Carbon Dioxide Methanation Enabled by Biochar-Nanocatalyst Composite Materials: A Mini-Review
Abstract
:1. Introduction
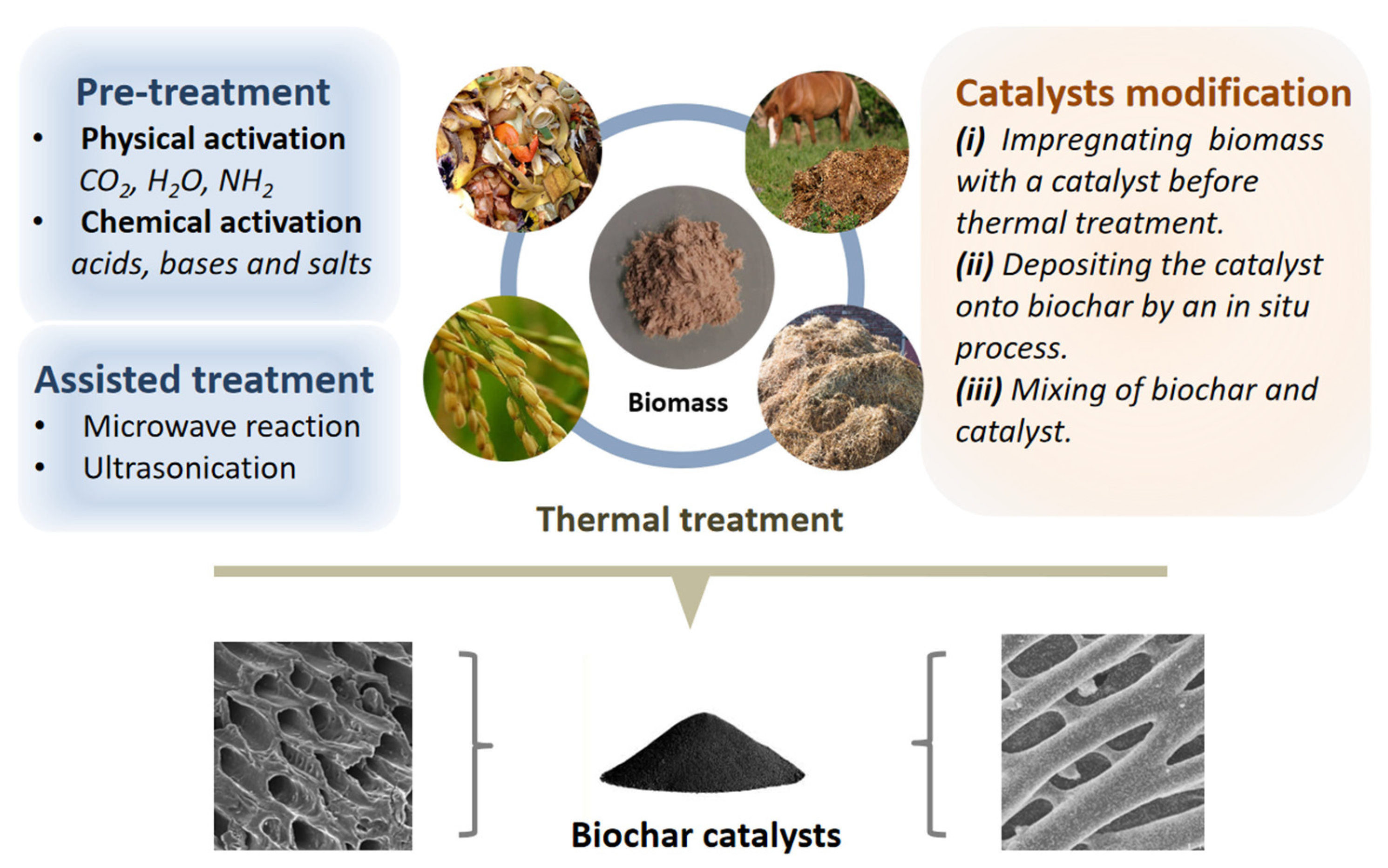
2. Biochar Catalyst Fabrication and Modification
2.1. Biomass Sources
2.2. Fabrication Process
2.3. Precursor Material Pretreatment
2.3.1. Physical Pretreatment
2.3.2. Chemical Pretreatment
2.4. Nanoparticle Loading
2.5. Sequence of Wetness Impregnation/Pyrolysis
2.6. Porous Properties of Biochar-Supported Materials
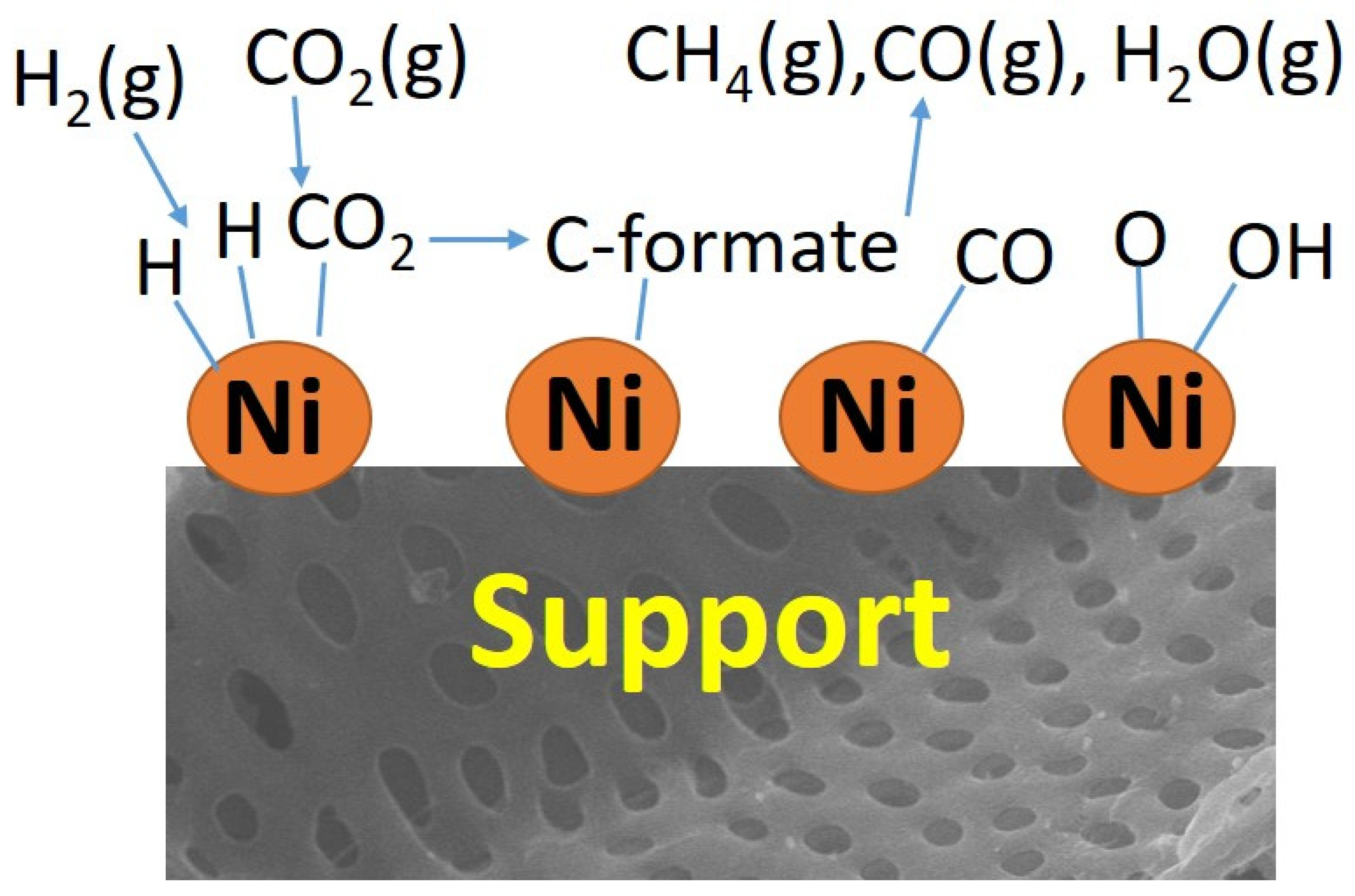
3. Case Studies for Catalyzed CO2 Methanation
3.1. The Role of Biochar Composite Preparation in CO2 Methanation
3.2. Effect of Surface Basic Sites
3.3. Effect of Nanometal Dispersion
3.4. Effect of Metal Loading
4. Challenges and Prospects of Biochar catalyst Composites in CO2 Methanation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Guetschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Le Quere, C. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Scientific Data 2023, 10, 155. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyzynska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Semyonov, O.V.; Dingemans, J.; Xu, W.; Zhuiykov, S.; Khan, A.; Verpoort, F. Progress on catalyst development for direct synthesis of dimethyl carbonate from CO2 and methanol. Chem. Afr. 2019, 2, 533–549. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Mao, Y.; Wang, W.; Sun, J.; Song, Z.; Sun, J.; Zhao, X. Enhanced dry reforming of methane by microwave-mediated confined catalysis over Ni-La/AC catalyst. Chem. Eng. J. 2023, 451, 138616. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Yan, P.H.; Peng, H.; Vogrin, J.; Rabiee, H.; Zhu, Z.H. Selective CO2 hydrogenation over zeolite-based catalysts for targeted high-value products. J. Mater. Chem. A 2023, 11, 17938–17960. [Google Scholar] [CrossRef]
- Xie, Y.; Wen, J.; Li, Z.; Chen, J.; Zhang, Q.; Ning, P.; Hao, J. Double-Edged Sword Effect of Classical Strong Metal–Support Interaction in Catalysts for CO2 Hydrogenation to CO, Methane, and Methanol. ACS Mater. Lett. 2023, 5, 2629–2647. [Google Scholar] [CrossRef]
- Malik, M.I.; Achouri, I.E.; Abatzoglou, N.; Gitzhofer, F. Intensified performance of methane dry reforming based on non-thermal plasma technology: Recent progress and key challenges. Fuel Process. Technol. 2023, 245, 107748. [Google Scholar] [CrossRef]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Khader, M.M.; Al-Marri, M.J.; Ali, S.; Abdelmoneim, A.G.; Kumar, A.; Saleh, M.A.H.; Soliman, A. Catalytic evaluation of Ni-based nano-catalysts in dry reformation of methane. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 1051–1055. [Google Scholar]
