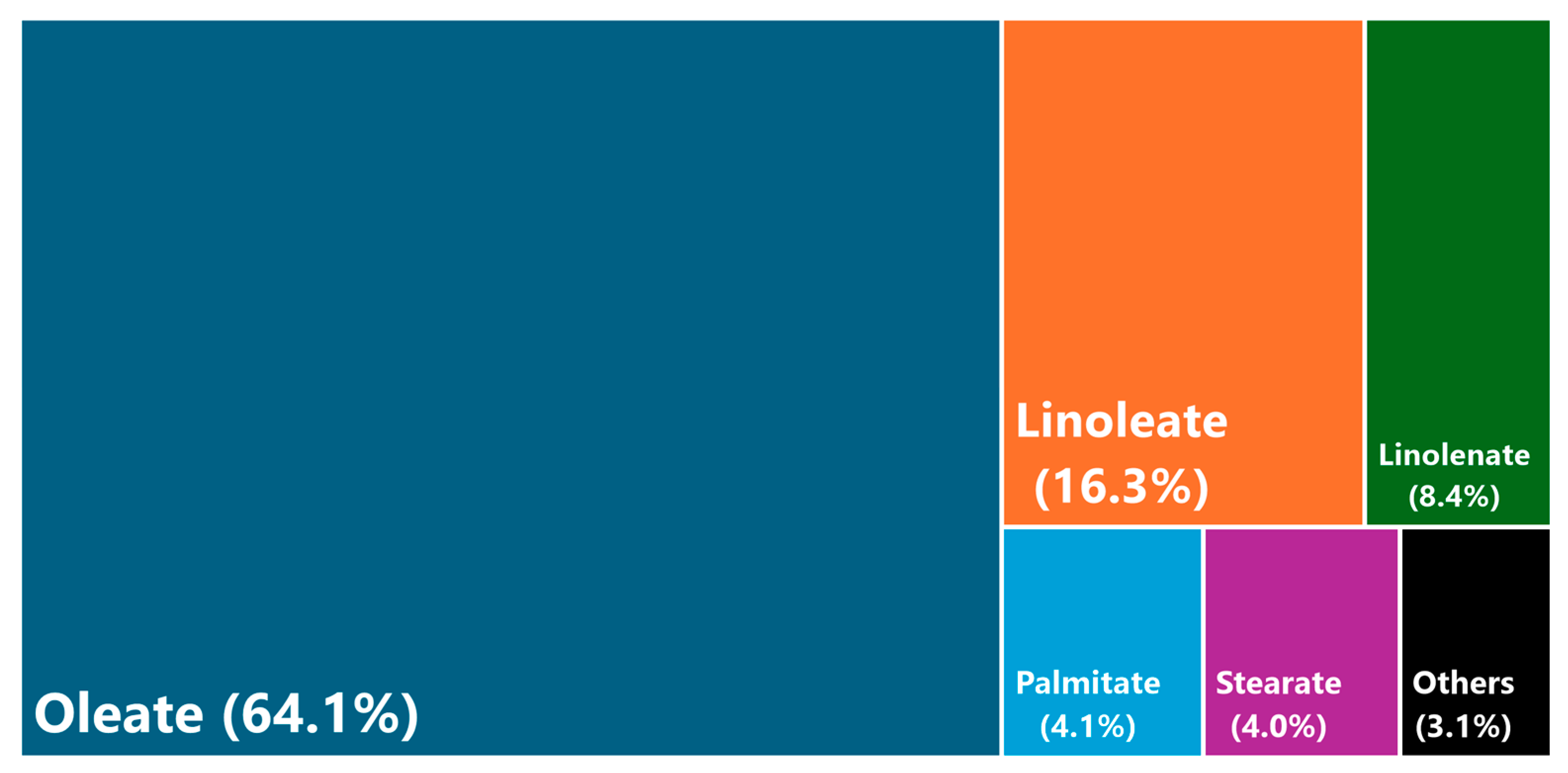
Figure 6.
FAME profile of canola biodiesel, indicating the different percentages of methyl esters.
As observed, the canola biodiesel had high percentages of methyl oleate, exceeding 60%, whereas the amount of methyl linoleate and linolenate was lower. These results were similar to those observed in the literature for canola biodiesel, especially concerning these three methyl esters [53], with oleate levels similar to other high-oleic vegetable oils, like sunflower, safflower or soybean oils [44]. According to previous works and the literature, the FAME profile is essential in understanding the main properties of biodiesel and its derivatives (like biolubricants).
This way, high percentages of saturated or mono-unsaturated FAMEs are highly recommended for higher oxidation stability values, whereas the cold flow properties could be compromised [24,54]. Regarding the properties of the canola biodiesel, Table 3 shows the main results obtained in this work:

Table 3.
Characterization of canola biodiesel and comparison with lower and upper limits of UNE-EN 14214 standard.
From this table, it can be inferred that the properties of this sample complied with most of the parameters established by the UNE-EN 14214 standard [45]. For instance, the viscosity and density values were within the upper and lower limits, assuring a suitable performance in diesel engines and avoiding blockages. Also, the FAME content was high (above 96.5%, the lower limit of the standard), implying a high purity and low amounts of different impurities. Equally, the acid value was low, which implies a low corrosion of containers and engines in contact with this fluid, whereas the iodine value was slightly above the upper limit. However, the oxidation stability was short, as the lower limit established by the standard is 8 h (480 min). This could be due to the presence of unsaturated FAMEs like methyl linoleate and linolenate (with two and three double bonds in their molecular structure, respectively), which normally implies the starting point for free radical generation through auto-oxidation [55]. Equally, the use of antioxidants (both from natural, extracts or synthetic) would be advisable to improve this property [56]. Also, the iodine number is related to oxidation stability, as it points out the presence of double bounds in a certain substance. Thus, these values were relatively high compared to other more stable biodiesel samples found in the literature, like palm biodiesel, whereas rapeseed biodiesel had similar values [57]. When it comes to the flash and fire points, they were high (exceeding 170 °C in both cases), assuring safety during their storage or handling.
These interesting properties have been covered in the literature, where a higher canola biodiesel proportion (compared to other biodiesel samples such as waste cooking oil) was recommended in biodiesel mixtures in order to improve the properties of the final product [53]. Indeed, these suitable properties have allowed for the use of canola biodiesel in mixtures for specific biodiesel formulations, whose optimum proportion showed good combustion performance and exhaust emission characteristics [58].
