TiO2/Zeolite Composites for SMX Degradation under UV Irradiation
Abstract
1. Introduction
2. Results
2.1. Characterization
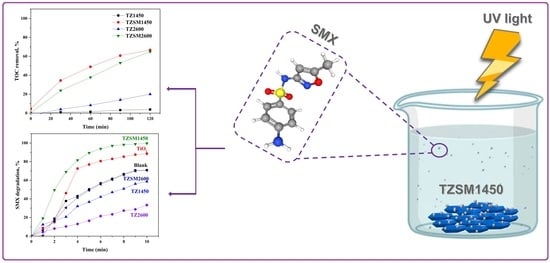
2.2. Photocatalytic Degradation and Mineralization of SMX
2.3. Electrical Energy Estimation
3. Materials and Methods
3.1. Materials
3.2. Preparation of TZ and TZSM Photocatalysts
3.2.1. Preparation of TZ1450 and TZSM1450
3.2.2. Preparation of TZ2600 andTZSM2600
3.3. Characterization
3.4. Photocatalytic Degradation and Mineralization of SMX
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oh, W.-D.; Dong, Z.; Ronn, G.; Lim, T.-T. Surface–Active Bismuth Ferrite as Superior Peroxymonosulfate Activator for Aqueous Sulfamethoxazole Removal: Performance, Mechanism and Quantification of Sulfate Radical. J. Hazard. Mater. 2017, 325, 71–81. [Google Scholar] [CrossRef]
- Guo, R.; Wang, Y.; Li, J.; Cheng, X.; Dionysiou, D.D. Sulfamethoxazole Degradation by Visible Light Assisted Peroxymonosulfate Process Based on Nanohybrid Manganese Dioxide Incorporating Ferric Oxide. Appl. Catal. B Environ. 2020, 278, 119297. [Google Scholar] [CrossRef]
- Ory, J.; Bricheux, G.; Togola, A.; Bonnet, J.L.; Donnadieu-Bernard, F.; Nakusi, L.; Forestier, C.; Traore, O. Ciprofloxacin Residue and Antibiotic-Resistant Biofilm Bacteria in Hospital Effluent. Environ. Pollut. 2016, 214, 635–645. [Google Scholar] [CrossRef]
- Haenni, M.; Dagot, C.; Chesneau, O.; Bibbal, D.; Labanowski, J.; Vialette, M.; Bouchard, D.; Martin-Laurent, F.; Calsat, L.; Nazaret, S.; et al. Environmental Contamination in a High-Income Country (France) by Antibiotics, Antibiotic-Resistant Bacteria, and Antibiotic Resistance Genes: Status and Possible Causes. Environ. Int. 2022, 159, 107047. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Liu, B.; Song, S. Reaction Mechanisms and Toxicity Evolution of Sulfamethoxazole Degradation by CoFe-N Doped C as Electro-Fenton Cathode. Sep. Purif. Technol. 2022, 298, 121655. [Google Scholar] [CrossRef]
- Doekhi-Bennani, Y.; Leilabady, N.M.; Fu, M.; Rietveld, L.C.; van der Hoek, J.P.; Heijman, S.G.J. Simultaneous Removal of Ammonium Ions and Sulfamethoxazole by Ozone Regenerated High Silica Zeolites. Water Res. 2021, 188, 116472. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, M.; Chen, Y.; Du, Y.; Feng, J.; Feng, H. Influence of Sulfamethoxazole on Anaerobic Digestion: Methanogenesis, Degradation Mechanism and Toxicity Evolution. J. Hazard. Mater. 2022, 431, 128540. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Xiong, Z.; Wang, Y.; Peng, S.; Wang, J.; Guo, Y. Lithium Cobalt Oxide with Excellent Electron Mobility: An Efficient Activator of Peroxymonosulfate for the Degradation of Sulfamethoxazole. Chem. Eng. J. 2022, 445, 136702. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, H.; Qi, C.; Zhao, Y.; Wen, Y.; Xu, C.; Zhong, Q.; Sun, D.; Zhou, S.; Yang, B.; et al. L-Cysteine Boosted Fe(III)-Activated Peracetic Acid System for Sulfamethoxazole Degradation: Role of L-Cysteine and Mechanism. Chem. Eng. J. 2023, 451, 138588. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, Y.; Liao, J.; Chen, J.; Du, Q. Efficient Removal of Sulfamethoxazole by Resin-Supported Zero-Valent Iron Composites with Tunable Structure: Performance, Mechanisms, and Degradation Pathways. Chemosphere 2021, 269, 128684. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and Persistence of Organic Emerging Contaminants and Priority Pollutants in Five Sewage Treatment Plants of Spain: Two Years Pilot Survey Monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring Release of Pharmaceutical Compounds: Occurrence and Environmental Risk Assessment of Two WWTP Effluents and Their Receiving Bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, A.; Eslami, A.; Massoudinejad, M.; Avazpour, M. Enhanced Degradation of Sulfamethoxazole Antibiotic from Aqueous Solution Using Mn-WO3/LED Photocatalytic Process: Kinetic, Mechanism, Degradation Pathway and Toxicity Reduction. Chem. Eng. J. 2020, 380, 122497. