Preparation of CaO@CeO2 Solid Base Catalysts Used for Biodiesel Production
Abstract
1. Introduction
2. Results
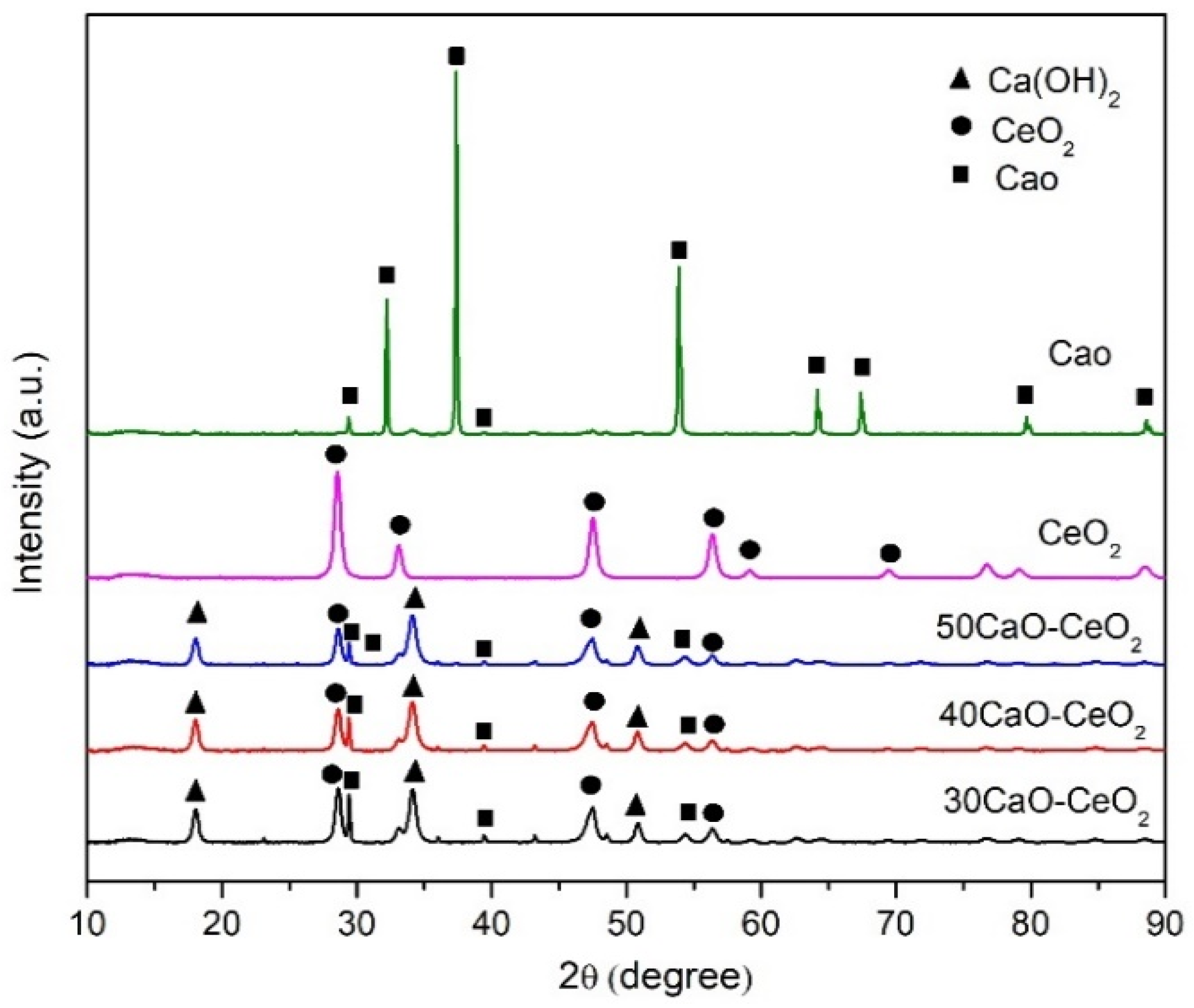
2.1. Catalysts Characterization
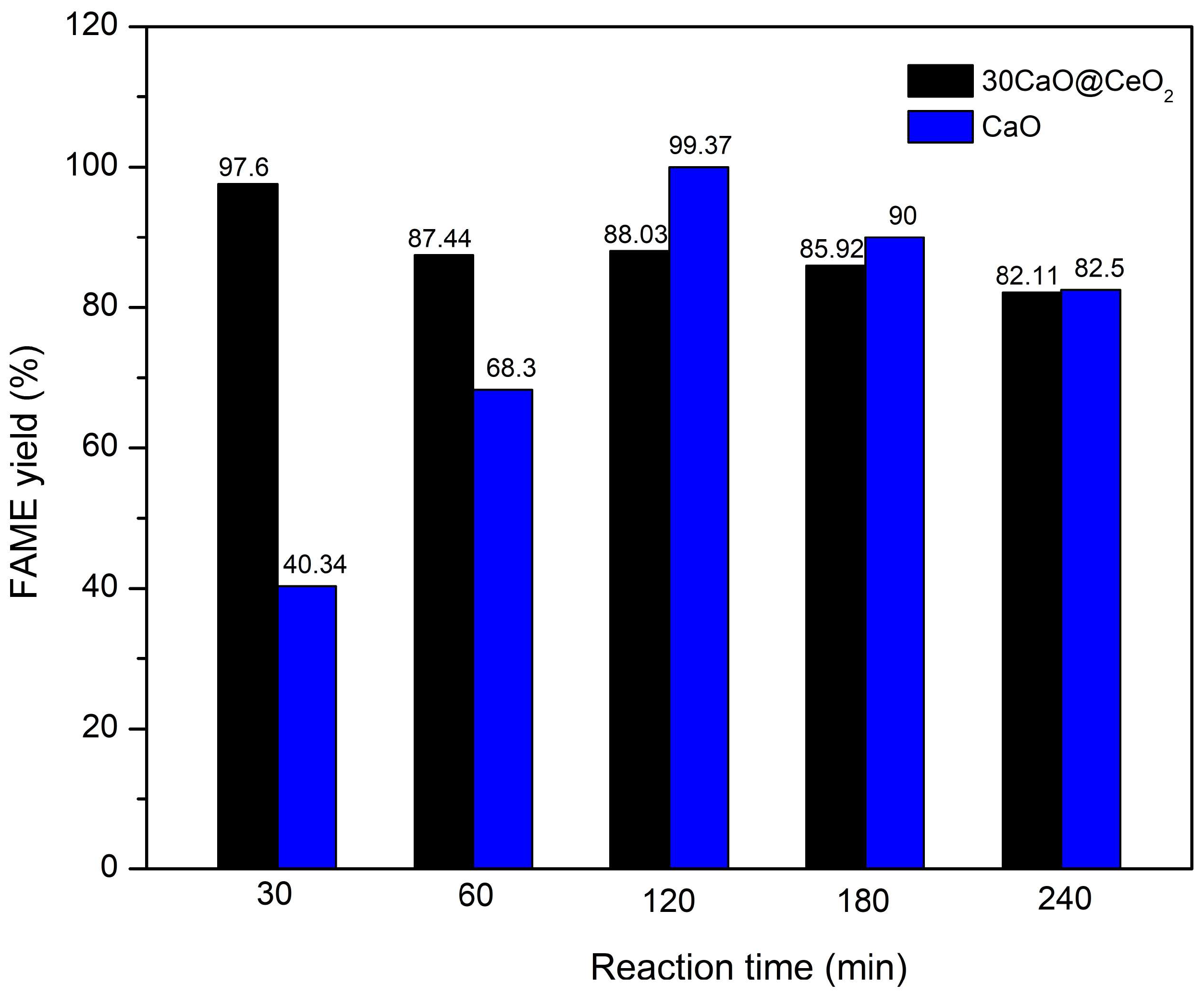
2.2. Catalytic Activity
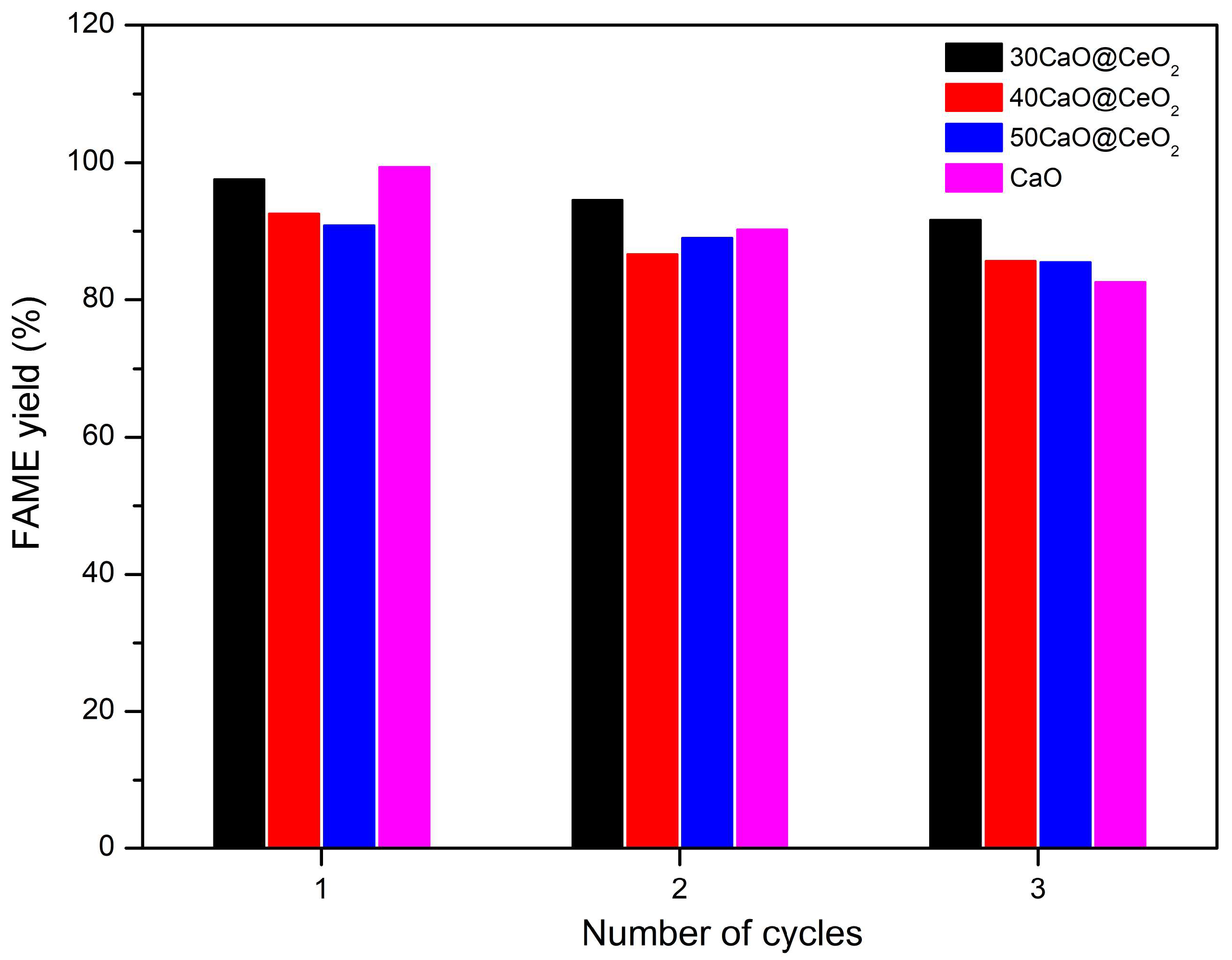
2.3. Reusability
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation and Characterization
3.3. Transesterification Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghahosseini, A.; Breyer, C. Assessment of geological resource potential for compressed air energy storage in global electricity supply. Energy Convers. Manag. 2018, 169, 161–173. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable energy in the sustainable development of electrical power sector: A review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Matthews, R.D.; Bian, Y.Z. Combustion and emission characteristics of ethanol–biodiesel–water micro-emulsions used in a direct injection compression ignition engine. Fuel 2010, 89, 958–964. [Google Scholar] [CrossRef]
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of catalytic transesterification methods for biodiesel production. Biofuels State Dev. 2018, 6, 93–119. [Google Scholar]
- López, D.E.; Goodwin, J.G., Jr.; Bruce, D.A.; Lotero, E. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; Kongpop, U. Optimization of biodiesel production using magnesium pyrophosphate. Chem. Eng. Sci. 2020, 226, 115884. [Google Scholar] [CrossRef]
- Vujicic, D.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel. 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Hattori, H.; Shima, M.; Kabashima, H. Alcoholysis of ester and epoxide catalyzed by solid base. Stud. Surf. Sci. Catal. 2000, 130, 3507–3512. [Google Scholar]
- Taufiq-Yap, Y.H.; Lee, H.V.; Yunus, R.; Juan, J.C. Transesterification of non-edible Jatropha curcas oil to biodiesel using binary Ca–Mg mixed oxide catalyst: Effect of stoichiometric composition. Chem. Eng. J. 2011, 178, 342–347. [Google Scholar] [CrossRef]