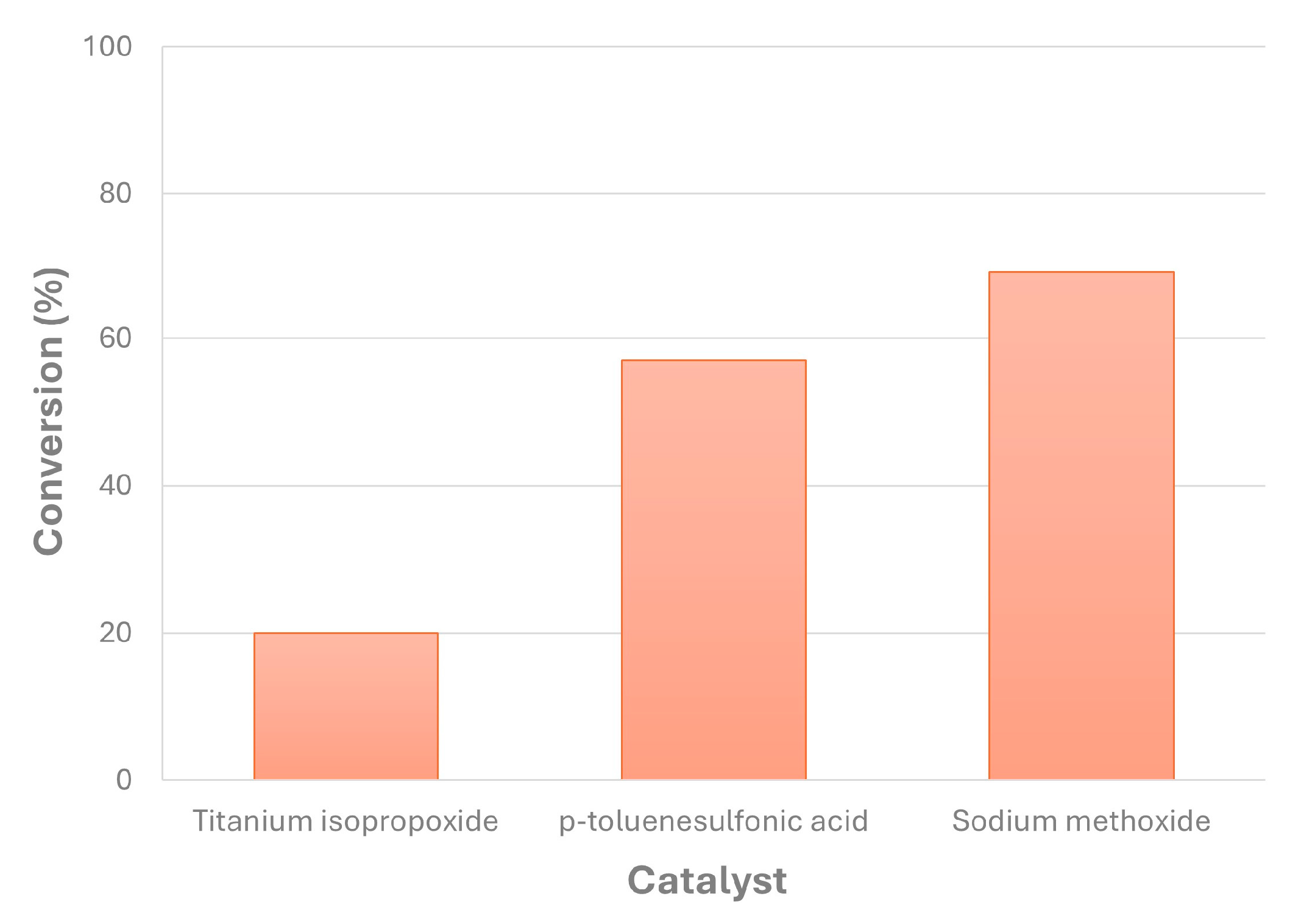
Concerning the selection of the homogeneous catalyst used in this work for biolubricant production from canola FAMEs and using NPG, there were differences in their performance under the same chemical conditions, as observed in Figure 7. This way, at 100 °C, sodium methoxide was the most effective catalyst, obtaining 69% conversion, followed by p-toluenesulfonic acid with less than 60%. However, titanium isopropoxide showed low conversion values, at around 20%. Thus, for the subsequent studies included in this work, sodium methoxide was the selected homogeneous catalyst for canola biolubricant production. According to the literature, the most valuable catalysts in terms of their price and effectiveness are sodium methoxide and hydroxide, with high conversions in general during the second transesterification step of FAMEs with superior alcohols [24]. Thus, previous studies showed that the transesterification of FAMEs with 2-ethyl-1-hexanol by using titanium isopropoxide offered conversions at around 90% with much higher temperatures (150 °C) [39], whereas other works offered the same conversions (exceeding 90%) under milder reaction conditions (80 °C, with vacuum) by using sodium methoxide [25]. In these experiments, the conversion of FAMEs was similar regardless of the kind of catalyst used and the stage of the reaction, as the profile of unreacted FAMEs was similar, only varying by a few % units for each experiment. This way, it can be assumed that the conversion of each kind of FAME was similar in this case.
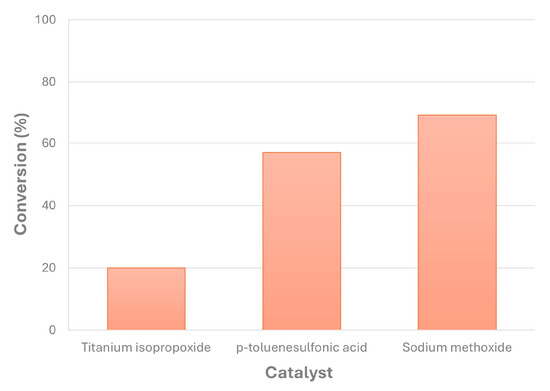
Figure 7.
Different catalysts used for transesterification of canola FAMEs with NPG and the corresponding conversion (catalyst percentage = 1% w/w; reaction time = 210 min; MR = 1; T = 100 °C).
Once the homogeneous catalyst was selected, the effect of different operating conditions, such as the NPG/FAME molar ratio (MR), reaction temperature or catalyst concentration (% MeONa in this case), were assessed. It should be noted that in the case of sodium methoxide, its addition for the subsequent experiment was reduced (to 0.25% w/w) in order to avoid a violent chemical reaction.
