Valorization of Reground Pasta By-Product through PHA Production with Phototrophic Purple Bacteria
Abstract
:1. Introduction
2. Results
2.1. RP Fermented Solution
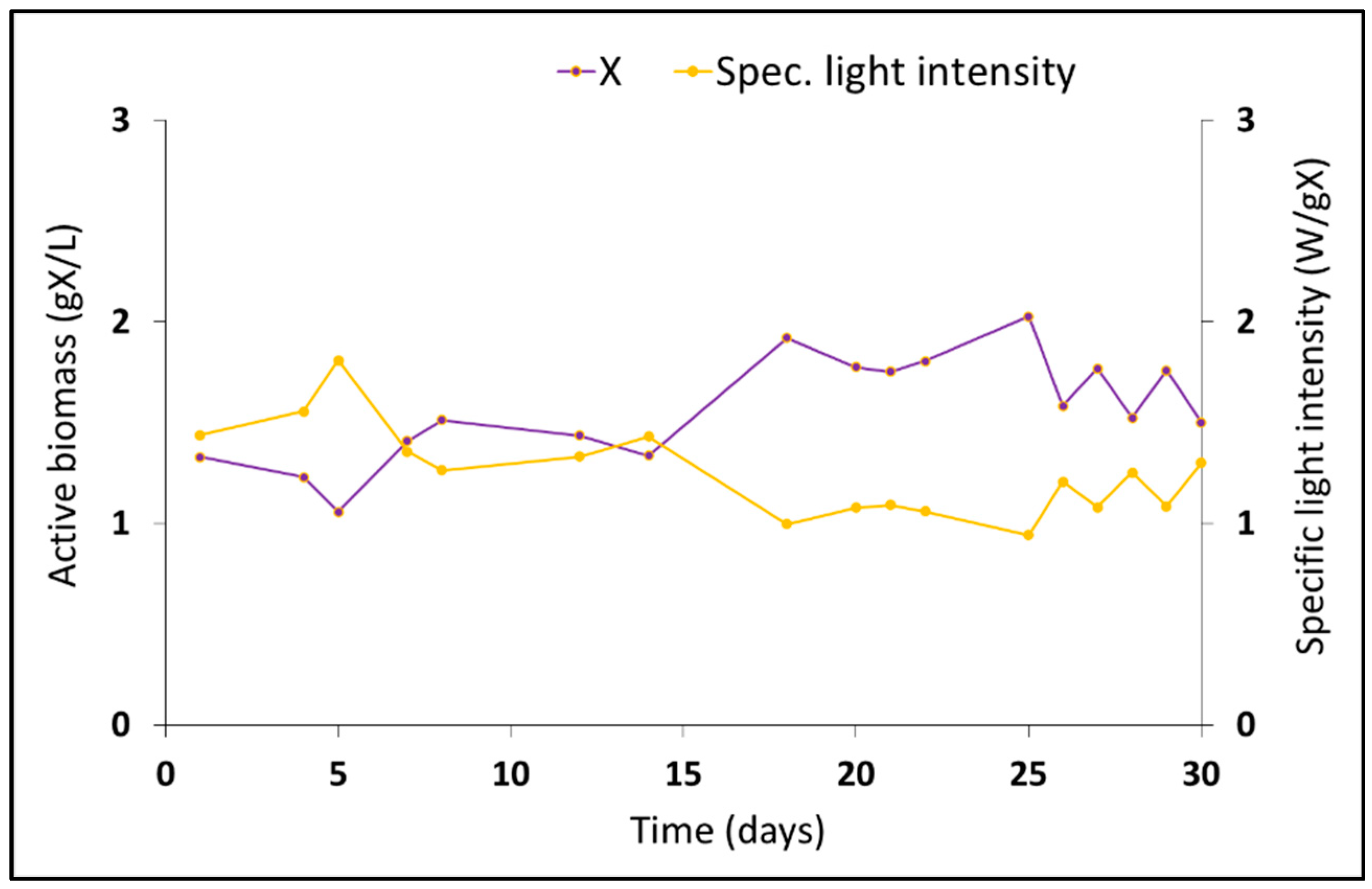
2.2. Operation of the Bioreactor with Phototrophic Purple Bacteria
2.3. PPB Microbial Characterization
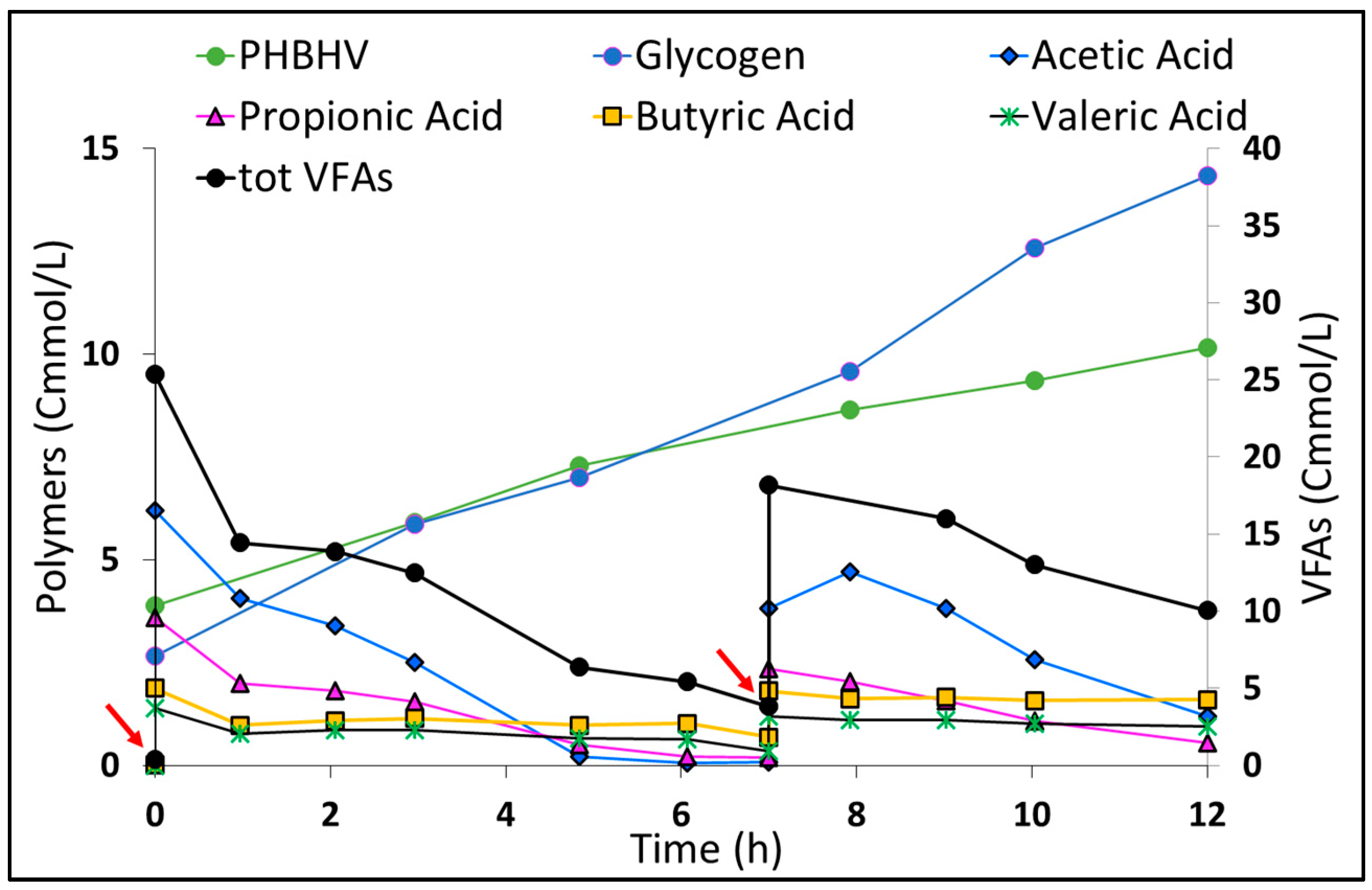
2.4. PPB-PHA Accumulation Capacity
3. Discussion
3.1. PPB Culture Performance
3.2. PPB Microbial Characteristics
4. Materials and Methods
4.1. Fermented Solution as a Carbon Source
4.2. Operation of PPB-PHA Selection Reactor
4.3. PHA Accumulation Test
4.4. Analytical Methods
4.5. Microbial and Metagenomic Analysys
4.6. Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keijser, T. Financial collateral arrangements in the European Union: Current state and the way forward. Unif. Law Rev. 2017, 22, 258–300. [Google Scholar] [CrossRef]
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; InTech Open: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Marchetti, A.; Salvatori, G.; Tayou Nguemna, L.; Grumi, M.; Ozan Basar, A.; Pardo-Figuerez, M.; Lagaron, J.M.; Prieto, C.; Marcoaldi, C.; Villano, M.; et al. Developing Bioplastics from Agro-Industrial Wastes for Applications in Food Packaging. In Developing Circular Agricultural Production Systems; Burleigh Dodds Science Publishing: London, UK, 2024; pp. 273–316. [Google Scholar]
- Sahai, S.; Sharma, C.; Singh, S.K.; Gupta, P.K. Assessment of Trace Gases, Carbon and Nitrogen Emissions from Field Burning of Agricultural Residues in India. Nutr. Cycl. Agroecosyst. 2011, 89, 143–157. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable Applications for the Valorization of Cereal Processing By-Products. Foods 2022, 11, 241. [Google Scholar] [CrossRef]
- Gottardo, M.; Bolzonella, D.; Adele Tuci, G.; Valentino, F.; Majone, M.; Pavan, P.; Battista, F. Producing Volatile Fatty Acids and Polyhydroxyalkanoates from Foods By-Products and Waste: A Review. Bioresour. Technol. 2022, 361, 127716. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From Sewage Sludge and Agri-Food Waste to VFA: Individual Acid Production Potential and up-Scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef] [PubMed]
- De Donno Novelli, L.; Moreno Sayavedra, S.; Rene, E.R. Polyhydroxyalkanoate (PHA) Production via Resource Recovery from Industrial Waste Streams: A Review of Techniques and Perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef] [PubMed]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile Fatty Acids as Carbon Sources for Polyhydroxyalkanoates Production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Salvatori, G.; Astolfi, M.L.; Fabiani, M.; Fradinho, J.; Reis, M.A.M.; Gianico, A.; Bolzonella, D.; Villano, M. Evaluation of the Acidogenic Fermentation Potential of Food Industry By-Products. Biochem. Eng. J. 2023, 199, 109029. [Google Scholar] [CrossRef]