- Wu, T.; Guo, R.T.; Li, C.F.; Pan, W.G. Recent progress of CeO2-based catalysts with special morphologies applied in air pollutants abatement: A review. J. Environ. Chem. Eng. 2023, 11, 109136. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I.; Tee, H.S. Biodiesel production via transesterification of palm oil by using CaO–CeO2 mixed oxide catalysts. Fuel 2015, 162, 288–293. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of heterogeneous catalysts for biodiesel production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef] [PubMed]
- Reyero, I.; Moral, A.; Bimbela, F.; Radosevic, J.; Sanz, O.; Montes, M.; Gandía, L.M. Metallic monolithic catalysts based on calcium and cerium for the production of biodiesel. Fuel 2016, 182, 668–676. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Kawi, S. An active and stable CaO–CeO2 catalyst for transesterification of oil to biodiesel. Green Chem. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, H.; Hu, R. The activity and stability of CeO2@CaO catalysts for the production of biodiesel. RSC Adv. 2018, 8, 32922–32929. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Nohair, B.; Shen, W.; Xu, H.; Kaliaguine, S. Promotional effects of CeO2 on stability and activity of CaO for the glycerolysis of triglycerides. Catal. Lett. 2016, 146, 1273–1282. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wen, Z.; Li, H.; Tu, S.T.; Yan, J. Transesterification of Pistacia chinensis oil for biodiesel catalyzed by CaO–CeO2 mixed oxides. Fuel 2011, 90, 1868–1874. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2022, 13, 2. [Google Scholar] [CrossRef]
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of pore size on the performance of immobilised enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010. [Google Scholar] [CrossRef]
- Maroa, S.; Inambao, F. A review of sustainable biodiesel production using biomass derived heterogeneous catalysts. Eng. Life Sci. 2021, 21, 790–824. [Google Scholar] [CrossRef] [PubMed]