- Song, L.; Wang, H.; Wang, S.; Qu, Z. Dual-site activation of H2 over Cu/ZnAl2O4 boosting CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2023, 322, 122137. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.A.; Izan, S.M.; Azami, M.S.; Kidam, K.; Ainirazali, N.; Ripin, A. Thermodynamic and experimental explorations of CO2 methanation over highly active metal-free fibrous silica-beta zeolite (FS@SiO2-BEA) of innovative morphology. Chem. Eng. Sci. 2021, 229, 116015. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Mateus, F.; Teixeira, P.; Lopes, J.M.; Henriques, C.; Bacariza, C. CO2 Methanation on Ni Catalysts Supported over Activated Carbons Derived from Cork Waste. Energy Fuels 2023, 37, 8552–8562. [Google Scholar] [CrossRef]
- Omiri, J.; Snoussi, Y.; Bhakta, A.K.; Truong, S.; Ammar, S.; Khalil, A.M.; Jouini, M.; Chehimi, M.M. Citric-Acid-Assisted Preparation of Biochar Loaded with Copper/Nickel Bimetallic Nanoparticles for Dye Degradation. Colloids Interfaces 2022, 6, 18. [Google Scholar] [CrossRef]
- Boubkr, L.; Bhakta, A.K.; Snoussi, Y.; Da Silva, C.M.; Michely, L.; Jouini, M.; Ammar, S.; Chehimi, M.M. Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture. Catalysts 2022, 12, 1475. [Google Scholar] [CrossRef]
- Bayoka, H.; Snoussi, Y.; Bhakta, A.K.; El Garah, M.; Khalil, A.M.; Jouini, M.; Ammar, S.; Chehimi, M.M. Evidencing the synergistic effects of carbonization temperature, surface composition and structural properties on the catalytic activity of biochar/ bimetallic composite. J. Anal. Appl. Pyrolysis 2023, 173, 106069. [Google Scholar] [CrossRef]
- Tang, M.; Snoussi, Y.; Bhakta, A.K.; El Garah, M.; Khalil, A.M.; Ammar, S.; Chehimi, M.M. Unusual, hierarchically structured composite of sugarcane pulp bagasse biochar loaded with Cu/Ni bimetallic nanoparticles for dye removal. Environ. Res. 2023, 232, 116232. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface Treatment of Biochar—Methods, Surface Analysis and Potential Applications: A Comprehensive Review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
- Gamal, A.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Khalil, A.M.; Jlassi, K.; Chehimi, M.M.; Ali, A.M.A. CO2 methanation using sugarcane bagasse biochar/nickel sustainable catalysts. Mater. Today Sustain. 2024, 25, 100627. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Cui, Z.-S.; Zhang, M.; Zhang, Z.-H. Biochar based functional materials as heterogeneous catalysts for organic reactions. Curr. Opin. Green Sustain. Chem. 2022, 38, 100713. [Google Scholar] [CrossRef]
- Velusamy, K.; Devanand, J.; Kumar, P.S.; Soundarajan, K.; Sivasubramanian, V.; Sindhu, J.; Vo, D.-V.N. A review on nano-catalysts and biochar-based catalysts for biofuel production. Fuel 2021, 306, 121632. [Google Scholar] [CrossRef]
- Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-supported biochar catalysts for sustainable biorefinery, electrocatalysis, and energy storage applications: A review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Dihingia, H.; Tiwari, D. Impact and implications of nanocatalyst in the Fenton-like processes for remediation of aquatic environment contaminated with micro-pollutants: A critical review. J. Water Process Eng. 2022, 45, 102500. [Google Scholar] [CrossRef]
