Production of 14α-Hydroxy Progesterone Using a Steroidal Hydroxylase from Cochliobolus lunatus Expressed in Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of a Functional Truncated Form of P-450lun (ΔP-450lun)
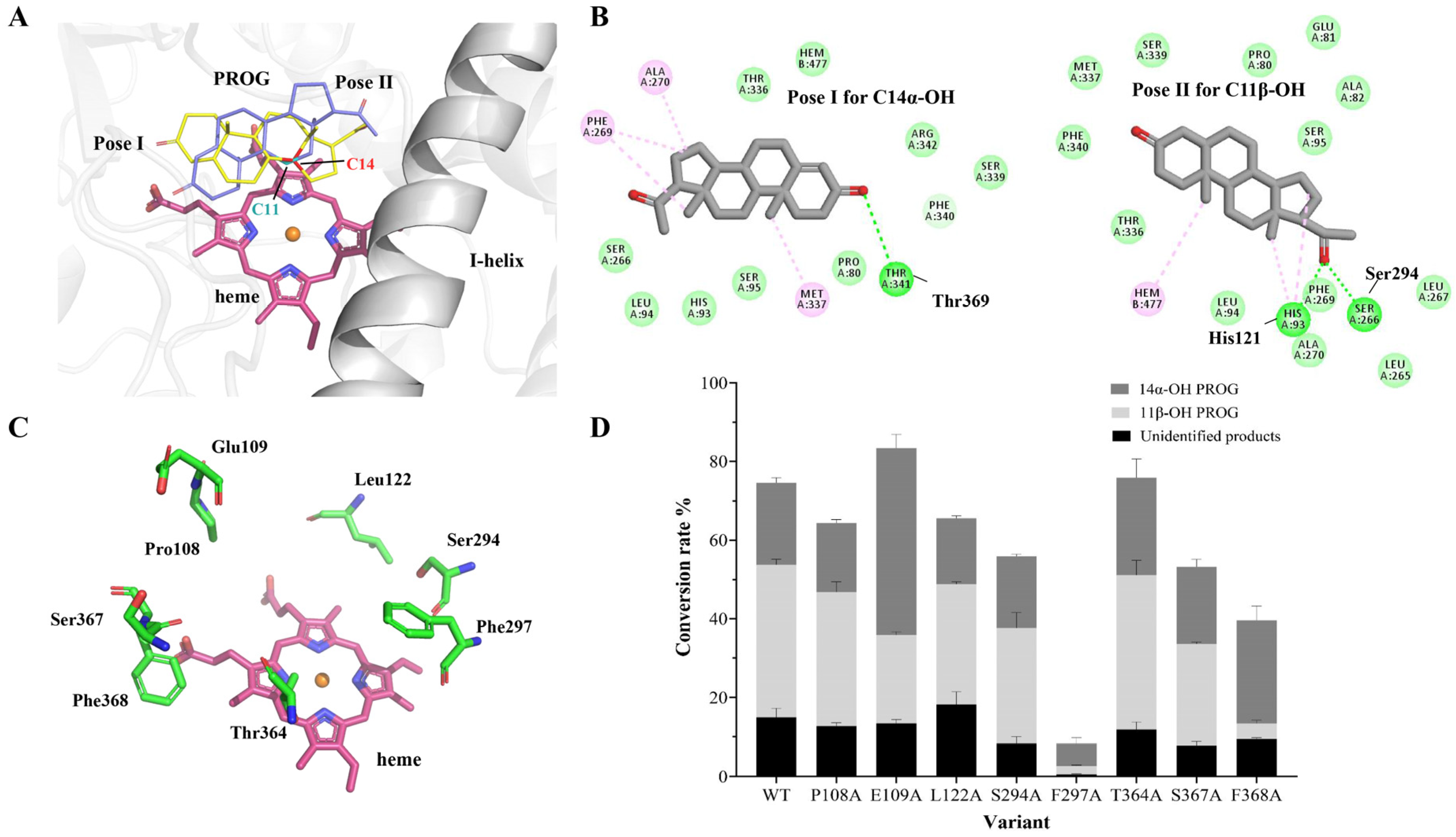
2.2. Engineering ΔP-450lun to Improve Its Regiospecificity and Catalytic Activity
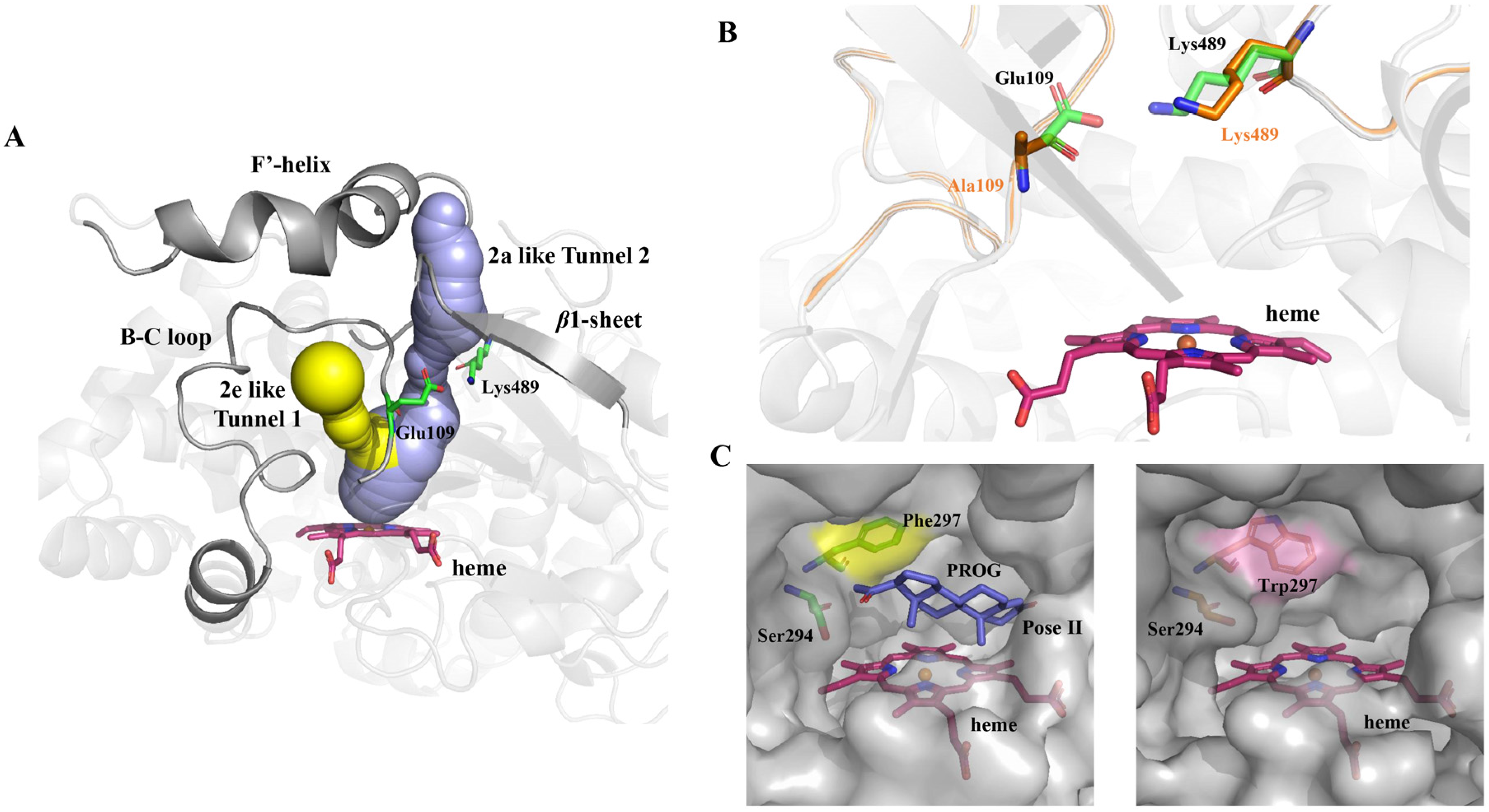
2.3. Computational Analysis of ΔP-450lun and Its Variants
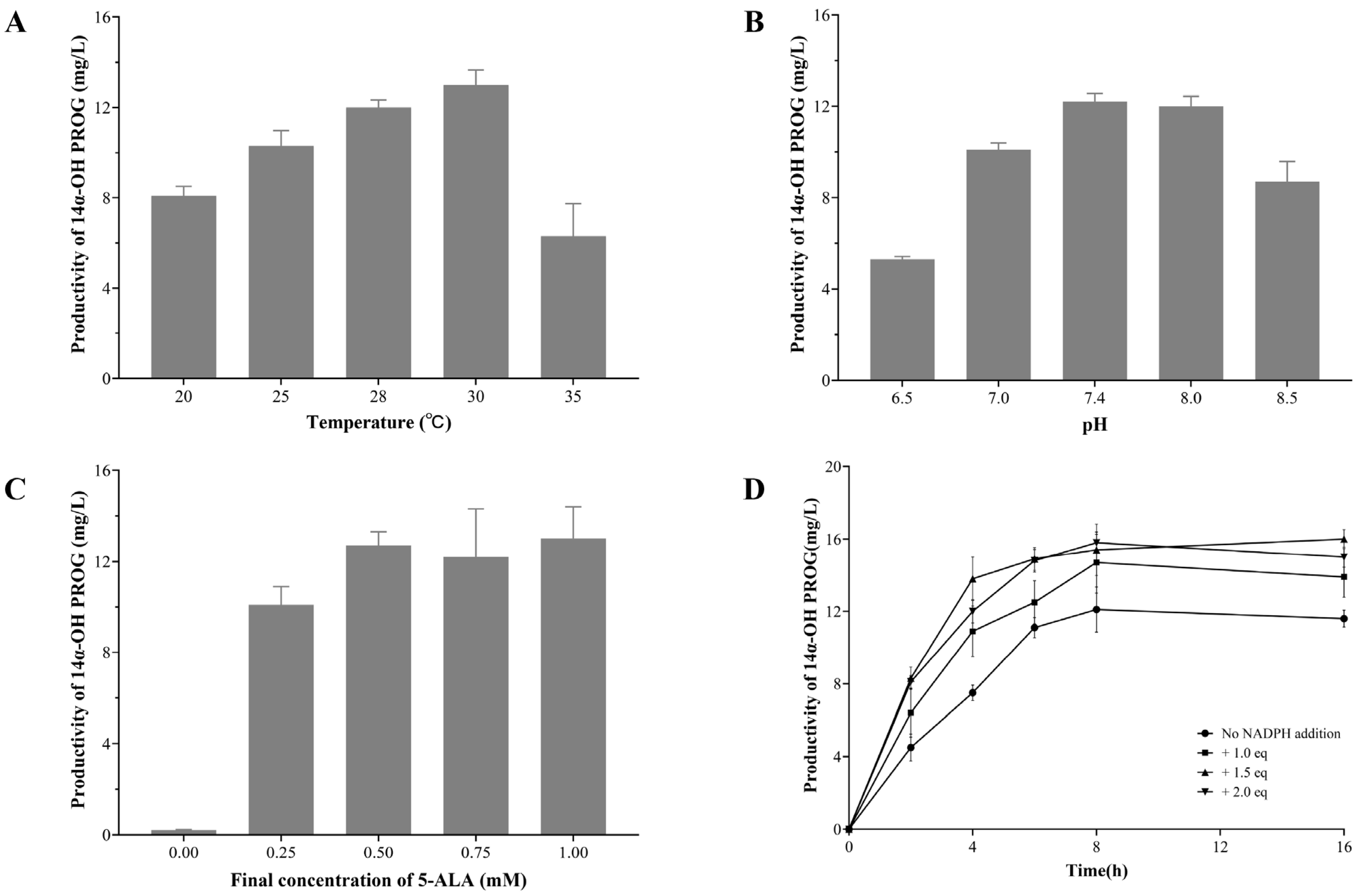
2.4. Optimizing the Conditions of Whole-Cell Biocatalyst
3. Materials and Methods
3.1. Strains and Reagents
3.2. Plasmid Construction and Protein Expression
3.3. Whole-Cell Conversion of Progesterone Using E. coli in the Erlenmeyer flask
3.4. Engineering of P-450lun for Improved Regioselectivity and Activity
3.5. Structural Modeling Analysis, Molecular Docking and Tunnel Analysis
3.6. Optimization of the Whole-Cell Biocatalytic Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutolo, M.; Straub, R.H. Sex steroids and autoimmune rheumatic diseases: State of the art. Nat. Rev. Rheumatol. 2020, 16, 628–644. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Cao, W.; Ji, J.; Wang, L.; Yang, Y.; Yang, H. The influence of corticosteroids, immunosuppressants and biologics on patients with inflammatory bowel diseases, psoriasis and rheumatic diseases in the era of COVID-19: A review of current evidence. Front. Immunol. 2021, 12, 677957. [Google Scholar] [CrossRef] [PubMed]
- Chasalow, F. An Introduction to Spiral Steroids. Int. J. Mol. Sci. 2022, 23, 9523. [Google Scholar] [CrossRef]
- Ogino, Y.; Sato, T.; Lguchi, T. Gonadal Steroids; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Chen, W.; Fisher, M.J.; Leung, A.; Cao, Y.; Wong, L.L. Oxidative diversification of steroids by nature-inspired scanning glycine mutagenesis of P450BM3 (CYP102A1). ACS Cat. 2020, 10, 8334–8343. [Google Scholar] [CrossRef]
