Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes
Abstract
:1. Introduction
2. Phytoremediation: Mechanisms and Plant Selection
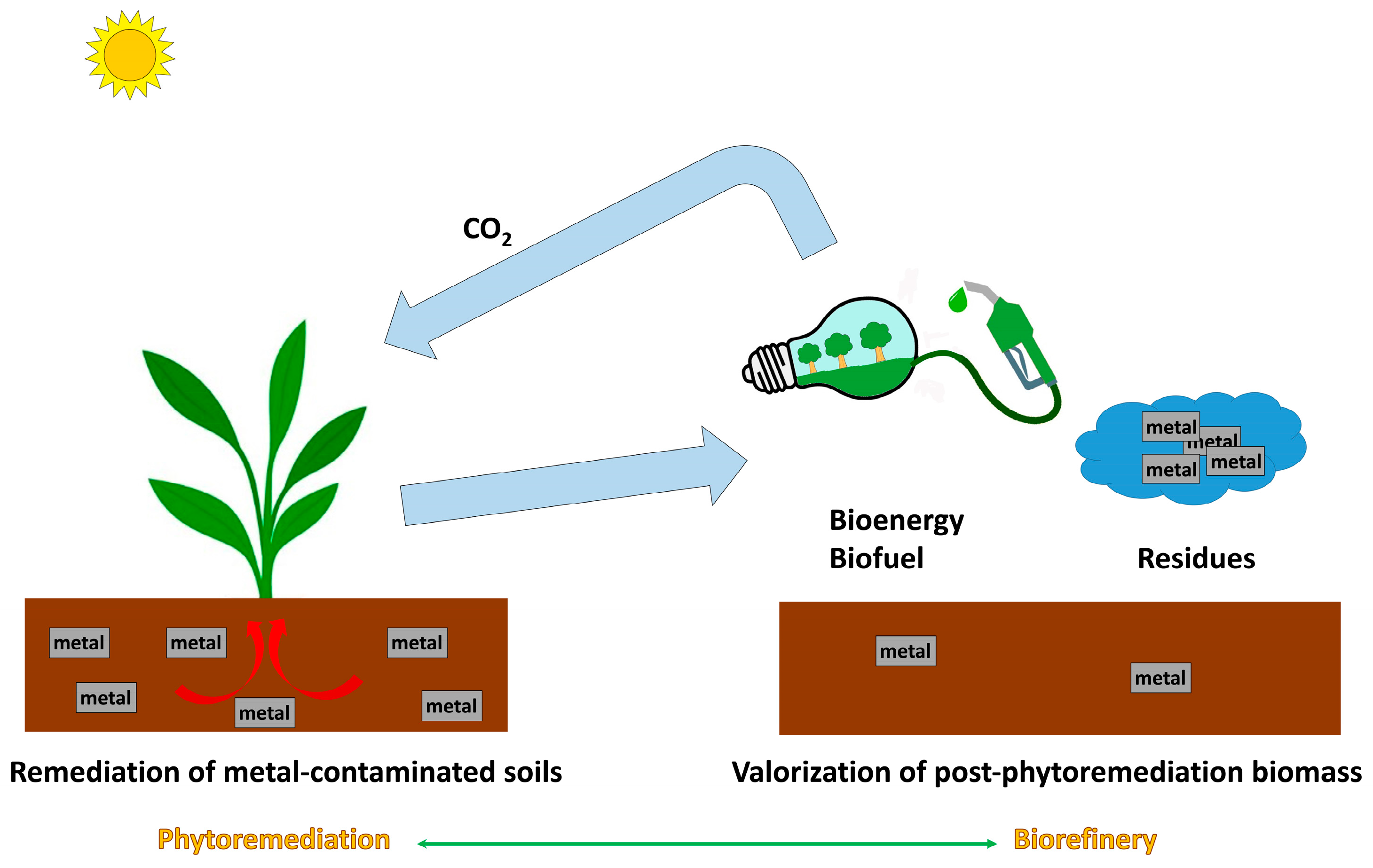
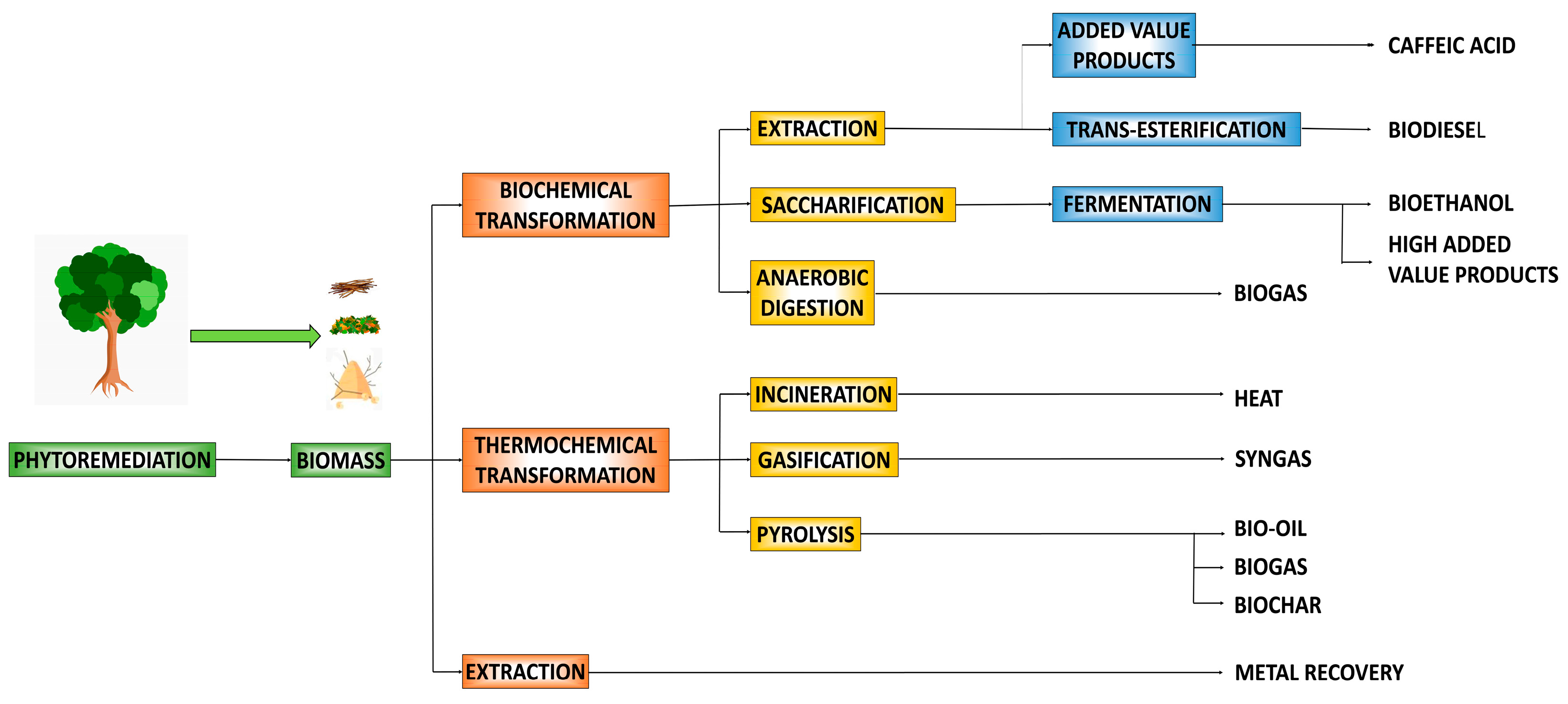
3. Valorization of Phytoremediation Byproducts
3.1. Thermochemical Methods
3.2. Biological Methods
4. Pretreatment Technology on Biomass from Phytoremediation
5. Biofuels and High Added Value Production
5.1. Bioethanol
5.2. Biogas
5.3. Biodiesel
6. Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Rai, S.; Gautam, K.; Sharma, S. Phytoremediation strategies of plants: Challenges and opportunities. In Plants and Their Interaction to Environmental Pollution. Damage Detection, Adaptation, Tolerance, Physiological and Molecular Responses; Husen, A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; pp. 211–229. [Google Scholar]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an effective remedy for removing trace elements from ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Lim, K.T.; Shukor, M.Y.; Wasoh, H.; Omri, A. Physical, chemical, and biological methods for the removal of arsenic compounds. BioMed Res. Int. 2014, 2014, 503784. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Haimi, J. Decomposer animals and bioremediation of soils. Environ. Pollut. 2000, 107, 233–238. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Ali, A.; Mohd, Q.; Haq, R. Prospects for Exploiting Bacteria for Bioremediation of Metal Pollution. Crit. Rev. Environ. Sci. Technol. 2014, 44, 519–560. [Google Scholar] [CrossRef]
- Arora, N.K. Bioremediation: A green approach for restoration of polluted ecosystems. Environ. Sustain. 2018, 1, 305–307. [Google Scholar] [CrossRef]
- Kafle, A.; Anil Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Antoniadis, V.; Sabry, M.; Shaheen, S.M.; Stärk, H.G.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Intern. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Amabogha, O.N.; Garelick, H.; Jones, H.; Purchase, D. Combining phytoremediation with bioenergy production: Developing a multi-criteria decision matrix for plant species selection. Environ. Sci. Pollut. Res. 2023, 30, 40698–40711. [Google Scholar] [CrossRef]
- Singh, B.S.M.; Singh, D.; Dhal, N.K. Enhanced phytoremediation strategy for sustainable management of heavy metals and radionuclides. Case Stud. Chem. Environ. Eng. 2022, 5, 100176. [Google Scholar] [CrossRef]
- Gordon, M.; Choe, N.; Duffy, J.; Ekuan, G.; Heilman, P.; Muiznieks, I.; Ruszaj, M.; Shurtleff, B.B.; Strand, S.; Wilmoth, J.; et al. Phytoremediation of trichloroethylene with hybrid poplars. Environ. Health Perspect. 1998, 106, 1001–1004. [Google Scholar]
- Burken, J.G.; Schnoor, J.L. Uptake and metabolism of atrazine by poplar trees. Environ. Sci. Technol. 1997, 31, 1399–1406. [Google Scholar] [CrossRef]
- Ancona, V.; Caracciolo, A.B.; Campanale, C.; Rascio, I.; Grenni, P.; Di Lenola, M.; Bagnuolo, G.; Uricchio, V.F. Heavy metal phytoremediation of a poplar clone in a contaminated soil in southern Italy. J. Chem. Technol. Biotechnol. 2020, 95, 940–949. [Google Scholar] [CrossRef]
- Bianconi, D.; De Paolis, M.R.; Agnello, A.C.; Lippi, D.; Pietrini, F.; Zacchini, M.; Polcaro, C.; Donati, S.E.; Paris, P.; Spina, S.; et al. Field-scale rhyzoremediation of a contaminated soil with hexachlorocyclohexane (HCH) isomers: The potential of poplars for environmental restoration and economical sustainability. In Handbook of Phytoremediation; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Chapter 31; pp. 1–12. ISBN 978161728753. [Google Scholar]
- Meers, E.; Ruttens, A.; Hopgood, M.; Lesage, E.; Tack, F.M. Potential of Brassica rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 2005, 61, 561–572. [Google Scholar] [CrossRef]
- Brunetti, G.; Farrag, K.; Soler-Rovira, P.; Nigro, F.; Senesi, N. Greenhouse and field studies on Cr, Cu, Pb and Zn phytoextraction by Brassica napus from contaminated soils in the apulia region, southern Italy. Geoderma 2011, 160, 517–523. [Google Scholar] [CrossRef]
- Banuelos, G.; Ajwa, H.; Terry, N.; Zayed, A. Phytoremediation of selenium laden soils: A new technology. J. Soil Water Conserv. 1997, 52, 426–430. [Google Scholar]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Robert, B.; Stewart, R.B.; Robinson, B.H. Phytofiltration of mercury-contaminated water: Volatilisation and plant-accumulation aspects. Environ. Exp. Bot. 2008, 62, 178–185. [Google Scholar] [CrossRef]
- Narayanan, M.; Davis, L.C.; Erickson, L.E. Fate of volatile chlorinated organic compounds in a laboratory chamber with alfalfa plants. Environ. Sci. Technol. 1995, 29, 2437–2444. [Google Scholar] [CrossRef]
- Mataruga, Z.; Jarić, S.; Kostić, O.; Marković, M.; Jakovljević, K.; Mitrović, M.; Pavlović, P. The potential of elm trees (Ulmus glabra Huds.) for the phytostabilisation of potentially toxic elements in the riparian zone of the Sava River. Environ. Sci. Pollut. Res. 2020, 27, 4309–4324. [Google Scholar] [CrossRef]