- Zhu, X.; Labianca, C.; He, M.; Luo, Z.; Wu, C.; You, S.; Tsang, D.C. Life-cycle assessment of pyrolysis processes for sustainable production of biochar from agro-residues. Bioresour. Technol. 2022, 360, 127601. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- Wan, J.; Liu, L.; Wang, G.; Sang, L.; Liang, W.; Zhang, W.; Peng, C.; Fu, R. Unveiling the mechanisms of carbon conversion and loss in biochars derived from characteristic lignocellulosic biomass. J. Environ. Chem. Eng. 2022, 10, 108403. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Tang, M.; Snoussi, Y.; Khalil, A.M.; Mascarenhas, R.J.; Mekhalif, Z.; Abderrabba, M.; Ammar, S.; Chehimi, M.M. Sweety, salty, sour, and romantic biochar-supported ZnO: Highly active composite catalysts for environmental remediation. Emergent Mater. 2023, 1–15. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Goyal, N.; Bobde, P.; Kwon, E.E.; Lin, K.Y.; Chen, W.H. A critical review on biochar production from pine wastes, upgradation techniques, environmental sustainability, and challenges. Bioresour. Technol. 2023, 387, 129632. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Liu, T.; Wang, D.; Wang, Y.; Chen, H.; Luo, G.; Zhou, Z.; Li, C.; Xu, M. Biochar as the effective adsorbent to combustion gaseous pollutants: Preparation, activation, functionalization and the adsorption mechanisms. Prog. Energy Combust. Sci. 2023, 99, 101098. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.P.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Ahuja, R.; Kalia, A.; Sikka, R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review. ACS Omega 2022, 7, 45825–45836. [Google Scholar] [CrossRef]
- Xia, C.; Pathy, A.; Paramasivan, B.; Ganeshan, P.; Dhamodharan, K.; Juneja, A.; Kumar, D.; Brindhadevi, K.; Kim, S.-H.; Rajendran, K. Comparative study of pyrolysis and hydrothermal liquefaction of microalgal species: Analysis of product yields with reaction temperature. Fuel 2022, 311, 121932. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Li, Q.; Zhang, Y.; Zhou, H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass. Mater. Today Sustain. 2022, 19, 100209. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: Performance and mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Iberahim, N.; Sethupathi, S.; Bashir, M.J.K.; Kanthasamy, R.; Ahmad, T. Evaluation of oil palm fiber biochar and activated biochar for sulphur dioxide adsorption. Sci. Total Environ. 2022, 805, 150421. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Chang, G.; Guo, Q.; Qin, Y. Adsorption-enrichment characterization of CO2 and dynamic retention of free NH3 in functionalized biochar with H2O/NH3•H2O activation for promotion of new ammonia-based carbon capture. Chem. Eng. J. 2021, 409, 128193. [Google Scholar] [CrossRef]