- Andryushina, V.A.; Voishvillo, N.E.; Druzhinina, A.V.; Stytsenko, T.S.; Yaderets, V.V.; Petrosyan, M.A.; Zeinalov, O.A. 14α-Hydroxylation of steroids by mycelium of the mold fungus Curvularia lunata (VKPM F-981) to produce precursors for synthesizing new steroidal drugs. Pharm. Chem. J. 2013, 47, 102–108. [Google Scholar] [CrossRef]
- Hu, S.H.; Genain, G.; Azerad, R. Microbial transformation of steroids: Contribution to 14α-hydroxylations. Steroids 1995, 60, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Takara, Y.; Masaaki, F.; Yasuko, F.; Jo, K.; Masamichi, N.; Makoto, Y.; Hiroji, O. Inhibitory effect of a new androstenedione derivative, 14α-hydroxy-4-androstene-3, 6, 17-trione (14α-OHAT) on aromatase activity of human uterine tumors. J. Steroid Biochem. 1990, 36, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Rieck, C.; Geiger, D.; Munkert, J.; Messerschmidt, K.; Petersen, J.; Strasser, J.; Meitinger, N.; Kreis, W. Biosynthetic approach to combine the first steps of cardenolide formation in Saccharomyces cerevisiae. MicrobiologyOpen 2019, 8, e925. [Google Scholar] [CrossRef]
- Sales, E.; Müller-Uri, F.; Nebauer, S.G.; Segura, J.; Kreis, W.; Arrillaga, I. Digitalis. In Wild Crop Relatives: Genomic and Breeding Resources: Plantation and Ornamental Crops; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–112. [Google Scholar] [CrossRef]
- Züst, T.; Mirzaei, M.; Jander, G. Erysimum cheiranthoides, an ecological research system with potential as a genetic and genomic model for studying cardiac glycoside biosynthesis. Phytochem. Rev. 2018, 17, 1239–1251. [Google Scholar] [CrossRef]
- Wife, R.L.; Kyle, D.; Mulheirn, L.J.; Volger, H.C. Selective hydrocarbon oxidation: Dry ozonation of steroids on silica. J. Chem. Soc. Chem. Commun. 1982, 5, 306–307. [Google Scholar] [CrossRef]
- Athelstan, L.J.B.; Thach, D. Regioselective oxidation of unactivated methylene and methine groups by dry ozonation: Similarity to microbiological oxidation. J. Chem. Soc. Chem. Comm. 1978, 9, 2473–2670. [Google Scholar] [CrossRef]
