Nanocomposite PVDF/TiO2 Photocatalytic Membranes for Micropollutant Removal in Secondary Effluent
Abstract
1. Introduction
2. Results and Discussion
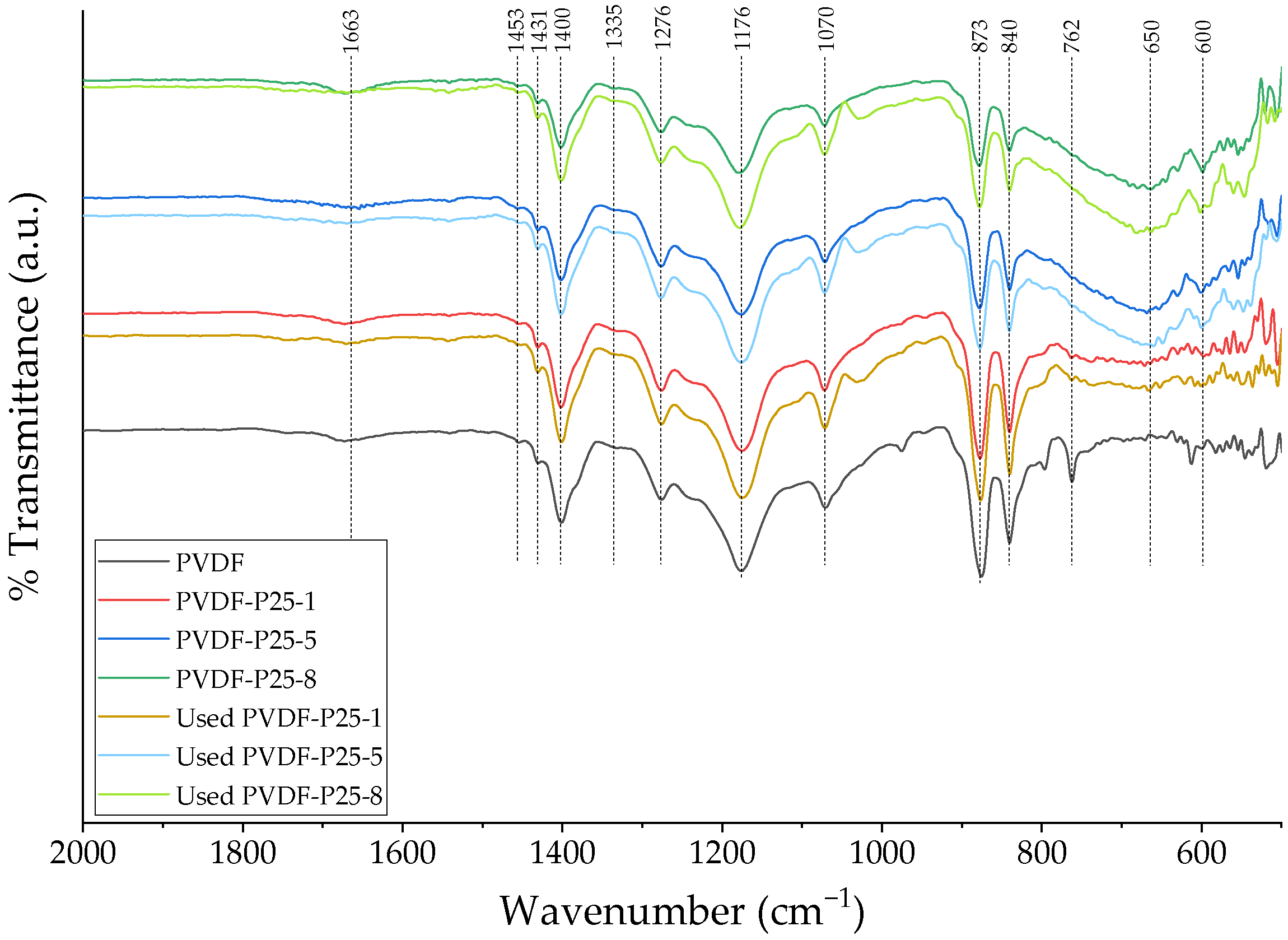
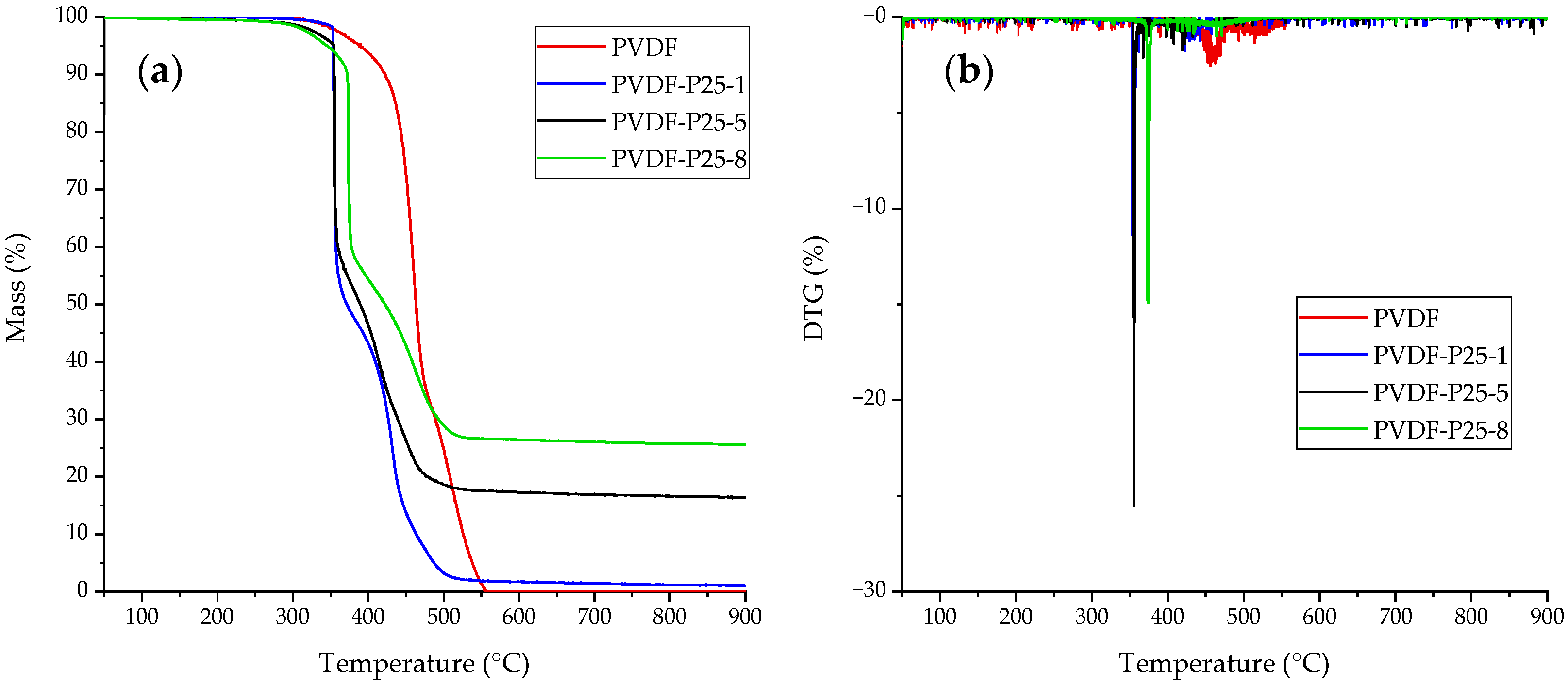
2.1. Membrane Characterization
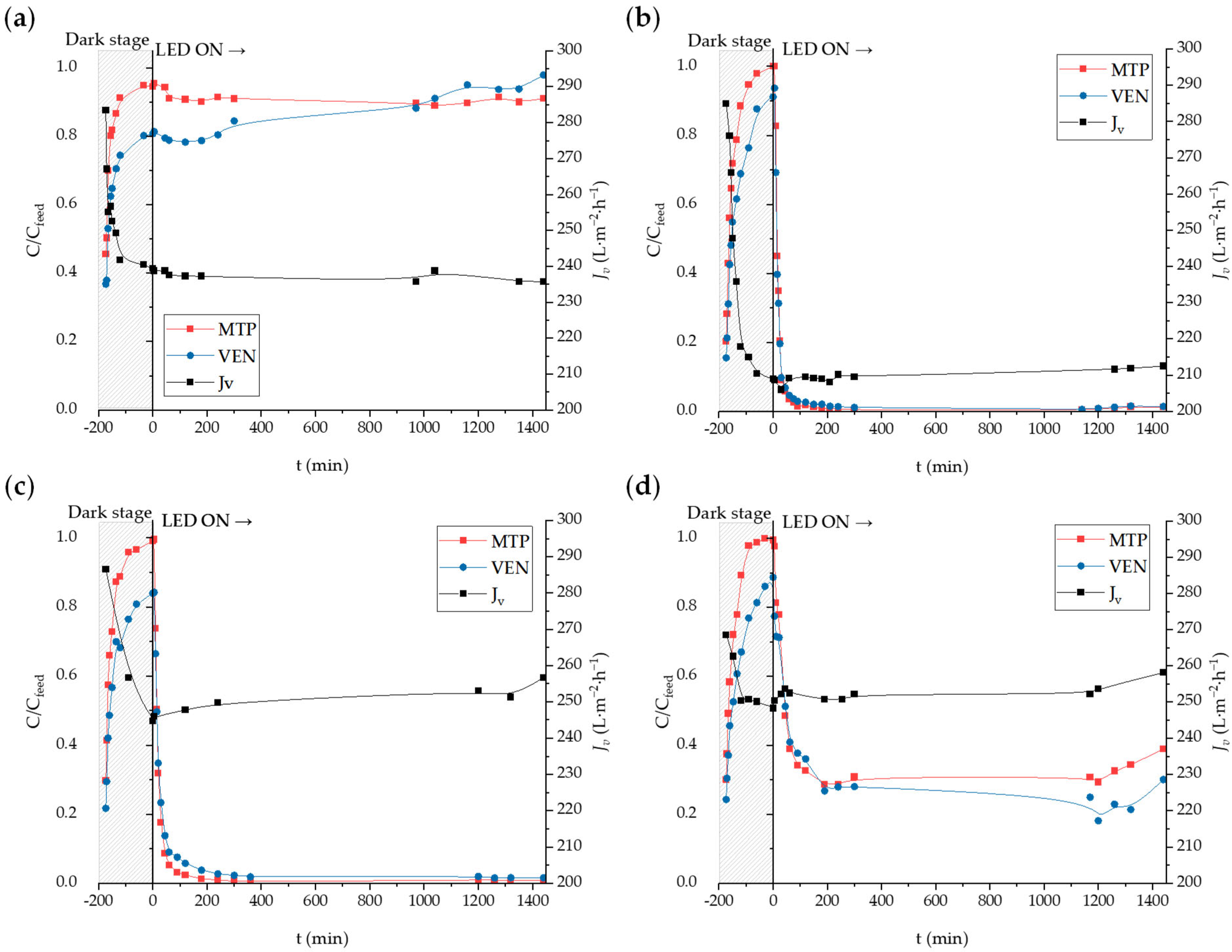
2.2. Ultrapure Water Filtration Experiments
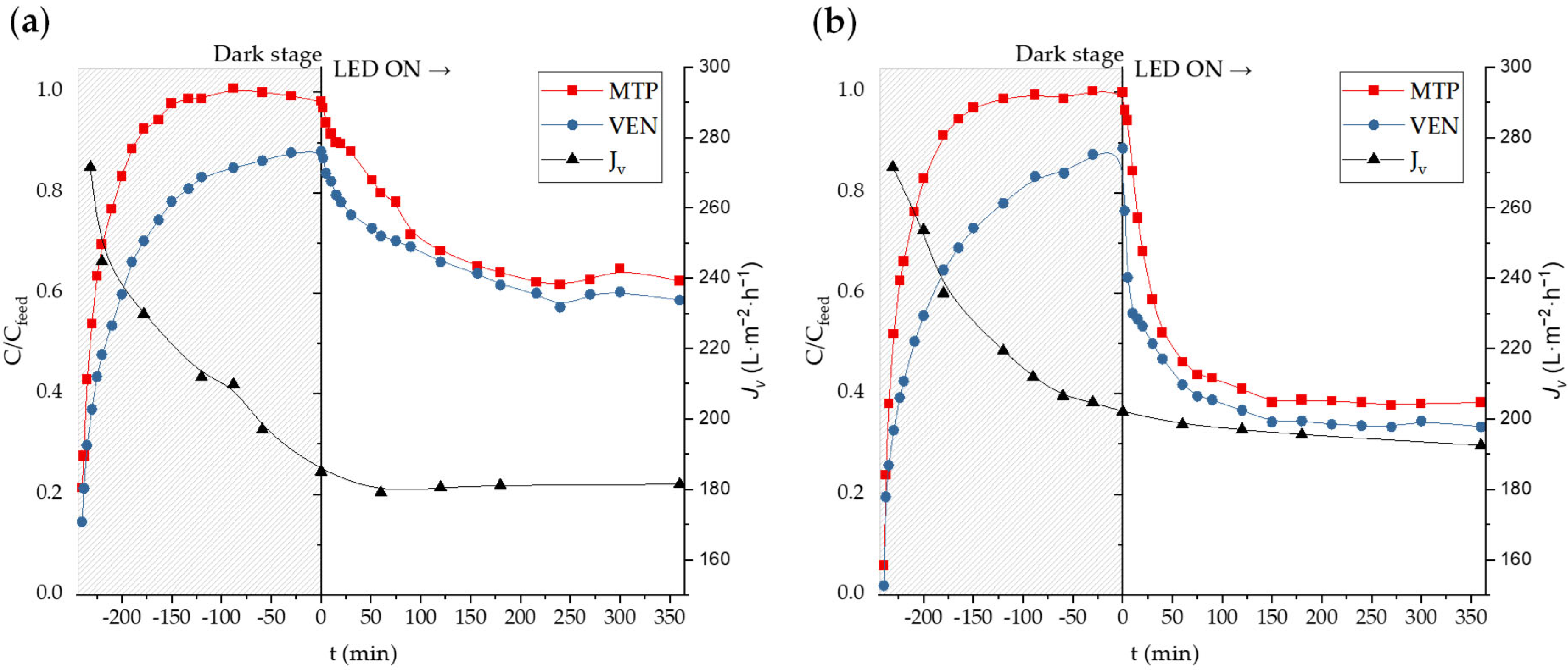
2.3. Secondary Effluent Filtration Experiments
3. Materials and Methods
3.1. Materials
3.2. Membrane Synthesis and Characterization
3.3. Experimental Procedure
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moratalla, Á.; Correia, S.E.; Lacasa, E.; Murillo, P.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Facing the Treatment of Polymedicated Effluents Using Gaseous Ozone Electrochemically Generated. J. Water Process Eng. 2023, 55, 104153. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M.; Ben Ayed, L.; Golomazou, E.; Karanis, P.; et al. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.H. Source, Fate, Transport and Modelling of Selected Emerging Contaminants in the Aquatic Environment: Current Status and Future Perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef] [PubMed]
- Bacci, F.; Campo, P. Emerging and Less Commonly Recognized Chemical Contaminants: Organic Micropollutants. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4, pp. 247–259. [Google Scholar]
- Lv, J.; Ou, C.; Fu, M.; Xu, Z. Characteristics and Transformation Pathways of Venlafaxine Degradation during Disinfection Processes Using Free Chlorine and Chlorine Dioxide. Chemosphere 2021, 276, 130147. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ramnarayanan, C.; Roopashree, T.S.; Anwer, M.K.; Sreeharsha, N.; Nair, A.B. Rapid, Precise and Affordable Estimation of Venlafaxine and Its Metabolites in Highly Polluted Effluent Waters: Proof-of-Concept for Methodology. Molecules 2020, 25, 4793. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.L.D.; Silva, C.P.; Otero, M. Dispersive Liquid-Liquid Microextraction for the Quantification of Venlafaxine in Environmental Waters. J. Environ. Manag. 2018, 217, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rutere, C.; Posselt, M.; Ho, A.; Horn, M.A. Biodegradation of Metoprolol in Oxic and Anoxic Hyporheic Zone Sediments: Unexpected Effects on Microbial Communities. Appl. Microbiol. Biotechnol. 2021, 105, 6103–6115. