Metal-Based Heterogeneous Catalysts for the Synthesis of Valuable Chemical Blends via Hydrodeoxygenation of Lignin-Derived Fractions
Abstract
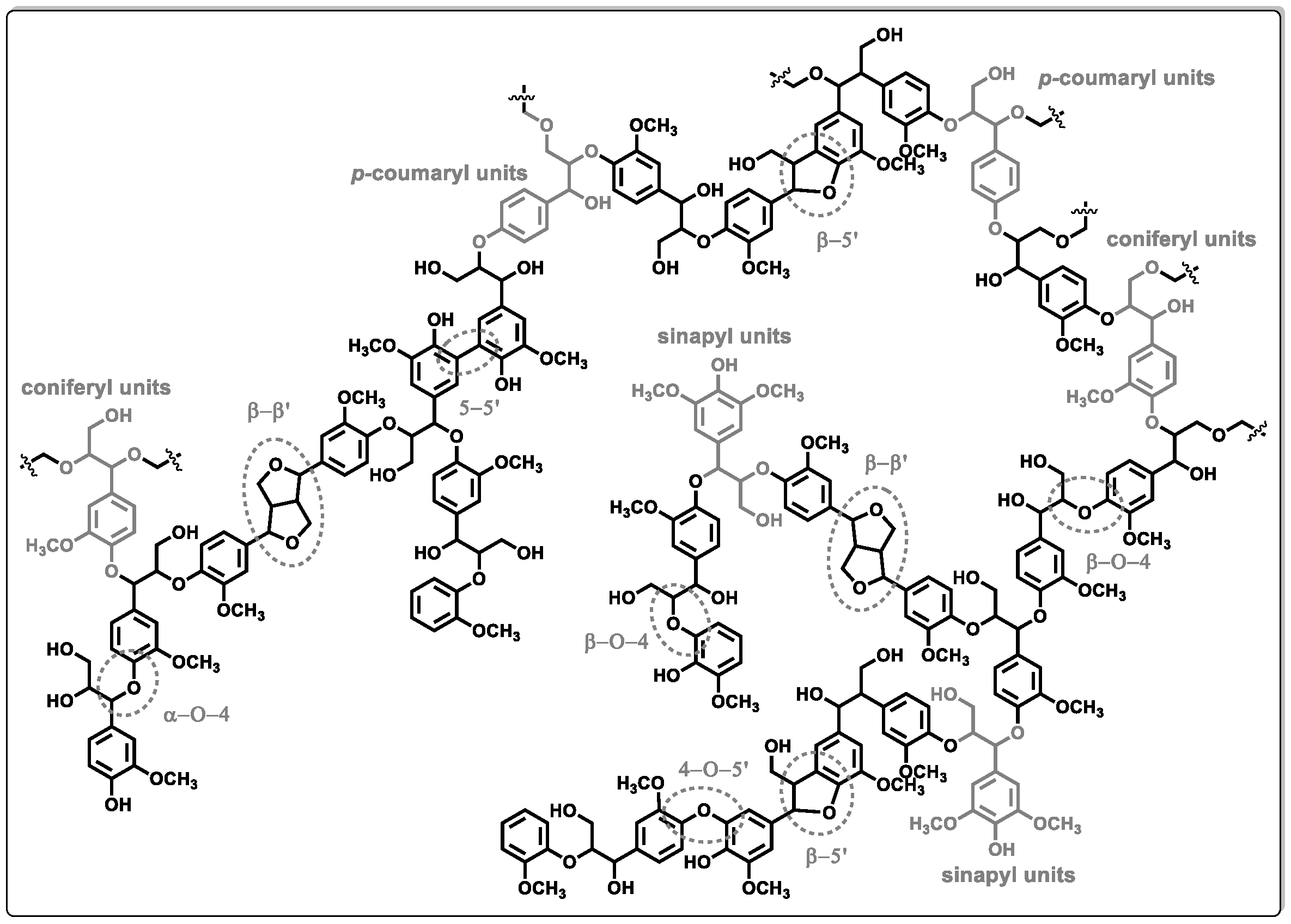
1. Introduction
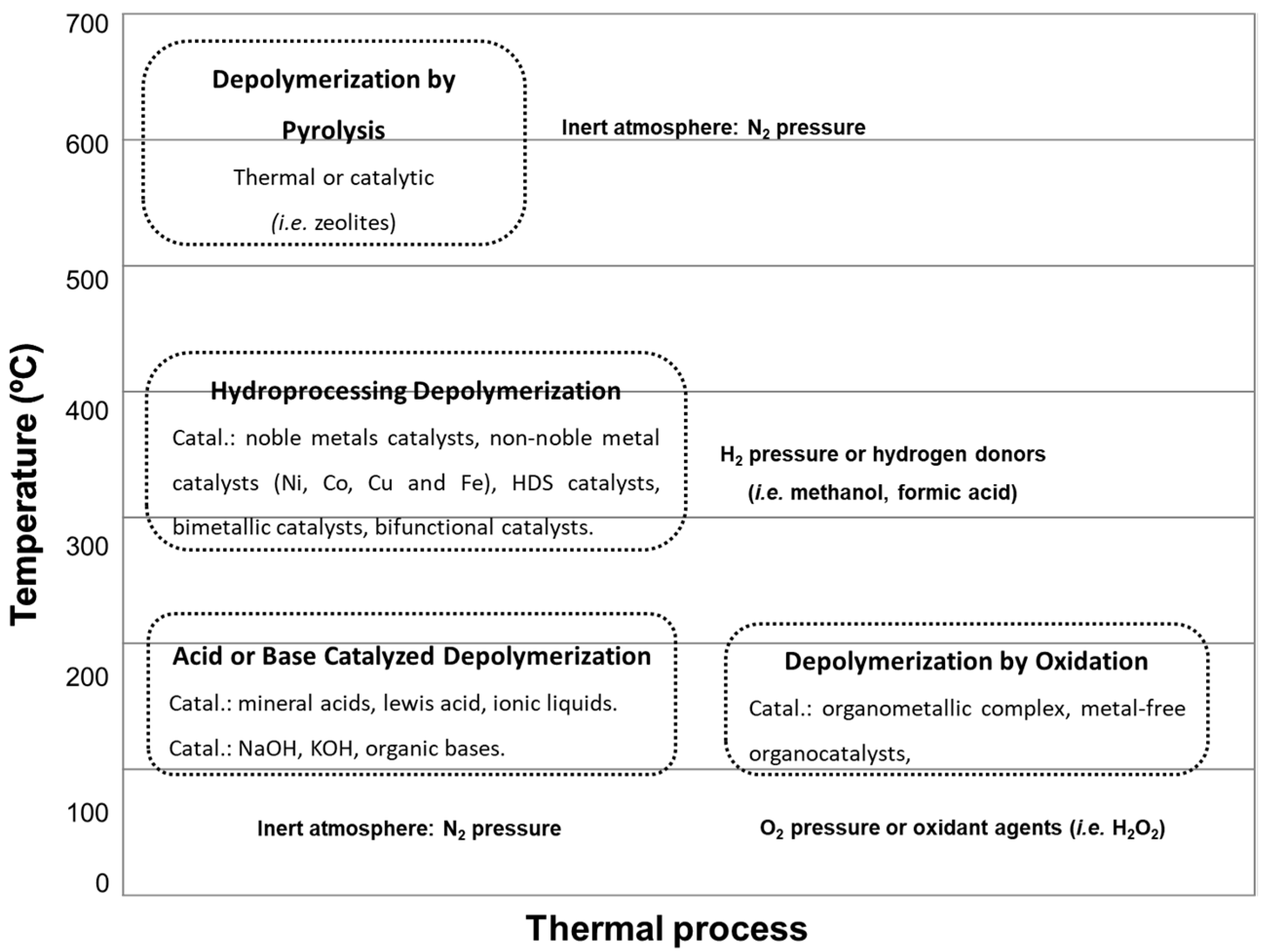
2. Hydrodeoxygenation for Upgrading of Lignin-Derived Fractions
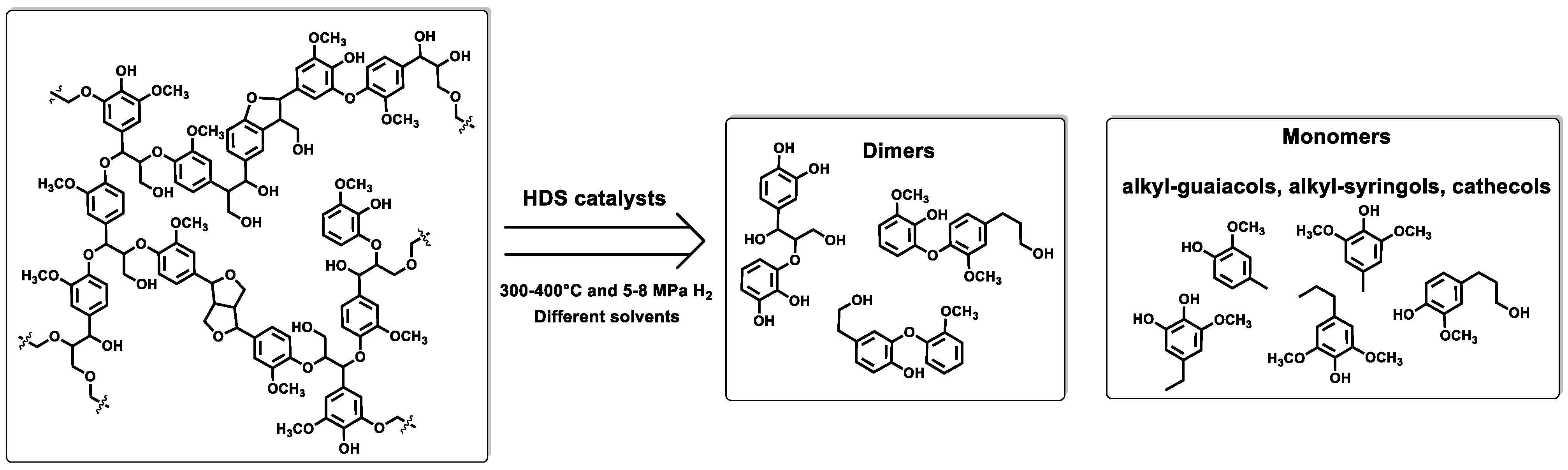
3. Hydrodesulfurization Catalysts (HDS)
| Hydrodesulfurization Catalyst | Type of Lignin or Lignin-Derived Fraction | Experimental Data | Main Results | Ref. |
|---|---|---|---|---|
| NiMoS/Al2O3 | Wheat straw soda lignin | Catalyst/lignin: 10 wt%, 350 °C, 8 MPa H2, 14 h reaction time. Tetraline as solvent and H2 donor. | Lignin conversion ≈ 85 wt.% Yields = 69 wt.% liquid fraction, and 25 wt.% oligomers Composition liquid fraction: 40 wt.% referred to alkyl-phenolics, guaiacols, catechols, aromatic hydrocarbons, naphthalenes, and alkanes. | [22] |
| CoS2/MoS2 | Alkali lignin Product: Lignin-derived bio-oil | Catalyst/lignin: 5 wt.%, 310 °C, 2.5 MPa H2, 1 h reaction time. Ethanol as a solvent | Lignin conversion = 91% Yield = 86% as bio-oil. Phenols, esters, and acids were identified as the main products in bio-oil. | [23] |
| NiMoS-SBA | Kraft lignin vs. Enzymatic hydrolysis lignin | Catalyst/lignin: 1/10 mass ratio, 400 °C, 8.0 MPa H2, 5 h reaction time. | Yield oil-phase = 65 wt.% (Kraft lignin) and ≈84 wt.% and (Enzymatic lignin) Yield total monomers = 47 wt.% (Kraft lignin) and 76 wt.% (Enzymatic lignin) | [27] |
| NiMoP/Al2O3 | Enzymatic hydrolysis lignin | Catalyst/lignin: 1/4–1/1 mass ratio, 320–380 °C, 4–7 MPa H2, Semi-continuous tubular reactor. | Yield organic phase = 15 wt.% Naphthenes (≈45 wt.%), aromatic (25 wt.%), and oxygenates (≈12 wt.%) as the main products in the organic phase | [30] |
| MoS2/γ-Al2O3 Promoters: Ni, Fe, Zn | Kraft lignin | Catalyst/lignin: 1/3 mass ratio, 340 °C, 4 MPa H2, 5 h reaction time. Hexadecane as solvent | NiMoS2/γ-Al2O3, selectivity to deoxygenated aromatic monomers (12%), and deoxygenated ciclo-alkane monomers (62%). | [31] |
| NiW/AC activated carbon | Kraft lignin | Catalyst/lignin: 1/4 mass ratio, 320 °C, 3.5 MPa H2, 8 h reaction time. Methanol as solvent | Yield methanol soluble products (MSPs) = 82% Monomeric product yield (MSPs) = 28.5 wt.% (based on lignin intake) | [32] |
| NiMo/MgO-La2O3 | Kraft lignin | Catalyst/lignin: 1/20 mass ratio, 350 °C, 10 MPa H2, 4 h reaction time. Solvent free | Lignin conversion = 87% Yield dichloromethane soluble products (DSPs) = 48% Monomeric product yield (DSPs) = 26.4 wt.% (based on lignin intake) | [33] |