- Laskar, I.B.; Deshmukhya, T.; Biswas, A.; Paul, B.; Changmai, B.; Gupta, R.; Chatterjee, S.; Rokhum, S.L. Utilization of biowaste-derived catalysts for biodiesel production: Process optimization using response surface methodology and particle swarm optimization method. Energy Adv. 2022, 1, 287–302. [Google Scholar] [CrossRef]
- Kingkam, W.; Nilgumhang, K.; Nuchdang, S.; Duangdee, B.; Puripunyavanich, V.; Rattanaphra, D. Study the Stable Diatomite-supported Catalyst for Biodiesel Production. Chiang Mai J. Sci. 2022, 49, 1332–1343. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, H.; Wu, R.A.; Zou, H. Recent advances of mesoporous materials in sample preparation. J. Chromatogr. A. 2012, 1228, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Mach, T.P.; Ding, Y.; Binder, J.R. Impact of Particle and Crystallite Size of Ba0.6Sr0.4TiO3 on the Dielectric Properties of BST/P (VDF-TrFE) Composites in Fully Printed Varactors. Polymers 2022, 14, 5027. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Jitjamnong, J.; Luengnaruemitchai, A.; Numwong, N.; Chollacoop, N.; Yoshimura, Y. Influence of Mg modifier on cis-trans selectivity in partial hydrogenation of biodiesel using different metal types. Appl. Catal. A Gen. 2016, 520, 170–177. [Google Scholar] [CrossRef]
- Anjalin, M.; Kanagathara, N.; Suganthi, A.R.B. A Brief Review on Aniline and Its Derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Sagadevan, S.; Oh, W.C.; Ameta, K.L. Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review. Chem. Eng. 2023, 7, 56. [Google Scholar] [CrossRef]
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Mazher, M.; Ishtiaq, M.; Hamid, B.; Haq, S.M.; Mazhar, A.; Bashir, F.; Mazhar, M.; Mahmoud, E.A.; Casini, R.; Alataway, A.; et al. Biosynthesis and characterization of Calcium Oxide nanoparticles from Citrullus colocynthis Fruit extracts; their biocompatibility and bioactivities. Materials 2023, 16, 2768. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Conv. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Manurung, R.; Parinduri, S.Z.D.M.; Hasibuan, R.; Tarigan, B.H.; Siregar, A.G.A. Synthesis of nano-CaO catalyst with SiO2 matrix based on palm shell ash as catalyst support for one cycle developed in the palm biodiesel process. Case Stud. Chem. Environ. Eng. 2023, 7, 100345. [Google Scholar] [CrossRef]
- Loy, C.W.; Amin Matori, K.; Lim, W.F.; Schmid, S.; Zainuddin, N.; Abdul Wahab, Z.; Nadakkavil Alassan, Z.; Mohd Zaid, M.H. Effects of calcination on the crystallography and nonbiogenic aragonite formation of ark clam shell under ambient condition. Adv. Mater. Sci. Eng. 2016, 2016, 2914368. [Google Scholar] [CrossRef]
- Habib, S.; Fayyad, E.; Nawaz, M.; Khan, A.; Shakoor, R.A.; Kahraman, R.; Abdullah, A. Cerium dioxide nanoparticles as smart carriers for self-healing coatings. Nanomaterials 2020, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Giammaria, G.; Lefferts, L. Catalytic effect of water on calcium carbonate decomposition. J. CO2 Util. 2019, 33, 341–356. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Brahma, S.; Hoque, M.; Das, B.K.; Selvaraj, M.; Brahma, S.; Basumatary, S. Advances in CaO-based catalysts for sustainable biodiesel synthesis. Green Energy Resour. 2023, 1, 100032. [Google Scholar] [CrossRef]
- Zheng, H.; He, Y.; Zhu, Y.; Liu, L.; Cui, X. Novel procedure of CO2 capture of the CaO sorbent activator on the reaction of one-part alkali-activated slag. RSC. Adv. 2021, 11, 12476–12483. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Heterogeneous catalysis of transesterification of jatropha curcas oil over calcium–cerium bimetallic oxide catalyst. RSC. Adv. 2014, 4, 48836–48847. [Google Scholar] [CrossRef]
- Pranata, D.E.; Syarif, A.; Yerizam, M. Characterization of Fly Ash Catalyst Using XRD Method for Biofuel Production from Used Cooking Oil. Indones. J. Fundam. Appl. Chem. 2021, 6, 90–95. [Google Scholar] [CrossRef]
- Poonanan, A.; Prukpaiboon, A.; Dim, P.E.; Termtanun, M. Using shrimp shells as based catalysts for FAME production from palm oil feedstock. J. Met. Mater. Miner. 2021, 31, 78–83. [Google Scholar] [CrossRef]