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-Catalytic Membrane Reactors for the Remediation of Persistent Organic Pollutants—A Review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Qing, W.; Liu, F.; Yao, H.; Sun, S.; Chen, C.; Zhang, W. Functional Catalytic Membrane Development: A Review of Catalyst Coating Techniques. Adv. Colloid Interface Sci. 2020, 282, 102207. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; El Aatik, A.; Aliste, M.; Navarro, G.; Fenoll, J.; Navarro, S. Removal of Contaminants of Emerging Concern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water Air Soil Pollut. 2023, 234, 55. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and Rutile in Evonik Aeroxide P25: Heterojunctioned or Individual Nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Tung, T.X.; Xu, D.; Zhang, Y.; Zhou, Q.; Wu, Z. Removing Humic Acid from Aqueous Solution Titanium Dioxide: A Review. Pol. J. Environ. Stud. 2018, 28, 529–542. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Mertes, A.; Vreuls, C.; Drot, S.; Smeets, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Lambert, S.D. Advanced Photocatalytic Oxidation Processes for Micropollutant Elimination from Municipal and Industrial Water. J. Environ. Manag. 2019, 250, 109561. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Maubane-Nkadimeng, M.S.; Moma, J.A. The Use of TiO2/Clay Heterostructures in the Photocatalytic Remediation of Water Containing Organic Pollutants: A Review. J. Environ. Chem. Eng. 2021, 9, 106546. [Google Scholar] [CrossRef]
- López, J.; Rey, A.; Viñuelas-Zahinos, E.; Álvarez, P.M. Preparation of a New Green Magnetic Fe3O4 @TiO2-P25 Photocatalyst for Solar Advanced Oxidation Processes in Water. J. Environ. Chem. Eng. 2023, 11, 109999. [Google Scholar] [CrossRef]
- Checa, M.; Montes, V.; Rivas, J.; Beltrán, F.J. Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water. Catalysts 2022, 12, 1587. [Google Scholar] [CrossRef]
- Zou, D.; Lee, Y.M. Design Strategy of Poly(Vinylidene Fluoride) Membranes for Water Treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J.; Shon, H.K. A Pilot-Scale Hybrid Municipal Wastewater Reclamation System Using Combined Coagulation and Disk Filtration, Ultrafiltration, and Reverse Osmosis: Removal of Nutrients and Micropollutants, and Characterization of Membrane Foulants. Bioresoure Technol. 