Improved Structural Description of Different γ-Al2O3 Materials Using Disordered δ5-Al2O3 Phase via X-ray Pair Distribution Function Analysis
Abstract
:1. Introduction
2. Results and Discussion
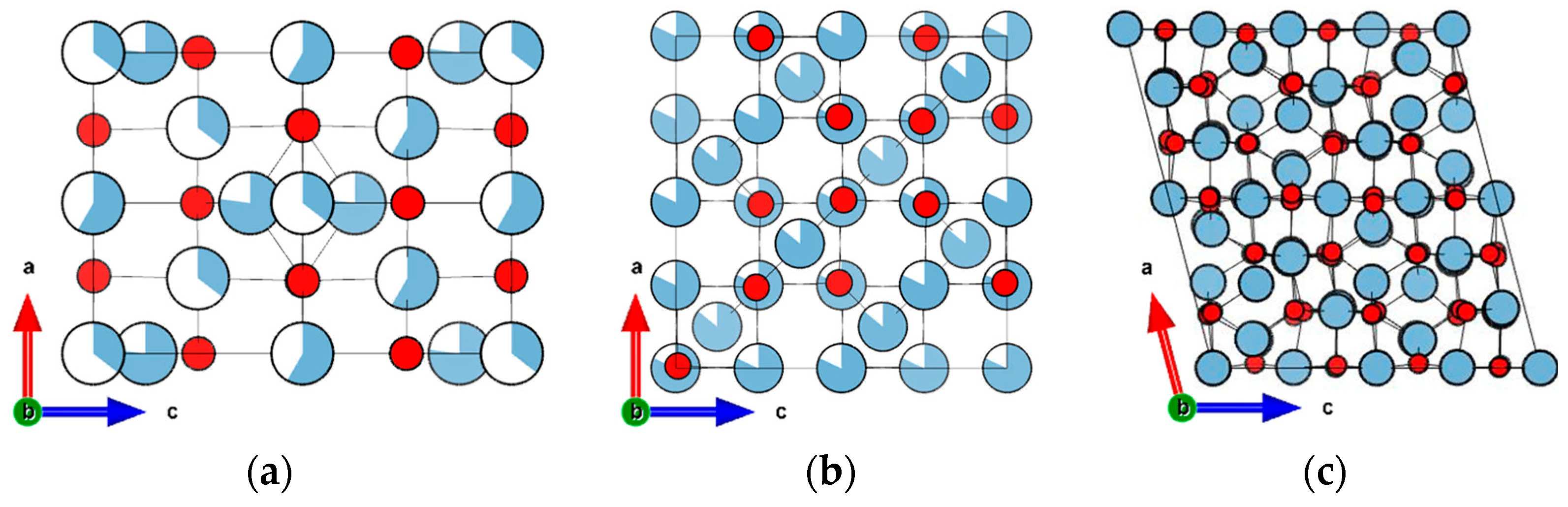
2.1. Improved Description of γ-Al2O3 Materials with δ5 Phase
2.2. Supported Catalysts—Ni/Al2O3
3. Experimental Section
3.1. Synthesis of Ni/Al2O3
3.2. Characterization
3.3. X-ray Total Scattering Experiments
3.4. PDF Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busca, G.; Spennati, E.; Riani, P.; Garbarino, G. Looking for an Optimal Composition of Nickel-Based Catalysts for CO2 Methanation. Energies 2023, 16, 5304. [Google Scholar] [CrossRef]
- Hu, F.; Ye, R.; Lu, Z.-H.; Zhang, R.; Feng, G. Structure–Activity Relationship of Ni-Based Catalysts toward CO2 Methanation: Recent Advances and Future Perspectives. Energy Fuels 2022, 36, 156–169. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ahn, J.Y.; Nguyen, D.D.; Chang, S.W.; Kim, S.S.; Lee, S.M. Role of oxide support in Ni based catalysts for CO2 methanation. RSC Adv. 2021, 11, 17648–17657. [Google Scholar] [CrossRef]
- Rascón, F.; Wischert, R.; Copéret, C. Molecular nature of support effects in single-site heterogeneous catalysts: Silica vs. alumina. Chem. Sci. 2011, 2, 1449. [Google Scholar] [CrossRef]
- Candia, R.; Sørensen, O.; Villadsen, J.; Topsøe, N.Y.; Clausen, B.S.; Topsøe, H. Effect of Sulfiding Temperature on Activity and Structures of CO-MO/Al2O3 Catalysts. ii. Bull. Sociétés Chim. Belg. 1984, 1984, 763–774. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, Q.; Li, W. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La–Al2O3 catalysts for NH3 decomposition. Appl. Catal. A Gen. 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Centeno, M.A.; Hadjiivanov, K.; Venkov, T.; Klimev, H.; Odriozola, J.A. Comparative study of Au/Al2O3 and Au/CeO2-Al2O3 catalysts. J. Mol. Catal. A Chem. 2006, 252, 142–149. [Google Scholar] [CrossRef]
- Weber, S.; Zimmermann, R.T.; Bremer, J.; Abel, K.L.; Poppitz, D.; Prinz, N.; Ilsemann, J.; Wendholt, S.; Yang, Q.; Pashminehazar, R.; et al. Digitization in Catalysis Research: Towards a Holistic Description of a Ni/Al2O3 Reference Catalyst for CO2 Methanation. ChemCatChem 2022, 14, e202101878. [Google Scholar] [CrossRef]
- Mutz, B.; Gänzler, A.; Nachtegaal, M.; Müller, O.; Frahm, R.; Kleist, W.; Grunwaldt, J.-D. Surface Oxidation of Supported Ni Particles and Its Impact on the Catalytic Performance during Dynamically Operated Methanation of CO2. Catalysts 2017, 7, 279. [Google Scholar] [CrossRef]