- Gupta, K.; Srivastava, S.; Saxena, G.; Kumar, A. Application of Pteris vittata L. for phytoremediation of arsenic and biomonitoring of the process through cyto-genetic biomarkers of Trigonella foenum-graecum L. Physiol. Mol. Biol. Plants 2022, 28, 91–106. [Google Scholar] [CrossRef]
- Tian, T.; Li, G.; Tang, W.; Zhu, Q.; Li, X.; Du, C.; Li, C.; Li, J.; Zhao, C.; Zhang, L. Role of Sedum alfredii and soil microbes in the remediation of ultra-high content heavy metals contaminated soil. Agric. Ecosys. Environ. 2022, 339, 108090. [Google Scholar] [CrossRef]
- Robinson, B.H.; Chiarucci, A.; Brooks, R.R.; Petit, D.; Kirkman, J.H.; Gregg, P.E.H.; De Dominicis, V. The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J. Geochem. Explor. 1997, 59, 75–86. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, N.; Golia, E.E. Preliminary investigation of the use of Silybum marianum (L.) Gaertn as a Cd accumulator in contaminated Mediterranean soils: The relationships among cadmium (Cd) soil fractions and plant Cd content. Euro-Mediterr. I Environ. Integr. 2023, 1–13. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.N. Phytoremediation of heavy metal-contaminated soils using the perennial energy crops Miscanthus spp. and Arundo donax L. Bioenerg. Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Durešová, Z.; Šuňovská, A.; Horník, M.; Pipíška, M.; Gubišová, M.; Gubiš, J.; Hosti, S. Rhizofiltration potential of Arundo donax for cadmium and zinc removal from contaminated wastewater. Chem. Pap. 2014, 68, 1452–1462. [Google Scholar] [CrossRef]
- Mirza, N.; Qaisar Mahmood, Q.; Pervez, A.; Raza Ahmad, R.; Farooq, R.; Shah, M.M.; Muhammad Rashid Azim, M.R. Phytoremediation potential of Arundo donax in arsenic-contaminated synthetic wastewater. Bioresour. Technol. 2010, 101, 5815–5819. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Phytoremediation Potential of Maize (Zea mays L.). A Review. Afr. J. Gen. Agric. 2010, 6, 275–287. [Google Scholar]
- Carvajal, L.; Benavides, L.C.L.; Rodriguez, R.; Serrezuela, W.F.R. Extraction in laboratory of heavy metals through rhizofiltration using the plant Zea mays (maize). Int. J. Appl. Environ. Sci. 2018, 13, 9–26. [Google Scholar]
- Herzig, R.; Nehnevajova, E.; Pfistner, C.; Schwitzguebel, J.-P.; Ricci, A.; Keller, C. Feasibility of labile Zn phytoextraction using enhanced tobacco and sunflower: Results of five-and one-year field-scale experiments in Switzerland. Int. J. Phytoremediat. 2014, 16, 735–754. [Google Scholar] [CrossRef]
- Hattab-Hambli, N.; Motelica-Heino, M.; Mench, M. Aided phytoextraction of Cu, Pb, Zn, and As in copper-contaminated soils with tobacco and sunflower in crop rotation: Mobility and phytoavailability assessment. Chemosphere 2016, 145, 543–555. [Google Scholar] [CrossRef]
- Liu, X.; Peng, K.; Wang, A.; Lian, C.; Shen, Z. Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere 2010, 78, 1136–1141. [Google Scholar] [CrossRef]
- McBride, M.; Zhou, Y. Cadmium and zinc bioaccumulation by Phytolacca americana from hydroponic media and contaminated soils. Int. J. Phytoremed. 2019, 21, 1215–1224. [Google Scholar] [CrossRef]
- Xiao, M.Z.; Sun, Q.; Hong, S.; Chen, W.J.; Pang, B.; Du, Z.Y.; Yang, W.B.; Sun, Z.; Yuan, T.Q. Sweet sorghum for phytoremediation and bioethanol production. J. Leather Sci. Eng. 2021, 3, 32–55. [Google Scholar] [CrossRef]
- Osman, H.E.; Fadhlallah, R.S.; Alamoudi, W.M.; Eid, E.M.; Abdelhafez, A.A. Phytoremediation potential of Sorghum as a bioenergy crop in Pb-amendment soil. Sustainability 2023, 15, 2178. [Google Scholar] [CrossRef]
- Dimitroula, H.; Syranidou, E.; Manousaki, E.; Nikolaidis, N.P.; Karatzas, G.P.; Kalogerakis, N. Mitigation measures for chromium-VI contaminated groundwater –The role of endophytic bacteria in rhizofiltration. J. Hazard. Mater. 2015, 281, 114–120. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Banach, A.M.; Banach, K.; Stępniewska, Z. Phytoremediation as a promising technology for water and soil purification: Azolla caroliniana willd. as a case study. Acta Agrophys. 2012, 19, 241–252. [Google Scholar]
- Daud, M.K.; Ali, S.; Abbas, Z.; Zaheer, I.E.; Riaz, M.A.; Malik, A.; Hussain, A.; Rizwan, M.; Zia-ur-Rehman, M.; Zhu, S.J. Potential of duckweed (Lemna minor) for the phytoremediation of landfill leachate. J. Chem. 2018, 2, 3951540. [Google Scholar] [CrossRef]
- Amjad, N.; Isbah Akhtar, I.; Hameed, A.; Ahmad, F.; Ali, F.; Ahmad, M.; Ullah, I.; Bibi, U.; Altaf, N.; Sanaullah; et al. Phytoremediation of copper and arsenic contaminated soil using Gladiolus (Gladiolus grandifloras) and Chrysanthemum (Chrysanthemum morifolium) plants. Asian J. Soil Sci. Plant Nutr. 2023, 9, 36–42. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Aery, N.C. Phytostabilization of mine waste: Growth and physiological responses of Vigna unguiculata (L.) Walp. J. Environ. Biol. 2006, 28, 651–654. [Google Scholar]