4. Noble Metal Catalysts
Combination of Different Catalysts and Stages (One-Pot Experiments)
5. Nickel-Based Catalysts
| Ni-Based Catalyst | Type of Lignin or Lignin-Derived Fraction | Experimental Data | Main Results | Ref. |
|---|---|---|---|---|
| Nickel Raney® | Organosolv lignin (Eucalyptus wood) | Catalyst/lignin: 1/30 mass ratio, 280 °C, 0.5 MPa H2, 1 h reaction time. 1-butanol as solvent. | Yield = 7.4 wt.% monomers (based on the initial mass of lignin) Composition liquid fraction: diphenyl-methane-4-ethyl, 2,4-dimethyl-3-(methoxycarbonyl)-5-ethyl furan, trimethoxy-benzene | [67] |
| Serie of Ni-based catalysts (10 wt% Ni loading) | Lignosulfonate | Catalyst/lignosulfonate: 1/10 mass ratio, 200 °C, 5 MPa H2. Ethylene glycol as solvent. | Lignosulfonate conversion > 60%. Selectivity of 75–95% for alkane-substituted guaiacols, dimers. | [69] |
| 5 wt.% Ni/activated carbon | Corncob lignin | Catalyst/lignin: 1/5 mass ratio, 240 °C, 3 MPa H2, 4 h reaction time. Methanol as solvent. | Yield = 12.1 wt.% monomers (based on the initial mass of lignin) Composition of liquid fraction: propyl/propenyl guaiacol and syrinol, mono-phenols of ethyl/vinyl phenol and guaiacol, and methyl coumarate/ferulate and derivatives. | [70] |
| 10 wt.% Ni/carbon | Organosolv lignin | Catalyst/lignin: 1/5 mass ratio, 200 °C, 5 MPa H2, 6 h reaction time. Methanol as solvent. | Lignin conversion = 42%. Selectivity to main monomers = 97 wt.% Main monomers: propyl-4-guaiacol, and propyl-4-syringol | [71] |
| 10 wt.% Ni/Al-SBA | Organosolv lignin (Olive tree pruning) | Catalyst/lignin: 1/1 mass ratio, 140 °C, 0.5 h reaction time. Tetraline as solvent and H2 donor. | Yield ≈ 17 wt.% oil fraction (based on the initial mass of lignin) | [72] |
| 5 wt.% Ni/Al-SBA-15 | Organosolv lignin | Catalyst/lignin: 1/2.5 mass ratio, 300 °C, 7 MPa H2, 8 h reaction time. Methylcyclohexane as solvent. | Lignin conversion = 84%. Selectivity to monomers = 99% (cycloalkanes) | [76] |
| Ni-Cu/H-Beta (Ni-Cu: 20–20 wt.% loading) | Kraft lignin | Catalyst/lignin: 1/2.5 mass ratio, 330 °C, 7 MPa H2, 3 h reaction time. Isopropanol as solvent. | Lignin conversion = 98 wt.% Yield to monomers ≈ 51 wt.% Monomers: aromatics, cyclic ketones/alcohols, cycloalkanes, alkanes. | [78] |
| 28 wt.% Ni/ASA (amorphous silica-alumina) | Kraft lignin | Catalyst/lignin: 1/8 mass ratio, 300 °C, 6 MPa H2, 160 min reaction time. Dodecane as solvent. | Yield to liquid fraction = 42.8 wt.% Selectivity: 96.7% base don cyclo-alkanes + bicyclo-alkanes. | [79] |