- Prins, R. Location of the Spinel Vacancies in γ-Al2O3. Angew. Chem. Int. Ed Engl. 2019, 58, 15548–15552. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Javier Garcia-Garcia, F.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Sun, M.; Nelson, A.E.; Adjaye, J. Examination of spinel and nonspinel structural models for gamma-Al2O3 by DFT and rietveld refinement simulations. J. Phys. Chem. B 2006, 110, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.J.W. The Crystal Structure of γ-Fe2O3 and γ-Al2O3. Z. Für Krist.-Cryst. Mater. 1935, 91, 65–69. [Google Scholar] [CrossRef]
- Ushakov, V.A.; Moroz, E.M. Structure of low-temperature γ- and η-Al2O3. React. Kinet. Catal. Lett. 1984, 24, 113–118. [Google Scholar] [CrossRef]
- Zhou, R.-S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Rudolph, M.; Motylenko, M.; Rafaja, D. Structure model of γ-Al2O3 based on planar defects. IUCrJ 2019, 6, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Smrcok, L.; Langer, V.; Krestan, J. Gamma-alumina: A single-crystal X-ray diffraction study. Acta Crystallogr. C 2006, 62, i83–i84. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Božin, E.S.; Billinge, S.J.L. Fine-Scale Nanostructure in γ-Al2O3. Chem. Mater. 2006, 18, 3242–3248. [Google Scholar] [CrossRef]
- Saalfeed, H. The Dehydration of Gibbsite and the Structure of a Tetragonal γ-Al2O3. Clay Miner. 1958, 3, 249–257. [Google Scholar] [CrossRef]
- Wilson, S.J. The dehydration of boehmite, γ-AlOOH, to γ-Al2O3. J. Solid State Chem. 1979, 30, 247–255. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hunter, B.A.; Hart, R.D.; Hanna, J.V.; Byrne, L.T. Tetragonal structure model for boehmite-derived γ-alumina. Phys. Rev. B 2003, 68, 144110. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Kocaefe, Y.; Cheng, J.; Liu, W. The Effect of Novel Synthetic Methods and Parameters Control on Morphology of Nano-alumina Particles. Nanoscale Res. Lett. 2016, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Billinge, S.J.L.; Kanatzidis, M.G. Beyond crystallography: The study of disorder, nanocrystallinity and crystallographically challenged materials with pair distribution functions. Chem. Commun. 2004, 749–760. [Google Scholar] [CrossRef]
- Billinge, S.J.L. Nanostructure studied using the atomic pair distribution function. Z. Kristallogr. Suppl. 2007, 26, 17–26. [Google Scholar] [CrossRef]
- Newton, M.A.; Di Michiel, M.; Ferri, D.; Fernàndez-Garcia, M.; Beale, A.M.; Jacques, S.D.M.; Chupas, P.J.; Chapman, K.W. Catalytic Adventures in Space and Time Using High Energy X-rays. Catal Surv Asia 2014, 18, 134–148. [Google Scholar] [CrossRef]
- Zimmerli, N.K.; Müller, C.R.; Abdala, P.M. Deciphering the structure of heterogeneous catalysts across scales using pair distribution function analysis. Trends Chem. 2022, 4, 807–821. [Google Scholar] [CrossRef]
- Chupas, P.J.; Chapman, K.W.; Chen, H.; Grey, C.P. Application of high-energy X-rays and Pair-Distribution-Function analysis to nano-scale structural studies in catalysis. Catal. Today 2009, 145, 213–219. [Google Scholar] [CrossRef]