- Liang, Y.; Meggo, M.; Hu, D.; Schnoor, J.L.; Mattes, T.E. Enhanced polychlorinated biphenyl removal in a switchgrass rhizosphere by bioaugmentation with Burkholderia xenovorans LB400. Ecol. Eng. 2014, 71, 215–222. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis jalapa L. in a greenhouse plot experiment. J. Hazard. Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design of a sustainable development process between phytoremediation and production of bioethanol with Eichhornia crassipes. Environ. Monit. Assess. 2019, 191, 221. [Google Scholar] [CrossRef]
- Khashij, S.; Karimi, B.; Makhdoumi, P. Phytoremediation with Festuca arundinacea: A Mini Review. Int. J. Health Life Sci. 2018, 4, e86625. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy production potential of phytoremediation plant biomass: Helianthus annuus and Silybum marianum. Ind. Crops Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Bernal, M.P.; Gómez, X.; Chang, R.; Arco-Lázaro, E.; Clemente, R. Strategies for the use of plant biomass obtained in the phytostabilisation of trace-element-contaminated soils. Biomass Bioenergy 2019, 126, 220–230. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Phytoremediation using willow in industrial contaminated soil. Sustainability 2022, 14, 8449. [Google Scholar] [CrossRef]
- Bart, S.; Motelica-Heino, M.; Miard, F.; Joussein, E.; Soubrand, M.; Bourgerie, S.; Morabito, D. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment. Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Selvaraj, C.; Li, J.; Singh, R.; Zhao, X.; Kim, D.; Kim, J.Y.; Kang, Y.C.; Lee, J.K. Phytoremediation of metal-contaminated soils by the hyperaccumulator canola (Brassica napus L.) and the use of its biomass for ethanol production. Fuel 2016, 183, 107–114. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Placido, D.F.; Lee, C.C. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Plants 2022, 11, 595. [Google Scholar] [CrossRef]
- Limmer, M.; Joel Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Yadav, A.K.; Pathak, B.; Fulekar, M.H. Rhizofiltration of heavy metals (cadmium, lead and zinc) from fly ash leachates using water hyacinth (Eichhornia crassipes). Int. J. Environ. 2015, 4, 179–196. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization. A green approach to contaminant containment. Adv. Agron. 2011, 112, 45–204. [Google Scholar]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Mechanisms of arsenic sequestration by Prosopis juliflora during the phytostabilization of metalliferous mine tailings. Environ. Sci. Technol. 2018, 52, 1156–1164. [Google Scholar] [CrossRef]
- Guo, S.; Li, W.; Zhang, L.; Peng, J.; Xia, H.; Zhang, S. Kinetics and equilibrium adsorption study of lead (II) onto the low cost adsorbent—Eupatorium adenophorum spreng. Process Saf. Environ. Prot. 2009, 87, 343–351. [Google Scholar] [CrossRef]
- Li, H.; Sheng, G.; Sheng, W.; Xu, O. Uptake of trifluralin and lindane from water by ryegrass. Chemosphere 2002, 48, 335–341. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, L.; Ling, W.; Gong, S.; Sun, B.; Zhang, Y. Desorption of phenanthrene and pyrene in soils by root exudates. Bioresour. Technol. 2010, 101, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Lu, M.; Peng, F.; Wan, Y.; Liao, M.-H. Remediation of polychlorinated biphenyl-contaminated soil by using a combination of ryegrass, arbuscular mycorrhizal fungi and earthworms. Chemosphere 2014, 106, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Agostini, E.; Coniglio, M.S.; Milrad, S.R.; Tigier, H.A.; Giulietti, A.M. Phytoremediation of 2,4-dichlorophenol by Brassica napus hairy root cultures. Biotechnol. Appl. Biochem. 2003, 37, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hannink, N.; Subramanian, N.; Rosser, S.J.; Basran, A.; Murray, J.A.H.; Shanks, J.V. Enhanced transformation of TNT by tobacco plants expressing a bacterial nitroreductase. Int. J. Phytorem. 2007, 9, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Correa-Garcıa, S.; Pande, P.; Armand, S.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of petroleum hydrocarbons: A model system for plant microbiome manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Cebron, A. Short-term rhizosphere effect on available carbon sources, phenanthrene degradation, and active microbiome in an aged-contaminated industrial soil. Front. Microbiol. 2016, 7, 92. [Google Scholar] [CrossRef]
- Bourceret, A.; Leyval, C.; Faure, P.; Lorgeoux, C.; Cébron, A. High PAH degradation and activity of degrading bacteria during alfalfa growth where a contrasted active community developed in comparison to unplanted soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 29556–29571. [Google Scholar] [CrossRef]
- Lopez-Echartea, E.; Michal Strejcek, M.; Mukherjee, S.; Uhlik, O.; Yrjala, K. Bacterial succession in oil-contaminated soil under phytoremediation with poplars. Chemosphere 2020, 243, 125242. [Google Scholar] [CrossRef] [PubMed]