2013, 141, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic Membrane-Based Oxidation-Filtration Systems for Organic Wastewater Purification: A Review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Gokulakrishnan, S.A.; Arthanareeswaran, G.; László, Z.; Veréb, G.; Kertész, S.; Kweon, J. Recent Development of Photocatalytic Nanomaterials in Mixed Matrix Membrane for Emerging Pollutants and Fouling Control, Membrane Cleaning Process. Chemosphere 2021, 281, 130891. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Fan, R.; Lewis, R. A Facile TiO2/PVDF Composite Membrane Synthesis and Their Application in Water Purification. J. Nanopart. Res. 2016, 18, 31. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.J.; Chou, H.H. Study the Self Cleaning, Antibacterial and Photocatalytic Properties of TiO2 Entrapped PVDF Membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Erusappan, E.; Thiripuranthagan, S.; Radhakrishnan, R.; Durai, M.; Kumaravel, S.; Vembuli, T.; Kaleekkal, N.J. Fabrication of Mesoporous TiO2/PVDF Photocatalytic Membranes for Efficient Photocatalytic Degradation of Synthetic Dyes. J. Environ. Chem. Eng. 2021, 9, 105776. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Huang, B.; Liu, G.; Guo, Y.; Zhu, H.; Yu, B. Fabrication of Anatase TiO2/PVDF Composite Membrane for Oil-in-Water Emulsion Separation and Dye Photocatalytic Degradation. Membranes 2023, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.R.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Polyvinylidene Fluoride (PVDF)/Ag@TiO2 Nanocomposite Membrane with Enhanced Fouling Resistance and Antibacterial Performance. Mater. Chem. Phys. 2021, 268, 124723. [Google Scholar] [CrossRef]
- Valenzuela, L.; Pedrosa, M.; Bahamonde, A.; Rosal, R.; Torres-Pinto, A.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Carbon Nitride—PVDF Photocatalytic Membranes for Visible-Light Degradation of Venlafaxine as Emerging Water Micropollutant. Catal. Today 2023, 418, 114042. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Torres-Pinto, A.; Pedrosa, M.; Munoz, M.; De Pedro, Z.M.; Silva, C.G.; Faria, J.L.; Casas, J.A.; Silva, A.M.T. Application of g-C3N4-PVDF Membrane for the Photocatalytic Degradation of Micropollutants in Continuous Flow Mode: Impact of Water Matrix. J. Environ. Chem. Eng. 2023, 11, 110586. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, X.; Li, Q.; Zheng, Y. A Novel PVDF-TiO2@g-C3N4 Composite Electrospun Fiber for Efficient Photocatalytic Degradation of Tetracycline under Visible Light Irradiation. Ecotoxicol. Environ. Saf 2021, 210, 111866. [Google Scholar] [CrossRef]