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y.; Cheng, J.; Chen, Y. Enhanced Performance of Sulfamethoxazole Degradation Using Achromobacter Sp. JL9 with in-Situ Generated Biogenic Manganese Oxides. Bioresour. Technol. 2021, 333, 125089. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.-Y.; Lee, D.S. Reduced Graphene oxide−TiO2/Sodium Alginate 3-Dimensional Structure Aerogel for Enhanced Photocatalytic Degradation of Ibuprofen and Sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Atabaev, T.S.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Photocatalytic Degradation of 4-Tert-Butylphenol Using Solar Light Responsive Ag2CO3. Catalysts 2022, 12, 1523. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Kumarov, A.; Atabaev, T.S.; Hapeshi, E.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Degradation of 4-Tert-Butylphenol in Water Using Mono-Doped (M1: Mo, W) and Co-Doped (M2-M1: Cu, Co, Zn) Titania Catalysts. Nanomaterials 2022, 12, 2326. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Hakkoum, H.; Comini, E. Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials 2023, 13, 1424. [Google Scholar] [CrossRef]
- Wan, X.; Mo, G.; Luo, J. Metal–Organic Frameworks-Derived TiO2 for Photocatalytic Degradation of Tetracycline Hydrochloride. Can. J. Chem. Eng. 2023, 101, 1358–1370. [Google Scholar] [CrossRef]
- Kamegawa, T.; Kido, R.; Yamahana, D.; Yamashita, H. Design of TiO2-Zeolite Composites with Enhanced Photocatalytic Performances under Irradiation of UV and Visible Light. Microporous Mesoporous Mater. 2013, 165, 142–147. [Google Scholar] [CrossRef]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of Air Photocatalytic Purification Using a Total Hazard Index: Effect of the Composite TiO2/Zeolite Photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Piedra López, J.G.; González Pichardo, O.H.; Pinedo Escobar, J.A.; de Haro del Río, D.A.; Inchaurregui Méndez, H.; González Rodríguez, L.M. Photocatalytic Degradation of Metoprolol in Aqueous Medium Using a TiO2/Natural Zeolite Composite. Fuel 2021, 284, 119030. [Google Scholar] [CrossRef]
- You-ji, L.; Wei, C. Photocatalytic Degradation of Rhodamine B Using Nanocrystalline TiO2–Zeolite Surface Composite Catalysts: Effects of Photocatalytic Condition on Degradation Efficiency. Catal. Sci. Technol. 2011, 1, 802–809. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Jácome-Francis, K. Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability 2021, 13, 6378. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Abitayev, Z.; Batyrbayeva, M.; Vakros, J.; Poulopoulos, S.G. Zeolite Supported TiO2 for the Degradation and Mineralization of Sulfamethoxazole under UV Light Irradiation. IOP Conf. Ser. Earth Environ. Sci. 2022, 1123, 012086. [Google Scholar] [CrossRef]
- Cieśla, J.; Franus, W.; Franus, M.; Kedziora, K.; Gluszczyk, J.; Szerement, J.; Jozefaciuk, G. Environmental-Friendly Modifications of Zeolite to Increase Its Sorption and Anion Exchange Properties, Physicochemical Studies of the Modified Materials. Materials 2019, 12, 3213. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Plutino, M.R. Sustainable Secondary-Raw Materials, Natural Substances and Eco-Friendly Nanomaterial-Based Approaches for Improved Surface Performances: An Overview of What They Are and How They Work. Int. J. Mol. Sci. 2023, 24, 5472. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Bahranifard, A. Application of TiO2-Zeolite as Photocatalyst for Photodegradation of Some Organic Pollutants. Iran. J. Catal. 2011, 1, 45–50. [Google Scholar]