- Ciaramella, A.; Minerdi, D.; Gilardi, G. Catalytically self-sufficient cytochromes P450 for green production of fine chemicals. Rend. Fis. Acc. Lincei 2016, 28, 169–181. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.L.; Xi, Y.Y.; Dai, Z.B.; Bi, C.H.; Chen, X.; Fan, F.Y.; Zhang, X.L. Production of 14α-hydroxysteroids by a recombinant Saccharomyces cerevisiae biocatalyst expressing of a fungal steroid 14α-hydroxylation system. Appl. Microbiol. Biotechnol. 2019, 103, 8363–8374. [Google Scholar] [CrossRef]
- Kristan, K.; Rizner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. 2012, 129, 79–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Sun, Z.Q.; Zhang, H.; Wang, W.; Wang, Z.R.; Guo, Z.K.; Yu, S.; Tan, R.X.; Ge, H.M. Biocatalytic C14-Hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids. ACS Cat. 2022, 12, 9839–9845. [Google Scholar] [CrossRef]
- Song, F.Z.; Zheng, M.M.; Wang, J.L.; Liu, H.H.; Lin, Z.; Liu, B.B.; Deng, Z.X.; Cong, H.J.; Zhou, Q.H.; Qu, X.D. Chemoenzymatic synthesis of C14-functionalized steroids. Nat. Synth. 2023, 2, 729–739. [Google Scholar] [CrossRef]
- Felpeto-Santero, C.; Galan, B.; Luengo, J.M.; Fernandez-Canon, J.M.; Del Cerro, C.; Medrano, F.J.; Garcia, J.L. Identification and expression of the 11beta-steroid hydroxylase from Cochliobolus lunatus in Corynebacterium glutamicum. Microb. Biotechnol. 2019, 12, 856–868. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, Y.; Gao, Y.; Zan, Z.; Wang, M. Efficient production of 14α-OH-AD by engineered Mycolicibacterium neoaurum via coupled cofactor and reconstructed electron transport system. Syst. Microbiol. Biomanuf. 2023, 3, 358–369. [Google Scholar] [CrossRef]
- Permana, D.; Niesel, K.; Ford, M.J.; Ichinose, H. Latent functions and applications of cytochrome P450 monooxygenases from Thamnidium elegans: A novel biocatalyst for 14alpha-hydroxylation of Testosterone. ACS Omega 2022, 7, 13932–13941. [Google Scholar] [CrossRef]