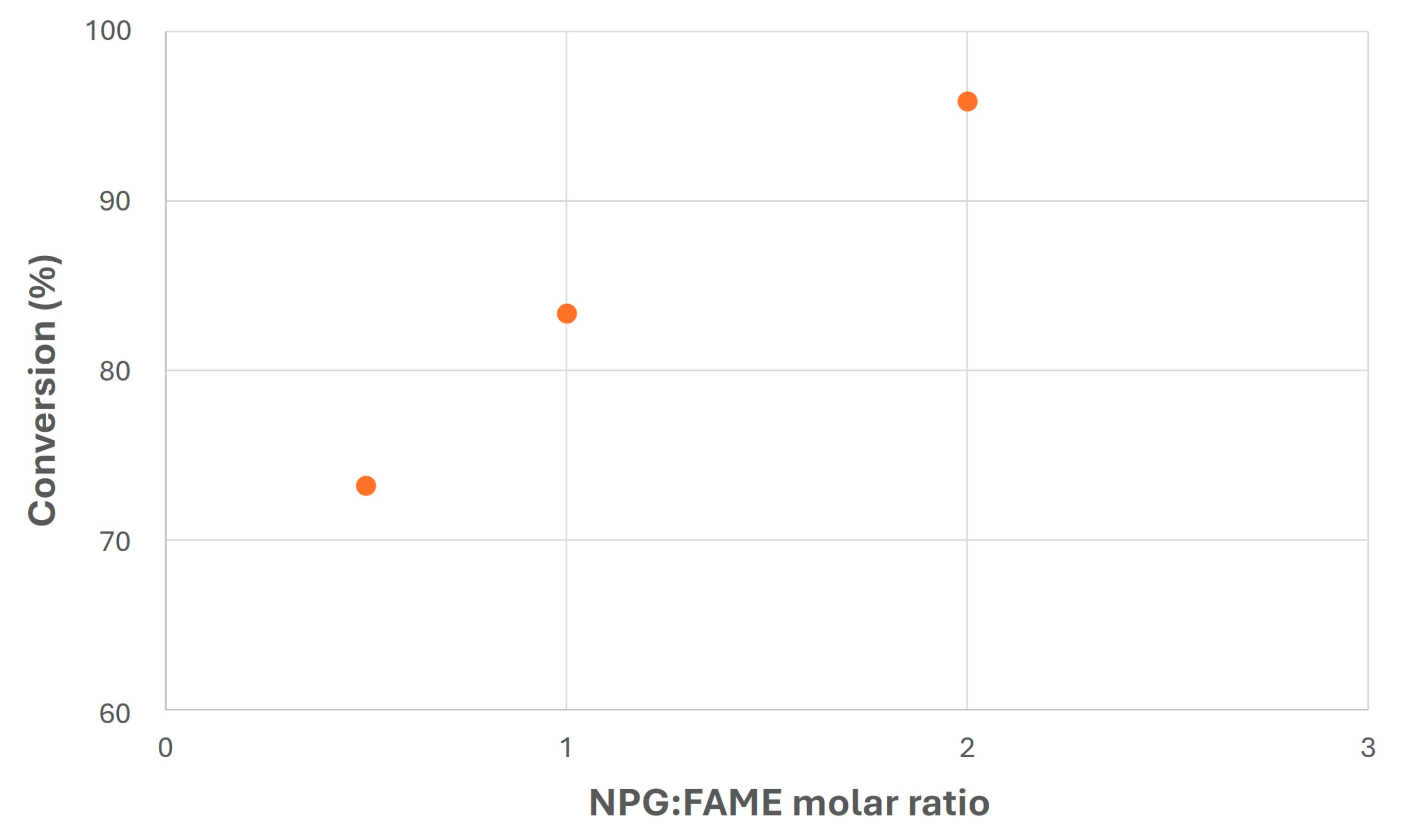
Regarding NPG/FAME ratio, Figure 8 shows the main results obtained in this experiment, where a considerable increase in FAME conversion with MR can be observed, exceeding 95% for MR = 2. This fact could be due to the higher presence of one of the reagents in the reaction medium, which could have shifted the chemical balance towards product generation. On the other hand, excess NPG would be problematic once the reaction is finished, as it would require further separation steps from the final biolubricant (mainly through filtration), as happened in previous studies with safflower biolubricant obtained with pentaerythritol and 2-ethyl-2-hydroxymethyl-1,3-propanediol [28,59]. In this sense, an average value would be advisable (MR = 1) in order to try to improve other operating conditions like catalyst addition.
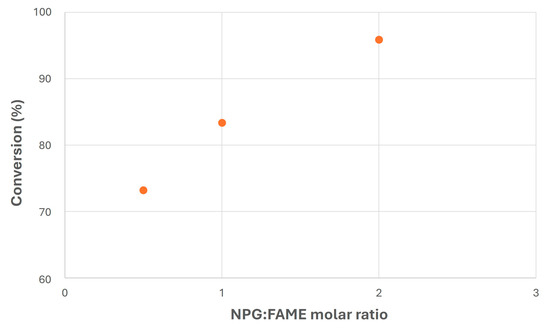
Figure 8.
Transesterification of canola FAMEs with NPG at different molar ratios (T = 120 °C; MeONa = 0.25% w/w).
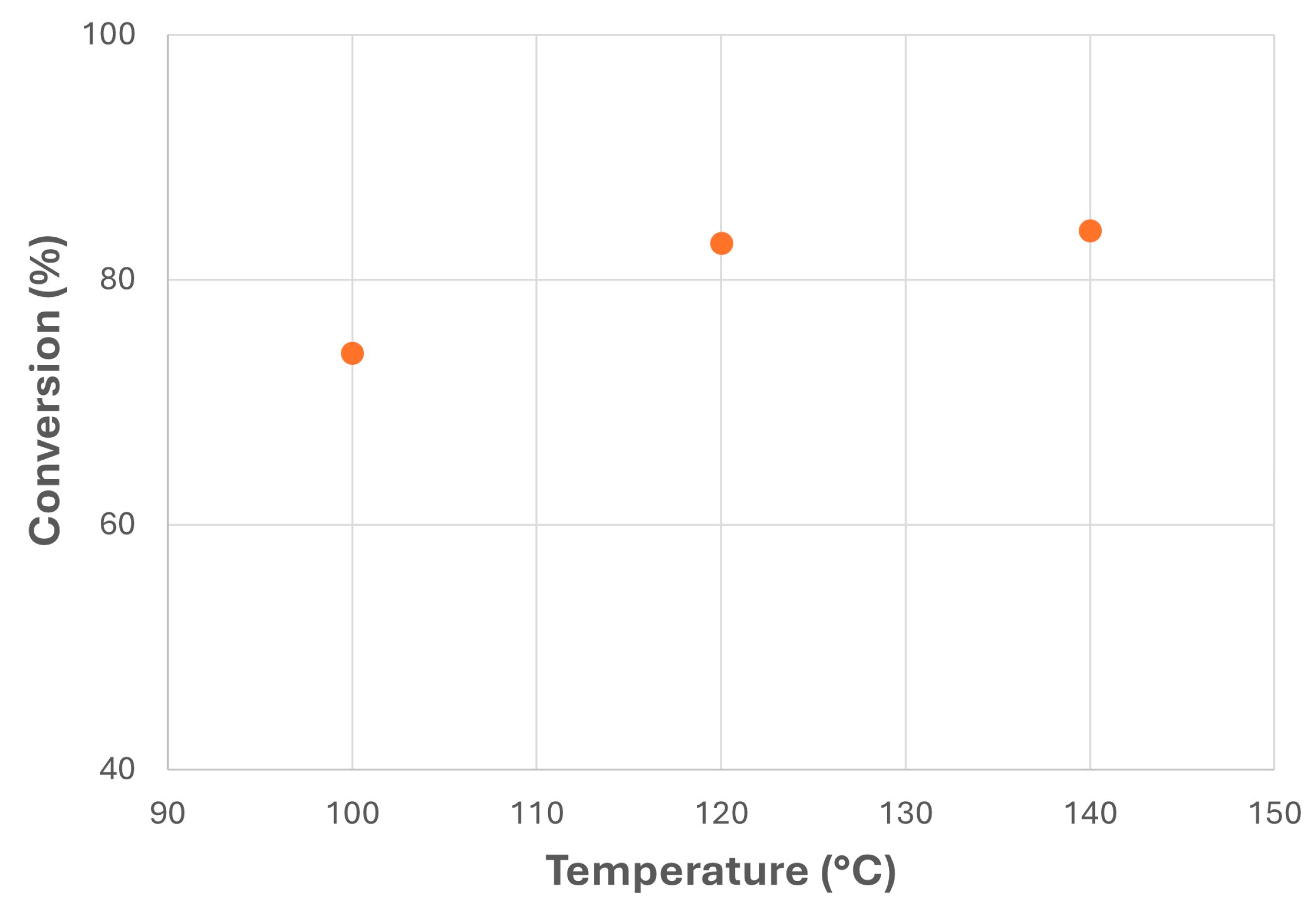
Temperature was another important factor in canola biolubricant production, as observed in Figure 9. Thus, relatively low increases in temperature showed a considerable increase in FAME conversion, from 74% at 100 °C to 83% at 120 °C. However, higher temperatures did not offer significant improvements in the conversion rate, thus recommending intermediate or lower reaction temperatures to avoid the loss of physico-chemical properties on account of oxidation [25].