- Marzulli, F.; Musivand, S.; Arengi, M.; De Caprariis, B.; De Filippis, P.; Marchetti, A.; Majone, M.; Villano, M. Coupled Biological and Thermochemical Process for Plastic Waste Conversion Into Biopolymers. Chem. Eng. Trans. 2023, 100, 2023. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Bengtsson, S.; Pisco, A.R.; Reis, M.A.M.; Lemos, P.C. Production of Polyhydroxyalkanoates from Fermented Sugar Cane Molasses by a Mixed Culture Enriched in Glycogen Accumulating Organisms. J. Biotechnol. 2010, 145, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Carvalho, G.; Reis, M.A.M. An Integrated Process for Mixed Culture Production of 3-Hydroxyhexanoate-Rich Polyhydroxyalkanoates from Fruit Waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Zeng, S.; Song, F.; Lu, P.; He, Q.; Zhang, D. Improving PHA Production in a SBR of Coupling PHA-Storing Microorganism Enrichment and PHA Accumulation by Feed-on-Demand Control. AMB Express 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA Production by Mixed Cultures and Renewable Waste Materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Betancourth, C.; Echeverri, S.; Rodriguez-Gonzalez, C.; Wist, J.; Combariza, M.Y.; Sanabria, J. Enhancement of PHA Production by a Mixed Microbial Culture Using VFA Obtained from the Fermentation of Wastewater from Yeast Industry. Fermentation 2022, 8, 180. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Kleerebezem, R.; Oehmen, A.; Ghimire, A.; Pikaar, I.; et al. Purple Phototrophic Bacteria for Resource Recovery: Challenges and Opportunities. Biotechnol. Adv. 2020, 43, 107567. [Google Scholar] [CrossRef]
- Comer, A.D.; Abraham, J.P.; Steiner, A.J.; Korosh, T.C.; Markley, A.L.; Pfleger, B.F. Enhancing Photosynthetic Production of Glycogen-Rich Biomass for Use as a Fermentation Feedstock. Front. Energy Res. 2020, 8, 93. [Google Scholar] [CrossRef]
- Vainshtein, M.B.; Gogotova, G.I.; Heinritz, N.-J. Removal of H2S by the Purple Sulphur Bacterium Ectothiorhodospira Shaposhnikovii. World J. Microbiol. Biotechnol. 1994, 10, 110–111. [Google Scholar] [CrossRef]