- Peter, A.; Chabot, B.; Loranger, E. Pre-and post-pyrolysis effects on iron impregnation of ultrasound pre-treated softwood biochar for potential catalysis applications. SN Appl. Sci. 2021, 3, 643. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, J.; Zhang, Y.; Du, Q.; Feng, D.; Dong, H.; Peng, Y.; Zhang, T.; Xie, M. Ultra-microporous biochar-based carbon adsorbents by a facile chemical activation strategy for high-performance CO2 adsorption. Fuel Process. Technol. 2023, 241, 107613. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A.; Ayinde, Y.T.; Yilmaz, M. African almond (Terminalia catappa L.) leaves biochar prepared through pyrolysis using H3PO4 as chemical activator for sequestration of methylene blue dye. Results Eng. 2022, 14, 100385. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Truong, Q.-M.; Chen, C.-W.; Doong, R.-A.; Chen, W.-H.; Dong, C.-D. Mesoporous and adsorption behavior of algal biochar prepared via sequential hydrothermal carbonization and ZnCl2 activation. Bioresour. Technol. 2022, 346, 126351. [Google Scholar] [CrossRef]
- Snoussi, Y.; Sifaoui, I.; El Garah, M.; Khalil, A.M.; Jouini, M.; Ammar, S.; Morales, J.L.; Chehimi, M.M. Green, zero-waste pathway to fabricate supported nanocatalysts and anti-kinetoplastid agents from sugarcane bagasse. Waste Manag. 2023, 155, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, E.; Pillay, K.; Brink, H. Hydrothermal synthesis of magnetic-biochar nanocomposite derived from avocado peel and its performance as an adsorbent for the removal of methylene blue from wastewater. Mater. Today Sustain. 2022, 18, 100123. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Lv, J.; Zhou, S.; Niu, J.; Li, Z.; Wang, X.; Wagberg, T.; Hu, G. Microwave-assisted synthesis of amorphous cobalt nanoparticle decorated N-doped biochar for highly efficient degradation of sulfamethazine via peroxymonosulfate activation. J. Water Process Eng. 2022, 50, 103226. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, D.; Yuan, G.; Zhang, R. Waste-derived chars as methanation catalyst support: Role of inorganics in the char and its guide to catalyst design. Fuel 2023, 349, 128574. [Google Scholar] [CrossRef]
- Marcinczyk, M.; Krasucka, P.; Bogusz, A.; Tomczyk, B.; Duan, W.; Pan, B.; Oleszczuk, P. Ecotoxicological characteristics and properties of zinc-modified biochar produced by different methods. Chemosphere 2023, 315, 137690. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Zhang, N.; Reguyal, F.; Praneeth, S.; Sarmah, A.K. A green approach of biochar-supported magnetic nanocomposites from white tea waste: Production, characterization and plausible synthesis mechanisms. Sci. Total Environ. 2023, 886, 163923. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Liu, K.; Wang, Q.; Chang, Q. Three-dimensional hierarchical pore biochar prepared from soybean protein and its excellent Cr (VI) adsorption. Sep. Purif. Technol. 2023, 304, 122295. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Cai, Y.; Zhao, Y.; Song, S.; Quintana, M. Engineering sulfuric acid-pretreated biochar supporting MnO2 for efficient toxic organic pollutants removal from aqueous solution in a wide pH range. J. Clean. Prod. 2023, 416, 137968. [Google Scholar] [CrossRef]