- Huang, J.; Hu, J.; Shi, Y.; Zeng, G.; Cheng, W.; Yu, H.; Gu, Y.; Shi, L.; Yi, K. Evaluation of Self-Cleaning and Photocatalytic Properties of Modified g-C3N4 Based PVDF Membranes Driven by Visible Light. J. Colloid Interface Sci. 2019, 541, 356–366. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Tailoring the Effects of Titanium Dioxide (TiO2) and Polyvinyl Alcohol (PVA) in the Separation and Antifouling Performance of Thin-Film Composite Polyvinylidene Fluoride (PVDF) Membrane. Membranes 2021, 11, 241. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In Situ Growth and Crystallization of TiO2 on Polymeric Membranes for the Photocatalytic Degradation of Diclofenac and 17α-Ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Liu, S.; Véron, E.; Lotfi, S.; Fischer, K.; Schulze, A.; Schäfer, A.I. Poly(Vinylidene Fluoride) Membrane with Immobilized TiO2 for Degradation of Steroid Hormone Micropollutants in a Photocatalytic Membrane Reactor. J. Hazard. Mater. 2023, 447, 130832. [Google Scholar] [CrossRef]
- Wang, X.; Pehkonen, S.O.; Rämö, J.; Väänänen, M.; Highfield, J.G.; Laasonen, K. Experimental and Computational Studies of Nitrogen Doped Degussa P25 TiO2: Application to Visible-Light Driven Photo-Oxidation of As(III). Catal. Sci. Technol. 2012, 2, 784–793. [Google Scholar] [CrossRef]
- Yadav, M.S.P.; Neghi, N.; Kumar, M.; Varghese, G.K. Photocatalytic-Oxidation and Photo-Persulfate-Oxidation of Sulfadiazine in a Laboratory-Scale Reactor: Analysis of Catalyst Support, Oxidant Dosage, Removal-Rate and Degradation Pathway. J. Environ. Manag. 2018, 222, 164–173. [Google Scholar] [CrossRef]
- Chen, L.; Hou, Z.; Lu, X.; Chen, P.; Liu, Z.; Shen, L.; Bian, X.; Qin, Q. Antifouling Microfiltration Membranes Prepared from Poly(Vinylidene Fluoride)-Graft-Poly(N-Vinyl Pyrrolidone) Powders Synthesized via Pre-Irradiation Induced Graft Polymerization. J. Appl. Polym. Sci. 2013, 128, 3949–3956. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane Surface Modification with TiO2–Graphene Oxide for Enhanced Photocatalytic Performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Construction of TiO2@Graphene Oxide Incorporated Antifouling Nanofiltration Membrane with Elevated Filtration Performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High Performance PVDF-TiO2 Membranes for Water Treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Medeiros, K.A.R.; Rangel, E.Q.; Sant’Anna, A.R.; Louzada, D.R.; Barbosa, C.R.H.; D’Almeida, J.R.M. Evaluation of the Electromechanical Behavior of Polyvinylidene Fluoride Used as a Component of Risers in the Offshore Oil Industry. Oil Gas Sci. Technol.