- Hülsen, T.; Barry, E.M.; Lu, Y.; Puyol, D.; Keller, J.; Batstone, D.J. Domestic Wastewater Treatment with Purple Phototrophic Bacteria Using a Novel Continuous Photo Anaerobic Membrane Bioreactor. Water Res. 2016, 100, 486–495. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; Zheng, Z.; Meng, F.; Du, T.; He, S. Bio-Conversion of Photosynthetic Bacteria from Non-Toxic Wastewater to Realize Wastewater Treatment and Bioresource Recovery: A Review. Bioresour. Technol. 2019, 278, 383–399. [Google Scholar] [CrossRef]
- Mondala, A.H. Direct Fungal Fermentation of Lignocellulosic Biomass into Itaconic, Fumaric, and Malic Acids: Current and Future Prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Trably, E.; Bernet, N.; Carrère, H. The Environmental Biorefinery: State-of-the-Art on the Production of Hydrogen and Value-Added Biomolecules in Mixed-Culture Fermentation. Green Chem. 2018, 20, 3159–3179. [Google Scholar] [CrossRef]
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for Energy and Nutrient Recovery from Domestic Wastewater: A Review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Almeida, J.R.; Serrano, E.; Fernandez, M.; Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates Production from Fermented Domestic Wastewater Using Phototrophic Mixed Cultures. Water Res. 2021, 197, 117101. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; León, E.S.; Rogalla, F.; Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates Production in Purple Phototrophic Bacteria Ponds: A Breakthrough in Outdoor Pilot-Scale Operation. Sci. Total Environ. 2024, 912, 168899. [Google Scholar] [CrossRef]
- Fradinho, J.C.; Reis, M.A.M.; Oehmen, A. Beyond Feast and Famine: Selecting a PHA Accumulating Photosynthetic Mixed Culture in a Permanent Feast Regime. Water Res. 2016, 105, 421–428. [Google Scholar] [CrossRef]
- Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Suzuki, K.; Ludwig, W.; Whitman, W.B. (Eds.) Bergey’s Manual® of Systematic Bacteriology; Springer: New York, NY, USA, 2012; ISBN 978-0-387-95043-3. [Google Scholar]
- Makowka, A.; Nichelmann, L.; Schulze, D.; Spengler, K.; Wittmann, C.; Forchhammer, K.; Gutekunst, K. Glycolytic Shunts Replenish the Calvin–Benson–Bassham Cycle as Anaplerotic Reactions in Cyanobacteria. Mol. Plant 2020, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Stevens, B.; Ebrahimi, S.; Alloul, A.; Vlaeminck, S.E.; Weissbrodt, D.G. Enrichment and Aggregation of Purple Non-Sulfur Bacteria in a Mixed-Culture Sequencing-Batch Photobioreactor for Biological Nutrient Removal From Wastewater. Front. Bioeng. Biotechnol. 2020, 8, 557234. [Google Scholar] [CrossRef]
- Stephanopoulos, G.N.; Aristidou, A.A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies; Academic Press: Cambridge, MA, USA, 1998; ISBN 0126662606. [Google Scholar]