| 15 wt.% Ni/ZrP-2.0 | Organosolv bagasse lignin | Catalyst/lignin: 1/5 mass ratio, 270 °C, 2 MPa H2, 4 h reaction time. Isopropanol as solvent. | Lignin conversion = 89 wt.% (based on lignin input). Yield to phenolic monomers ≈ 15 wt.% Yield to biochar = 8.1 wt.% (based on lignin input). | [80] |
6. Summary and Conclusions
- Hydrodesulfurization catalysts, such as NiMoS and CoMoS, originally tailored for oil refinery stream upgrading, have been repurposed for lignin-derived fraction upgrading. Despite yielding positive outcomes in the liquid fraction, characterized by dominant dimers and alkyl phenolic compounds, these catalysts require an external sulfur source and are constrained by the demanding experimental conditions.
- Noble metal catalysts, notably Pd, Ru, and Pt supported on solid oxide surfaces, originally designed for hydrogenation or dehydrogenation, have been investigated for lignin depolymerization. The intricate balance between the Lewis and Brønsted acid sites plays a pivotal role in the selective cleavage of C−O bonds. While showing higher hydrogenation and hydrogenolysis activity under moderate conditions, challenges persist in controlling intrinsic catalytic activity, leading to increased hydrogen consumption and the preferential formation of cyclic hydrocarbons over aromatics.
- Nickel-based catalysts, serving as cost-effective alternatives to noble metals, exhibit hydrogenation activity conducive to lignin-derived fraction upgrading. Operating under elevated experimental conditions compared with noble metals, these catalysts yield a liquid fraction predominantly composed of dimers, alkyl phenolic compounds, and aromatic hydrocarbons (BTX). The synergy between the solid support and the intrinsic activity of nickel, coupled with the influence of the support acid properties on mechanistic reactions, underscores the significance of these catalysts in lignin HDO.
7. Future Outlooks
- Lignin utilization in a biorefinery requires a one-pot strategy, and researchers have explored the synergistic effects of combining two different types of catalysts. Examples include a) the combination of noble-metal-supported catalysts (i.e., Pd/C) with solid acid catalysts (i.e., zeolites) and b) the combination of noble-metal-supported catalysts (i.e., Ru/ZrO2)
- With homogeneous alkaline catalysts (i.e., NaOH), however, the mechanisms underlying these processes are still unclear and lower conversion and selectivity have been observed.