- Ismail, S.; Ahmed, A.S.; Anr, R.; Hamdan, S. Biodiesel production from castor oil by using calcium oxide derived from mud clam shell. J. Renew. Energy 2016, 2016, 5274917. [Google Scholar] [CrossRef]
- Yang, F.X.; Su, Y.Q.; Li, X.H.; Zhang, Q.; Sun, R.C. Preparation of biodiesel from Idesia polycarpa var. vestita fruit oil. Ind. Crops. Prod. 2009, 29, 622–628. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Wei, Q.; Zheng, J.; Zhang, J. Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalyst. Fuel Process Technol. 2013, 109, 13–18. [Google Scholar] [CrossRef]
- Sohn, J.R.; Park, E.H. Acidic properties of caosio2 binary oxide catalyst and Activity for acid catalysis. Korean J. Chem. Eng. 1997, 14, 192–197. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, M.; Hidaka, J.S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Oueda, N.; Bonzi-Coulibaly, Y.L.; Ouédraogo, I.W. Deactivation processes, regeneration conditions and reusability performance of CaO or MgO based catalysts used for biodiesel production—A review. Mater. Sci. Appl. 2016, 8, 94–122. [Google Scholar] [CrossRef][Green Version]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Tercini, A.C.B.; Pinesi, M.; Calera, G.C.; Sequinel, R.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Ultrafast gas chromatographic method for quantitative determination of total FAMEs in biodiesel: An analysis of 90 s. Fuel 2018, 222, 792–799. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Rocha, A.C.P.; Nelson, D.L.; de Freitas, M.S.; Mestre, A.A.F.; Klein, S.I.; Clososki, G.C.; Caires, F.J.; Flumignan, D.L.; Dos Santos, L.K.; et al. Catalytic transformation of triglycerides to biodiesel with SiO2-SO3H and quaternary ammonium salts in toluene or DMSO. Molecules 2022, 27, 953. [Google Scholar] [CrossRef] [PubMed]









| Formular | Concentration (%) | ||||
|---|---|---|---|---|---|
| CaO | CeO2 | 30CaO@CeO2 | 40CaO@CeO2 | 50CaO@CeO2 | |