- Marchetti, A.; Lorini, L.; Salvatori, G.; Gianico, A.; Majone, M.; Villano, M. Polyhydroxyalkanoates Production by Mixed Microbial Cultures in Sequencing Batch Reactors Operated Under Different Feeding Conditions. Chem. Eng. Trans. 2022, 93, 163–168. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) Production by a Mixed Microbial Culture Using Sugar Molasses: Effect of the Influent Substrate Concentration on Culture Selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Majone, M.; Vallini, G.; Di Gregorio, S.; Beccari, M. Effect of the Applied Organic Load Rate on Biodegradable Polymer Production by Mixed Microbial Cultures in a Sequencing Batch Reactor. Biotechnol. Bioeng. 2006, 93, 76–88. [Google Scholar] [CrossRef]
- APHA. Washington DC. Standard methods for the examination of water and wastewater. Part 5. Am. J. Public Health 1995, 85, P.164–P.203. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for Efficiently Selecting PHA Producing Mixed Microbial Cultures Using Complex Feedstocks: Feast and Famine Regime and Uncoupled Carbon and Nitrogen Availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.V.R. Wastewater and Biomass Characterization for the Activated Sludge Model No. 2: Biological Phosphorus Removal. Water Sci. Technol. 1995, 31, 13–23. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of Polyhydroxyalkanoates by Activated Sludge Treating a Paper Mill Wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Nguyen, H.T.T.; Mcilroy, S.J.; Mielczarek, A.T.; Seviour, R. Identification of Polyphosphate-Accumulating and Glycogen-Accumulating Organisms by FISH. In FISH Handbook for Biological Wastewater Treatment; IWA Publishing: London, UK, 2009; pp. 25–31. [Google Scholar]
- Hugenholtz, P.; Tyson, G.W.; Webb, R.I.; Wagner, A.M.; Blackall, L.L. Investigation of Candidate Division TM7, a Recently Recognized Major Lineage of the Domain Bacteria, with No Known Pure-Culture Representatives. Appl. Environ. Microbiol. 2001, 67, 411–419. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Jumpstart Consortium Human Microbiome Project Data Generation Working Group. Evaluation of 16S RDNA-Based Community Profiling for Human Microbiome Research. PLoS ONE 2012, 7, e39315. [Google Scholar]


| COD 1 | gCOD/L | % (solCOD/totCOD) |
|---|---|---|
| Total COD | 25.0 | - |
| Soluble COD | 18.0 | 72.0 |
| Sugars | g/L | % (gsugars/totCOD); (gsugars/solCOD) |
| Total sugar | 0.7 | 2.8; 3.9 |
| Soluble sugar | 0.1 | 0.4; 0.6 |
| Volatile Fatty Acids (VFAs) | gCOD/L | % (CODVFAs/totCOD); (CODVFAs/sol COD) |