- Shang, H.; Hu, W.; Li, Y.; Zhang, Q.; Feng, Y.; Xu, Y.; Yu, Y. Biochar-supported magnesium oxide as high-efficient lead adsorbent with economical use of magnesium precursor. Environ. Res. 2023, 229, 115863. [Google Scholar] [CrossRef]
- Anthonysamy, S.I.; Ahmad, M.A.; Yahaya, N.K.E. Insight the mechanism of MgAl/layered double hydroxide supported on rubber seed shell biochar for Remazol Brilliant Violet 5R removal. Arab. J. Chem. 2023, 16, 104643. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Chen, X.; Li, D.; Jin, Q. Enhanced oxidation and removal of As (Ⅲ) from water using biomass-derived porous carbon-supported nZVI with high iron utilization and fast adsorption. J. Environ. Chem. Eng. 2023, 11, 109038. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Wang, Y.; Cheng, Z.; Chen, X.; Gao, X.; Guo, M. Porous spherical Cu2O supported by wood-based biochar skeleton for the adsorption-photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 611, 155744. [Google Scholar]
- Yan, P.; Azreena, I.N.; Peng, H.; Rabiee, H.; Ahmed, M.; Weng, Y.; Zhu, Z.; Kennedy, E.M.; Stockenhuber, M. Catalytic hydropyrolysis of biomass using natural zeolite-based catalysts. Chem. Eng. J. 2023, 476, 146630. [Google Scholar] [CrossRef]
- El-Sabban, H.A.; Attia, S.Y.; Mostafa, H.Y.; Mohamed, S.G. Rational design of MoS2 nanoflowers grafted highly porous functionalized woody pulp-derived biochar for sustainable energy storage devices. Fuel 2024, 359, 130485. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, L.; Yin, Z.; Jiang, H. Facile microwave assisted one-pot solid-state construction of Co-Fe spinel oxide/porous biochar for highly efficient 4-nitrophenol degradation: Effect of chemical blowing and surface vulcanization. Sep. Purif. Technol. 2024, 328, 125033. [Google Scholar] [CrossRef]
- Gonzalez-Castano, M.; Morales, C.; Navarro de Miguel, J.C.; Boelte, J.H.; Klepel, O.; Flege, J.I.; Arellano-Garcia, H. Are Ni/ and Ni5Fe1/biochar catalysts suitable for synthetic natural gas production? A comparison with g-Al2O3 supported catalysts. Green Energy Environ. 2023, 8, 744–756. [Google Scholar] [CrossRef]
- Renda, S.; Di Stasi, C.; Manya, J.J.; Palma, V. Biochar as support in catalytic CO2 methanation: Enhancing effect of CeO2 addition. J. CO2 Util. 2021, 53, 101740. [Google Scholar] [CrossRef]