—Rev. D’ifp Energ. Nouv. 2018, 73, 48. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Lanceros-Méndez, S.; Mano, J.F.; Costa, A.M.; Schmidt, V.H. FTIR and DSC Studies of Mechanically Deformed β-PVDF Films. J. Macromol. Sci. Part B 2001, 40, 517–527. [Google Scholar] [CrossRef]
- Boccaccio, T.; Bottino, A.; Capannelli, G.; Piaggio, P. Characterization of PVDF Membranes by Vibrational Spectroscopy. J. Membr. Sci. 2002, 210, 315–329. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P. A Two Dimensional Infrared Correlation Spectroscopic Study on the Structure Changes of PVDF during the Melting Process. Polymer 2004, 45, 5295–5299. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-Doped Carbon Quantum Dots/TiO2 Composite with Improved Photocatalytic Activity. Appl. Catal. B 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Sriwong, C.; Choojun, K.; Sriwong, S. High Photocatalytic Performance of 3D Porous-Structured TiO2@natural Rubber Hybrid Sheet on the Removal of Indigo Carmine Dye in Water. SN Appl. Sci. 2019, 1, 864. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Ramujana, S.; Katwire, D.M.; Taziwa, R.T. Synthesis of a Novel Visible Light Responsive γ-Fe2O3/SiO2/C-TiO2 Magnetic Nanocomposite for Water Treatment. Water Sci. Technol. 2018, 78, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, E.; Maréchal, W.; Lorain, O.; Lamaa, L.; Peruchon, L.; Brochier, C.; Mendret, J.; Mericq, J.P.; Brosillon, S.; Faur, C.; et al. Understanding Aging Mechanisms in the Context of UV Irradiation of a Low Fouling and Self-Cleaning PVDF-PVP-TiO2 Hollow-Fiber Membrane. Membranes 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Liu, H.; Ding, Y.; Lu, B.; Zhang, M. Fabrication of Micro-Structures on a PVDF/TiO2 Nano-Composite Film Using Photocatalytic Lithography. Appl. Surf. Sci. 2012, 258, 5052–5055. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Šťastná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef]
- Chinh, V.D.; Broggi, A.; Di Palma, L.; Scarsella, M.; Speranza, G.; Vilardi, G.; Thang, P.N. XPS Spectra Analysis of Ti2+, Ti3+ Ions and Dye Photodegradation Evaluation of Titania-Silica Mixed Oxide Nanoparticles. J. Electron. Mater. 2018, 47, 2215–2224. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, Y.M. Preparation and Investigation of PVDF/PMMA/TiO2 Composite Film. J. Mater. Sci. 2009, 44, 2977–2984. [Google Scholar] [CrossRef]
