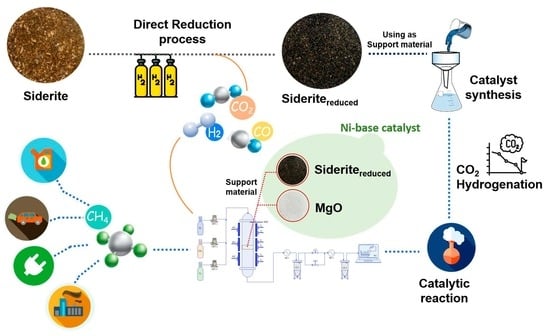
Reduced Siderite Ore Combined with Magnesium Oxide as Support Material for Ni-Based Catalysts; An Experimental Study on CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Effect of Reduced Siderite Ore and MgO
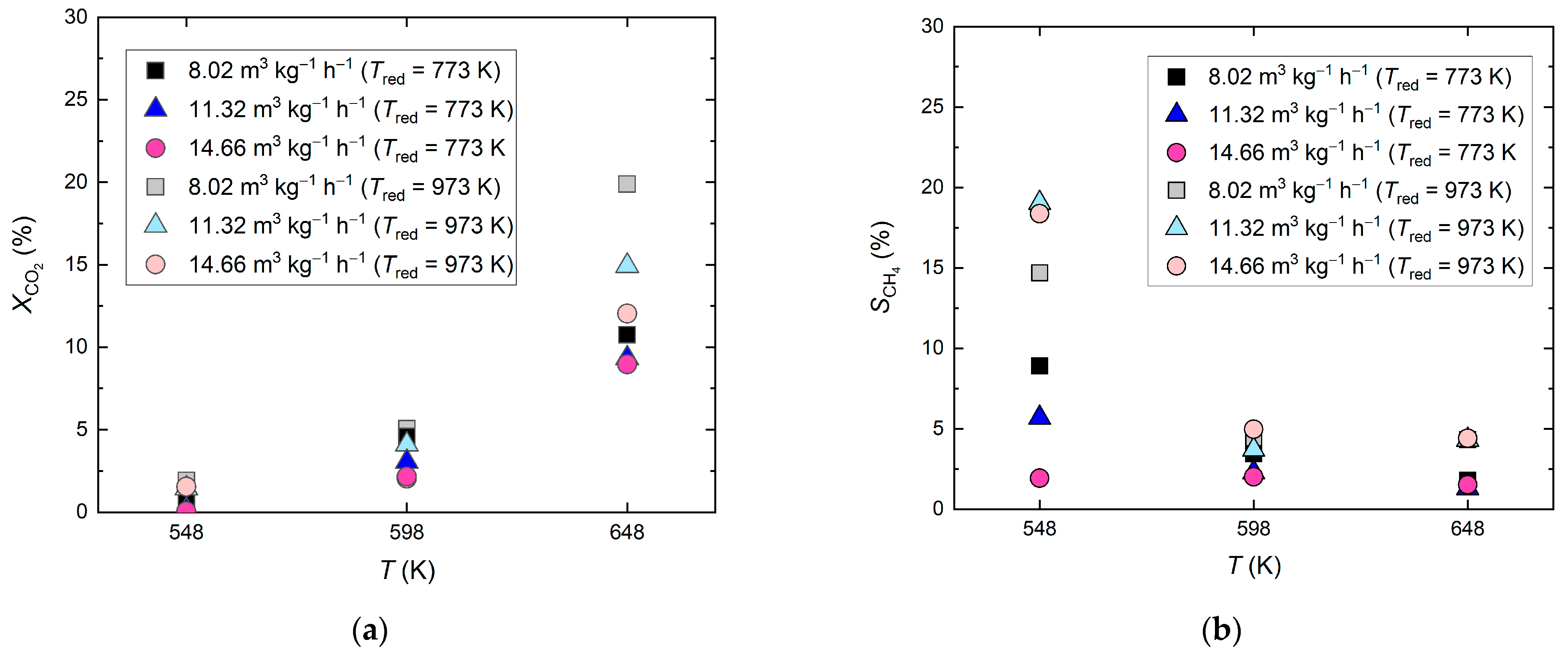
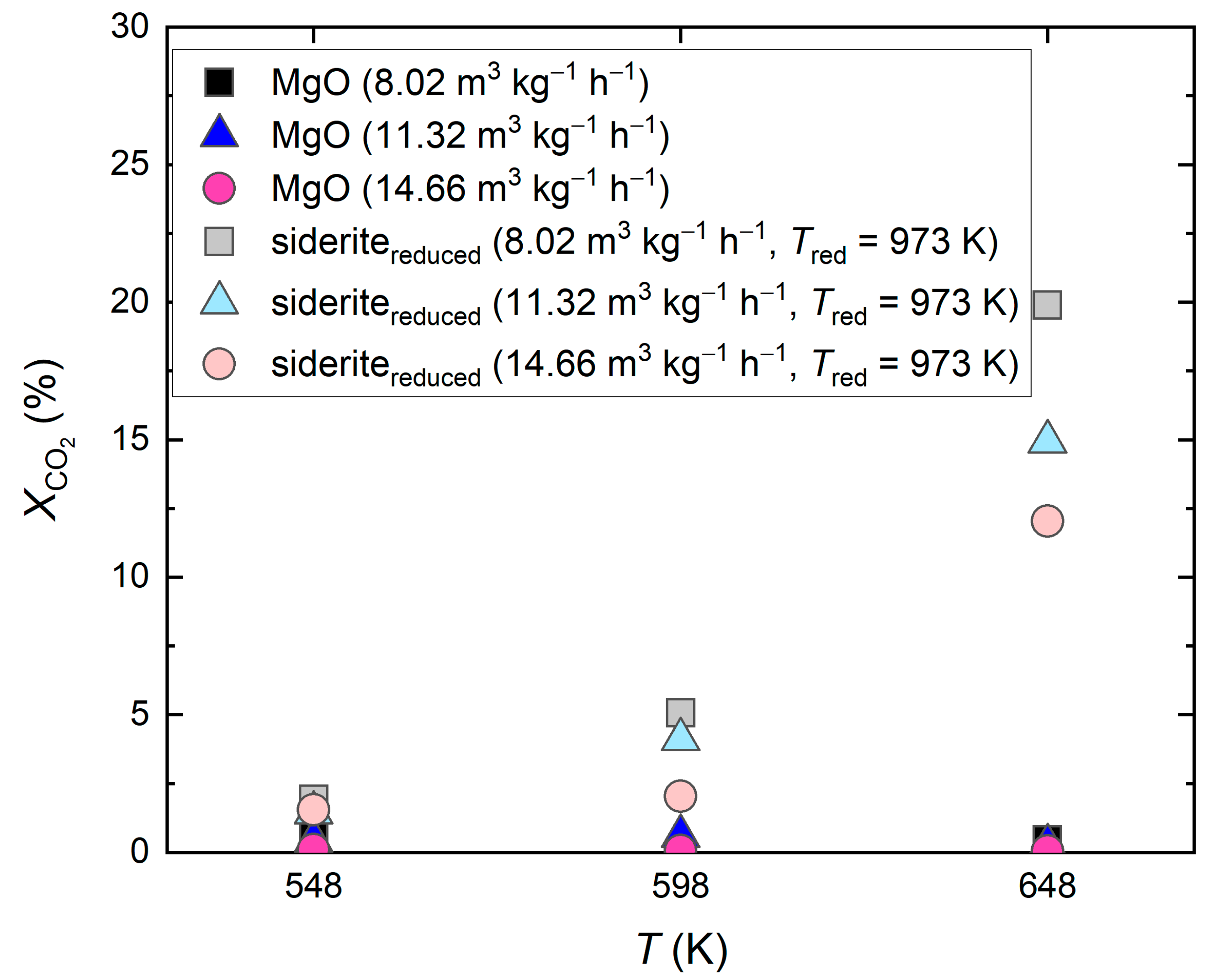
2.1.1. Reduced Siderite Ore as a Catalyst
2.1.2. Undoped MgO
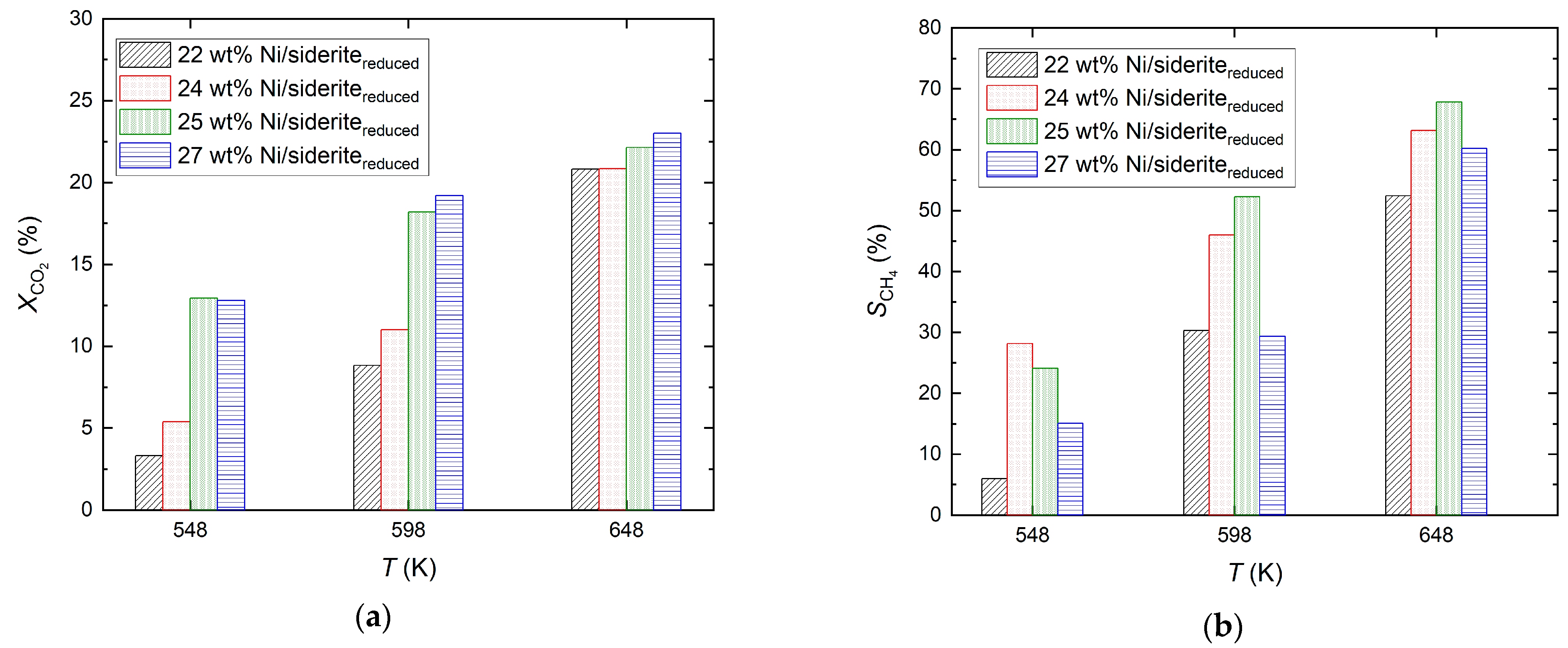
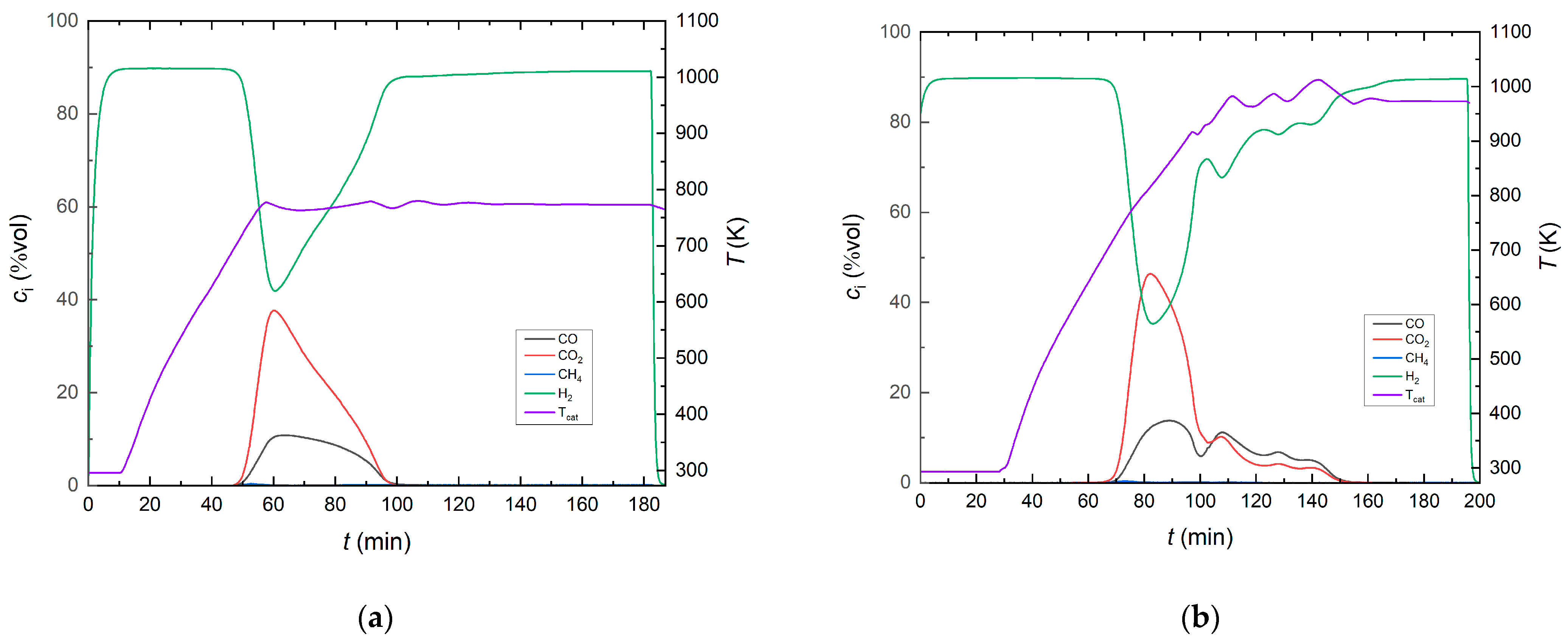
2.2. Ni-Based Catalysts on Reduced Siderite Ore as Support Material
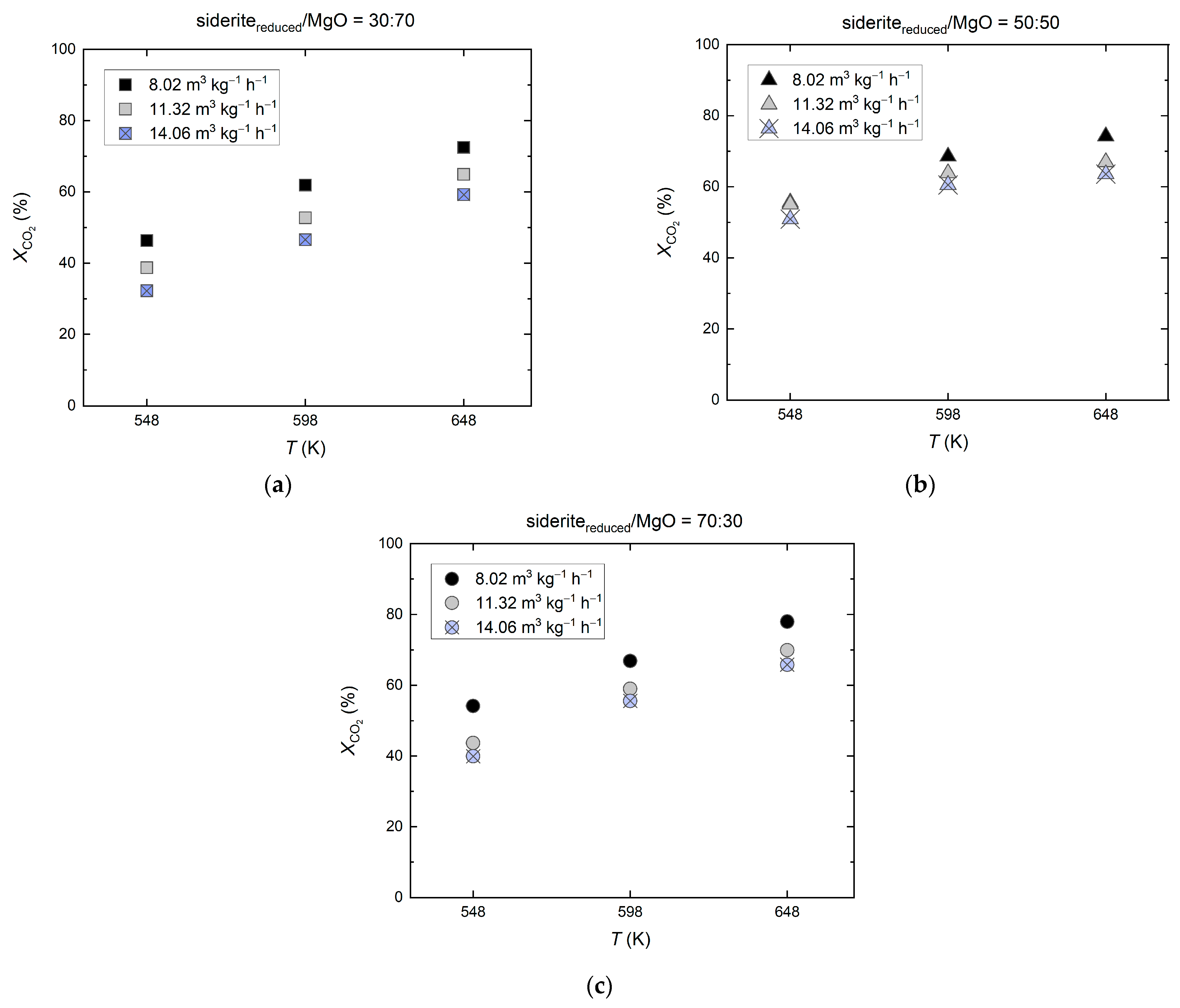
2.3. Mixed Reduced Siderite Ore/Magnesium Oxide as Support Material for Ni-Based Catalysts
2.4. Catalyst Characterization
2.4.1. X-ray Fluorescence Spectrometry
2.4.2. X-ray Diffraction
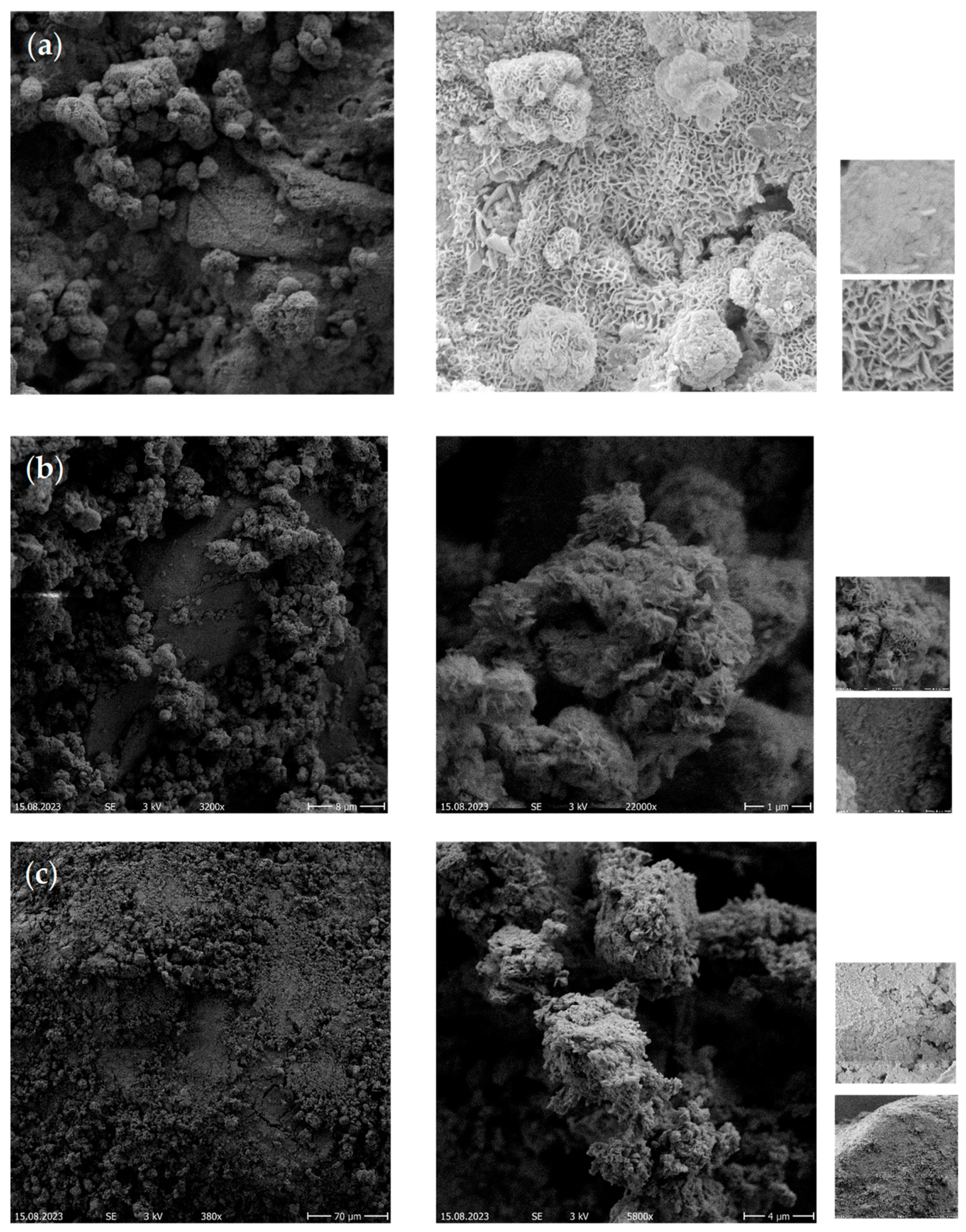
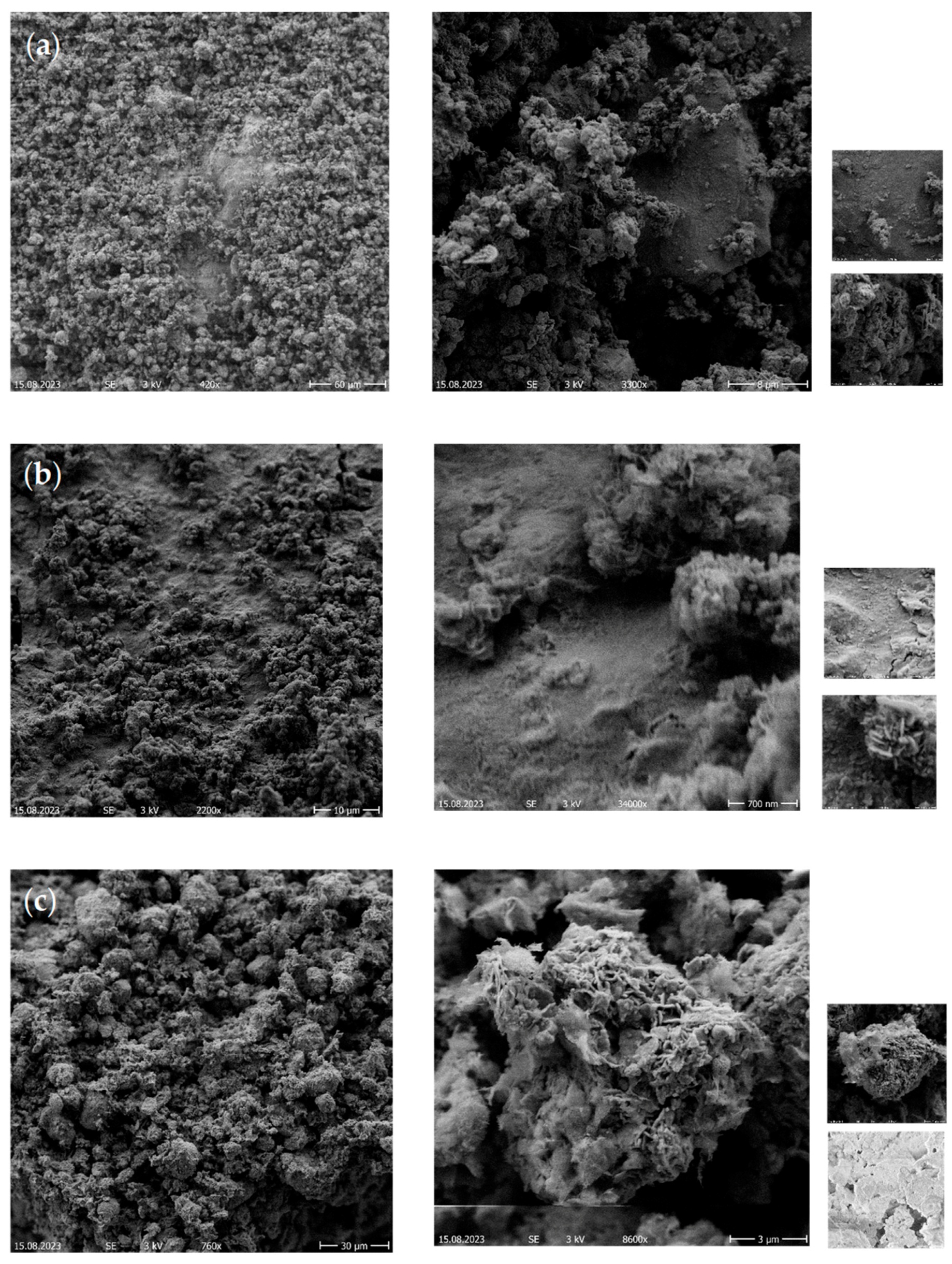
2.4.3. Scanning Electron Microscopy (SEM) Analysis
3. Materials and Methods
3.1. Materials and Catalyst Preparation
- (i)
- (ii)
- impregnation of the support material with nickel nitrate,
- (iii)
- thermal decomposition of nickel nitrate to nickel oxide (Equations (8)–(10)) [71], andNi(NO3)2∙6 H2O ⇌ NiO +2 NO2 + 0.5 O2 + 6 H2OMgO + Ni(NO3)2 → (Mg1−xNix)(OH)2(Mg1−xNix)(OH)2 → Mg1−xNixO + H2O
- (iv)
- (i)
- Preparation of the support material:
- (ii)
- Impregnation:
- (iii)
- Thermal deposition:
- (iv)
- Reduction with hydrogen/activation:
3.2. Siderite Ore Reduction
3.3. Catalyst Characterization
3.3.1. X-ray Diffraction
3.3.2. X-ray Fluorescence Spectrometry
3.3.3. Scanning Electron Microscopy
3.3.4. AAS Analysis
3.4. Experimental Setup and Experimental Procedure of the Methanation Experiments
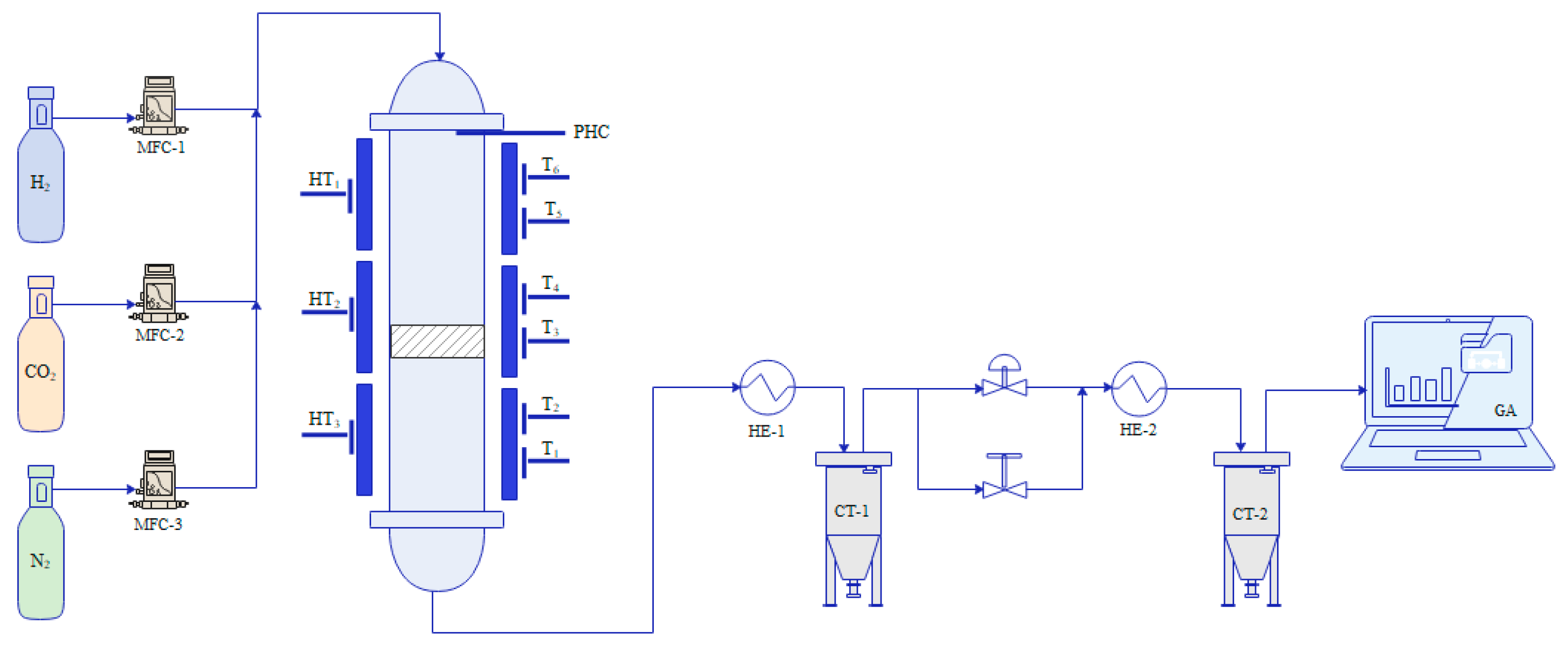
3.4.1. Experimental Setup
3.4.2. Experimental Procedure
3.4.3. Determination of the Chemical Composition of the Reduced Siderite Ore
- (i)
- Measurement of the weight increase in air (equaling the reactivity of the sample in air) and determination of the oxidation state by loss on ignition (LOI, fully oxidized and in a neutral atmosphere);
- (ii)
- Combustion analysis via the Leco method to determine the total (residual) carbon content;
- (iii)
- Selective dissolution of metallic iron from iron oxides in bromine/methanol to determine elemental iron and for the determination of dissolved iron as FeII via the Zimmermann–Reinhardt method;