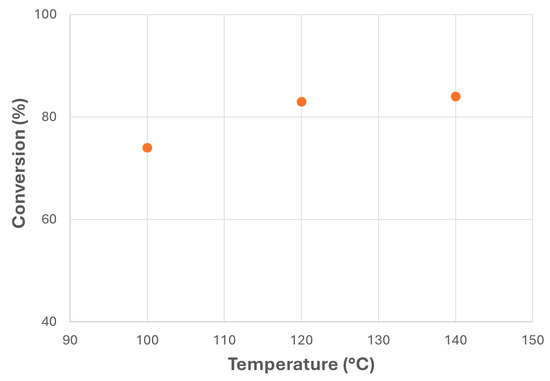
Figure 9.
Transesterification of canola FAMEs with NPG at different reaction temperatures (MR = 1; MeONa = 0.25% w/w).
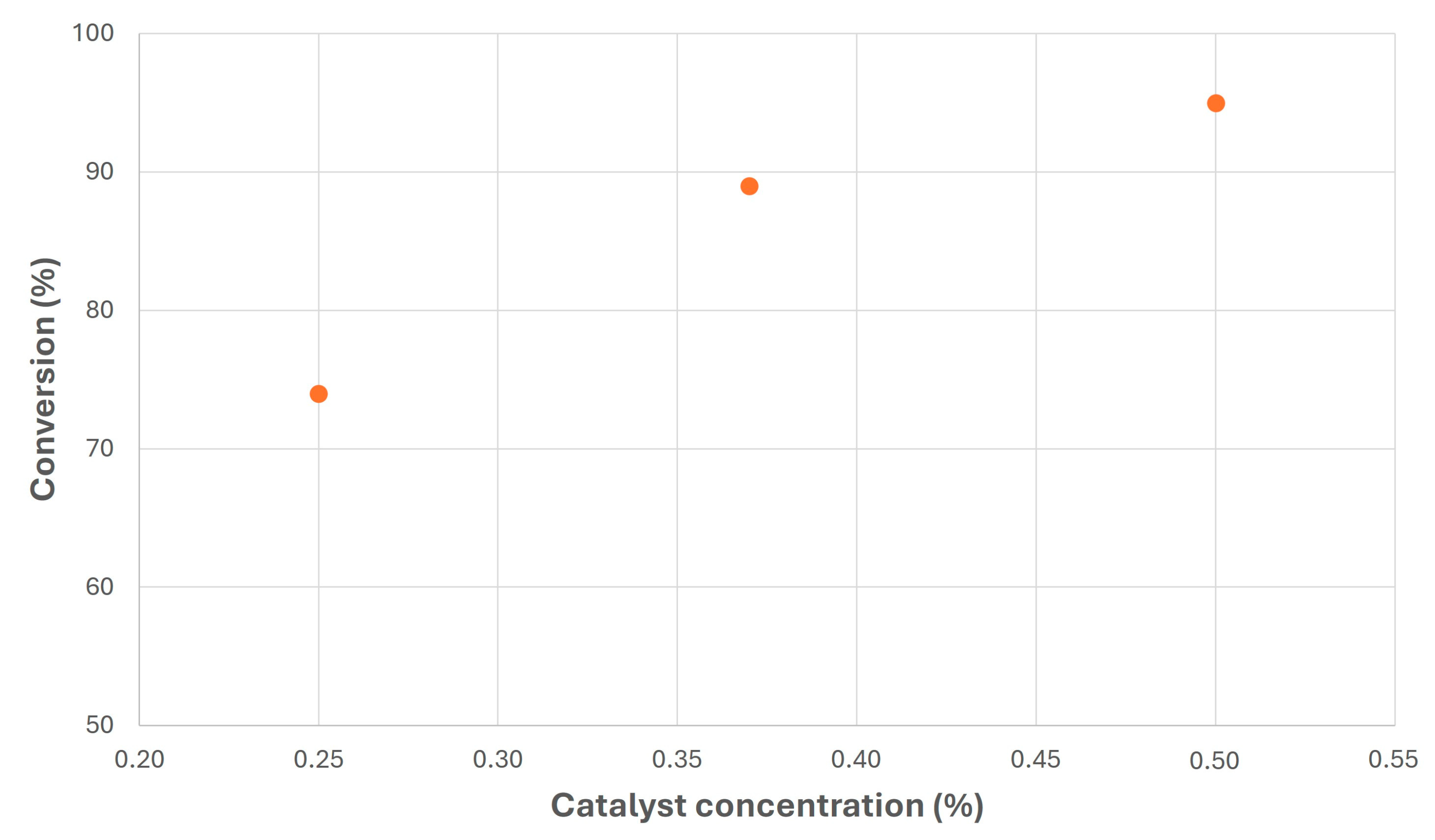
Finally, the effect of the catalyst is shown in Figure 10. As can be inferred, there was a continuous increase in conversion with the catalyst concentration, meaning that the chemical reaction, under these conditions, was not complete for 0.25 and 0.37% MeONa concentrations. Thus, only the highest concentration, with a conversion above 95%, assured the complete conversion of the catalyst at this reaction time (210 min) due to the increase in the reaction kinetics.
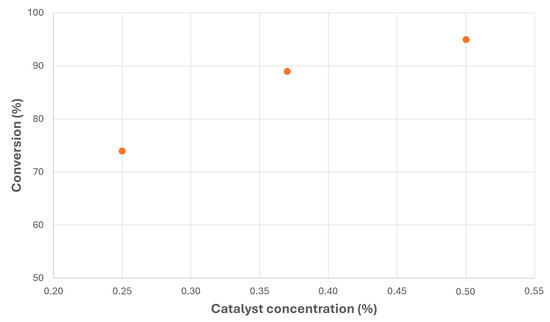
Figure 10.