- As an alternative, a tandem process has been researched, which generally comprises two stages. In the first stage, the depolymerization of lignin is facilitated by an alkaline homogeneous catalyst (i.e., NaOH), resulting in a liquid fraction containing a complex mixture of lignin-derived oligomers. Subsequently, the resulting liquid fraction is hydrodeoxygenated in the second stage with a bifunctional catalyst. Although both strategies offer advantages and disadvantages, a common challenge is the accurate control of hydrogen consumption and recombination of the fragmented components.
- During the hydrodeoxygenation process, the reaction still relies on harsh conditions and noble metal catalysts. A key goal is to first develop milder experimental conditions and then replace the expensive precious metals with more economical and environmentally friendly metal-based catalysts. Research into waste-based catalysts is a promising way to tackle this problem. Understanding the reactivity of isolated lignin is crucial. Natural lignins contain free radicals that are activated by degradation reactions and self-condensation. In contrast, isolated lignin (especially industrial lignin waste) exhibits lower activity and unpredictable quality as the aromatic structures are partially and non-selectively broken down during industrial processing. Therefore, improving the quality and reactivity of industrial lignin by-products has emerged as a key focus for subsequent valorization and reaction.
8. Further Improvements of Metal Oxide Catalysts
- Do oxygen vacancies play an important role? Much research has been carried out to find out how oxygen vacancies can affect catalytic behavior, especially in HDO reactions [84]. Based on the current state of research on vacancies in lignin-derived oxygenates, it is clarified that vacancies in the HDO reaction can act as acidic sites, promote substrate adsorption, and regulate product distribution, while vacancies in catalysts can increase stability and reducibility, improve metal dispersion and increase redox capacity [85]. The role of oxygen vacancies is to act as acid sites and regulate product distribution in the reaction. However, it should be noted that the improvement of deoxidation performance by oxygen vacancies is not always given. After the concentration has reached the extreme value, the improvement effect is rather flat. In addition, oxygen vacancies can also improve the stability and reducibility of the catalyst. However, according to the current state of research, vacancies can be further improved in the catalytic conversion of lignin and some improvements to the state of the art should implemented:
- (a)
- There are few methods for the preparation of vacancies, and there is a lack of safe and simple methods. Developing more convenient, safe, and energy-saving preparation methods is crucial. As this concept is in the field of photocatalysis, a multidisciplinary team could develop a more suitable method for oxide surface engagement and characterization.
- (b)
- There is a lack of knowledge about how a vacancy is generated. A deeper understanding of the mechanism should provide intelligent methods to generate this vacancy. Moreover, it is still not clear what role they play in upgrading the lignin fraction. Even though the improvements in the use of low-oxygen oxides are known, the reaction mechanisms are not clear.
- (c)
- A few real lignin raw materials are also used as research objects, but the majority of tests are focused on oxygen-deficient molecules as lignin model compounds. In order to represent real lignin objectively, model compounds cannot be used anymore, or as alternative a mixture of compounds shall be as close as possible to real lignin, should be used. It is imperative to understand the interactions between the various components of real lignin to develop a method for converting it into a high-value chemical.
- Is the active surface area so important in driving the reaction? Conventionally is believed that a higher surface area leads to a higher catalytic performance, [86], and this is true for some types of reactions, but for HDO the composition of this surface is more important than its active surface area, based on the BET area. The oxide can serve as a support for other materials containing the active sites (i.e., metal nanoparticles), but can also catalyze reactions alone or in combination with active sites from other phases (i.e., at the interfaces between metal nanoparticles and metal oxides). According to some authors, [87] controlling the layering of this surface will lead to higher catalytic activity. However, the mechanisms of interaction with the substrate and the formation of active sites in the layers are still unclear and further research should be conducted in this direction.
- Is the interaction of the metal-support interface a key parameter or is the acidity of the support? It is generally recognized that the HDO of lignin derivatives requires a bifunctional catalyst in which hydrogenation at a metal site is followed by sequential dehydrogenation/deoxygenation at the support. However, the role of the support and the metal-support interface is controversial. For example, some authors suggest that the deoxygenation reaction occurs at the acid sites, [88] while others claim that the defect sites of the support are responsible for the deoxygenation activity [89]. More research needs to be carried out to find out the critical aspect of the surface of the metal oxide, as these types of catalysts can be easily modified to become more acidic or have more defects. If we knew that aspects have the biggest impact, we could develop a better catalyst that increases activity and selectivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging Challenges on Viability and Commercialization of Lignin in Biobased Polymers for Food Packaging: A Review. Food Packag. Shelf Life 2022, 34, 100969. [Google Scholar] [CrossRef]