- Ancona, V.; Rascio, I.; Aimola, G.; Campanale, C.; Grenni, P.; Di Lenola, M.; Garbini, G.L.; Uricchio, V.F.; Caracciolo, A.B. Poplar-assisted bioremediation for recovering a PCB and heavy-metal-contaminated area. Agriculture 2021, 11, 689. [Google Scholar] [CrossRef]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffré, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable valorization of biomass: From assisted phytoremediation to green energy production. In Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology, 1st ed.; Prasad, M.N.V., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2021; pp. 29–51. [Google Scholar]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Santiago-Herrera, M.; Ibanez, J.; Yousaf, S.; Iqbal, M.; Martel-Martín, S.; Barros, R. Sustainability of phytoremediation: Post-harvest stratagems and economic opportunities for the produced metals contaminated biomass. J. Environ. Manag. 2023, 326, 116700. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.Z.; Li, J.X.; Yang, X.-G.; Chen, Z.-W.; Huang, R.; Chen, Z.-X.; Zhou, S.-G.; Chen, Z. Preparation of high-performance CdS@C catalyst using Cd-enriched biochar recycled from plating wastewater. Front. Chem. 2020, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhu, X.; Chi, T.; Liu, B.; Du, C.; Yu, G.; Wu, H.; Chen, H. Reutilization of manganese enriched biochar derived from Phytolacca acinosa Roxb. residue after phytoremediation for lead and tetracycline removal. Bioresour. Technol. 2022, 345, 126546. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, J.; Wang, X.; Pan, M.; Lin, Q.; Khan, K.Y.; Yan, B.; Li, T.; He, Z.; Yang, X.; et al. A Review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products. J. Hazard. Mater. 2021, 405, 123832. [Google Scholar] [CrossRef]
- Cui, X.; Shen, Y.; Yang, Q.; Kawi, S.; He, Z.; Yang, X.; Wang, C.-H. Simultaneous syngas and biochar production during heavy metal separation from Cd/Zn hyperaccumulator (Sedum alfredii) by gasification. J. Chem. Eng. 2018, 347, 543–551. [Google Scholar] [CrossRef]
- Qian, F.; Zhu, X.; Liu, Y.; Shi, Q.; Wu, L.; Zhang, S.; Chen, J.; Ren, Z.J. Influences of temperature and metal on subcritical hydrothermal liquefaction of hyperaccumulator: Implications for the recycling of hazardous hyperaccumulators. Environ. Sci. Technol. 2018, 52, 2225–2234. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Morel, J.L.; Simonnot, M.-O.; Echevarria, G.; van der Ent, A.; Baker, A.J.M. Conclusions and outlook for agromining. In Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants; van der Ent, A., Baker, A.J.M., Echevarria, G., Simonnot, M.-O., Morel, J.L., Eds.; Mineral Resource Reviews; Springer International Publishing: Cham, Switzerland, 2021; pp. 485–489. ISBN 978-3-030-58904-2. [Google Scholar]
- Chaney, R.L.; Baker, A.J.M.; Morel, J.L. The long road to developing agromining/phytomining. In Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants; van der Ent, A., Baker, A.J.M., Echevarria, G., Simonnot, M.-O., Morel, J.L., Eds.; Mineral Resource Reviews; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–22. ISBN 978-3-030-58904-2. [Google Scholar]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels: Their characteristics and analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 277–325. [Google Scholar]
- Ko, C.-H.; Yu, F.-C.; Chang, F.-C.; Yang, B.-Y.; Chen, W.-H.; Hwang, W.-S.; Tu, T.-C. Bioethanol production from recovered napier grass with heavy metals. J. Environ. Manag. 2017, 203, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, M.; Barra, S.; Sagnelli, F.; Zitella, P. Biomass-to-electricity: Analysis and optimization of the complete pathway steam explosion—Enzymatic hydrolysis—Anaerobic digestion with ICE vs SOFC as biogas users. Bioresour. Technol. 2012, 123, 430–438. [Google Scholar] [CrossRef]
- Zieminski, K.; Frac, M. Methane fermentation process as anaerobic digestion of biomass: Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar]
- Tian, Y.; Zhang, H. Producing biogas from agricultural residues generated during phytoremediation process: Possibility, threshold, and challenges. Int. J. Green Energy 2016, 13, 1556–1563. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y.; Cho, J.; Kim, J.Y. Releasing characteristics and fate of heavy metals from phytoremediation crop residues during anaerobic digestion. Chemosphere 2018, 191, 520–526. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.R.; Jeong, S.; Cho, J.; Kim, J.Y. Long-Term performance of anaerobic digestion for crop residues containing heavy metals and response of microbial communities. Waste Manag. 2017, 59, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–588. [Google Scholar] [CrossRef]
- Tandon, M.; Vasudevan, P.; Naik, S.N.; Davies, P. Oil bearing seasonal crops in India: Energy and phytoremediation potential. Int. J. Energy Sect. Manag. 2013, 7, 338–354. [Google Scholar] [CrossRef]
- Fermeglia, M.; Perisic, M. Nature-based solution to man-made problems: Fostering the uptake of phytoremediation and low-iluc biofuels in the EU. J. Eur. Environ. Plan. Law 2023, 20, 145–167. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef] [PubMed]
- EIA—Energy Information Administration. International Energy Outlook 2013; Energy Information Administration: Washington, DC, USA, 2013. Available online: www.eia.gov/forecasts/ieo/pdf/0484(2013).pdf (accessed on 11 October 2023).