Transesterification of canola FAMEs with NPG at different MeONa concentrations (T = 100 °C; MR = 1).
Nevertheless, the highest concentration of catalyst used in this experiment was within the range of catalyst concentration used in the literature, with catalyst loads of from 0.5 to 2% in some cases [28,59].
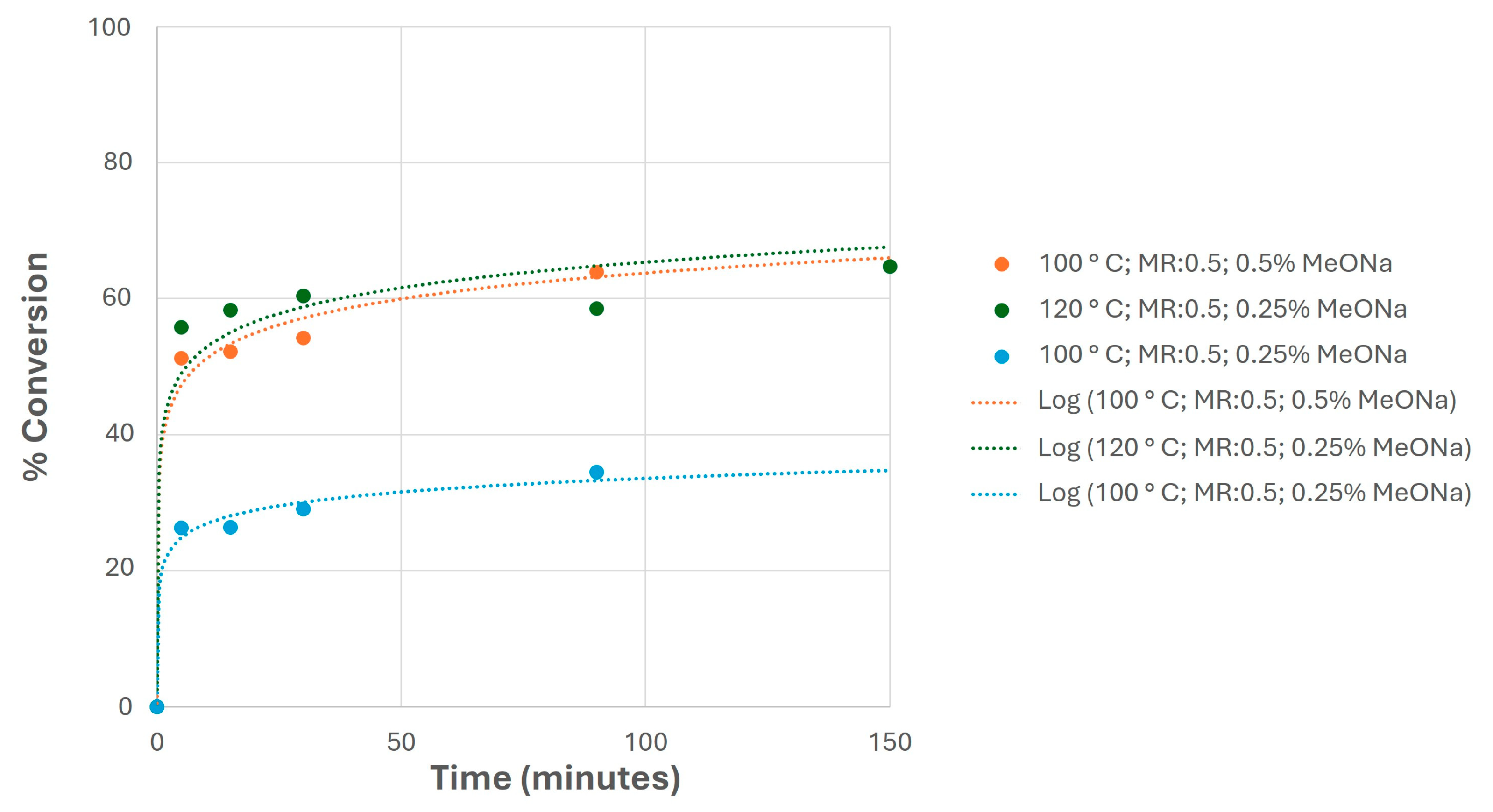
In this sense, a selection of previous studies is shown in Figure 11, showing the conversion evolution with the reaction time. As observed, the use of relatively low catalyst loads allowed for similar conversion values at the end of these experiments compared to higher reaction temperatures, obtaining milder reaction conditions that are desirable in terms of maintaining some of the properties of the final biolubricant (mainly the oxidation stability, which determines the maintenance of the viscosity and acidity during biolubricant storage) [25].
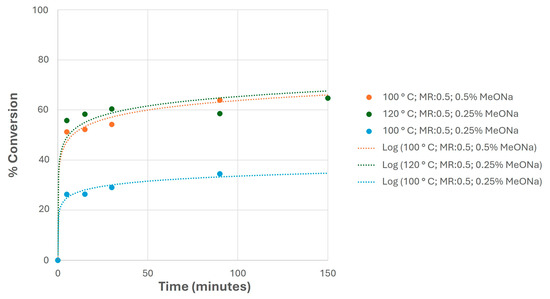
Figure 11.
Conversion evolution of selected experiments at different reaction temperatures and catalyst loads (sodium methoxide, MeONa), keeping the same MR in all cases.
As a consequence, the selected operating conditions required to produce the final lubricant were as follows: 0.5% sodium methoxide, T = 120 °C and MR = 1. For these criteria, efficiency factors were considered, using intermediate conditions to avoid the degradation of the sample (which was highly affected by the temperature and Na concentration, especially regarding oxidation stability). The reaction time was 210 min, with a stirring rate of 700 rpm. The main characteristics of the final product (purified biolubricant) are included in Table 4.

Table 4.
Characterization of canola biolubricant.
As observed in this table, a high conversion was achieved at around 95%, which assured a product with constant properties regardless of the presence of considerable amounts of impurities (mainly unconverted biodiesel). This way, the viscosity, which is one of the most influential parameters in determining the industrial use of a biolubricant, was relatively low compared to biolubricants produced with other more complex alcohols such as trimethylolpropane or pentaerythritol (exceeding 80 cSt in many cases) due to the higher molecular complexity of the latter, which allows for more intermolecular interactions that provoke an increase in viscosity [24,60]. Nevertheless, it was relatively similar to other equivalent biolubricants, such as soybean–NPG biolubricant obtained through esterification (10–11 cSt) [61] or Malaysian crude palm oil biolubricant synthesized with NPG (around 50 cSt) [62]. Regarding the density, it was lower compared to other studies included in the literature, where values of 911 kg·m−3 were found for a biolubricant obtained through the esterification of palm fatty acid distillate and NPG [63].
The flash and fire points were high enough to assure safety during its storage and handling, observing values from 190 to 260 °C for different biolubricants based on multiple alcohols [24,62].
This proposal to produce biodiesel and biolubricant from canola oil could contribute to the valorization of this crop. In this sense, other works have produced biolubricants from canola biodiesel through epoxidation, offering high thermal stability (above 190 °C) and viscosity (116 cSt at 40 °C) [18].