- Tian, Z.; Song, Y.; Tao, K.; Liu, N.; Qin, S.; Yang, J.; Li, J.; Cui, Z. Preparation of TiO2-Ag Heterostructure via Tannic Acid-Assistance and Its Immobilization on PVDF Membrane for the Degradation of Dye under Visible Light. Appl. Surf. Sci. 2023, 625, 157195. [Google Scholar] [CrossRef]
- Abdelmaksoud, M.; Mohamed, A.; Sayed, A.; Khairy, S. Physical Properties of PVDF-GO/Black-TiO2 Nanofibers and Its Photocatalytic Degradation of Methylene Blue and Malachite Green Dyes. Environ. Sci. Pollut. Res. 2021, 28, 30613–30625. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of Photoluminescence Performance of Nano-Sized Semiconductor Materials and Its Relationships with Photocatalytic Activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of Emerging Contaminants by Simultaneous Application of Membrane Ultrafiltration, Activated Carbon Adsorption, and Ultrasound Irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Lambropoulou, D.; Evgenidou, E.; Saliverou, V.; Kosma, C.; Konstantinou, I. Degradation of Venlafaxine Using TiO2/UV Process: Kinetic Studies, RSM Optimization, Identification of Transformation Products and Toxicity Evaluation. J. Hazard. Mater. 2017, 323, 513–526. [Google Scholar] [CrossRef]
- Leyva, E.; Moctezuma, E.; López, M.; Baines, K.M.; Zermeño, B. Photocatalytic Degradation of β-Blockers in TiO2 with Metoprolol as Model Compound. Intermediates and Total Reaction Mechanism. Catal. Today 2019, 323, 14–25. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Gracia-Pinilla, M.; Pillai, S.C.; O’Shea, K. UV and Visible Light-Driven Production of Hydroxyl Radicals by Reduced Forms of N, F, and P Codoped Titanium Dioxide. Molecules 2019, 24, 2147. [Google Scholar] [CrossRef]
- Guo, Y.; Zhan, J.; Yu, G.; Wang, Y. Evaluation of the Concentration and Contribution of Superoxide Radical for Micropollutant Abatement during Ozonation. Water Res. 2021, 194, 116927. [Google Scholar] [CrossRef]
- Kovácsa, K.; Tóth, T.; Wojnárovits, L. Evaluation of Advanced Oxidation Processes for β-Blockers Degradation: A Review. Water Sci. Technol. 2022, 85, 685–705. [Google Scholar] [CrossRef]
- de Souza, L.P.; Sanches-Neto, F.O.; Junior, G.M.Y.; Ramos, B.; Lastre-Acosta, A.M.; Carvalho-Silva, V.H.; Teixeira, A.C.S.C. Photochemical Environmental Persistence of Venlafaxine in an Urban Water Reservoir: A Combined Experimental and Computational Investigation. Process Saf. Environ. Prot. 2022, 166, 478–490. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W. Comparative Assessment of Hydrophobic and Hydrophilic Membrane Fouling in Wastewater Applications. J. Membr. Sci. 2009, 339, 93–99. [Google Scholar] [CrossRef]