- Pessôa, M.T.C.; Barbosa, L.A.; Villar, J.A.F. Synthesis of cardiac steroids and their role on heart failure and cancer. Stud. Nat. Prod. Chem. 2018, 57, 79–113. [Google Scholar] [CrossRef]
- Boer, E.; Steinborn, G.; Kunze, G.; Gellissen, G. Yeast expression platforms. Appl. Microbiol. Biotechnol. 2007, 77, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Alder, N.N.; Johnson, A.E. Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 22787–22790. [Google Scholar] [CrossRef] [PubMed]
- Freigassner, M.; Pichler, H.; Glieder, A. Tuning microbial hosts for membrane protein production. Microb. Cell Fact. 2009, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.; Horsefield, R.; Swarts, H.G.; de Pont, J.J.; Neutze, R.; Snijder, A. Effective high-throughput overproduction of membrane proteins in Escherichia coli. Protein Expr. Purif. 2008, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.; Shich, E.; Makhova, A.; Kuzikov, A.; Archakov, A. Cytochrome P450 enzymes and electrochemistry: Crosstalk with electrodes as redox partners and electron sources. Adv. Exp. Med. Biol. 2015, 851, 229–246. [Google Scholar] [CrossRef]
- Li, S.; Du, L.; Bernhardt, R. Redox partners: Function modulators of bacterial P450 enzymes. Trends Microbiol. 2020, 28, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, J.J.; Xie, Q.; Ames, R.S.; Lu, Q. Production of protein complexes via co-expression. Protein Expr. Purif. 2011, 75, 1–14. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Xu, Z.; Ishii-Watabe, A.; Uchida, E.; Hayakawa, T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, H.E.; Lee, K.H.; Han, W.; Yi, M.J.; Jeong, J.; Oh, B.H. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. 2004, 13, 1698–1703. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Yu, K.; Liu, C.; Kim, B.G.; Lee, D.Y. Synthetic fusion protein design and applications. Biotechnol. Adv. 2015, 33, 155–164. [Google Scholar] [CrossRef] [PubMed]