- (iv)
- Digestion of the filter cake in boiling hydrochloric acid (HCl) to determine bivalent FeII and trivalent FeIII iron using the Zimmermann–Reinhardt method;
- (v)
- X-ray diffraction of the residual elements.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Jinlong, G. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Hao, W.; Vall, M.; Shi, Y.; Wang, Q.; Strømme, M.; Yan, X.; Cheung, O.; Li, R. Novel Ni/MgO Catalysts from Mesoporous MgCO3 for Highly Efficient CO Methanation: Effects of Al and Si Stabilization. ChemRxiv, 2020; preprint. [Google Scholar]
- Park, S.-E.; Yoo, J.S. New CO2 chemistry–Recent advances in utilizing CO2 as an oxidant and current understanding on its role. Carbon Dioxide Utilization for Global Sustainability. In Proceedings of the 7th International Conference on Carbon Dioxide Utilization, Seoul, Republic of Korea, 12–16 October 2004; Elsevier: Amsterdam, The Netherlands, 2004; pp. 303–314. [Google Scholar]
- Downing, C.A.; Sokol, A.A.; Catlow, C.R.A. The reactivity of CO2 on the MgO(100) surface. Phys. Chem. Chem. Phys. 2014, 16, 184–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandey, D.; Ray, K.; Bhardwaj, R.; Bojja, S.; Chary, K.; Deo, G. Promotion of unsupported nickel catalyst using iron for CO2 methanation. Int. J. Hydrog. Energy 2018, 43, 4987–5000. [Google Scholar] [CrossRef]
- Yin, L.; Chen, X.; Sun, M.; Zhao, B.; Chen, J.; Zhang, Q.; Ning, P. Insight into the role of Fe on catalytic performance over the hydrotalcite-derived Ni-based catalysts for CO2 methanation reaction. Int. J. Hydrog. Energy 2022, 47, 7139–7149. [Google Scholar] [CrossRef]
- Huynh, H.L.; Zhu, J.; Zhang, G.; Shen, Y.; Tucho, W.M.; Ding, Y.; Yu, Z. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J. Catal. 2020, 392, 266–277. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Lux, S. The reaction kinetics of CO2 methanation on a bifunctional Ni/MgO catalyst. J. Ind. Eng. Chem. 2020, 85, 196–207. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Aldana, P.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, J.; Du, X.; Wang, P.; Chen, Y.; Zhuang, W.; Song, M.; Sun, L.; Tao, X.; Li, J.; et al. Constructing the highly efficient Ni/ZrO2/SiO2 catalyst by a combustion-impregnation method for low-temperature CO2 methanation. J. Environ. Chem. Eng. 2022, 10, 108476. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, H.; Du, X.; Yao, B.; Wei, S.; Chen, Y.; Zhuang, W.; Yang, H.; Sun, L.; Tao, X.; et al. Highly active Ni/CeO2/SiO2 catalyst for low-temperature CO2 methanation: Synergistic effect of small Ni particles and optimal amount of CeO2. Fuel Process. Technol. 2022, 236, 107418. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Xing, Y.; Xu, S.; Xie, H.; Xiong, K. CO2 hydrogenation to methane over mesoporous Co/SiO2 catalysts: Effect of structure. J. CO2 Util. 2018, 26, 221–229. [Google Scholar] [CrossRef]
- Da Silva, D.C.; Letichevsky, S.; Borges, L.E.; Appel, L.G. The Ni/ZrO2 catalyst and the methanation of CO and CO2. Int. J. Hydrog. Energy 2012, 37, 8923–8928. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Park, J.-N. Bifunctional mechanism of CO2 methanation on Pd-MgO/SiO2 catalyst: Independent roles of MgO and Pd on CO2 methanation. J. Phys. Chem. C 2010, 114, 7128–7131. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Q. Mesostructured cellular foam silica supported bimetallic LaNi1-xCoxO3 catalyst for CO2 methanation. Int. J. Hydrog. Energy 2020, 45, 4417–4426. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, X.; Han, J.; Wang, H.; Ge, Q. Rhenium-promoted selective CO2 methanation on Ni-based catalyst. J. CO2 Util. 2018, 26, 8–18. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Song, M.; Shi, L.; Xu, X.; Du, X.; Chen, Y.; Zhuang, W.; Tao, X.; Sun, L.; Xu, Y. Ni/M/SiO2 catalyst (M=La, Ce or Mg) for CO2 methanation: Importance of the Ni active sites. J. CO2 Util. 2022, 64, 102150. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Wang, X.; Fang, X.; Wang, H.; Xu, X. New insights into CO2 methanation mechanisms on Ni/MgO catalysts by DFT calculations: Elucidating Ni and MgO roles and support effects. J. CO2 Util. 2019, 33, 55–63. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline γ-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Li, J.; Wei, S.; Gao, X.; Wang, P. Combustion-impregnation preparation of Ni/SiO2 catalyst with improved low-temperature activity for CO2 methanation. Int. J. Hydrog. Energy 2021, 46, 20919–20929. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Zhong, Z.; Teo, J.; Gofer, Y.; Gedanken, A. Methanation of Carbon Dioxide on Ni Catalysts on Mesoporous ZrO2 Doped with Rare Earth Oxides. Catal. Lett. 2009, 130, 455–462. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/MgO catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Bette, N.; Thielemann, J.; Schreiner, M.; Mertens, F. Methanation of CO2 over a (Mg,Al)Ox Supported Nickel Catalyst Derived from a (Ni,Mg,Al)-Hydrotalcite-like Precursor. ChemCatChem 2016, 8, 2903–2906. [Google Scholar] [CrossRef]
- Fedoročková, A.; Raschman, P. Effects of pH and acid anions on the dissolution kinetics of MgO. Chem. Eng. J. 2008, 143, 265–272. [Google Scholar] [CrossRef]
- Ho, K.; Jin, S.; Zhong, M.; Vu, A.-T.; Lee, C.-H. Sorption capacity and stability of mesoporous magnesium oxide in post-combustion CO2 capture. Mater. Chem. Phys. 2017, 198, 154–161. [Google Scholar] [CrossRef]
- Takezawa, N.; Terunuma, H.; Shimokawabe, M.; Kobayashib, H. Methanation of carbon dioxide: Preparation of Ni/MgO catalysts and their performance. Appl. Catal. 1986, 23, 291–298. [Google Scholar] [CrossRef]
- Varun, Y.; Sreedhar, I.; Singh, S.A. Highly stable M/NiO–MgO (M = Co, Cu and Fe) catalysts towards CO2 methanation. Int. J. Hydrog. Energy 2020, 45, 28716–28731. [Google Scholar] [CrossRef]
- Baldauf-Sommerbauer, G.; Lux, S.; Aniser, W.; Bitschnau, B.; Letofsky-Papst, I.; Siebenhofer, M. Steady-state and controlled heating rate methanation of CO2 on Ni/MgO in a bench-scale fixed bed tubular reactor. J. CO2 Util. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Suksumrit, K.; Kleiber, S.; Lux, S. The Role of Carbonate Formation during CO2 Hydrogenation over MgO-Supported Catalysts: A Review on Methane and Methanol Synthesis. Energies 2023, 16, 2973. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Promotional effects in alumina and silica supported bimetallic Ni–Fe catalysts during CO2 hydrogenation. J. Mol. Catal. A Chem. 2014, 382, 23–30. [Google Scholar] [CrossRef]
- Franken, T.; Heel, A. Are Fe based catalysts an upcoming alternative to Ni in CO2 methanation at elevated pressure? J. CO2 Util. 2020, 39, 101175. [Google Scholar] [CrossRef]