- Alpatova, A.; Kim, E.-S.; Dong, S.; Sun, N.; Chelme-Ayala, P.; Gamal El-Din, M. Treatment of Oil Sands Process-Affected Water with Ceramic Ultrafiltration Membrane: Effects of Operating Conditions on Membrane Performance. Sep. Purif. Technol. 2014, 122, 170–182. [Google Scholar] [CrossRef]
- García-Ivars, J.; Iborra-Clar, M.-I.I.; Alcaina-Miranda, M.I.M.-I.; Mendoza-Roca, J.-A.A.; Pastor-Alcañiz, L.; Garcia-Ivars, J.; Iborra-Clar, M.-I.I.; Alcaina-Miranda, M.I.M.-I.; Mendoza-Roca, J.-A.A.; Pastor-Alcañiz, L. Treatment of Table Olive Processing Wastewaters Using Novel Photomodified Ultrafiltration Membranes as First Step for Recovering Phenolic Compounds. J. Hazard. Mater. 2015, 290, 51–59. [Google Scholar] [CrossRef]
- Acero, J.L.; Von Gunten, U. Influence of Carbonate on the Ozone/Hydrogen Peroxide Based Advanced Oxidation Process for Drinking Water Treatment. Ozone Sci. Eng. 2000, 22, 305–328. [Google Scholar] [CrossRef]
- Chávez, A.M.; Solís, R.R.; Beltrán, F.J. Magnetic Graphene TiO2-Based Photocatalyst for the Removal of Pollutants of Emerging Concern in Water by Simulated Sunlight Aided Photocatalytic Ozonation. Appl. Catal. B 2020, 262, 118275. [Google Scholar] [CrossRef]
- Vieira, O.; Ribeiro, R.S.; Pedrosa, M.; Lado Ribeiro, A.R.; Silva, A.M.T. Nitrogen-Doped Reduced Graphene Oxide—PVDF Nanocomposite Membrane for Persulfate Activation and Degradation of Water Organic Micropollutants. Chem. Eng. J. 2020, 402, 126117. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W., Braun-Howland, E., Baxter, T., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]



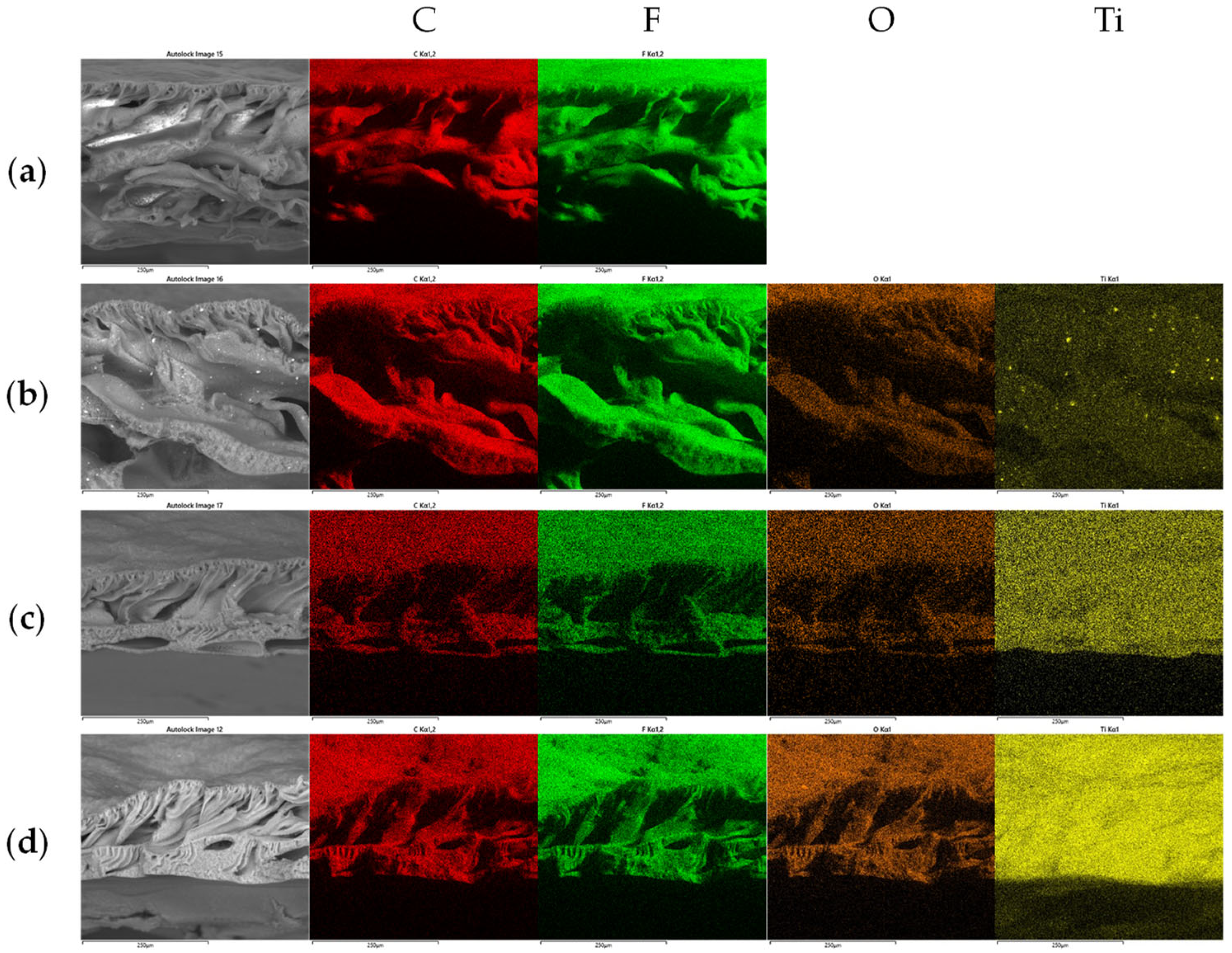




| Membrane | Specific Surface Area (m2·g−1) | Pore Volume (cm3/g) | Water Contact Angle (°) | Membrane Thickness (µm) |
|---|---|---|---|---|
| PVDF | 11.5 | 0.025 | 67.2 ± 3.6 | 312.8 ± 4.7 |
| PVDF-P25-1 | 12.4 | 0.031 | 66.3 ± 3.1 | 389.1 ± 6.0 |
| PVDF-P25-5 | 15.0 | 0.039 | 66.6 ± 4.8 | 180.6 ± 3.6 |
| PVDF-P25-8 | 14.1 | 0.036 | 67.6 ± 1.6 | 184.5 ± 4.2 |
| Membrane | Elemental Composition (wt.%) | |||
|---|---|---|---|---|
| C | O | F | Ti | |
| PVDF | 54.27 ± 0.06 | 1.14 ± 0.13 | 44.59 ± 0.09 | - |
| PVDF-P25-1 | 50.56 ± 0.27 | 3.87 ± 0.47 | 44.13 ± 0.28 | 1.45 ± 0.03 |
| PVDF-P25-5 | 42.50 ± 0.15 | 10.70 ± 0.04 | 39.67 ± 0.03 | 7.12 ± 0.15 |