- Padoan, E.; Passarella, I.; Prati, M.; Bergante, S.; Facciotto, G.; Ajmone-Marsan, F. The suitability of short rotation coppice crops for phytoremediation of urban soils. Appl. Sci. 2020, 10, 307. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Nandita Singh, N. Energy crops in sustainable phytoremediation. Renew. Sust. Energ. Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Kidd, P.S.; Bani, A.; Benizri, E.; Gonnelli, C.; Hazotte, C.; Kisser, J.; Konstantinou, M.; Kuppens, T.; Kyrkas, D.; Laubie, B.; et al. Developing sustainable agromining systems in agricultural ultramafic soils for nickel recovery. Front. Environ. Sci. 2018, 6, 44. [Google Scholar] [CrossRef]
- Marcolongo, L.; La Cara, F.; Del Monaco, G.; Paixão, S.M.; Alves, L.; Marques, I.P.; Ionata, E. A Novel β-Xylosidase from Anoxybacillus sp. 3M towards an improved agro-industrial residues saccharification. Int. J. Biol. Macromol. 2019, 122, 1224–1234. [Google Scholar] [CrossRef]
- Tusher, T.R.; Chang, J.J.; Saunivalu, M.I.; Wakasa, S.; Li, W.H.; Huang, C.C.; Inoue, C.; Chien, M.F. Second-generation bioethanol production from phytomass after phytoremediation using recombinant bacteria-yeast co-culture. Fuel 2022, 326, 124975. [Google Scholar] [CrossRef]
- Ojo, A.O. An overview of lignocellulose and its biotechnological importance in high-value product production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A Review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Liu, Z.; Tran, K.-Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicol. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Han, L.; Gao, C.; Xiao, W.; Zhang, Y.; Cao, Y. Quantitative approaches for illustrating correlations among the mechanical fragmentation scales, crystallinity and enzymatic hydrolysis glucose yield of rice straw. Bioresour. Technol. 2017, 241, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Halder, P.; Kundu, S.; Patel, S.; Marzbali, M.H.; Parthasarathy, R.; Shah, K. Investigation of reaction mechanism and the effects of process parameters on ionic liquid–based delignification of sugarcane straw. Bioenerg. Res. 2020, 13, 1144–1158. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Menana, Z.; Chrusciel, L.; Chalot, M.; Bert, V.; Brosse, N. Steam explosion pretreatment of willow grown on phytomanaged soils for bioethanol production. Ind. Crops Prod. 2019, 140, 111722. [Google Scholar] [CrossRef]
- Cai, W.; Chen, T.; Lei, M.; Wan, X. Effective strategy to recycle Arsenic-accumulated biomass of Pteris vittata with high benefits. Sci. Total Environ. 2021, 756, 143890. [Google Scholar] [CrossRef]
- da Silva, E.B.; Mussoline, W.A.; Wilkie, A.C.; Ma, L.Q. Arsenic removal and biomass reduction of As-hyperaccumulator Pteris vittata: Coupling ethanol extraction with anaerobic digestion. Sci. Total Environ. 2019, 666, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Menana, Z.; Ziegler-Devin, I.; Bert, V.; Chalot, M.; Herzig, R.; Mench, M.; Brosse, N. Pretreatment of trace element-enriched biomasses grown on phytomanaged soils for bioethanol production. Ind. Crops Prod. 2017, 107, 63–72. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Yu, L.; Tang, S.; Xia, T.; Kang, H.; Xu, C.; Gao, H.; Madadi, M.; Alam, A.; et al. A mechanism for efficient cadmium phytoremediation and high bioethanol production by combined mild chemical pretreatments with desirable rapeseed stalks. Sci. Total Environ. 2020, 708, 135096. [Google Scholar] [CrossRef]
- Xiao, M.-Z.; Hong, S.; Shen, X.; Du, Z.-Y.; Yuan, T.-Q. In vivo Cadmium-assisted dilute acid pretreatment of the phytoremediation sweet sorghum for enzymatic hydrolysis and cadmium enrichment. Environ. Pollut. 2023, 324, 121372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; Yang, S.; Liu, Z.; Zhang, L.; He, H.; Qiu, W.; Zhuo, R.; Xu, J.; Wang, L. Valorization of heavy metal enriched phytoremediation biomass using a deep eutectic solvent (DES). Green Chem. 2023, 25, 771–778. [Google Scholar] [CrossRef]
- Hennequin, L.M.; Tan, S.; Jensen, E.; Fennell, P.; Hallett, J.P. Combining phytoremediation and biorefinery: Metal extraction from lead contaminated Miscanthus during pretreatment using the ionoSolv process. Ind. Crops Prod. 2022, 176, 114259. [Google Scholar] [CrossRef]
- Yao, J.G.; Tan, S.; Metcalfe, P.I.; Fennell, P.S.; Kelsall, G.H.; Hallett, J.P. Demetallization of Sewage sludge using low-cost ionic liquids. Environ. Sci. Technol. 2021, 55, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Scelsi, E.; Ancona, V.; Aimola, G.; Pastore, C. Performic acid pre-treatment of poplar biomasses grown on a contaminated area for enhanced enzymatic digestibility: A viable route to obtain fine-products and recovery of contaminants. J. Clean. Prod. 2022, 369, 133346. [Google Scholar] [CrossRef]
- Marcolongo, L.; Ionata, E.; La Cara, F.; Amore, A.; Giacobbe, S.; Pepe, O.; Faraco, V. The effect of Pleurotus ostreatus arabinofuranosidase and its evolved variant in lignocellulosic biomasses conversion. Fungal Genet. Biol. 2014, 72, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Jana, A.K. Sweet sorghum bagasse pretreatment by Coriolus versicolor in mesh tray bioreactor for selective delignification and improved saccharification. Waste Biomass Valor. 2019, 10, 2689–2702. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-economic bottlenecks of the fungal pretreatment of lignocellulosic biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef]