- Sehested, J.; Larsen, K.E.; Kustov, A.L.; Frey, A.M.; Johannessen, T.; Bligaard, T.; Andersson, M.P.; Nørskov, J.K.; Christensen, C.H. Discovery of technical methanation catalysts based on computational screening. Top. Catal. 2007, 45, 9–13. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.-A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.-D. Potential of an Alumina-Supported Ni3Fe Catalyst in the Methanation of CO2: Impact of Alloy Formation on Activity and Stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Serrer, M.-A.; Kalz, K.F.; Saraҫi, E.; Lichtenberg, H.; Grunwaldt, J.-D. Role of Iron on the Structure and Stability of Ni3.2Fe/Al2O3 during Dynamic CO2 Methanation for P2X Applications. ChemCatChem 2019, 11, 5018–5021. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni–Fe/(Mg,Al)Ox catalysts for CO2 methanation–Tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Jiang, L.; Wang, C. Nickel supported on iron-bearing olivine for CO2 methanation. Int. J. Hydrog. Energy 2016, 41, 12910–12919. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Da Diniz Costa, J.C. Influence of water on high-temperature CO2 capture using layered double hydroxide derivatives. Ind. Eng. Chem. Res. 2008, 47, 2630–2635. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Synthesis of nanocrystalline mesoporous Ni/Al2O3 single bond SiO2 catalysts for CO2 methanation reaction. Int. J. Hydrog. Energy 2018, 43, 19038–19046. [Google Scholar] [CrossRef]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Seo, J.G.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Al2O3 catalysts prepared by a coprecipitation method: Effect of precipitation agent. J. Ind. Eng. Chem. 2013, 19, 2016–2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Liu, Q. Green synthesis of MCM-41 derived from renewable biomass and construction of VOx-modified nickel phyllosilicate catalyst for CO2 methanation. Int. J. Hydrog. Energy 2021, 46, 32003–32016. [Google Scholar] [CrossRef]
- Gao, W.; Meng, X.; Jin, D.; Xu, B.; Dai, W.; Zhao, R.; Xin, Z. Polyol-pretreated SBA-16 supported Ni-Fe bimetallic catalyst applied in CO methanation at low temperature. Mol. Catal. 2021, 512, 111769. [Google Scholar] [CrossRef]
- González-Castaño, M.; Navarro de Miguel, J.C.; Boelte, J.-H.; Centeno, M.A.; Klepel, O.; Arellano-García, H. Assessing the impact of textural properties in Ni–Fe catalysts for CO2 methanation performance. Microporous Mesoporous Mater. 2021, 327, 111405. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Kumar Prabhakar, J.; Apte, P.A.; Deo, G. The kinetics of Ni/Al2O3 and Ni-Fe/Al2O3 catalysts for the CO2 methanation reaction and the reasons for promotion. Chem. Eng. J. 2023, 471, 144252. [Google Scholar] [CrossRef]