| PVDF-P25-8 | 40.84 ± 0.59 | 15.84 ± 0.39 | 34.19 ± 1.17 | 9.14 ± 0.22 |
| Membrane | Jw (L·m−2·h−1) | |
|---|---|---|
| LED Off | LED On | |
| PVDF | 240.8 | 238.4 |
| PVDF-P25-1 | 210.2 | 204.8 |
| PVDF-P25-5 | 256.0 | 258.3 |
| PVDF-P25-8 | 292.6 | 286.2 |
| MP | Molecular Formula | Molecular Weight (g·mol−1) | pKa | log Kow | Molar Volume (cm3) |
|---|---|---|---|---|---|
| Venlafaxine (VEN) | C17H27NO2 | 277.40 | 9.50 | 3.20 | 261.6 ± 3.0 |
| Metoprolol (MTP) | C15H25NO3 | 267.36 | 9.56 | 1.88 | 258.7 ± 3.0 |
| Membrane | Jvss (L·m−2·h−1) | Jvss/Jw | MRR (mg·m−2·h−1) | |
|---|---|---|---|---|
| MTP | VEN | |||
| PVDF | 236.6 | 0.988 | - | - |
| PVDF-P25-1 | 211.1 | 1.017 | 48.3 | 45.0 |
| PVDF-P25-5 | 253.7 | 0.986 | 66.3 | 65.3 |
| PVDF-P25-8 | 254.7 | 0.880 | 45.9 | 49.0 |
| Matrix | Jvss (L·m−2·h−1) | Jvss/Jw | MRR (mg·m−2·h−1) | |
|---|---|---|---|---|
| MTP | VEN | |||
| UP | 211.1 | 1.017 | 48.3 | 45.0 |
| SE1 | 181.0 | 0.872 | 17.3 | 18.5 |
| SE2 | 195.8 | 0.944 | 30.7 | 32.2 |
| Parameter | SE1 | SE2 |
|---|---|---|
| pH | 7.67 | 7.37 |
| Electric conductivity (μS·cm−1) | 1171 | 1136 |
| Turbidity (NTU) | 2.31 | 2.16 |
| COD (mg·L−1) | 21 | 24 |
| TOC (mg·L−1) | 6.05 | 6.29 |
| TIC (mg·L−1) | 89.7 | 9.55 |
| Absorbance (254 nm) | 0.119 | 0.123 |
| Total N (mg·L−1) | 5.2 | 5.0 |
| Total P (mg·L−1) | 0.29 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldana, J.C.; Pedrosa, M.; Silva, A.M.T.; Faria, J.L.; Acero, J.L.; Álvarez, P.M. Nanocomposite PVDF/TiO2 Photocatalytic Membranes for Micropollutant Removal in Secondary Effluent. Catalysts 2024, 14, 109. https://doi.org/10.3390/catal14020109
Aldana JC, Pedrosa M, Silva AMT, Faria JL, Acero JL, Álvarez PM. Nanocomposite PVDF/TiO2 Photocatalytic Membranes for Micropollutant Removal in Secondary Effluent. Catalysts. 2024; 14(2):109. https://doi.org/10.3390/catal14020109
Chicago/Turabian StyleAldana, Juan C., Marta Pedrosa, Adrián M. T. Silva, Joaquim L. Faria, Juan L. Acero, and Pedro M. Álvarez. 2024. "Nanocomposite PVDF/TiO2 Photocatalytic Membranes for Micropollutant Removal in Secondary Effluent" Catalysts 14, no. 2: 109. https://doi.org/10.3390/catal14020109
APA StyleAldana, J. C., Pedrosa, M., Silva, A. M. T., Faria, J. L., Acero, J. L., & Álvarez, P. M. (2024). Nanocomposite PVDF/TiO2 Photocatalytic Membranes for Micropollutant Removal in Secondary Effluent. Catalysts, 14(2), 109. https://doi.org/10.3390/catal14020109