Other studies on epoxidized canola oil offered similar results, with high viscosity values (151 cSt) and thermal stabilities above 300 °C [64]. Alternatively, a similar double transesterification of canola oil (but changing the alcohol to trimethylol propane) was carried out, with viscosity values of around 30 cSt at 40 °C [65]. In this sense, canola oil and its derivatives could be an interesting starting point to produce a wide range of biolubricants, with variable properties that could be easily adaptable to specific industrial usages.
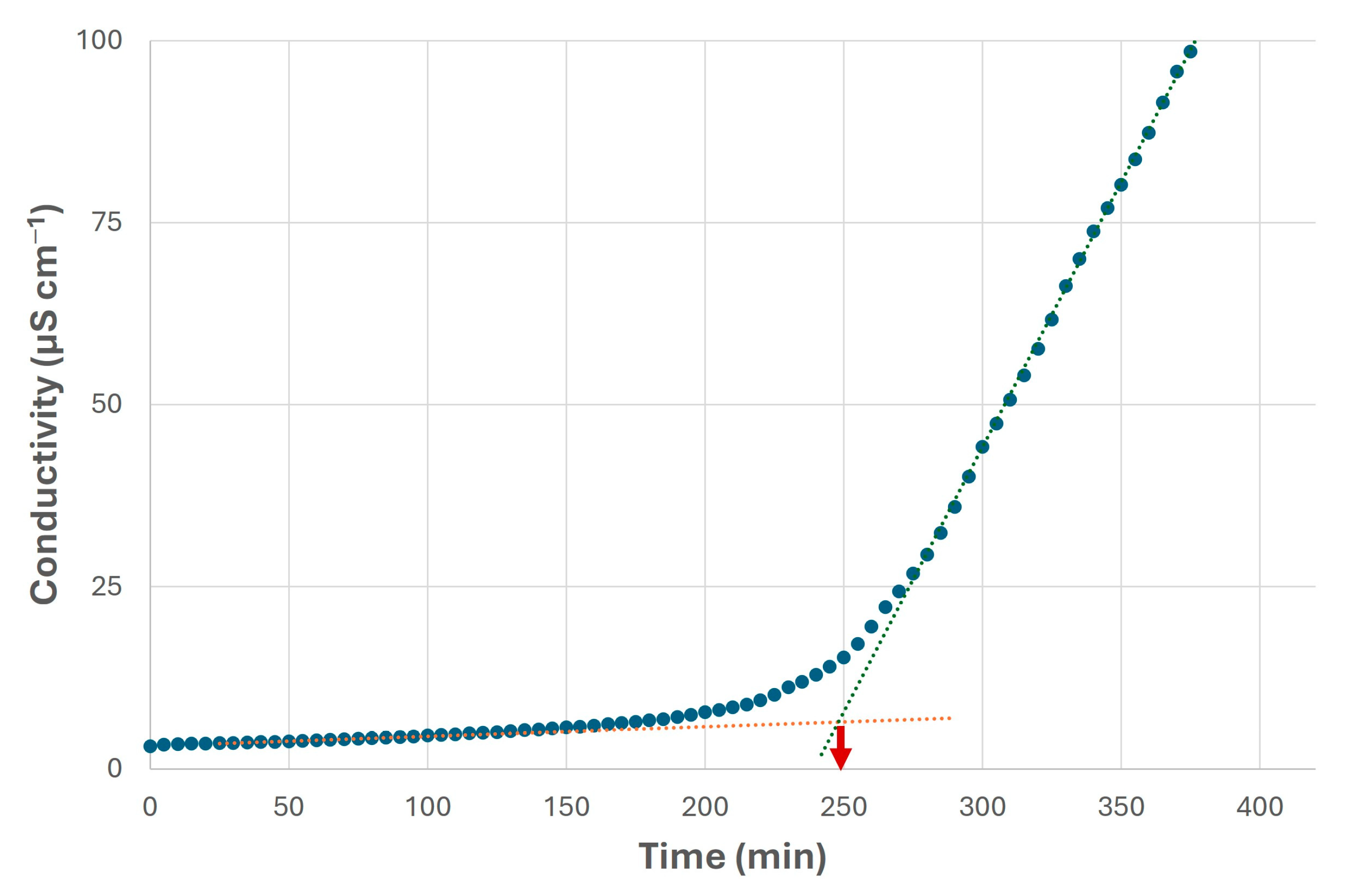
It should be noted the oxidation stability of the canola biolubricant, which was not as high as in the case of the canola biodiesel, possibly due to the presence of metal traces (on account of the homogeneous catalyst and the difficulty in removing it from the reaction medium), and further thermal treatments that could reduce this property. Nevertheless, this value (around 250 min, see Figure 12) was higher than those included in the literature [40,66,67], possibly requiring lower amounts of antioxidants to improve this property. As a consequence, higher stability during storage or oxidation processes will be obtained, thus maintaining the main properties of this product (viscosity and acidity, among others) over time [24]. Thus, the traces could be removed by using ion exchange columns, which could be interesting to avoid a decrease in oxidation stability due to the presence of Na [25,68].
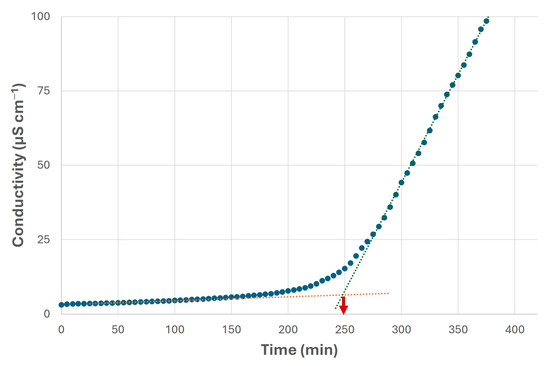
Figure 12.
Oxidation stability of final biolubricant obtained from canola oil through double transesterification with methanol and NPG under selected conditions. Blue dots indicate conductivity values, whereas red dashed line indicates the stable stage and blue dashed line indicates the unstable stage. Their intersection points out the oxidation stability (in this case, at 250 min).
4. Conclusions
The main findings of this work are as follows:
- Biodiesel and biolubricant production from canola oil through double transesterification with methanol and neopentyl glycol was carried out. For this purpose, homogeneous catalysts were used, with high conversion levels obtained in these two stages (97.0 and 94.7%, respectively).
- Specifically, relatively small amounts of catalysts were necessary to exceed 90% conversions to produce the biolubricants, proving the suitability of these catalysts for this purpose.
- In this sense, canola oil, along with other vegetable oils, could be an interesting starting point in a biorefinery context, where different products (in this case, glycerol, biodiesel and biolubricant) can be obtained with a high atom economy. Specifically, this work proposed the production of a biolubricant with unique properties conferred by the nature of canola oil (especially concerning its oxidation stability) and the use of NPG during the second transesterification step (with a strong influence on viscosity).