- Lan, P.-W.; Wang, C.-C.; Chen, C.-Y. Effect of Ni/Fe ratio in Ni–Fe catalysts prepared under external magnetic field on CO2 methanation. J. Taiwan Inst. Chem. Eng. 2021, 127, 166–174. [Google Scholar] [CrossRef]
- Tsuji, M.; Kodama, T.; Yoshida, T.; Kitayama, Y.; Tamaura, Y. Preparation and CO2 methanation activity of an ultrafine Ni(II) ferrite catalyst. J. Catal. 1996, 164, 315–321. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Sdiri, A.; Gálvez, M.; Zidi, H.; Da Costa, P.; Ben Zina, M. Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering 2018, 2, 29. [Google Scholar] [CrossRef]
- Wei, Y.; Gui, K.; Liu, X.; Liang, H.; Gu, S.; Ren, D. Performance of Mn-Ce co-doped siderite catalysts in the selective catalytic reduction of NOx by NH3. J. Fuel Chem. Teshimachnol. 2019, 47, 1495–1503. [Google Scholar] [CrossRef]
- Görmez, Ö.; Saçlı, B.; Çağlayan, U.; Kalderis, D.; Gözmen, B. Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone. Molecules 2022, 27, 8056. [Google Scholar] [CrossRef]
- Boehm, A.; Boehm, M.; Kogelbauer, A. Neutrons for Mineral Processing–Thermo Diffractometry to Investigate Mineral Selective Magnetizing Flash Roasting. Chem. Ing. Tech. 2014, 86, 883–890. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, L.; Peng, J.; Hu, T.; Yang, L. Microwave roasting of siderite and the catalytic combustion effects on anthracite. Appl. Therm. Eng. 2017, 117, 668–674. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Böhm, A.; Lux, S. Clean iron production through direct reduction of mineral iron carbonate with low-grade hydrogen sources; the effect of reduction feed gas composition on product and exit gas composition. Clean. Eng. Technol. 2021, 5, 100345. [Google Scholar] [CrossRef]
- Loder, A.; Santner, S.; Siebenhofer, M.; Böhm, A.; Lux, S. Reaction kinetics of direct reduction of mineral iron carbonate with hydrogen: Determination of the kinetic triplet. Chem. Eng. Res. Des. 2022, 188, 575–589. [Google Scholar] [CrossRef]