- Tillou, J.G.; Ezeorah, C.J.; Kuchta, J.J.; Dissanayake Mudiyanselage, S.C.D.; Sitter, J.D.; Vannucci, A.K. A Review on Recent Trends in Selective Hydrodeoxygenation of Lignin Derived Molecules. RSC Sustain. 2023, 1, 1608–1633. [Google Scholar] [CrossRef]
- Lang, M.; Li, H. Toward Mild Synthesis of Functional Chemicals from Lignin-Derived Phenolics via Emerging Catalytic Technology. Chem Catal. 2023, 3, 100609. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Li, C.; Tang, B.; Peng, C. Catalytic Conversion of Lignin and Its Derivatives to Alkanes over Multifunctional Catalysts: A Review. Fuel 2024, 361, 130726. [Google Scholar] [CrossRef]
- Ročnik, T.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M. Catalytic Lignin Valorisation by Depolymerisation, Hydrogenation, Demethylation and Hydrodeoxygenation: Mechanism, Chemical Reaction Kinetics and Transport Phenomena. Chem. Eng. J. 2022, 448, 137309. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A Guide to Lignin Valorization in Biorefineries: Traditional, Recent, and Forthcoming Approaches to Convert Raw Lignocellulose into Valuable Materials and Chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C.; et al. Kraft Lignin: A Valuable, Sustainable Resource, Opportunities and Challenges. ChemSusChem 2023, 16, e202300492. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Agrawal, R.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Ramakumar, S.S.V. Next Generation Applications of Lignin Derived Commodity Products, Their Life Cycle, Techno-Economics and Societal Analysis. Int. J. Biol. Macromol. 2022, 197, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Nikkhah Dafchahi, M.; Acharya, B. Transforming Lignin into Renewable Fuels, Chemicals, and Materials: A Review. Bioresour. Technol. Reports 2023, 22, 101463–101487. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A Review on Lignin Pyrolysis: Pyrolytic Behavior, Mechanism, and Relevant Upgrading for Improving Process Efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Domine, M.E.; Chávez-Sifontes, M. Catalytic Processes for Lignin Valorization into Fuels and Chemicals (Aromatics). Curr. Catal. 2019, 8, 20–40. [Google Scholar] [CrossRef]
- Zheng, J.L.; Wei, Q. Improving the Quality of Fast Pyrolysis Bio-Oil by Reduced Pressure Distillation. Biomass Bioenergy 2011, 35, 1804–1810. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic Lignin: A Promising Biorefinery Feedstock for the Production of Fuels and Valuable Chemicals. Green Chem. 2022, 24, 4680–4702. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Valorization of Lignocellulosic Biomass. ChemCatChem 2016, 8, 1422–1423. [Google Scholar]
- Phan, D.P.; Lee, E.Y. Controlled Hydrogenolysis over Heterogeneous Catalysts for Lignin Valorization. Catal. Rev. Sci. Eng. 2020, 62, 607–630. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Yu, J.L.; Savage, P.E. Decomposition of Formic Acid under Hydrothermal Conditions. Ind. Eng. Chem. Res. 1998, 37, 2–10. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yan, Z.; Li, Q.; Zhang, Y. Catalysts with Metal-Acid Dual Sites for Selective Hydrodeoxygenation of Lignin Derivatives: Progress in Regulation Strategies and Applications. Appl. Catal. A Gen. 2023, 662, 119266. [Google Scholar] [CrossRef]
- Kumar, A.; Jindal, M.; Maharana, S.; Thallada, B. Lignin Biorefinery: New Horizons in Catalytic Hydrodeoxygenation for the Production of Chemicals. Energy Fuels 2021, 35, 16965–16994. [Google Scholar] [CrossRef]
- Prabhudesai, V.S.; Gurrala, L.; Vinu, R. Catalytic Hydrodeoxygenation of Lignin-Derived Oxygenates: Catalysis, Mechanism, and Effect of Process Conditions. Energy Fuels 2022, 36, 1155–1188. [Google Scholar] [CrossRef]
- Joffres, B.; Nguyen, M.T.; Laurenti, D.; Lorentz, C.; Souchon, V.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C. Lignin Hydroconversion on MoS2-Based Supported Catalyst: Comprehensive Analysis of Products and Reaction Scheme. Appl. Catal. B Environ. 2016, 184, 153–162. [Google Scholar] [CrossRef]
- Li, N.; Wei, L.; Bibi, R.; Chen, L.; Liu, J.; Zhang, L.; Zheng, Y.; Zhou, J. Catalytic Hydrogenation of Alkali Lignin into Bio-Oil Using Flower-like Hierarchical MoS2-Based Composite Catalysts. Fuel 2016, 185, 532–540. [Google Scholar] [CrossRef]
- Wu, K.; Sang, Y.; Kasipandi, S.; Ma, Y.; Jiao, H.; Liu, Q.; Chen, H.; Li, Y. Catalytic Roles of Mo-Based Sites on MoS2 for Ethanolysis of Enzymatic Hydrolysis Lignin into Aromatic Monomers. Catal. Today 2023, 408, 211–222. [Google Scholar] [CrossRef]
- Mukundan, S.; Chowdari, R.K.; Beltramini, J. External Solvent-Free Catalytic Hydrodeoxygenation of Softwood Lignin to Aromatics over Carbon–ZrO2 Supported Ni/MoS2 Catalysts. Adv. Sustain. Syst. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Abdus Salam, M.; Wayne Cheah, Y.; Hoang Ho, P.; Bernin, D.; Achour, A.; Nejadmoghadam, E.; Öhrman, O.; Arora, P.; Olsson, L.; Creaser, D. Elucidating the Role of NiMoS-USY during the Hydrotreatment of Kraft Lignin. Chem. Eng. J. 2022, 442, 136216. [Google Scholar] [CrossRef]
- Achour, A.; Bernin, D.; Creaser, D.; Olsson, L. Evaluation of Kraft and Hydrolysis Lignin Hydroconversion over Unsupported NiMoS Catalyst. Chem. Eng. J. 2022, 453, 139829. [Google Scholar] [CrossRef]
- Shabtai, J.S.; Zmierczak, W.W.; Chornet, E. Process for Conversion of Lignin to Reformulated Hydrocarbon Gasoline. US 5,959,167 A, 4 March 1999. [Google Scholar]