- The characteristics of the NPG-based biolubricant were analyzed, considering the use of this product as engine oil (SAE 5W), with viscosity and density values of 20.7 cSt at 40 °C and 855 kg·m−3, respectively. However, the oxidation stability was relatively low (around 4 h), requiring the use of antioxidants to maintaining its properties during storage.
- Further studies, like studies on the use of different kinds of catalysts (including improved heterogeneous ones, which should present high reusability in order to make the process sustainable), tribological studies or studies on the economic feasibility of the process, are suggested.
Author Contributions
Conceptualization, S.N.-D. and J.F.G.G.; methodology, M.A.-S. and S.N.-D.; validation, S.N.-D.; formal analysis, M.A.-S. and S.N.-D.; investigation, M.A.-S. and S.N.-D.; resources, S.N.-D. and J.F.G.G.; data curation, M.A.-S. and S.N.-D.; writing—original draft preparation, M.A.-S. and S.N.-D.; writing—review and editing, S.N.-D.; visualization, M.A.-S., S.N.-D. and J.F.G.G.; supervision, S.N.-D. and J.F.G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors appreciate the vegetable oil provided by “Finca La Orden”, belonging to CICYTEX (Technological and Scientific Research Center of Extremadura) and their continuous support to our work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bazilian, M.; Bradshaw, M.; Gabriel, J.; Goldthau, A.; Westphal, K. Four Scenarios of the Energy Transition: Drivers, Consequences, and Implications for Geopolitics. Wiley Interdiscip. Rev. Clim. Change 2020, 11, e625. [Google Scholar] [CrossRef]
- Di Foggia, G.; Beccarello, M.; Jammeh, B. A Global Perspective on Renewable Energy Implementation: Commitment Requires Action. Energies 2024, 17, 5058. [Google Scholar] [CrossRef]
- Tick, A.; Akaev, A.; Devezas, T.C.; Sarygulov, A.; Petryakov, A.; Evgenevich, A.I. Assessing Decarbonization Approaches across Major Economies. Energies 2024, 17, 4381. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Granjo, J.F.O.; Duarte, B.P.M.; Oliveira, N.M.C. Integrated Production of Biodiesel in a Soybean Biorefinery: Modeling, Simulation and Economical Assessment. Energy 2017, 129, 273–291. [Google Scholar] [CrossRef]
- Gottumukkala, L.D.; Haigh, K.; Collard, F.X.; van Rensburg, E.; Görgens, J. Opportunities and Prospects of Biorefinery-Based Valorisation of Pulp and Paper Sludge. Bioresour. Technol. 2016, 215, 37–49. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of Food Waste Biorefinery: A Review on Valorisation Pathways, Techno-Economic Constraints, and Environmental Assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef]
- Ubando, A.T.; Anderson, S.; Ng, E.; Chen, W.H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae Biorefinery: An Integrated Route for the Sustainable Production of High-Value-Added Products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- Igwebuike, C.M.; Awad, S.; Andrès, Y. Renewable Energy Potential: Second-Generation Biomass as Feedstock for Bioethanol Production. Molecules 2024, 29, 1619. [Google Scholar] [CrossRef]
- Moncada, B.J.; Aristizábal, M.V.; Cardona, A.C.A. Design Strategies for Sustainable Biorefineries. Biochem. Eng. J. 2016, 116, 122–134. [Google Scholar] [CrossRef]
- Bauer, F.; Coenen, L.; Hansen, T.; McCormick, K.; Palgan, Y.V. Technological Innovation Systems for Biorefineries: A Review of the Literature. Biofuels Bioprod. Biorefining 2017, 11, 534–548. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in Fatty Acid and Mineral Composition of Rapeseed (Brassica Napus Ssp. Oleifera L.) Oil with Seed Sizes. Ind. Crops. Prod. 2019, 129, 10–14. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224. [Google Scholar] [CrossRef]
- U. S. Department of Agriculture. USDA Open Data Catalog. Available online: https://www.usda.gov/content/usda-open-data-catalog (accessed on 20 October 2024).
- Appiah, G.; Tulashie, S.K.; Akpari, E.E.A.; Rene, E.R.; Dodoo, D. Biolubricant Production via Esterification and Transesterification Processes: Current Updates and Perspectives. Int. J. Energy Res. 2022, 46, 3860–3890. [Google Scholar] [CrossRef]
- Sharma, R.V.; Somidi, A.K.R.; Dalai, A.K. Preparation and Properties Evaluation of Biolubricants Derived from Canola Oil and Canola Biodiesel. J. Agric. Food Chem. 2015, 63, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized Production and Advanced Assessment of Biodiesel: A Review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Álvez-Medina, C.M.; González González, J.F. Biorefinery Based on Multiple Raw Materials and Wastes for the Production of Energy: A Proposal Tailored to Southwestern Europe. Encyclopedia 2024, 4, 1381–1395. [Google Scholar] [CrossRef]
- Di Fidio, N.; Carmassi, L.; Kasmiarti, G.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M.; Antonetti, C. Chemical and Enzymatic Hydrolysis of Waste Wheat Bran to Sugars and Their Simultaneous Biocatalytic Conversion to Valuable Carotenoids and Lipids. Catal. Today 2024, 442, 114941. [Google Scholar] [CrossRef]
- Di Fidio, N.; Minonne, F.; Antonetti, C.; Raspolli Galletti, A.M. Cutaneotrichosporon Oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes. Catalysts 2021, 11, 1291. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Sánchez Ocaña, M. Use of Mild Reaction Conditions to Improve Quality Parameters and Sustainability during Biolubricant Production. Biomass Bioenergy 2022, 161, 106456. [Google Scholar] [CrossRef]
- Samuel, J.; Kaisan, M.U.; Sanusi, Y.S.; Narayan, S.; Menacer, B.; Valenzuela, M.; Salas, A.; Oñate, A.; Mahroogi, F.O.; Tuninetti, V. Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants 2024, 12, 282. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Natural and Synthetic Antioxidant Additives for Improving the Performance of New Biolubricant Formulations. J. Agric. Food Chem. 2011, 59, 12917–12924. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arab. J. Chem. 2022, 15, 103796. [Google Scholar] [CrossRef]
- Kim, I.; Won, K.J. Method for Producing Neopentyl Glycol Diester as a Biolubricant Using Enzymatic Reaction. KR102564510B1, 4 August 2023. [Google Scholar]
- Cavalcanti da Silva, J.A.; Guimaraes Freire, D.M.; Habert, A.C.; Ferreira Soares, V. Method for Manufacturing a Bio Lubricant from Methyl Biodiesel and the Bio Lubricant Obtained by the Method. JP2015059176A, 30 March 2015. [Google Scholar]