- Lux, S.; Baldauf-Sommerbauer, G.; Ottitsch, B.; Loder, A.; Siebenhofer, M. Iron Carbonate Beneficiation Through Reductive Calcination-Parameter Optimization to Maximize Methane Formation. Eur. J. Inorg. Chem. 2019, 2019, 1748–1758. [Google Scholar] [CrossRef]
- Bock, S.; Pauritsch, M.; Lux, S.; Hacker, V. Natural iron ores for large-scale thermochemical hydrogen and energy storage. Energy Convers. Manag. 2022, 267, 115834. [Google Scholar] [CrossRef]
- Lux, S.; Baldauf-Sommerbauer, G.; Siebenhofer, M. Hydrogenation of Inorganic Metal Carbonates: A Review on Its Potential for Carbon Dioxide Utilization and Emission Reduction. ChemSusChem 2018, 11, 3357–3375. [Google Scholar] [CrossRef]
- Reller, A.; Emmenegger, R.; Padeste, C.; Oswald, H.-R. Thermochemical Reactivity of Metal Carbonates. Chimia 1991, 45, 262. [Google Scholar] [CrossRef]
- Kleiber, S.; Loder, A.; Siebenhofer, M.; Böhm, A.; Lux, S. Direct reduction of siderite ore combined with catalytic CO/CO2 hydrogenation to methane and methanol: A technology concept. Chem. Ing. Tech. 2022, 94, 701–711. [Google Scholar] [CrossRef]
- Baldauf-Sommerbauer, G.; Lux, S.; Wagner, J.; Siebenhofer, M. Determination of the kinetic triplet by an isoconversional and a regression method applied to the decomposition of mineral iron carbonate in nitrogen. Thermochim. Acta 2017, 649, 1–12. [Google Scholar] [CrossRef]

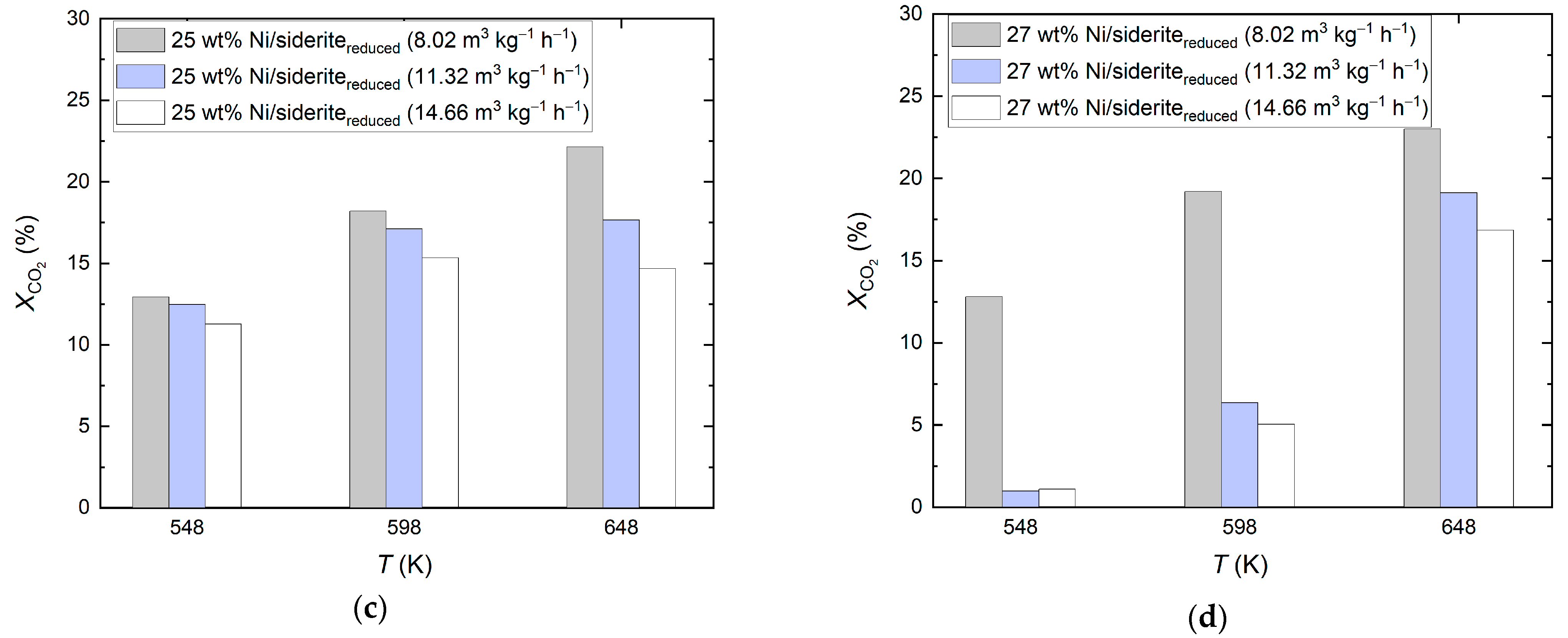


| Catalyst Composition | Preparation Method | Operation Conditions | Performance | Ref. |
|---|---|---|---|---|
| Ni/MgO (wNi = 0–27 wt%) | wet impregnation | T = 533–648 K GHSV = 3.7 m3 kg−1 h−1 H2:CO2:N2 = 4:1:5 | = 87% = 99% | [9] |
| Mg-Al-CO3 LDH 1 catalyst | coprecipitation | T = 473–573 K CO2:O2:N2 = 14:4:82 | CO2 sorption: 2.72% (dry sorption), 3.14% (wet condition, 12% water) | [48] |
| Ni/CeO2 | wet impregnation | T = 573 K F = 3000 mL min−1 | > 90% > 99.5% | [49] |
| Ni/Al2O3SiO2 | sol-gel | T = 625 K H2:CO2 = 3.5:1 GHSV = 12,000 mL g−1 h−1 | = 77% = 98% | [50] |
| Ni/ZrO2, Ni-K/ZrO2 and Ni-La/ZrO2 | wetness impregnation | T = 523–723 K H2:CO2 = 12.5:1 F = 50–100 mL min−1 | = 20–60% = 89–99% | [51] |
| Ni-FeAl-(NH4)2CO3 | co-precipitation | T = 493 K CO2:H2:N2 = 1:4:1.7 WHSV = 9600 mL g−1 h−1 | = 58.5% = 64% | [52] |
| Ni/MCM-41 with VOx-modified | T = 673 K WHSV = 60,000 mL g−1 h−1 | = 81.4% = 72.8% | [53] | |