| CaO | 97.48 | 0.30 | 79.92 | 84.14 | 85.28 |
| CeO2 | - | 99.02 | 17.92 | 13.33 | 12.28 |
| MgO | 0.93 | - | 0.66 | 0.71 | 0.76 |
| P2O5 | 0.38 | - | 0.53 | 0.51 | 0.43 |
| SO3 | 0.37 | - | 0.39 | 0.39 | 0.41 |
| SiO2 | 0.35 | 0.19 | 0.37 | 0.38 | 0.40 |
| Al2O3 | 0.20 | 0.06 | - | 0.29 | 0.20 |
| Fe2O3 | 0.19 | 0.03 | 0.17 | 0.18 | 0.18 |
| K2O | 0.05 | - | 0.02 | 0.04 | 0.03 |
| SrO | 0.04 | - | 0.02 | 0.03 | 0.03 |
| ZnO | - | 0.06% | - | - | - |
| PbO | - | 0.03% | - | - | - |
| Samples | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Width (nm) | Crystallite Size (nm) | Average Particle Size (nm) |
|---|---|---|---|---|---|
| CaO | 37.40 | 0.09 | 8.90 | 39.10 | 160.40 |
| 30CaO@CeO2 | 34.49 | 0.11 | 10.06 | 24.64 | 173.93 |
| 40CaO@CeO2 | 46.91 | 0.15 | 9.77 | 19.85 | 127.87 |
| 50CaO@CeO2 | 53.30 | 0.16 | 9.17 | 18.87 | 112.56 |
| CeO2 | 39.68 | 0.09 | 8.46 | 15.24 | 151.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingkam, W.; Maisomboon, J.; Khamenkit, K.; Nuchdang, S.; Nilgumhang, K.; Issarapanacheewin, S.; Rattanaphra, D. Preparation of CaO@CeO2 Solid Base Catalysts Used for Biodiesel Production. Catalysts 2024, 14, 240. https://doi.org/10.3390/catal14040240
Kingkam W, Maisomboon J, Khamenkit K, Nuchdang S, Nilgumhang K, Issarapanacheewin S, Rattanaphra D. Preparation of CaO@CeO2 Solid Base Catalysts Used for Biodiesel Production. Catalysts. 2024; 14(4):240. https://doi.org/10.3390/catal14040240
Chicago/Turabian StyleKingkam, Wilasinee, Jirapa Maisomboon, Khemmanich Khamenkit, Sasikarn Nuchdang, Kewalee Nilgumhang, Sudarat Issarapanacheewin, and Dussadee Rattanaphra. 2024. "Preparation of CaO@CeO2 Solid Base Catalysts Used for Biodiesel Production" Catalysts 14, no. 4: 240. https://doi.org/10.3390/catal14040240
APA StyleKingkam, W., Maisomboon, J., Khamenkit, K., Nuchdang, S., Nilgumhang, K., Issarapanacheewin, S., & Rattanaphra, D. (2024). Preparation of CaO@CeO2 Solid Base Catalysts Used for Biodiesel Production. Catalysts, 14(4), 240. https://doi.org/10.3390/catal14040240