- van Rosmalen, M.; Krom, M.; Merkx, M. Tuning the flexibility of Glycine-Serine linkers to allow rational design of multidomain proteins. Biochemistry 2017, 56, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Snijder, H.J.; Hakulinen, J. Membrane protein production in E. coli for applications in drug discovery. Adv. Exp. Med. Biol. 2016, 896, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhao, X.; Wang, E.; Zhou, J.; Li, J.; Chen, J.; Du, G. Efficient heterologous expression of cytochrome P450 enzymes in microorganisms for the biosynthesis of natural products. Crit. Rev. Biotechnol. 2023, 43, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Cosme, J.; Johnson, E.F. Engineering Microsomal Cytochrome P450 2C5 to Be a Soluble, Monomeric Enzyme: Mutations That Alter Aggregation, Phospholipid Dependence of Catalysis, and Membrane Binding. J. Biol. Chem. 2000, 275, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Salamanca-Pinzon, S.G.; Wu, Z.L.; Xiao, Y.; Guengerich, F.P. Human cytochrome P450 4F11: Heterologous expression in bacteria, purification, and characterization of catalytic function. Arch. Biochem. Biophys. 2010, 494, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C.; Too, H.P. Multienzyme biosynthesis of dihydroartemisinic acid. Molecules 2017, 22, 1422. [Google Scholar] [CrossRef] [PubMed]
- Zelasko, S.; Palaria, A.; Das, A. Optimizations to achieve high-level expression of cytochrome P450 proteins using Escherichia coli expression systems. Protein Expr. Purif. 2013, 92, 77–87. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Sevrioukova, I.F. High-Level Production and Properties of the Cysteine-Depleted Cytochrome P450 3A4. Biochemistry 2017, 56, 3058–3067. [Google Scholar] [CrossRef]