| Ni-Fe/S16 | mesoporous silica molecular sieve | T = 473–573 K H2:CO:N2 = 3:1:1 WHSV = 15,000 mL g−1 h−1 | XCO = 100% (at 503 K) > 90% | [54] |
| Ni-Fe/olivine ((MgxFe1−x)2 SiO4) | wet impregnation | T = 673 K H2:CO2 = 6:1 GHSV = 11,000 h−1 | = 98% = 99% | [47] |
| 1−10 wt% Fe/13X | wet impregnation | T = 473–823 K P = 1–15 bar | = 74% (T = 823 K, P = 15 bar) = 76% (P = 5–15 bar) | [42] |
| 10Ni–Fe/CA-C 2 | wet impregnation | T = 473–773 K H2:CO2 = 4:1 GHSV = 12,000 h−1 | = 5–74% > 90% | [55] |
| γ-Fe2O3(n) and | commercial from Sigma-Aldrich | T = 523–723 K H2:CO2 = 4:1 F = 500 mL min−1 GHSV = 120,000 h−1 | = 45–65% (65% at 723 K) = 45–77% (77% at 623 K) | [56] |
| α-Fe2O3(PVA) | polyvinyl alcohol (PVA) route | T = 523–723 K H2:CO2 = 4:1 F = 500 mL min−1 GHSV = 120,000 h−1 | = 96% (723 K) = 11% (673 K) |
| Component | wt% |
|---|---|
| Fe | 33.56 |
| CaO | 6.88 |
| MgO | 3.72 |
| Mn | 1.91 |
| SiO2 | 5.19 |
| Al2O3 | 1.02 |
| others (including CO2) | 47.72 |
| Component | wt% |
|---|---|
| Siderite | 79.04 |
| Calcite | 8.91 |
| Quartz | 5.19 |
| Ankerite | 3.96 |
| Dolomite | 1.80 |
| others (including CO2) | 47.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suksumrit, K.; Hauzenberger, C.A.; Santitharangkun, S.; Lux, S. Reduced Siderite Ore Combined with Magnesium Oxide as Support Material for Ni-Based Catalysts; An Experimental Study on CO2 Methanation. Catalysts 2024, 14, 206. https://doi.org/10.3390/catal14030206
Suksumrit K, Hauzenberger CA, Santitharangkun S, Lux S. Reduced Siderite Ore Combined with Magnesium Oxide as Support Material for Ni-Based Catalysts; An Experimental Study on CO2 Methanation. Catalysts. 2024; 14(3):206. https://doi.org/10.3390/catal14030206
Chicago/Turabian StyleSuksumrit, Kamonrat, Christoph A. Hauzenberger, Srett Santitharangkun, and Susanne Lux. 2024. "Reduced Siderite Ore Combined with Magnesium Oxide as Support Material for Ni-Based Catalysts; An Experimental Study on CO2 Methanation" Catalysts 14, no. 3: 206. https://doi.org/10.3390/catal14030206
APA StyleSuksumrit, K., Hauzenberger, C. A., Santitharangkun, S., & Lux, S. (2024). Reduced Siderite Ore Combined with Magnesium Oxide as Support Material for Ni-Based Catalysts; An Experimental Study on CO2 Methanation. Catalysts, 14(3), 206. https://doi.org/10.3390/catal14030206