- Schlicher, S.; Prinz, N.; Bürger, J.; Omlor, A.; Singer, C.; Zobel, M.; Schoch, R.; Lindner, J.K.N.; Schünemann, V.; Kureti, S.; et al. Quality or Quantity? How Structural Parameters Affect Catalytic Activity of Iron Oxides for CO Oxidation. Catalysts 2022, 12, 675. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Khivantsev, K.; Kwak, J.H.; Szanyi, J. Structural complexity of γ-Al2O3: The nature of vacancy ordering and the structure of complex antiphase boundaries. Acta Mater. 2024, 266, 119639. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Shi, D.; Szanyi, J.; Peden, C.H.F. Structural Intergrowth in δ-Al2O3. J. Phys. Chem. C 2019, 123, 9454–9460. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Shi, D.; Washton, N.M.; Andersen, A.; Hu, J.Z.; Lee, J.; Szanyi, J.; Kwak, J.-H.; Peden, C.H.F. Unraveling the Origin of Structural Disorder in High Temperature Transition Al2O3: Structure of θ-Al2O3. Chem. Mater. 2015, 27, 7042–7049. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Genc, A.; Szanyi, J.; Peden, C.H.F.; Kwak, J.H. Structure of δ-Alumina: Toward the Atomic Level Understanding of Transition Alumina Phases. J. Phys. Chem. C 2014, 118, 18051–18058. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J.-D. Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions. ChemCatChem 2017, 9, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Zhou, X.D. Computer-assisted area detector masking. J. Synchrotron Radiat. 2017, 24, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Juhás, P.; Davis, T.; Farrow, C.L.; Billinge, S.J.L. PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef]
- Juhás, P.; Farrow, C.L.; Yang, X.; Knox, K.R.; Billinge, S.J.L. Complex modeling: A strategy and software program for combining multiple information sources to solve ill posed structure and nanostructure inverse problems. Acta Crystallogr. A Found. Adv. 2015, 71, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.F.; Božin, E.S.; Proffen, T.; Billinge, S.J.L. Improved measures of quality for the atomic pair distribution function. J Appl. Crystallogr. 2003, 36, 53–64. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoni, F.; Fahl, B.M.D.; Zobel, M. Improved Structural Description of Different γ-Al2O3 Materials Using Disordered δ5-Al2O3 Phase via X-ray Pair Distribution Function Analysis. Catalysts 2024, 14, 238. https://doi.org/10.3390/catal14040238
Manzoni F, Fahl BMD, Zobel M. Improved Structural Description of Different γ-Al2O3 Materials Using Disordered δ5-Al2O3 Phase via X-ray Pair Distribution Function Analysis. Catalysts. 2024; 14(4):238. https://doi.org/10.3390/catal14040238
Chicago/Turabian StyleManzoni, Fabio, Benjamin M. D. Fahl, and Mirijam Zobel. 2024. "Improved Structural Description of Different γ-Al2O3 Materials Using Disordered δ5-Al2O3 Phase via X-ray Pair Distribution Function Analysis" Catalysts 14, no. 4: 238. https://doi.org/10.3390/catal14040238