- Waghmare, P.R.; Watharkar, A.D.; Jeon, B.-H.; Govindwar, S.P. Bio-ethanol production from waste biomass of Pogonatherum crinitum Phytoremediator: An eco-friendly strategy for renewable energy. 3 Biotech 2018, 8, 158. [Google Scholar] [CrossRef]
- Grzegórska, A.; Rybarczyk, P.; Rogala, A.; Zabrocki, D. Phytoremediation—From environment cleaning to energy generation—Current status and future perspectives. Energies 2020, 13, 2905. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wang, L.; Zhang, L.; Dai, L. Ecophysiological characteristics and biogas production of cadmium-contaminated crops. Bioresour. Technol. 2013, 146, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, S.; Wang, T.; Chang, Z.; Shen, Z.; Chen, Y. Using contaminated plants involved in phytoremediation for anaerobic digestion. Int. J. Phytoremediat. 2015, 17, 201–207. [Google Scholar] [CrossRef]
- Verma, V.K.; Singh, Y.P.; Rai, J.P.N. Biogas production from plant biomass used for phytoremediation of industrial wastes. Bioresour. Technol. 2007, 98, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Li, J.; Hou, Y.; Shi, L.; Lian, C.; Shen, Z.; Chen, Y. Evaluation of biogas production potential of trace element-contaminated plants via anaerobic digestion. Ecotoxicol. Environ. Saf. 2021, 208, 111598. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.S.; Zhao, X.; Li, J.; Kim, D.; Kalia, V.C.; Kim, I.-W.; Kim, J.Y.; Lee, J.-K. Metal Accumulation by sunflower (Helianthus annuus L.) and the efficacy of its biomass in enzymatic saccharification. PLoS ONE 2017, 12, e0175845. [Google Scholar] [CrossRef] [PubMed]
- Sotenko, M.; Coles, S.; Barker, G.; Song, L.; Jiang, Y.; Longhurst, P.; Romanova, T.; Shuvaeva, O.; Kirwan, K. Phytoremediation-biorefinery tandem for effective clean-up of metal contaminated soil and biomass valorisation. Int. J. Phytoremediat. 2017, 19, 965–975. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Bhuptani, J.; Beyer, J.; Ismawati, Y.; Radu, T. Anaerobic digestion of mercury phytoextraction crops with intermediary stage bio-waste polymer treatment. Int. J. Phytoremediat. 2020, 22, 1431–1439. [Google Scholar] [CrossRef]
- Xin, L.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P.; Feng, W.; Xu, W. Feasibility of anaerobic digestion on the release of biogas and heavy metals from rice straw pretreated with sodium hydroxide. Environ. Sci. Pollut. Res. 2019, 26, 19434–19444. [Google Scholar] [CrossRef]
- Vintila, T.; Negrea, A.; Barbu, H.; Sumalan, R.; Kovacs, K. Metal distribution in the process of lignocellulosic ethanol production from heavy metal contaminated sorghum biomass. J. Chem. Technol. Biotechnol. 2016, 91, 1607–1614. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Li, H.-L.; Zeng, X.-J.; Lu, C.; Fu, J.-Y.; Guo, L.-J.; Kimani, W.M.; Yan, H.-L.; He, Z.-Y.; Hao, H.-Q.; et al. Coupling phytoremediation of cadmium-contaminated soil with safe crop production based on a sorghum farming system. J. Clean. Prod. 2020, 275, 123002. [Google Scholar] [CrossRef]
- Van Slycken, S.; Witters, N.; Meers, E.; Peene, A.; Michels, E.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Wierinck, I.; et al. Safe use of metal-contaminated agricultural land by cultivation of energy maize (Zea mays). Environ. Pollut. 2013, 178, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.M.; Caetano, N.S.; Marques, A.P.G.C. The potential of bioaugmentation-assisted phytoremediation derived maize biomass for the production of biomethane via anaerobic digestion. Plants 2023, 12, 3623. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodríguez, V.; García-Gutiérrez, A.; Canales, J.; Cañas, R.A.; Kirby, E.G.; Avila, C.; Cánovas, F.M. Poplar trees for phytoremediation of high levels of nitrate and applications in bioenergy. Plant Biotechnol. J. 2016, 14, 299–312. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, J.; Tong, G.; Ding, H.; Ouyang, J.; Zhou, Q.; Fu, Y.; Zhong, M. Emerging disposal technologies of harmful phytoextraction biomass (HPB) containing heavy metals: A review. Chemosphere 2022, 290, 133266. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Rivera, A.; Paredes, M.G.; Güereca, L.P. A systematic review of the sustainability assessment of bioenergy: The case of gaseous biofuels. Biomass Bioenergy 2019, 125, 79–94. [Google Scholar] [CrossRef]
- Debnath, D. From Biomass to biofuel economics. In Biofuels, Bioenergy and Food Security; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–60. [Google Scholar]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Boda, R.K.; Majeti, N.V.P.; Suthari, S. Ricinus communis L. (Castor Bean) as a potential candidate for revegetating industrial waste contaminated sites in peri-urban greater hyderabad: Remarks on seed oil. Environ. Sci. Pollut. Res. 2017, 24, 19955–19964. [Google Scholar] [CrossRef]