- Aziztyana, A.; Wardhani, S.; Prananto, Y.; Purwonugroho, D.; Darjito. Optimisation of Methyl Orange Photodegradation Using TiO2 -Zeolite Photocatalyst and H2O2 in Acid Condition. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042047. [Google Scholar] [CrossRef]
- An, Y.; de Ridder, D.J.; Zhao, C.; Schoutteten, K.; Bussche, J.V.; Zheng, H.; Chen, G.; Vanhaecke, L. Adsorption and Photocatalytic Degradation of Pharmaceuticals and Pesticides by Carbon Doped-TiO2 Coated on Zeolites under Solar Light Irradiation. Water Sci. Technol. 2016, 73, 2868–2881. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Guo, W.; Xi, B. Performance and Mechanism into TiO2/Zeolite Composites for Sulfadiazine Adsorption and Photodegradation. Chem. Eng. J. 2018, 350, 131–147. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced Photocatalytic Activity of TiO2/Zeolite Composite for Abatement of Pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Guo, Q.; Wang, X.; Zhang, L.; Zhu, W.; Luo, Y. Activation of Peroxymonosulfate by the CoFe/ZSM-5 for Efficient Sulfamethoxazole Degradation. J. Environ. Chem. Eng. 2022, 10, 107012. [Google Scholar] [CrossRef]
- Chi, H.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M.; Li, X.; Pu, M. ZSM-5-(C@Fe) Activated Peroxymonosulfate for Effectively Degrading Ciprofloxacin: In-Depth Analysis of Degradation Mode and Degradation Path. J. Hazard. Mater. 2020, 398, 123024. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.M.; Alvarado, J.; Soto, B.S.; Hernandez, M.A. Synthesis of TiO2 Nanoparticles and TiO2-Zeolite Composites and Study of Optical Properties and Structural Characterization. Optik 2018, 169, 137–146. [Google Scholar] [CrossRef]
- Aini, N.; Sagita, F.E.; Diyanah, K.; Arifah, A.; Chasanah, S.N.; Prasetyo, A. Structural and Photocatalytic Properties of TiO2/Zeolite Synthesized Using Sol-Gel Method. Alchemy J. Chem. 2019, 7, 25–30. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite–TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Fu, J.; Wang, J.; Fan, P.; Yang, L.; Yuan, Z. Direct Synthesis and Characterization of Pore-Broadened Al-SBA-15. Microporous Mesoporous Mater. 2017, 239, 316–327. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Chen, G.; Zhang, J.; Liu, J. The Relationship between Acidity, Dispersion of Nickel, and Performance of Ni/Al-SBA-15 Catalyst on Eugenol Hydrodeoxygenation. Renew. Energy 2020, 149, 609–616. [Google Scholar] [CrossRef]
- Ahmed, O.; Pons, M.-N.; Lachheb, H.; Houas, A.; Zahraa, O. Degradation of Sulfamethoxazole by Photocatalysis Using Supported TiO2. Sustain. Environ. Res. 2014, 24, 381–387. [Google Scholar]
- Karimi-Shamsabadi, M.; Nezamzadeh-Ejhieh, A. Comparative Study on the Increased Photoactivity of Coupled and Supported Manganese-Silver Oxides onto a Natural Zeolite Nano-Particles. J. Mol. Catal. Chem. 2016, 418–419, 103–114. [Google Scholar] [CrossRef]
- Anandan, S.; Ikuma, Y.; Niwa, K. An Overview of Semi-Conductor Photocatalysis: Modification of TiO2 Nanomaterials. Solid State Phenom. 2010, 162, 239–260. [Google Scholar] [CrossRef]