- Gricman, L.; Vogel, C.; Pleiss, J. Conservation analysis of class-specific positions in cytochrome P450 monooxygenases: Functional and structural relevance. Proteins 2014, 82, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.H.; Lee, G.R.; Heo, L.; Lee, H.; Seok, C.J.B.D. Prediction of protein structure and interaction by GALAXY protein modeling programs. BioDesign 2014, 2, 1–11. [Google Scholar]
- Seok, C.; Baek, M.; Steinegger, M.; Park, H.; Lee, G.R.; Won, J. Accurate protein structure prediction: What comes next? BioDesign 2021, 9, 47–50. [Google Scholar] [CrossRef]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A method to identify protein sequences that fold into a known three-dimensional structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Pleiss, J. Identification of selectivity-determining residues in cytochrome P450 monooxygenases: A systematic analysis of the substrate recognition site 5. Proteins 2009, 74, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Khatri, Y.; Jozwik, I.K.; Ringle, M.; Ionescu, I.A.; Litzenburger, M.; Hutter, M.C.; Thunnissen, A.W.H.; Bernhardt, R. Structure-Based engineering of steroidogenic CYP260A1 for stereo- and regioselective hydroxylation of progesterone. ACS Chem. Biol. 2018, 13, 1021–1028. [Google Scholar] [CrossRef]
- Kokkonen, P.; Bednar, D.; Pinto, G.; Prokop, Z.; Damborsky, J. Engineering enzyme access tunnels. Biotechnol. Adv. 2019, 37, 107386. [Google Scholar] [CrossRef]
- Banerjee, R.; Lipscomb, J.D. Small-molecule tunnels in metalloenzymes viewed as extensions of the active site. Acc. Chem. Res. 2021, 54, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, E.; Pavelka, A.; Benes, P.; Strnad, O.; Brezovsky, J.; Kozlikova, B.; Damborsky, J. CAVER 3.0: A tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 2012, 8, e1002708. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Winn, P.J.; Wade, R.C. The ins and outs of cytochrome P450s. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Y.; Cui, W.J.; Liu, Z.M.; Zhou, L.; Wang, M.; Kobayashi, M.; Zhou, Z.M. A switch in a substrate tunnel for directing regioselectivity of nitrile hydratases towards α, ω-dinitriles. Catal. Sci. Technol. 2016, 6, 1292–1296. [Google Scholar] [CrossRef]