- Santos, J.L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na. J. Carbon Res. 2018, 4, 47. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.; Zhu, X.; Zhu, L.; Wang, S. Experimental study and life cycle assessment of CO2 methanation over biochar supported catalysts. Appl. Energy 2020, 280, 115919. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Di Stasi, C.; Renda, S.; Greco, G.; Gonzalez, B.; Palma, V.; Manya, J.J. Wheat-Straw-Derived Activated Biochar as a Renewable Support of Ni-CeO2 Catalysts for CO2 Methanation. Sustainability 2021, 13, 8939. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 120592. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, X.; Xu, Y.; Xiang, W.; Wang, R.; Ding, F.; Hong, P.; Gao, B. Straw and wood based biochar for CO2 capture: Adsorption performance and governing mechanisms. Sep. Purif. Technol. 2022, 287, 120592. [Google Scholar] [CrossRef]
- Shafawi, A.N.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Recent advances in developing engineered biochar for CO2 capture: An insight into the biochar modification approaches. J. Environ. Chem. Eng. 2021, 9, 106869. [Google Scholar] [CrossRef]
- Dekkar, S. Dry Reforming of Methane Over Ni/ZrO2, Ni/CeO2 and Ni/La2O3 Catalysts: Role of Support Nature and its Synthesis by Microemulsion Method. Chem. Afr. 2024, 7, 1–11. [Google Scholar] [CrossRef]

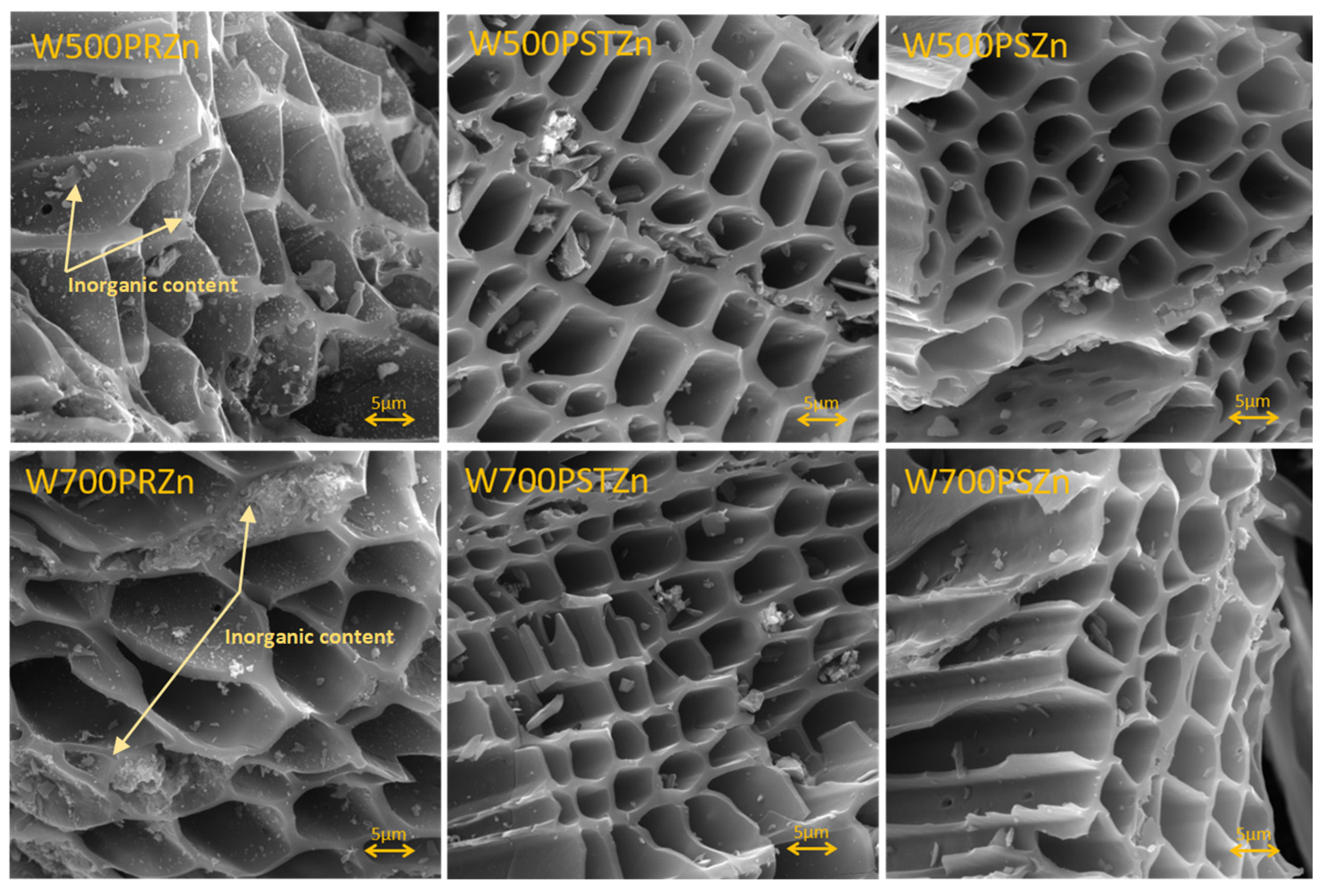
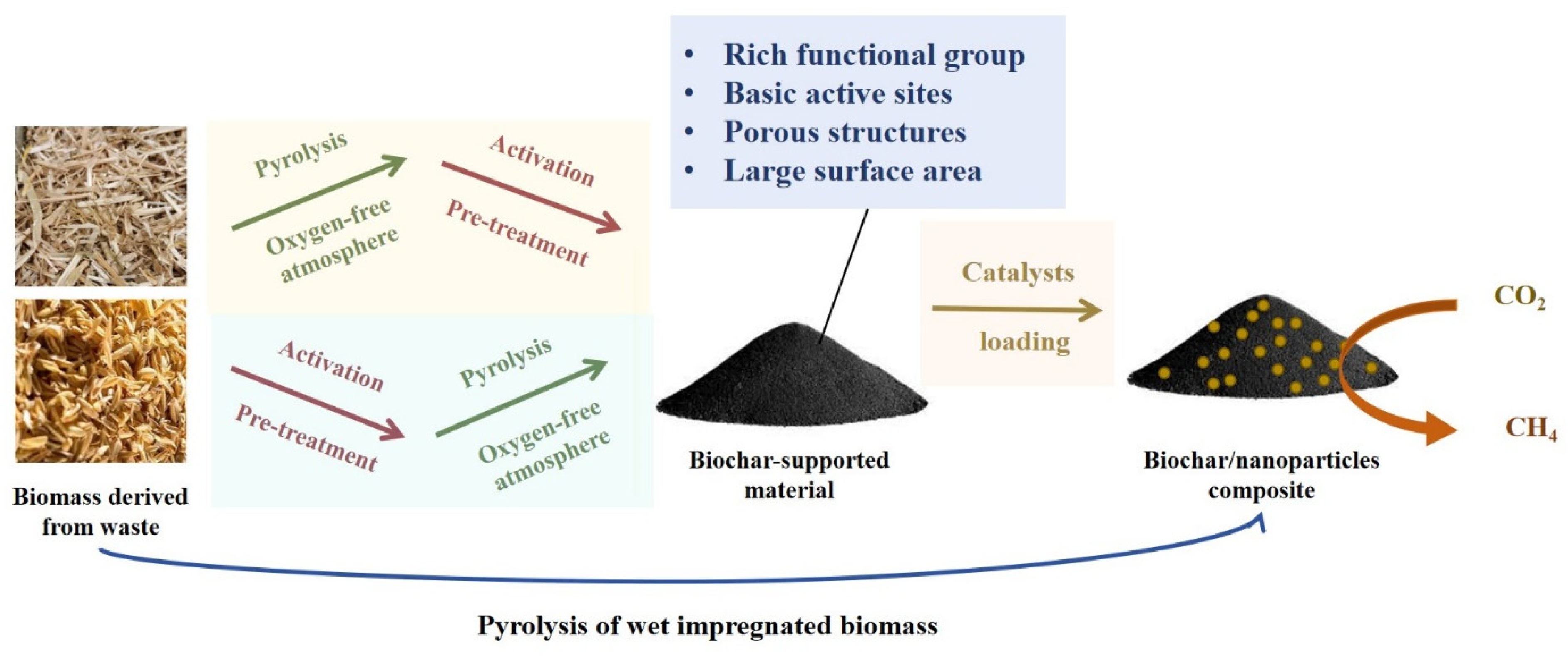
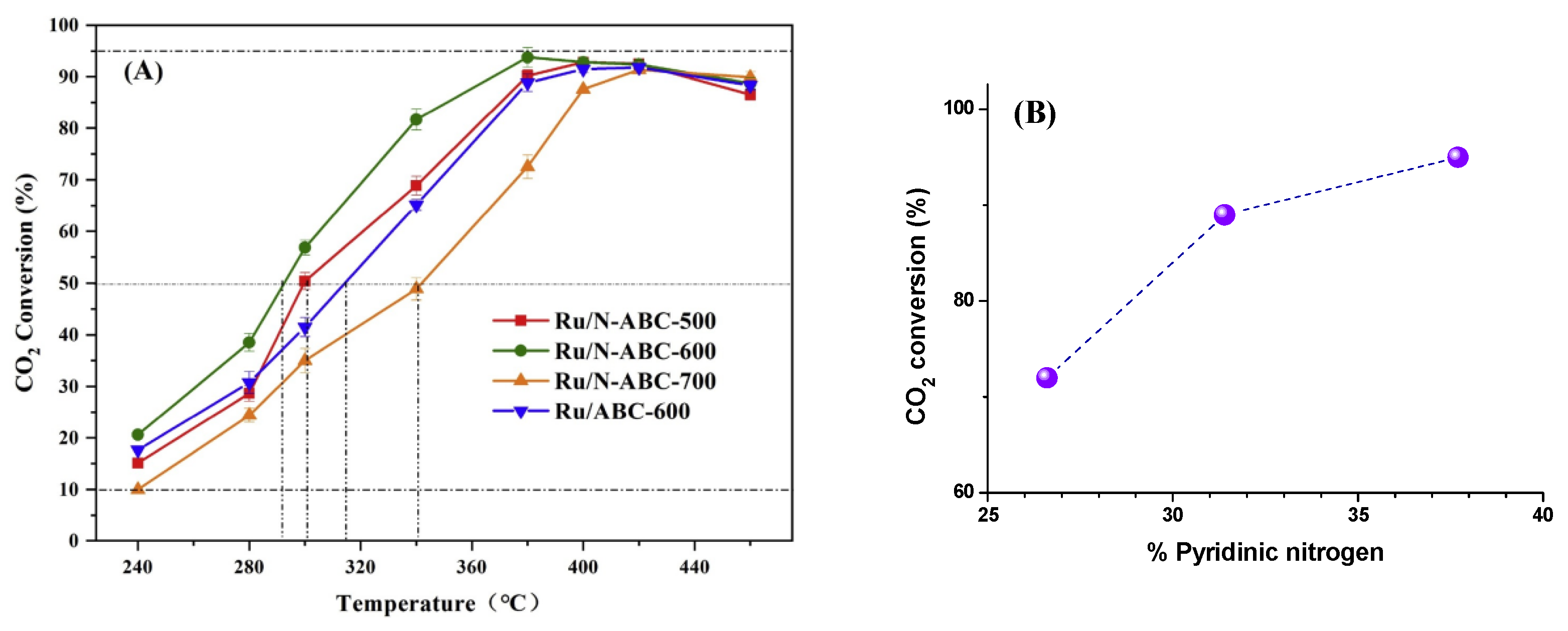


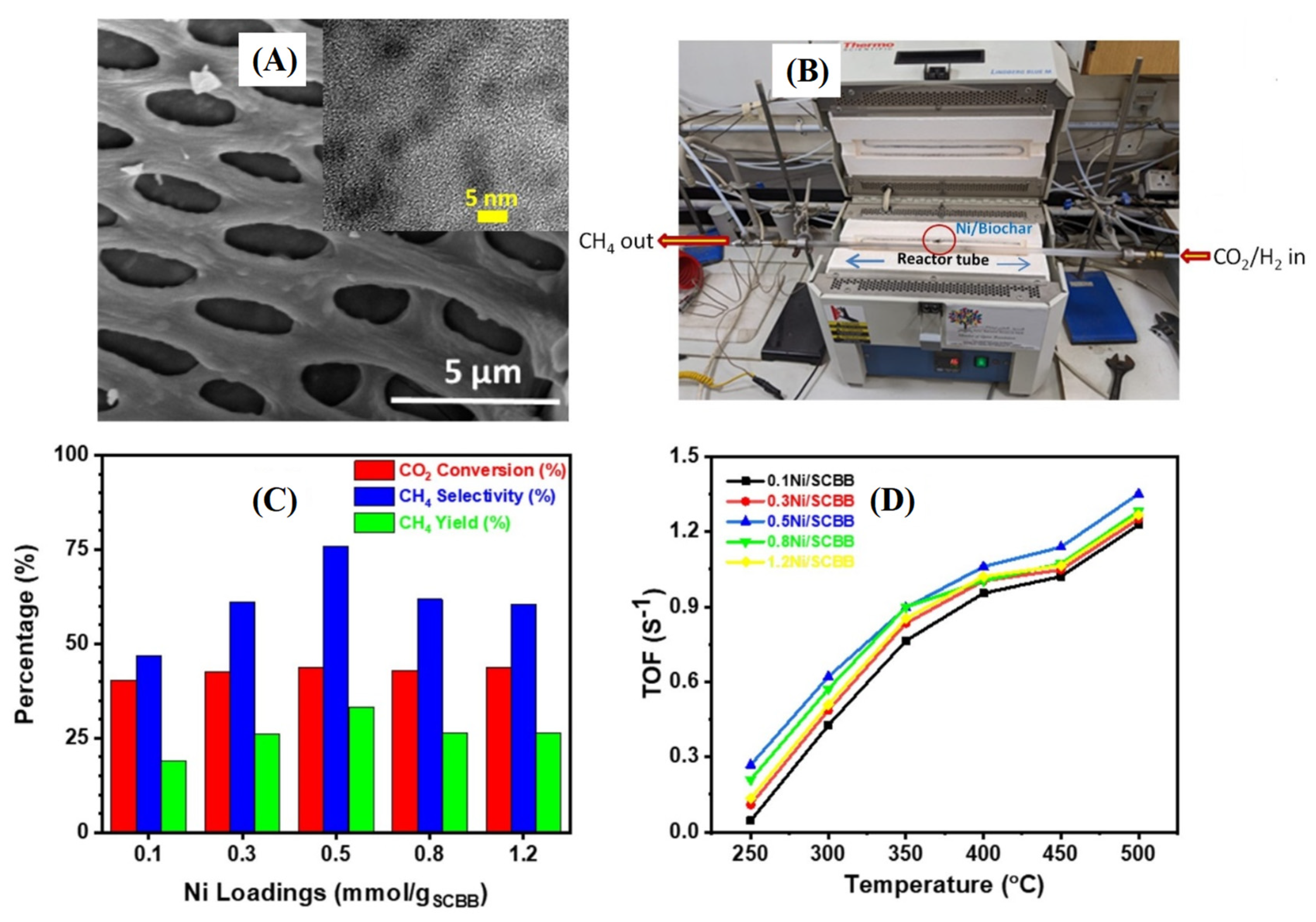
| Biomass | Samples | Synthesis Methods | Surface Area (m2/g) | Total Pore (cm3/g) | Ref. |
|---|---|---|---|---|---|
| White tea waste | Fe3O4/BC | Pyrolysis and co-precipitation | 52.2 | 0.118 | [54] |
| BC | Pyrolysis | 36.6 | 0.0123 | ||
| White tea waste | Biomass without any treatment | 0.196 | 0.0006 | ||
| soybean protein | Activated BC | pre-carbonization and KOH activation | 2788 | 1.65 | [55] |
| BC | pyrolysis | 145 | 0.073 | ||
| Peanut shell | SO3H modified biochar-supported MnO2 | Sulfuric acid pretreatment, pyrolysis, and co-precipitation for loading MnO2 | 52.27 | 0.56 | [56] |
| SO3H modified biochar | Sulfuric acid pretreatment and pyrolysis | 83.39 | 0.10 | ||
| Biochar | pyrolysis | 32.27 | 0.03 | ||
| Corncob-to-xylose residue | BC/MgO | Impregnation and pyrolysis | 407.7 | - | [57] |
| BC | pyrolysis | 232.9 | - | ||
| Rubber seed shell | MgAl/LDH-BC | Impregnation and pyrolysis | 132.40 | 0.0732 | [58] |
| BC | pyrolysis | 114.32 | 0.0617 | ||
| Rice husks | BPC/nano zero-valent iron | liquid-phase reduction of BPC | 1430.37 | 1.303 | [59] |
| BPC | KOH activation and pyrolysis | 1503.65 | 1.315 | ||
| BC/nano zero-valent iron | liquid-phase reduction of BC | 574.64 | 0.370 | ||
| BC | Pyrolysis | 420.61 | 0.310 | ||
| Balsa wood powder | MBC Cu2O | Impregnation of MBC | 30.3436 | 0.070830 | [60] |
| MBC | KOH activation and pyrolysis | 837.6070 | 0.430967 | ||
| BC | pyrolysis | 492.2085 | 0.206011 | ||
| Cu2O | - | 15.7583 | 0.040900 | ||
| Eucalyptus globulus leaves | Ni-Fe/Escott-BEA/BC | Impregnation and pyrolysis | 96 | 5.0 | [61] |
| Ni-Fe/BEA/BC | 97 | 5.1 | |||
| Ni-Fe/Escott | 107 | 0.17 | |||
| BC | Pyrolysis | 74 | 4.8 | ||