- Shabtai, J.S.; Zmierczak, W.W.; Chornet, E. Process for Conversion of Lignin to Reformulated, Partially Oxygenated Gasoline. US 6,172,272 B1, 9 January 2001. [Google Scholar]
- Horáček, J.; Homola, F.; Kubičková, I.; Kubička, D. Lignin to Liquids over Sulfided Catalysts. Catal. Today 2012, 179, 191–198. [Google Scholar] [CrossRef]
- Cheah, Y.W.; Salam, M.A.; Arora, P.; Öhrman, O.; Creaser, D.; Olsson, L. Role of Transition Metals on MoS2-Based Supported Catalysts for Hydrodeoxygenation (HDO) of Propylguaiacol. Sustain. Energy Fuels 2021, 5, 2097–2113. [Google Scholar] [CrossRef]
- Narani, A.; Chowdari, R.K.; Cannilla, C.; Bonura, G.; Frusteri, F.; Heeres, H.J.; Barta, K. Efficient Catalytic Hydrotreatment of Kraft Lignin to Alkylphenolics Using Supported NiW and NiMo Catalysts in Supercritical Methanol. Green Chem. 2015, 17, 5046–5057. [Google Scholar] [CrossRef]
- Kumar, C.R.; Anand, N.; Kloekhorst, A.; Cannilla, C.; Bonura, G.; Frusteri, F.; Barta, K.; Heeres, H.J. Solvent Free Depolymerization of Kraft Lignin to Alkyl-Phenolics Using Supported NiMo and CoMo Catalysts. Green Chem. 2015, 17, 4921–4930. [Google Scholar] [CrossRef]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild Hydrogenolysis of In-Situ and Isolated Pinus Radiata Lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Fan, J.; Chang, J. Selective Production of 4-Ethylphenolics from Lignin via Mild Hydrogenolysis. Bioresour. Technol. 2012, 118, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.-F.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the Lignin Oil OH-Content with Ru and Pd Catalysts during Lignin Hydrogenolysis on Birch Wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive Lignocellulose Fractionation into Soluble Lignin-Derived Phenolic Monomers and Dimers and Processable Carbohydrate Pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Li, H.; Song, G. Ru-Catalyzed Hydrogenolysis of Lignin: Base-Dependent Tunability of Monomeric Phenols and Mechanistic Study. ACS Catal. 2019, 9, 4054–4064. [Google Scholar] [CrossRef]
- Xu, W.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Depolymerization and Hydrodeoxygenation of Switchgrass Lignin with Formic Acid. ChemSusChem 2012, 5, 667–675. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic Depolymerisation of Isolated Lignins to Fine Chemicals Using a Pt/Alumina Catalyst: Part 1-Impact of the Lignin Structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- McVeigh, A.; Bouxin, F.P.; Jarvis, M.C.; Jackson, S.D. Catalytic Depolymerisation of Isolated Lignin to Fine Chemicals: Part 2—Process Optimisation. Catal. Sci. Technol. 2016, 6, 4142–4150. [Google Scholar] [CrossRef]
- Hita, I.; Deuss, P.J.; Bonura, G.; Frusteri, F.; Heeres, H.J. Biobased Chemicals from the Catalytic Depolymerization of Kraft Lignin Using Supported Noble Metal-Based Catalysts. Fuel Process. Technol. 2018, 179, 143–153. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective Production of Arenes via Direct Lignin Upgrading over a Niobium-Based Catalyst. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Ji, N.; Yin, J.; Rong, Y.; Li, H.; Yu, Z.; Lei, Y.; Wang, S.; Diao, X. More than a Support: The Unique Role of Nb2O5 in Supported Metal Catalysts for Lignin Hydrodeoxygenation. Catal. Sci. Technol. 2022, 12, 3751–3766. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.; Xie, J.; Zhao, L.; Zhang, C.; Zhao, X.; Zhao, Y.; Zhang, J. Catalytic Hydrodeoxygenation of Lignin and Its Model Compounds to Hydrocarbon Fuels over a Metal/Acid Ru/HZSM—5 Catalyst. Energy Fuels 2021, 35, 19543–19552. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, R.; Singh, N.; Tripathi, D.; Natte, K.; Narani, A. Hydrogenation of Lignin-Derived Feedstocks and Bio-Oil Using Active and Stable Ruthenium Catalyst. Catal. Today 2023, 408, 139–149. [Google Scholar] [CrossRef]
- Domine, M.E.; Chávez-Sifontes, M.; Gutierrez, A.; Vilonen, K.; Strengell, T.; Jokela, P.; Eilos, I. Process for Converting Lignocellulosic Materials. WO 2018/015608 A1, 25 January 2018. [Google Scholar]
- Domine, M.E.; Chávez-Sifontes, M.; Gutierrez, A.; Vilonen, K.; Strengell, T.; Jokela, P.; Eilos, I. Simple Process for Converting Lignocellulosic Materials. WO 2018/015610 A1, 25 January 2018. [Google Scholar]
- Dong, L.; Lin, L.; Han, X.; Si, X.; Liu, X.; Guo, Y.; Lu, F.; Rudić, S.; Parker, S.F.; Yang, S.; et al. Breaking the Limit of Lignin Monomer Production via Cleavage of Interunit Carbon–Carbon Linkages. Chem 2019, 5, 1521–1536. [Google Scholar] [CrossRef]
- Chen, S.S.; Yan, P.; Yu, X.; Zhu, W.; Wang, H.; Yu, X.; Wang, H. Conversion of Lignin to High Yields of Aromatics over Ru–ZnO/SBA-15 Bifunctional Catalysts. Renew. Energy 2023, 215, 118919. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic Hydrotreatment of Kraft Lignin into Aromatic Alcohols over Nickel-Rhenium Supported on Niobium Oxide Catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Pan, X.; Ji, J.; Ren, Y.; Wang, H.; Ji, N.; Liu, Q.; Li, C. One-Pot Conversion of Lignin into Naphthenes Catalyzed by a Heterogeneous Rhenium Oxide-Modified Iridium Compound. ChemSusChem 2020, 13, 4409–4419. [Google Scholar] [CrossRef]
- Parsell, T.H.; Owen, B.C.; Klein, I.; Jarrell, T.M.; Marcum, C.L.; Haupert, L.J.; Amundson, L.M.; Kenttamaa, H.I.; Ribeiro, F.; Miller, J.T.; et al. Cleavage and Hydrodeoxygenation (HDO) of C-O Bonds Relevant to Lignin Conversion Using Pd/Zn Synergistic Catalysis. Chem. Sci. 2013, 4, 806–813. [Google Scholar] [CrossRef]