- Meng, S.Q.; An, R.P.; Li, Z.Y.; Schwaneberg, U.; Ji, Y.; Davari, M.D.; Wang, F.; Wang, M.; Qin, M.; Nie, K.; et al. Tunnel engineering for modulating the substrate preference in cytochrome P450BsβHI. Bioresour. Bioprocess. 2021, 8, 26. [Google Scholar] [CrossRef]
- Feng, C.; Pan, M.; Tang, L. 5-Aminolevulinic acid level and dye-decolorizing peroxidase expression regulate heme synthesis in Escherichia coli. Biotechnol. Lett. 2022, 44, 271–277. [Google Scholar] [CrossRef]
- Quehl, P.; Hollender, J.; Schüürmann, J.; Brossette, T.; Maas, R.; Jose, J. Co-expression of active human cytochrome P450 1A2 and cytochrome P450 reductase on the cell surface of Escherichia coli. Microb. Cell Fact. 2016, 15, 26. [Google Scholar] [CrossRef]



| Mutants | Conversion (%) 1 | Selectivity (%) | ||
|---|---|---|---|---|
| 14α | 11β | Others | ||
| WT | 75 | 28 | 52 | 20 |
| E109A | 84 | 57 | 27 | 16 |
| F297A | 9 | 69 | 26 | 6 |
| F297W | 41 | 93 | 6 | 1 |
| E109A/F297W | 71 | 97 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Liu, H.; Tian, W.; Chang, Z. Production of 14α-Hydroxy Progesterone Using a Steroidal Hydroxylase from Cochliobolus lunatus Expressed in Escherichia coli. Catalysts 2024, 14, 247. https://doi.org/10.3390/catal14040247
Chang Y, Liu H, Tian W, Chang Z. Production of 14α-Hydroxy Progesterone Using a Steroidal Hydroxylase from Cochliobolus lunatus Expressed in Escherichia coli. Catalysts. 2024; 14(4):247. https://doi.org/10.3390/catal14040247
Chicago/Turabian StyleChang, Yaowen, Han Liu, Wei Tian, and Zunxue Chang. 2024. "Production of 14α-Hydroxy Progesterone Using a Steroidal Hydroxylase from Cochliobolus lunatus Expressed in Escherichia coli" Catalysts 14, no. 4: 247. https://doi.org/10.3390/catal14040247