| Acetate | 2.3 | 9.2; 12.7 |
| Propionate | 4.1 | 16.4; 22.7 |
| Butyrate | 2.3 | 9.2; 12.7 |
| Valerate | 1.8 | 7.0; 10.0 |
| Total VFA content | 10.5 | 42.0; 58.0 |
| Nutrients | PO43− (mgP/L) | NH4+ (mgN/L) |
| 14.60 | 164.3 |
 less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.
less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.
 less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.
less abundant to more abundant. Here, P indicates the phylum, C the class, and G the genus of bacteria.| Day 0 | Day 30 | |||
|---|---|---|---|---|
| 0.1 | 5.9 | (G) Rhodopsuedomonas | (C) Alphaproteobacteria; | (P) Proteobacteria |
| 84.8 | 37.4 | (G) Rhodomicrobium | ||
| 0.6 | 0.2 | (G) Rhodobacter | ||
| 0.2 | 0.3 | (G) Rhizobium | ||
| 9.7 | 47.1 | (G) Bastochloris |
| Ref | Feed | Selection | Operating Conditions | Culture Performance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light Intensity (W/L) | Cycle | SRT (d) | OLR (1) | Enrichment Reactor | Accumulation Test (6) | ||||||||
| PHA Content (2) | −qs (3) | Yx (4) | YPHA (5) | max PHA Content | YPHA | qPHA (7) | |||||||
| This work | RP fermented solution | PF | 1.9 | 12 h light/12 h dark | 6 | 14–19 | 4.30 ± 0.35; max 8.3% (≈50 HB/50 HV) | 0.23 ± 0.02 | 0.84 ± 0.07 | 0.02 ± 0.001 | 10 (≈60 HB/40 HV) | 0.32 ± 0.08 | 0.35 ± 0.1 |
| [28] | Fermented domestic wastewater supplemented with molasses | PF | 1.1 | 12 h light/12 h dark | 6 | 7–12 (9) | ≈2%, max 18% (≈60 HB/40 HV) | 0.48 ± 0.05 | 0.42 | 0.30 ± 0.09 | n.d. | n.d. | n.d. |
| FF (8) | 1.9 | 7–10 (9) | 1–6%, max 26% (≈63 HB/37 HV) | 0.84 | 0.36 | 0.74 | 30.8 (10) 24.6 (11) (85 HB/15 HV) | 0.75 (10) 0.50 (11) | 1.84 (10) 1.07 (11) | ||||
| [30] | Synthetic acetate solution | PF | 1.8 | 24 h light | 3 | 40 | 3–5 (100% PHB) | 0.69 ± 0.08 | 0.64 ± 0.18 | 0.07 ± 0.05 | 40 (100 PHB) (12) | 0.67 ± 0.01 | 0.73 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, A.; Palhas, M.; Villano, M.; Fradinho, J. Valorization of Reground Pasta By-Product through PHA Production with Phototrophic Purple Bacteria. Catalysts 2024, 14, 239. https://doi.org/10.3390/catal14040239
Marchetti A, Palhas M, Villano M, Fradinho J. Valorization of Reground Pasta By-Product through PHA Production with Phototrophic Purple Bacteria. Catalysts. 2024; 14(4):239. https://doi.org/10.3390/catal14040239
Chicago/Turabian StyleMarchetti, Angela, Miguel Palhas, Marianna Villano, and Joana Fradinho. 2024. "Valorization of Reground Pasta By-Product through PHA Production with Phototrophic Purple Bacteria" Catalysts 14, no. 4: 239. https://doi.org/10.3390/catal14040239