- Torkian, N.; Bahrami, A.; Hosseini-Abari, A.; Momeni, M.M.; Abdolkarimi-Mahabadi, M.; Bayat, A.; Hajipour, P.; Amini Rourani, H.; Abbasi, M.S.; Torkian, S.; et al. Synthesis and Characterization of Ag-Ion-Exchanged Zeolite/TiO2 Nanocomposites for Antibacterial Applications and Photocatalytic Degradation of Antibiotics. Environ. Res. 2022, 207, 112157. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, J.; Hu, G.; Yang, C.; Chen, Y.; Liu, Q.; Ren, S.; Li, J. Optimization of TiO2/ZSM-5 Photocatalysts: Energy Band Engineering by Solid State Diffusion Method with Calcination. J. Environ. Chem. Eng. 2021, 9, 105563. [Google Scholar] [CrossRef]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic Oxidation of Sulfamethoxazole in the Presence of TiO2: Effect of Matrix in Aqueous Solution on Decomposition Mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Sahoo, C.; Gupta, A.K.; Pal, A. Photocatalytic Degradation of Methyl Red Dye in Aqueous Solutions under UV Irradiation Using Ag+ Doped TiO2. Desalination 2005, 181, 91–100. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Q.; Gu, C.; Luo, X.; Yu, C.; Wu, M. Photocatalytic Degradation of Two Different Types of Dyes by Synthesized La/Bi 2 WO 6. RSC Adv. 2016, 6, 85852–85859. [Google Scholar] [CrossRef]
- Shi, W.; Liu, X.; Zhang, T.; Wang, Q.; Zhang, L. Magnetic Nano-Sized Cadmium Ferrite as an Efficient Catalyst for the Degradation of Congo Red in the Presence of Microwave Irradiation. RSC Adv. 2015, 5, 51027–51034. [Google Scholar] [CrossRef]
- Dianati, R.A.; Mengelizadeh, N.; Zazouli, M.A.; Yazdani Cherati, J.; Balarak, D.; Ashrafi, S. Photocatalytic Degradation of Bisphenol A by GO-TiO2 Nanocomposite under Ultraviolet Light: Synthesis, Effect of Parameters and Mineralisation. Int. J. Environ. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Chen, B.; Wang, L.; Zhu, R.; Yang, J. Hydrogen Peroxide Formation in Water during the VUV/UV Irradiation Process: Impacts and Mechanisms of Selected Anions. Environ. Res. 2021, 195, 110751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mathur, N. Photocatalytic Degradation of Aniline at the Interface of TiO2 Suspensions Containing Carbonate Ions. J. Colloid Interface Sci. 2006, 300, 244–252. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-Assisted Photocatalytic Degradation of Sulfadiazine Using MgO@CNT Heterojunction Composite: Effective Factors, Pathway and Biodegradability Studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Wen, X.-J.; Qian-Lu; Lv, X.-X.; Sun, J.; Guo, J.; Fei, Z.-H.; Niu, C.-G. Photocatalytic Degradation of Sulfamethazine Using a Direct Z-Scheme AgI/Bi4V2O11 Photocatalyst: Mineralization Activity, Degradation Pathways and Promoted Charge Separation Mechanism. J. Hazard. Mater. 2020, 385, 121508. [Google Scholar] [CrossRef]
- Joseph, C.; Liew, S.; Bono, A.; Teng, L. Photodegradation of Indigo Dye Using TiO2 and TiO2/Zeolite System. Asian J. Chem. 2013, 25, 8402–8406. [Google Scholar] [CrossRef]
- Yang, L.; Hao, X.; Yu, D.; Zhou, P.; Peng, Y.; Jia, Y.; Zhao, C.; He, J.; Zhan, C.; Lai, B. High Visible-Light Catalytic Activity of Bis-PDI-T@TiO2 for Activating Persulfate toward Efficient Degradation of Carbamazepine. Sep. Purif. Technol. 2021, 263, 118384. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Chen, C.; Wang, X. Enhanced Photocatalytic Degradation of Methylene Blue under Visible Irradiation on graphene@TiO2 Dyade Structure. Appl. Catal. B Environ. 2012, 111–112, 303–308. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Vishnu Priya, S.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Photocatalytic Degradation of Aqueous Propoxur Solution Using TiO2 and Hβ Zeolite-Supported TiO2. J. Hazard. Mater. 2009, 161, 336–343. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Z.; Ji, B.; Liu, Y.; Jiang, Y.; Duan, J.; Zhao, W. Comparative Analysis of Conventional Light Source and LED Array Combined with the Catalyst for Degradation of Antibiotics. Process Saf. Environ. Prot. 2021, 149, 915–926. [Google Scholar] [CrossRef]
- Zambrano, J.; García-Encina, P.A.; Jiménez, J.J.; López-Serna, R.; Irusta-Mata, R. Photolytic and Photocatalytic Removal of a Mixture of Four Veterinary Antibiotics. J. Water Process Eng. 2022, 48, 102841. [Google Scholar] [CrossRef]