- Echeverri, D.; Perez, W.; Rios, L. Synthesis of maleated-castor oil glycerides from biodiesel-derived crude glycerol. Ind. Crops Prod. 2013, 49, 299–303. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenerg. 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sust. Energ. Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative stability and cold flow behavior of Palm, Sacha-Inchi, Jatropha and Castor Oil biodiesel blends. Fuel Process. Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Moon, H.; Park, Y.-K.; Rinklebe, J.; Park, C.-J.; Kwon, E.E. Valorization of phytoremediation byproduct via synthesis of biodiesel from cockspur grass (Echinochloa crus-galli) seed. ACS Sustain. Chem. Eng. 2020, 8, 11588–11595. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Biodiesel production (FAEEs) by heterogeneous combi-lipase biocatalysts using wet extracted lipids from microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef]
- Edgar, V.-N.; Fabián, F.-L.; Mario, P.-C.J.; Ileana, V.-R. Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high-value by-products—A review. Appl. Sci. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel production from lignocellulosic biomass using oleaginous microbes: Prospects for integrated biofuel production. Front. Microbiol. 2021, 12, 658284. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Mench, M.; Garbisu, C.; Kidd, P.; Castro, P.M.L. Phytomanagement of metal(loid)-contaminated soils: Options, efficiency and value. Front. Environ. Sci. 2021, 9, 661423. [Google Scholar] [CrossRef]
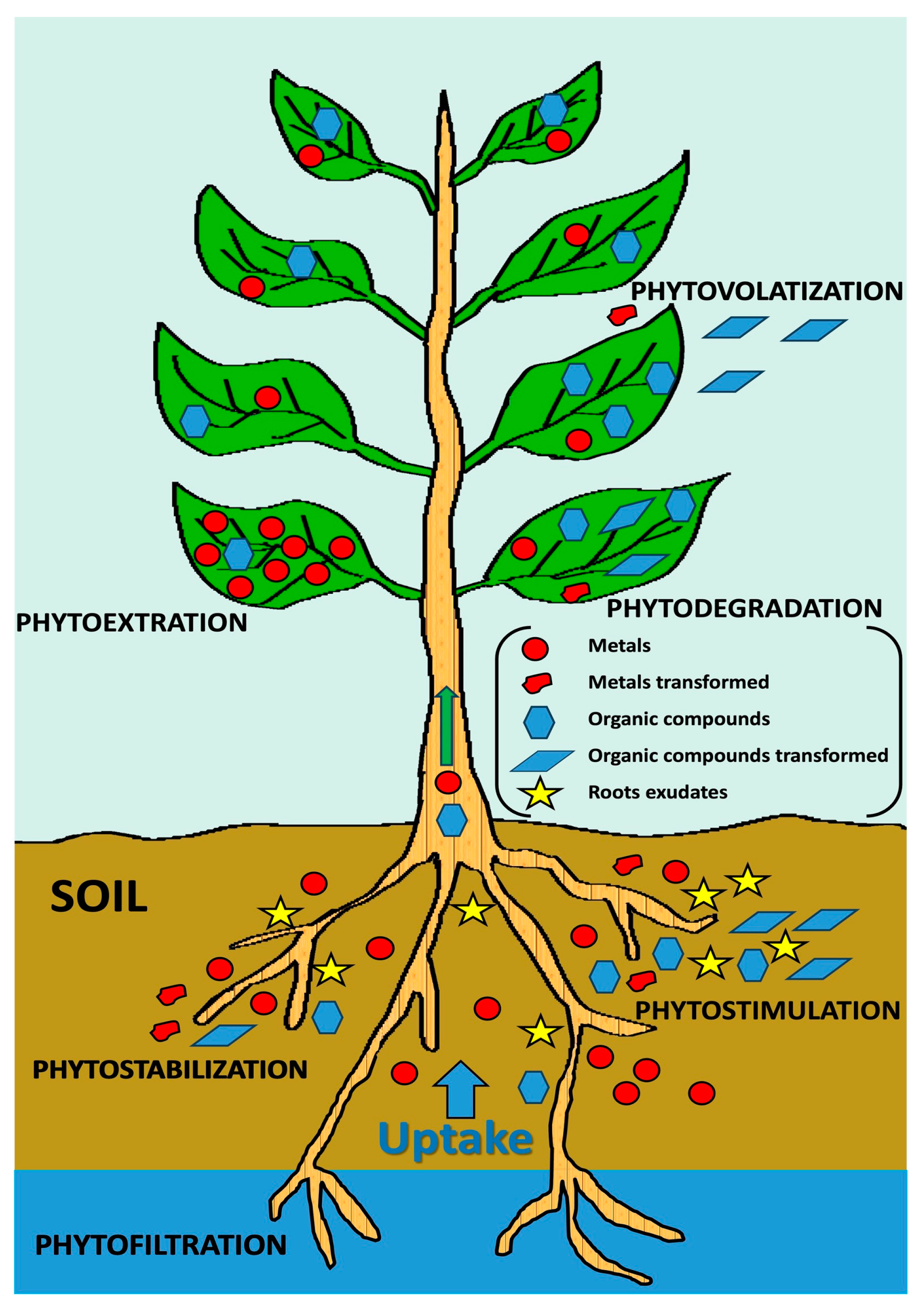
| Species | Life Cycle | Mechanism | Contaminants | References |
|---|---|---|---|---|
| Populus spp. | Perennial | Phystimulation, Phytodegradation Phytofiltration | a TCE, Atrazine, b PCBs, c HCH Pb, Ni, | [14,15,16] [17] |
| Phytostabilization | V, Cr, Sn, Pb | [16] | ||
| Phytovolatilization | d TCA | [14] | ||
| Cannabis sativa | Annual | Phytoextraction | Ni, Pd, Cd | [18] |
| Brassica napus | Annual | Phytoextraction Phytovolatilization | Cd, Cr, Cu, Ni, Pb, Zn Se | [19] [20] |
| Brassica juncea | Annual | Phytoextraction Phytovolatilization Phytofiltration | Cd, Pb Hg | [21] [22] |
| Medicago sativa | Perennial | Phytovolatilization Phytodegradation | TCA | [23] |
| Ulmus glabra | Perennial | Phytostabilization | As, Cu, Cr, Ni, Pb | [24] |
| Pteris vittata | Perennial | Phytoextraction | As | [25] |
| Sedum alfredii | Perennial | Phytoextraction | Zn, Cd, As, Pd, Cu | [26] |
| Alyssum bertolonii | Perennial | Phytoextraction | Ni, Co | [27] |
| Thlaspi caerulescens | Perennial | Phytoextraction | Pb, Cd, Ni, Zn, Co, Mn | [28] |
| Sylibum marianus | Annual | Phytoextraction | Cd | [29] |
| Miscanthus spp | Perennial | Phytextraxtion Phytostabilization | Zn | [30] [31] |
| Arundo donax | Perennial | Phytoextraction Phytostabilization | Cd, Zn, As Zn, Cr, Pb | [32] [33] [30] |
| Zea mays | Annual | Phytoextraction Phytofiltration | Cd, Cu, Zn, Pb Hg, Cr, Pb | [34] [35] |
| Nicotiana tabacum | Annual | Phytoextraction | Cu, Pb, As, Zn | [36] [37] |
| Phytolacca americana | Perennial | Phytoextraction Phytofiltration | Cd, Zn Cd | [38] [39] |
| Sorghum bicolor | Annual | Phytoextraction Phytostabilization | Cd Pb | [40] [41] |
| Juncus acutus | Perennial | Phytofiltration | Cr VI | [42] |
| Fhragmites australis | Perennial | Phytofiltration | Al, Mn, Zn, Cu, Pb, Ni, Cr, Hg | [43] |
| Azolla caroliniana | Annual | Phytofiltration | Hg, Cd, Pb, Cr, As, Ag, Pt, Au | [44] |
| Lemna minor | Perennial | Phytofiltration | Cu, Zn, Fe, Ni | [45] |
| Gladiolus grandiflorus | Perennial | Phytostabilization | Cu, As | [46] |
| Vigna unguiculata | Phytostabilization | Pb, Zn | [47] | |
| Panicum virgatum | Phytodegradation | b PCB | [48] | |
| Mirabilis jalapa | Phytodegradation | e TPHs | [49] | |
| Eichhornia crassipex | Phytofiltration | Cd, Zn, Pb, Cr | [50] | |
| Festuca arundinacea | Phytofiltration, Phytodegradation Phytostabilization | f PAHs, g TBA, anthracene, pyrene Ni, Pb | [51] | |
| Helianthus annus | Annual | Phytoextraction Phytostabilization Phytofiltration | As, Cu, Pb, Zn, Cd, Fe As, Cu, Pb, Zn, Hg Cd, Ni | [18] [52] [53] |
| Salix spp. | Perennial | Phytodegradation Phytovolatilization | f PAHs, b PCBs a TCE, h PCE | [54] [14] |
| Phytostabilization | As, Sb, Pb | [55] | ||
| Phytoextraction | Cd, Zn, Cu, Pb, Ni | [54] |
| Pretreatment | Biomass | Advantages | Disadvantages | Ref | |
|---|---|---|---|---|---|
| Physico-chemical | Steam explosion–sulfuric acid | Willow, Napier grass | Cellulose enrichment. Lignin transformation. High rate of metals removal | High operating temperature. Generation of toxic compounds. | [112] |
| Nitric acid | P. vittata | Lignin solubilization. Cellulose crystallinity reduction | Cost associated with acids and recovery | [105] | |
| Sulfuric acid | N. tabacum L., S. viminalis, B. pendula, B. juncea L., Sweet sorghum bagasse | Efficient extraction of the metals (80% As, up to 90% Cd). Glucan enrichment. | Hemicelluloses degradation. Formation of inhibitors, lignin breakdown products. Cost associated with acids and recovery | [115,117,118] | |
| Sodium hydroxide | N. tabacum L., S. viminalis, B. pendula, P. vittata, B. juncea L. | Lignin removal (up to 80%). Easy sugar recovery. | Low metal extraction. Expensive | [105,115,117] | |