- Klein, I.; Marcum, C.; Kenttamaa, H.; Abu-Omar, M.M. Mechanistic Investigation of the Zn/Pd/C Catalyzed Cleavage and Hydrodeoxygenation of Lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective Degradation of Wood Lignin over Noble-Metal Catalysts in a Two-Step Process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Liguori, L.; Barth, T. Palladium-Nafion SAC-13 Catalysed Depolymerisation of Lignin to Phenols in Formic Acid and Water. J. Anal. Appl. Pyrolysis 2011, 92, 477–484. [Google Scholar] [CrossRef]
- Laskar, D.D.; Tucker, M.P.; Chen, X.; Helms, G.L.; Yang, B. Noble-Metal Catalyzed Hydrodeoxygenation of Biomass-Derived Lignin to Aromatic Hydrocarbons. Green Chem. 2014, 16, 897–910. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, H.; Pei, H.; Wang, H.; Chen, X.; Tucker, M.P.; Cort, J.R.; Yang, B. Biomass-Derived Lignin to Jet Fuel Range Hydrocarbons via Aqueous Phase Hydrodeoxygenation. Green Chem. 2015, 17, 5131–5135. [Google Scholar] [CrossRef]
- Wang, H.; Ben, H.; Ruan, H.; Zhang, L.; Pu, Y.; Feng, M.; Ragauskas, A.J.; Yang, B. Effects of Lignin Structure on Hydrodeoxygenation Reactivity of Pine Wood Lignin to Valuable Chemicals. ACS Sustain. Chem. Eng. 2017, 5, 1824–1830. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, H.; Feng, M.; Qin, Y.; Job, H.; Luo, L.; Wang, C.; Engelhard, M.H.; Kuhn, E.; Chen, X.; et al. One-Pot Process for Hydrodeoxygenation of Lignin to Alkanes Using Ru-Based Bimetallic and Bifunctional Catalysts Supported on Zeolite Y. ChemSusChem 2017, 10, 1846–1856. [Google Scholar] [CrossRef]
- Kollman, M.S.; Jiang, X.; Sun, R.; Zhang, X.; Li, W.; Chang, H.; Jameel, H. Towards Jet Fuel from Technical Lignins: Feedstock-Catalyst-Product Interactions Revealed during Catalytic Hydrogenolysis. Chem. Eng. J. 2023, 451, 138464. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Boot, M.D.; Hensen, E.J.M. Efficient Conversion of Pine Wood Lignin to Phenol. ChemSusChem 2020, 13, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Weixiang, G.; Tsang, C.-W.; Haoquan, H.; Changhai, L. Lignin Valorizations with Ni Catalysts for Renewable. Catalysts 2019, 9, 488. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.C.; Lee, H.V. A Review on Catalytic Hydrodeoxygenation of Lignin to Transportation Fuels by Using Nickel-Based Catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. Solvent Effects on the Hydrogenolysis of Diphenyl Ether with Raney Nickel and Their Implications for the Conversion of Lignin. ChemSusChem 2012, 5, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Strüven, J.O.; Meier, D. Hydrocracking of Organosolv Lignin in Subcritical Water to Useful Phenols Employing Various Raney Nickel Catalysts. ACS Sustain. Chem. Eng. 2016, 4, 3712–3721. [Google Scholar] [CrossRef]
- Morgana, M.; Viola, E.; Zimbardi, F.; Cerone, N.; Romanelli, A.; Valerio, V. Depolymerization and Hydrogenation of Organosolv Eucalyptus Lignin by Using Nickel Raney Catalyst. Processes 2021, 9, 1093. [Google Scholar] [CrossRef]
- Forchheim, D.; Hornung, U.; Kempe, P.; Kruse, A.; Steinbach, D. Influence of RANEY Nickel on the Formation of Intermediates in the Degradation of Lignin. Int. J. Chem. Eng. 2012, 2012, 589749–589757. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of Lignosulfonate into Phenols over Heterogeneous Nickel Catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, W.; Xiao, L.P.; Shi, J.; Sun, R.C.; Song, G. Hydrogenolysis of Biorefinery Corncob Lignin into Aromatic Phenols over Activated Carbon-Supported Nickel. Sustain. Energy Fuels 2019, 3, 401–408. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin Depolymerization (LDP) in Alcohol over Nickel-Based Catalysts via a Fragmentation-Hydrogenolysis Process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Pineda, A.; Romero, A.A.; Luque, R.; Labidi, J. Microwave-Assisted Depolymerisation of Organosolv Lignin via Mild Hydrogen-Free Hydrogenolysis: Catalyst Screening. Appl. Catal. B Environ. 2014, 145, 43–55. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Kong, J.; He, M.; Lercher, J.A.; Zhao, C. Direct Production of Naphthenes and Paraffins from Lignin. Chem. Commun. 2015, 51, 17580–17583. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Li, B.; Zhao, C. Tuning Ni Nanoparticles and the Acid Sites of Silica-Alumina for Liquefaction and Hydrodeoxygenation of Lignin to Cyclic Alkanes. RSC Adv. 2016, 6, 71940–71951. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. Bifunctional Ni Catalysts for the One-Pot Conversion of Organosolv Lignin into Cycloalkanes. Catal. Today 2016, 269, 48–55. [Google Scholar] [CrossRef]
- Kasakov, S.; Shi, H.; Camaioni, D.M.; Zhao, C.; Baráth, E.; Jentys, A.; Lercher, J.A. Reductive Deconstruction of Organosolv Lignin Catalyzed by Zeolite Supported Nickel Nanoparticles. Green Chem. 2015, 17, 5079–5090. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and Controllable Alcoholysis of Kraft Lignin Catalyzed by Porous Zeolite-Supported Nickel-Copper Catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, J.; Ma, B.; Wang, Z.; Huang, J.; Zhao, C. Liquefaction and Hydrodeoxygenation of Polymeric Lignin Using a Hierarchical Ni Microreactor Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 2158–2166. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Zhao, W.; Li, L.; Liu, S.; Long, J.; Li, X. Selective Depolymerization of Lignin Catalyzed by Nickel Supported on Zirconium Phosphate. Green Chem. 2019, 21, 658–668. [Google Scholar] [CrossRef]
- Chen, B.; Rao, R.; Cao, M.; He, C.; Qian, Y.; Qiu, X.; Ouyang, X. Mild Hydrodeoxygenation of Lignin-Derived Bio-Oils to Hydrocarbons over Bifunctional ZrP2O7-Ni12P5 Catalysts. Fuel 2022, 313, 123044. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Guo, H.; Xiu, P.; Chen, J.; Qin, Y.; Robin, H.M.; Xu, C.; Gu, X. Effective Depolymerization of Alkali Lignin into Phenolic Monomers over ZrP Catalysts Promoted by Ni and W. Biomass Convers. Biorefinery 2021, 13, 5943–5955. [Google Scholar] [CrossRef]