- Mustafa, A. Lipase Catalyzed Reactions: A Promising Approach for Clean Synthesis of Oleochemicals. In Sustainable Solutions for Environmental Pollution; Wiley: Hoboken, NJ, USA, 2021; pp. 417–447. [Google Scholar]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the Time Finally Come for Green Oleochemicals and Biodiesel Production Using Large-Scale Enzyme Technologies? Current Status and New Developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Greco-Duarte, J.; Cavalcanti-Oliveira, E.D.; Da Silva, J.A.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Two-Step Enzymatic Production of Environmentally Friendly Biolubricants Using Castor Oil: Enzyme Selection and Product Characterization. Fuel 2017, 202, 196–205. [Google Scholar] [CrossRef]
- Cavalcanti, E.D.C.; Aguieiras, É.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved Production of Biolubricants from Soybean Oil and Different Polyols via Esterification Reaction Catalyzed by Immobilized Lipase from Candida Rugosa. Fuel 2018, 215, 705–713. [Google Scholar] [CrossRef]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess Development for Biolubricant Production Using Microbial Oil Derived via Fermentation from Confectionery Industry Wastes. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Fernandez-Lafuente, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic Synthesis of Biolubricants from By-Product of Soybean Oil Processing Catalyzed by Different Biocatalysts of Candida Rugosa Lipase. Catal. Today 2021, 362, 122–129. [Google Scholar] [CrossRef]
- Pucko, I.; Crnjac, K.; Faraguna, F. Lauric Acid-Based Polyol Esters as Potential Bio-Based Lubricants for Diesel Fuel. Chem. Biochem. Eng. Q. 2023, 37, 143–151. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, B.H.; Kim, Y.; Lee, M.W.; Im, D.J.; Kim, I.H. Lipase-Mediated Synthesis of Neopentyl Glycol Diester Using a Combination of Reduced and Standard Pressure. JAOCS J. Am. Oil Chem. Soc. 2021, 98, 1001–1007. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from Rapeseed and Castor Oil Transesterification by Using Titanium Isopropoxide as a Catalyst: Production and Characterization. Catalysts 2020, 10, 366. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon Biolubricant through Double Transesterification: Assessment of Its Oxidative, Thermal and Storage Stability. Mater. Lett. 2021, 302, 130454. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and Biolubricant Production from Different Vegetable Oils through Transesterification. Eng. Rep. 2020, 2, e12190. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of Its Oxidative Stability by Using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Zapata-Hernandez, C.; Santa, J.F.; Buitrago-Sierra, R. Palm Oil as a Biolubricant: Literature Review of Processing Parameters and Tribological Performance. J. Ind. Eng. Chem. 2022, 107, 31–44. [Google Scholar] [CrossRef]
- Kania, D.; Yunus, R.; Omar, R.; Abdul Rashid, S.; Mohamad Jan, B. A Review of Biolubricants in Drilling Fluids: Recent Research, Performance, and Applications. J. Pet. Sci. Eng. 2015, 135, 177–184. [Google Scholar] [CrossRef]
- UNE-EN 14214:2013 V2+A1:2018; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. Asociacion Espanola de Normalizacion: Madrid, Spain, 2018.
- UNE-EN ISO 3104/AC:1999; Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994). German Institute for Standardisation: Berlin, Germany, 1999.
- UNE-EN-ISO 3675; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method. Asociacion Espanola de Normalizacion: Madrid, Spain, 1999.
- UNE-EN ISO 12966-2:2011; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. Asociacion Espanola de Normalizacion: Madrid, Spain, 2011.
- UNE-EN 14104:2003; Oil and Fat Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Acid Value. British Standards Institution: London, UK, 2003.
- UNE-EN 14111:2003; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value. British Standards Institution: London, UK, 2003.
- UNE-EN 14112; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Oxidation Stability (Accelerated Oxidation Test). Asociacion Espanola de Normalizacion: Madrid, Spain, 2017.
- UNE-EN 51023:1990; Petroleum Products. Determination of Flash and Fire Points. Cleveland Open Cup Method. Asociacion Espanola de Normalizacion: Madrid, Spain, 1990.
- Issariyakul, T.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K.; Bakhshi, N.N. Biodiesel Production from Mixtures of Canola Oil and Used Cooking Oil. Chem. Eng. J. 2008, 140, 77–85. [Google Scholar] [CrossRef]
- Ajala, O.E.; Aberuagba, F.; Odetoye, T.E.; Ajala, A.M. Biodiesel: Sustainable Energy Replacement to Petroleum-Based Diesel Fuel—A Review. ChemBioEng Rev. 2015, 2, 145–156. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative Stability of Biodiesel: Causes, Effects and Prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renew. Sustain. Energy Rev. 2021, 145, 111109. [Google Scholar] [CrossRef]
- Caldeira, C.; Freire, F.; Olivetti, E.A.; Kirchain, R. Fatty Acid Based Prediction Models for Biodiesel Properties Incorporating Compositional Uncertainty. Fuel 2017, 196, 13–20. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González Cortés, Á. High Oleic Safflower Oil as a Feedstock for Stable Biodiesel and Biolubricant Production. Ind. Crops. Prod. 2021, 170, 113701. [Google Scholar] [CrossRef]
- Sanjurjo, C.; Rodríguez, E.; Viesca, J.L.; Battez, A.H. Influence of Molecular Structure on the Physicochemical and Tribological Properties of Biolubricants: A Review. Lubricants 2023, 11, 380. [Google Scholar] [CrossRef]
- Aguieiras, É.C.G.; Cavalcanti, E.D.C.; da Silva, P.R.; Soares, V.F.; Fernandez-Lafuente, R.; Bessa Assunção, C.L.; da Silva, J.A.C.; Freire, D.M.G. Enzymatic Synthesis of Neopentyl Glycol-Bases Biolubricants Using Biodiesel from Soybean and Castor Bean as Raw Materials. Renew. Energy 2020, 148, 689–696. [Google Scholar] [CrossRef]
- Nor, N.M.; Salih, N.; Salimon, J. Optimization and Lubrication Properties of Malaysian Crude Palm Oil Fatty Acids Based Neopentyl Glycol Diester Green Biolubricant. Renew. Energy 2022, 200, 942–956. [Google Scholar] [CrossRef]
- Ng, B.Y.S.; Ong, H.C.; Lau, H.L.N.; Ishak, N.S.; Elfasakhany, A.; Lee, H.V. Production of Sustainable Two-Stroke Engine Biolubricant Ester Base Oil from Palm Fatty Acid Distillate. Ind. Crops. Prod. 2022, 175, 114224. [Google Scholar] [CrossRef]
- Madankar, C.S.; Dalai, A.K.; Naik, S.N. Green Synthesis of Biolubricant Base Stock from Canola Oil. Ind. Crops. Prod. 2013, 44, 139–144. [Google Scholar] [CrossRef]
- Kamyab, B.; Beims, R.F.; Chio, C.; Qin, W.; Chambers, D.W.; Xu, C.C. Synthesis of TMP Esters as a Biolubricant from Canola Oil via a Two-Step Transesterification–Transesterification Process. Can. J. Chem. Eng. 2024, 102, 35–52. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring Tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27, 8931. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Investigation of Metals and Antioxidants on Stability Characteristics of Biodiesel. Mater. Today Proc. 2015, 2, 3196–3202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).