| Chemical | Sodium hydroxide + Sulfuric acid | B. juncea L. | Complete metal (Cd) release (99%). Cellulose crystallinity reduction | High costs | [117] |
| Ethanol extraction | P. vittata | Low soluble carbon reduction. Efficient Metals extraction (As 93%). | Expensive | [114] | |
| Ethanol organosolv | N. tabacum L., S. viminalis, B. pendula, | High rate of lignin solubilization. | Metals extraction is low. High production costs. | [115] | |
| Deep eutectic solvent (DES) | S. alfredii | Lignin (90%) and hemicellulose removal. Cellulose enrichment. | Low cellulose-rich pulp obtainment. High viscosity at room temperature. Toxicity. | [118] | |
| IonoSolv | Miscanthus | Biomass enriched in cellulose. Lignin removal. Effective extraction of HMs | Degradation of hemicelluloses. Costly solvents. | [121] | |
| Organosolv (formic acid + hydrogen peroxide) | Poplar | Complete removal of lignin. Obtainment of a clean cellulose pulp. Dissolution of metals | Xylan removal. Not be applied to softwoods. External energy requirement. | [122] | |
| Biological | C. versicolor | Sweet sorghum bagasse | Low cost. Environmentally friendly. No formation of inhibitors | Not usable with high HM content. Long treatment. Low hydrolysis rate. | [124] |
| Feedstock | Pretreatment | Metal Detected | Product Target | References |
|---|---|---|---|---|
| Avena sativa L. | Mechanical treatment Anaerobic digestion | Cd | Biogas | [128] |
| Betula pendula | 2% H2SO4 15% NaOH Ethanol organosolv | Zn, Mn Trace elements Trace elements | Bioethanol | [115] |
| Brassica juncea L. | 1.0%, 2.0%, 4.0%, 8.0% NaOH 2.0%, 4.0%, 8.0%, 12%, 16% H2SO4 4.0% NaOH + 2.0%, 4.0%, 8.0% H2SO4 | Cd | Bioethanol | [117] |
| Brassica napus | Mechanical treatment Anaerobic digestion | Cd, Cu | Biogas | [129] |
| Eichhornia crassipes | 1% NaOH—3% H2SO4 Mechanical milling | Cu, Cr | Bioethanol Biohydrogen Biogas | [50] [130] |
| Elsholtziahaichowensis | Anaerobic digestion | Cu, Pb, Zn, Cd, Mn, As | Biogas | [131] |
| Elsholtzia splendens Nakai | Anaerobic digestion | Cu | Biogas | [129] |
| Helianthus annuus | 2% NaOH at 50 °C Phanerochaete chrysosporium Anaerobic digestion Aerobic digestion | Ni, As, Pb, Cu, Cd, Zn Ni Trace elements | Bioethanol Value-added products Biogas Compost | [132] [133] [52] [53] [95] |
| Lepidium sativum L. | Anaerobic digestion | Hg | Biogas | [134] |
| Mentha spicata | Anaerobic digestion | Hg | Biogas | [134] |
| Miscanthus sinensis OPM-10 | 1-methylimidazolium chloride | Pb, Zn, Fe, Cu, Cr, Ni, As, Cd | Biorefinery | [120] |
| Nicotiana glauca | Aerobic digestion Anaerobic digestion | Trace elements | Biogas Compost | [53] |
| Nicotiana tabacum L. | 2% H2SO4 15% NaOH Ethanol organosolv | Zn, Mn Trace elements Trace elements | Bioethanol | [115] |
| Oenothera biennis L., | Anaerobic digestion | Cu | Biogas | [129] |
| Oryza sativa L. | 6% NaOH | Cd, Pb, Cu, Zn | Biogas | [135] |
| Pennisetum purpureum | Acid (3% H2SO4) Steam explosion | Zn, Cd, Cr | Bioethanol | [90] |
| Phytolacca americana L. | Anaerobic digestion | Cu, Pb, Zn, Cd, Mn, As | Biogas | [129,131] |
| Piptatherum miliaceum | Aerobic digestion Anaerobic digestion | Trace elements | Biogas Compost | [53] |
| Pogonatherum crinitum | B. pumilus | Bioethanol | [126] | |
| Populus nigra | 1.5–7 mol/L Performic Acid | Trace elements | Levulinic acid | [122] |
| Pteris vittata | ultrapure water, 1% HNO3, 1% NaOH, shaking (200 rpm) and ultrasonication (40 kHz). 35% Ethanol—anaerobic digestion | As, Mg | Bioethanol Biogas | [105,114] |
| Salix viminalis W | Steam explosion 2% H2SO4 15% NaOH Ethanol organosolv | Trace elements Zn, Mn | Bioethanol | [112] [115] |
| Sedum alfredii | choline chloride/lactic acid (deep eutetic solvent) Anaerobic digestion | Cd, Cu, Pb, Zn, Cd, Mn, As | Value-added products Biogas | [119,131] |
| Silybum marianum | Anaerobic digestion | Trace elements | Biogas | [52,53] |
| Sinapis alba | Phanerochaete chrysosporium. | Ni | Value-added products | [133] |
| Solanum nigrum | Anaerobic digestion | Cu, Pb, Zn, Cd, Mn, As | Biogas | [131] |
| Sorghum bicolor | 0.5, 0.75, 1.0, 1.5% H2SO4 Mechanical milling Alkaline (2% NaOH) Steam explosion HNO3 | Pb, Cu, Zn, Cd | Bioethanol Biogas Biofuels Organic fertilizer | [136,137] |
| Trapa bispinnosa | Mechanical milling | Cu, Cr | Biogas | [130] |
| Zea mays | Anaerobic digestion Rhizophagus irregularis and Cupriavidus sp. | Cu, Cd, Zn | Biogas | [129,138,139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionata, E.; Caputo, E.; Mandrich, L.; Marcolongo, L. Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes. Catalysts 2024, 14, 118. https://doi.org/10.3390/catal14020118
Ionata E, Caputo E, Mandrich L, Marcolongo L. Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes. Catalysts. 2024; 14(2):118. https://doi.org/10.3390/catal14020118
Chicago/Turabian StyleIonata, Elena, Emilia Caputo, Luigi Mandrich, and Loredana Marcolongo. 2024. "Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes" Catalysts 14, no. 2: 118. https://doi.org/10.3390/catal14020118
APA StyleIonata, E., Caputo, E., Mandrich, L., & Marcolongo, L. (2024). Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes. Catalysts, 14(2), 118. https://doi.org/10.3390/catal14020118