- Jing, Y.; Dong, L.; Guo, Y.; Liu, X.; Wang, Y. Chemicals from Lignin: A Review of Catalytic Conversion Involving Hydrogen. ChemSusChem 2019, 13, 4181–4198. [Google Scholar] [CrossRef]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Jiang, S.; Ji, N.; Diao, X.; Li, H.; Rong, Y.; Lei, Y.; Yu, Z. Vacancy Engineering in Transition Metal Sulfide and Oxide Catalysts for Hydrodeoxygenation of Lignin-Derived Oxygenates. ChemSusChem 2021, 14, 4377–4396. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, H.; Xu, Z.J. Impact of Surface Area in Evaluation of Catalyst Activity. Joule 2018, 2, 1024–1027. [Google Scholar] [CrossRef]
- Jenkins, A.H.; Medlin, J.W. Controlling Heterogeneous Catalysis with Organic Monolayers on Metal Oxides. Acc. Chem. Res. 2021, 54, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, M.M.-J.; Kennedy, E.; Adesina, A.; Zhao, G.; Setiawan, A.; Stockenhuber, M. The Role of Acid and Metal Sites in Hydrodeoxygenation of Guaiacol over Ni/Beta Catalysts. Catal. Sci. Technol. 2020, 10, 810–825. [Google Scholar] [CrossRef]
- Teles, C.A.; de Souza, P.M.; Braga, A.H.; Rabelo-Neto, R.C.; Teran, A.; Jacobs, G.; Resasco, D.E.; Noronha, F.B. The Role of Defect Sites and Oxophilicity of the Support on the Phenol Hydrodeoxygenation Reaction. Appl. Catal. B Environ. 2019, 249, 292–305. [Google Scholar] [CrossRef]




| Noble-Metal-Based Catalyst | Type of Lignin or Lignin-Derived Fraction | Experimental Data | Main Results | Ref. |
|---|---|---|---|---|
| 5 wt.% Pd/C | Enzymatic hydrolysis lignin | Catalyst/lignin: 0.18/0.67 (mass ratio), 195 °C, 3.45 MPa H2, 24 h reaction time. Dioxane/water (1:1 vol.) as solvent | Yield = 89 wt.% oil fraction (based on lignin input). Composition oil fraction: 21 wt.%, dihydro-coniferyl alcohol and 4-n-propyl guaiacol as the main products | [34] |
| 5 wt.% Ru/C | Enzymatic hydrolysis lignin | Catalyst/lignin: 1/5 mass ratio, 250 °C and 2.0 MPa H2, 3 h reaction time Ethanol/water (65 vol.%) as solvent | Yield ≈ 72 wt.% to liquid products (EtOAc soluble phase) (based on initial lignin). Composition liquid fraction: ethyl-4-guaiacol and ethyl-4-phenol as the primary products. | [35] |
| Pd/C vs. Ru/C (5 wt.%) | Extracted birch sawdust (19.5 wt% Klason lignin) | Catalyst/lignin: 1/10 mass ratio, 250 °C and 3.0 MPa H2, 3 h reaction time Methanol/water (65 vol.%) as solvent | Yield = 49 (C%) Pd and 48 (C%) Ru to monomers (based on the weight of lignin oil). Selectivity to main products: Pd (propanol-4-guaiacol + propanol-4-syringol) vs. Ru (n-propyl-4-guaiacol + n-propyl-4-syringol) | [36,37] |
| 20 wt.% Pt/C | Organosolv lignin (switchgrass) | Catalyst/lignin: 1/10 mass ratio, 350 °C and formic acid (H2 donor), 4 h reaction time Ethanol as solvent | Yield = 21 wt.% total of identified products Composition liquid fraction: ethyl-4-phenol and propyl-4-guaiacol as the main products. | [39] |
| 5 wt.% M/Al2O3 M = Ru, Pd, Pt, and Rh | Kraft lignin | Catalyst/lignin: 1/20 mass ratio, 450 °C, 10 MPa H2, 4 h reaction time Methanol/water (1:1 vol.) as solvent | Yields to organic phase = 30.4 wt.% (Ru), 40.3 wt.% (Pt), 37.5 wt.% (Pd), and 41.5 wt.% (Rh). Composition of organic phase: lignin oil and soluble fractions in dichloromethane or acetone. | [42] |
| 2 wt.% Ru/Nb2O5 | Birch lignin | Catalyst/lignin: 2/1 mass ratio, 250 °C, 0.7 MPa H2, 20 h reaction time Water as solvent | Yield to organic phase = 35.5 wt.% Composition: arenes (59.5 wt.%), cycloalkanes (24.2 wt.%), dicyclic arenes + dicyclic cycloalkanes (6.3 wt.%), aliphatic alkanes (1.7 wt.%). | [43] |
| 2.8 wt.% Ru-Nanocarbon/SiO2-Al2O3 | Alkali lignin bio-oil | Catalyst/lignin bio-oil: 50 mg/0.5 mL mass ratio, 120 °C, 3 MPa H2, 48 h reaction time t-butyl-alcohol as solvent | The catalyst was able to hydrogenate the aromatic rings of the lignin-derived compounds included in the bio-oil. From aromatic to cycloalkanes. | [46] |
| Ru-10ZnO/SBA-15 | Enzymatic hydrolysis lignin | Catalyst/lignin: 1/1 mass ratio, 220–240 °C, 2 MPa H2, 4 h reaction time Methanol as solvent | Yield to organic phase ≈ 42.5 mol% (220 °C) and 51.3 mol% (240 °C) | [50] |
| Ir-ReOx/SiO2 (1 wt% Ir and 5 wt% Re) | Different types of lignins | Catalyst/lignin: 1/1 mass ratio, 260 °C, 4 MPa H2, 10 h reaction time n-hexane as solvent | Yield to lignin oil = 15.3 C mol% (organosolv), 14.2 C mol% (enzymatic hydrolysis), 16.6 C mol% (alkaline) Yield to monomers = 29 C mol% (organosolv), 14.6 C mol% (enzymatic hydrolysis), 9.3 C mol% (alkaline) | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Sifontes, M.; Ventura, M. Metal-Based Heterogeneous Catalysts for the Synthesis of Valuable Chemical Blends via Hydrodeoxygenation of Lignin-Derived Fractions. Catalysts 2024, 14, 146. https://doi.org/10.3390/catal14020146
Chávez-Sifontes M, Ventura M. Metal-Based Heterogeneous Catalysts for the Synthesis of Valuable Chemical Blends via Hydrodeoxygenation of Lignin-Derived Fractions. Catalysts. 2024; 14(2):146. https://doi.org/10.3390/catal14020146
Chicago/Turabian StyleChávez-Sifontes, Marvin, and María Ventura. 2024. "Metal-Based Heterogeneous Catalysts for the Synthesis of Valuable Chemical Blends via Hydrodeoxygenation of Lignin-Derived Fractions" Catalysts 14, no. 2: 146. https://doi.org/10.3390/catal14020146
APA StyleChávez-Sifontes, M., & Ventura, M. (2024). Metal-Based Heterogeneous Catalysts for the Synthesis of Valuable Chemical Blends via Hydrodeoxygenation of Lignin-Derived Fractions. Catalysts, 14(2), 146. https://doi.org/10.3390/catal14020146