| Woody pulp | f-WPB | Water and Microwave treatment followed by pyrolysis and HNO3 | 31.38 | 0.0381 | [62] |
| MoS2-NFs | 27.02 | 0.0727 | |||
| MoS2-NFs/f-WPB | One-pot hydrothermal method | 15.95 | 0.0487 | ||
| Avocado seeds | Biochar | Pretreatment and pyrolysis | 12 | 0.03 | [63] |
| 5 wt% Ca loaded | Precipitation method | 28 | 0.04 | ||
| 10 wt% Ca loaded | 16 | 0.04 | |||
| 20 wt% Ca loaded | 253 | 0.19 | |||
| α-cellulose | S1-CF PC | Microwave | 61.045 | 0.060 | [64] |
| S1-CF C | Microwave | 27.320 | 0.032 | ||
| S1-CF C500 | Pyrolysis | 42.420 | 0.053 |
| Biomass | Biochar/Catalyst (Yield%) | Synthesis Procedure | CO2 Methanation Performance | Refs. |
|---|---|---|---|---|
| Sucrose (Silica gel as a template) | Biochar/Ni-Fe | Biochar was prepared in two steps: pyrolysis at 600 °C in N2 followed by heat treatment again of the resulting biochar at 900 °C. NiFe bimetallic catalyst was prepared from mixture of nickel and iron nitrates. Biochar was wet-impregnated in NiFe solution. The mixture was carbonized at 300 °C for 8 h under H2/N2 atmosphere for the biochar/NiFe. | CO2 conversion: 40% CH4 selectivity: 90% (400 °C under H2:CO2 ratio of 4) | [65] |
| Municipal solid waste (MSW, includes kitchen waste 25 wt%, paper 10 wt%, cloth and fiber 25 wt%, plastic 20 wt%, residue 20 wt%) | Biochar/Ni | A total of 100 g MSW was pyrolyzed at 600 °C in N2 (100 mL/min). A total of 20 g biochar was wet-impregnated into the 200 mL ethanol solution with 24.8 g nickel nitrate. Then, the mixture was calcined in N2 at 400 °C for 2 h and then calcined with H2 at 400 °C for 2 h for biochar/Ni. | CO2 conversion: ≥90% CH4 selectivity: ≥95% (1 MPa and 400 °C for 10 h) | [50] |
| Wheat straw pellets (9 mm OD and 10−13 mm long) | Biochar/Ni-CeO2 | Biochar was produced via a two-step process: pyrolysis of biomass under N2 at 0.1 MPa and 500 °C (heating rate of 5 °C/min), and subsequent physical activation with CO2 at 1.0 MPa and 700 °C. CeO2-doped biochar support (BBCe) was prepared via wet impregnation in Ce(NO3)3⋅6H2O solution and calcination at 500 °C in an Ar atmosphere. Then, the nickel was deposited on BCCe supports via wet impregnation with Ni(NO3)2⋅6H2O solution and calcined in air at 500 °C for biochar/NiCeO2. | CO2 conversion: ≥60% CH4 selectivity: ≥90% (0.1 MPa and 375 °C for 10 h) | [66] |
| Commercial microcrystalline cellulose | Biochar/Pt Biochar/Pt-Na | Pyrolysis of biomass prepared at 500 °C for 2 h (heating rate 10 °C/min) in a reductant flow (1:1 nitrogen/hydrogen, 200 mL·min−1). Pt and Pt-Na-promoted catalysts were prepared by wetness impregnation. The aqueous solution of Pt(NH3)2(NO2)2 alone or with Na2CO3 was dropped on the biochar support, and the mixture was maintained under continuous stirring for 1 h (metal loading 1 wt% for platinum and 5 wt% for sodium). Then, the solid was dried and reduced at 350 °C for 1 h in N2/H2 flow. | CO methanation reaction | [67] |