- Autin, O.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. Comparison of UV/TiO2 and UV/H2O2 Processes in an Annular Photoreactor for Removal of Micropollutants: Influence of Water Parameters on Metaldehyde Removal, Quantum Yields and Energy Consumption. Appl. Catal. B Environ. 2013, 138–139, 268–275. [Google Scholar] [CrossRef]
- Sahraeian, S.; Alipour, V.; Heidari, M.; Rahmanian, O.; Karimi Abdolmaleki, M. Application of Photocatalytic Process Using UV/TiO2 for Degradation of Cefepime: A Comparison between Photocatalytic and Photolytic. Iran. J. Chem. Chem. Eng. 2021, 40, 796–803. [Google Scholar] [CrossRef]
- Raashid, M.; Kazmi, M.; Ikhlaq, A.; Iqbal, T.; Sulaiman, M.; Zafar, A.M.; Aly Hassan, A. Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts. Water 2023, 15, 1283. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Wang, Q.; Wang, X.; Jiang, W.; Zhang, Z.; Yang, Y. A Novel Double-Cylindrical-Shell Photoreactor Immobilized with Monolayer TiO2-Coated Silica Gel Beads for Photocatalytic Degradation of Rhodamine B and Methyl Orange in Aqueous Solution. Sep. Purif. Technol. 2014, 123, 130–138. [Google Scholar] [CrossRef]
- Subtil, G.W.; Vicentini, J.C.M.; de Oliveira, D.M.; de Castro-Hoshino, L.V.; Yassue-Cordeiro, P.H.; Vicentino, R.C.; Scaliante, M.H.N.O. The Influence of Different Zeolitic Supports on Hydrogen Production and Waste Degradation. Can. J. Chem. Eng. 2023, 101, 1345–1357. [Google Scholar] [CrossRef]
- Saadati, F.; Keramati, N.; Mehdipour Ghazi, M. Synthesis of Nanocomposite Based on Semnan Natural Zeolite for Photocatalytic Degradation of Tetracycline under Visible Light. Adv. Environ. Technol. 2016, 2, 63–70. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I. Zeolite Supported TiO2 with Enhanced Degradation Efficiency for Organic Dye under Household Compact Fluorescent Light. Mater. Res. Express 2019, 6, 095506. [Google Scholar] [CrossRef]












| Catalyst | SBET (m2/g) | Si/Al Ratio |
|---|---|---|
| TiO2 | 52.4 | - |
| TZ1450 | 10.7 | 0.99 |
| TZ2600 | 47.1 | 0.91 |
| TZSM1450 | 361.7 | 18.25 |
| TZSM2600 | 109.9 | 18.00 |
| System | Organic Pollutant | Concentration (mg·L−1) | Wavelength (nm)/Power (W) | EE/O (kWh m−3 Order−1) | Ref. |
|---|---|---|---|---|---|
| UV/TiO2 | SMX | 1 | 257.7/36 | 62.1 | [60] |
| UV/TiO2 | Metaldehyde | 0.01 | 253.7/45 | 4.9 | [61] |
| UV/TiO2 | Cefepime | 20 | 247.3/125 | 344.09 | [62] |
| UV/TiO2-Fe-Zeolite/O3 | Sulfoxaflor | 100 | 253.7/38 | 42.9 | [63] |
| UV/Y-TiO2-ZSM-5 | Methyl orange | 8.17 | 320–440/120 | 1.56 × 1010 | [64] |
| UV/TiO2/ZSM-5 | Reactive blue dye (CI250) | 10 | 254/7 | 74.75 | [65] |
| UV/TZSM1450 | SMX | 30 | 365/500 | 68.53 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergenbayeva, S.; Abitayev, Z.; Batyrbayeva, M.; Vakros, J.; Mantzavinos, D.; Atabaev, T.S.; Poulopoulos, S.G. TiO2/Zeolite Composites for SMX Degradation under UV Irradiation. Catalysts 2024, 14, 147. https://doi.org/10.3390/catal14020147
Mergenbayeva S, Abitayev Z, Batyrbayeva M, Vakros J, Mantzavinos D, Atabaev TS, Poulopoulos SG. TiO2/Zeolite Composites for SMX Degradation under UV Irradiation. Catalysts. 2024; 14(2):147. https://doi.org/10.3390/catal14020147
Chicago/Turabian StyleMergenbayeva, Saule, Zhanibek Abitayev, Milana Batyrbayeva, John Vakros, Dionissios Mantzavinos, Timur Sh. Atabaev, and Stavros G. Poulopoulos. 2024. "TiO2/Zeolite Composites for SMX Degradation under UV Irradiation" Catalysts 14, no. 2: 147. https://doi.org/10.3390/catal14020147
APA StyleMergenbayeva, S., Abitayev, Z., Batyrbayeva, M., Vakros, J., Mantzavinos, D., Atabaev, T. S., & Poulopoulos, S. G. (2024). TiO2/Zeolite Composites for SMX Degradation under UV Irradiation. Catalysts, 14(2), 147. https://doi.org/10.3390/catal14020147