| Pinus sylvestris | Biochar/Ni-Ce | Pinus sylvestris powder was added into a cerium nitrate solution until dried. The powder product was then ground uniformly with NaHCO3. The mixture was heated up to 600 °C under N2 for 1 h. The black powder was impregnated with HNO3 (0.5 M) and then rinsed with deionized water until the filtered water was neutral. The resulting sample was then dried. The Ce-ABC was added in ethanol within Ni(NO3)2·6H2O. The obtained solid sample was then dried and calcined at 500 °C for 4 h to give biochar/NiCe. | CO2 conversion: 88.6% CH4 selectivity: 92.3% (360 °C and 1 MPa) | [68] |
| Pinus sylvestris | Biochar/Ru-N | Biomass, urea, and NaHCO3 were mixed (with mass ratio of 1:4:3). The mixture was heated at 500–700 °C for 1 h under N2 atmosphere. The biochar was then impregnated with HNO3 and washed with deionized water and then dried to give N-doped biochar. A modified wet-impregnation method was used to prepare a Ru-based catalyst by adding N-doped biochar to ethanol containing RuCl3·xH2O (Ru loading is 3 wt%). The mixture was oil-bath-treated and dried before being calcined at 480 °C for 4 h. | CO2 conversion: 93.8% CH4 selectivity: 99.7% (1 MPa and 460 °C, n(H2)/n(CO2) = 4) | [69] |
| Sugarcane bagasse | Biochar/Ni | Biomass impregnated into aqueous solution with nickel nitrate. The mixture underwent pyrolysis at 500 °C for 1 h under N2 atmosphere to obtain Ni-doped biochar. | CO2 conversion: 44% CH4 selectivity: 76% (1 Mpa and 400 °C) | [24] |
| Surface Area (m2 g−1) | Ni Particle Dispersion (%) | Ni Particle Size (nm) | Basic Site Density (μmol m−2) | CO2 Adsorption (μmol gcat−1) | TOF (103 s−1) | |
|---|---|---|---|---|---|---|
| Ni/Al | 168 | 13 | 8 | 1.89 | 308.5 | 0.81 |
| NiFe/Al | 162 | 18 | 6 | 2.26 | 379.1 | 1.52 |
| Ni/biochar | 754 | 9 | 13 | – | 120.9 | 0.63 |
| NiFe/biochar | 712 | 10 | 11 | 0.2 | 140.8 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Gamal, A.; Bhakta, A.K.; Jlassi, K.; Abdullah, A.M.; Chehimi, M.M. Carbon Dioxide Methanation Enabled by Biochar-Nanocatalyst Composite Materials: A Mini-Review. Catalysts 2024, 14, 155. https://doi.org/10.3390/catal14020155
Tang M, Gamal A, Bhakta AK, Jlassi K, Abdullah AM, Chehimi MM. Carbon Dioxide Methanation Enabled by Biochar-Nanocatalyst Composite Materials: A Mini-Review. Catalysts. 2024; 14(2):155. https://doi.org/10.3390/catal14020155
Chicago/Turabian StyleTang, Mengqi, Ahmed Gamal, Arvind K. Bhakta, Khouloud Jlassi, Aboubakr M. Abdullah, and Mohamed M. Chehimi. 2024. "Carbon Dioxide Methanation Enabled by Biochar-Nanocatalyst Composite Materials: A Mini-Review" Catalysts 14, no. 2: 155. https://doi.org/10.3390/catal14020155
APA StyleTang, M., Gamal, A., Bhakta, A. K., Jlassi, K., Abdullah, A. M., & Chehimi, M. M. (2024). Carbon Dioxide Methanation Enabled by Biochar-Nanocatalyst Composite Materials: A Mini-Review. Catalysts, 14(2), 155. https://doi.org/10.3390/catal14020155