Abstract
To cope with global warming and increasing carbon emissions, the chemical looping process has attracted attention due to its excellent ability to convert fossil fuel and capture CO2. In this case, chemical looping partial oxidation technology has become the focus of attention due to its advantages in the production of syngas and hydrogen, especially with respect to the design and selection of oxygen carriers, which directly affect the efficiency of the production of syngas and hydrogen. In particular, the conversion of methane can reach 95% in the chemical looping partial oxidation of methane, and the selectivity of syngas, in the range of 700 °C to 900 °C at atmospheric pressure, can reach 99% for twenty or more cycles. In this review, from the perspective of metal oxide selection and structure regarding the chemical looping partial oxidation process, we discuss the role of oxygen carriers in the chemical looping partial oxidation cycle, in which the specific surface area, the lattice oxygen mobility, and the thermal stability are understood as the important factors affecting reactivity. We hope to summarize the design and development of efficient oxygen carriers with high oxygen-carrying capacity and syngas selectivity, as well as contribute to the selection, design, optimization, and redox reaction mechanism of redox catalysts.
1. Introduction
The substantial and alarming rise in CO2 emissions and its consequential impact on climate change have spurred extensive research efforts aimed at controlling atmospheric CO2 levels. Consequently, significant endeavors have been dedicated to executing energy and material conversions via compact, environmentally friendly, and energy-efficient process technologies over an extended period. A strategy for achieving this target involves chemical looping, where a reaction or separation is deconstructed into several sub-reactions, which take place in separate steps or environments. In other words, chemical looping can offer distinct reaction pathways within oxygen carriers, where these carriers act as matrices for lattice oxygen transfer. They oxidize reactants and subsequently undergo a reduction in separate reactions. Hence, chemical looping is distinguished not only as a promising technology for converting carbonaceous fuels, thereby boasting manageable CO2 capture, but also as a transformative method for cleanly and efficiently converting fuels into hydrogen, syngas, and other forms of energy [1,2].
In recent years, the chemical looping process has gained prominence as a promising avenue for converting a broad spectrum of feedstocks into hydrogen and syngas [3]. Unlike traditional fossil fuel gasification and reforming methods, chemical looping partial oxidation (CLPO) removes the necessity for an air separation unit, a water–gas shift reactor, or molecular oxygen from an air separation unit (ASU), thus resulting in increased gas efficiency and decreased fuel consumption [4]. Accordingly, CLPO can directly generate syngas with desirable H2:CO ratios and reduced CO2 content [5]. Compared to conventional technology, CLPO technology is capable of reducing investment into equipment and preventing the risk of explosions from pre-mixed methane and oxygen combinations. Moreover, basic investigations into the application of CLPO methodologies have also been explored in renewable energy systems, including the thermo-chemical decomposition of CO2/H2O to generate CO/H2 and O2. As the sources of raw materials are particularly prominent in industrial production, a wide range of feedstock sources, such as shale gas and natural gas, makes the chemical looping partial oxidation of methane (CLPOM) attractive compared to other fuels. The reaction process is as follows:
Moreover, CLPOM can obtain a favorable hydrogen-to-carbon ratio of syngas, which facilitates the synthesis, such as Fischer–Tropsch synthesis, of subsequent chemicals. Although there have been many review works on the stability and activity of CLPO, works discussing the design and activity of oxygen carriers (OCs) on the basis of methane are scarce.
This review examines the recent research into the redox chemistry of OCs within the framework of CLPOM, and it is driven by the imperative for environmentally friendly and effective approaches to material and energy transformations. We pay particular attention to analyzing and summarizing the selection and design of OCs to provide a more open and effective way through which to promote CLPOM.
2. Fundamentals of CLPO and CLPOM
CLPO involves partially oxidizing fuels through using oxygen from the air to generate syngas. In this process, the fuels undergo partial oxidation in the reducer reactor with the OCs, thereby resulting in the production of high-purity syngas. The reduced OCs are then regenerated in a fluidized bed combustor through oxidation reactions with air [4]. After regeneration by air, the OCs are replenished with oxygen and release heat, thereby allowing them to be recycled back to the previous step. In this case, the partial oxidation products include syngas and lower oxygen-containing hydrocarbons, such as olefins and aromatics, which are crucial intermediates in the field of energy and chemical engineering [6].
Refinement of the CLPO methodology represents a relatively modern advancement that has emerged from practices dating back to the early 1900s. This period saw the utilization of fixed beds containing iron ore, which were subjected to alternating cycles of syngas and steam, thereby marking the inception of commercial hydrogen production. During the 1950s, CLPO improved the interaction between solid iron oxide and steam when employed by fluidized beds. In the 1970s, the CO2 acceptor gasification process made significant progress, and this innovative approach involved using a gasifier equipped with CaO to convert coal into syngas. Not only did this facilitate the in situ removal of CO2, but it also enhanced hydrogen production through the water–gas shift reaction [7]. Thermochemical studies on the splitting of H2O and CO2 began to gain momentum in the 1980s, initiating a series of investigations into different thermochemical cycles. During the 1990s, researchers discovered that the lattice oxygen atoms from cerium oxides could enhance methane undergoing partial oxidation to produce syngas [8,9,10]. An additional proposition suggested leveraging solar energy to heat fluidized iron oxides, thus driving methane to undergo partial oxidation in order to produce syngas. This concept shares a similar foundational direction with the development of chemical looping [11,12]. Both General Electric and Alstom have independently researched systems with three reactors to utilize coal in order to produce hydrogen with O2 carriers and CO2 carriers [13]. Starting in 2018, a 1 MWth chemical looping gasification pilot unit was upscaled in China by the ENN Energy Research Institute, whose target is to produce syngas with rich hydrogen [14]. At the same time, Ohio State University has produced hydrogen from coal-derived syngas and natural gas feedstocks in a high-pressure chemical looping plant [15]. In this setup, moving beds are used as the reducer and oxidizer functions, while a fluidized bed is used as the combustor operates. They are also driving bench and sub-pilot scale experiments to progress coal-to-syngas and shale-gas-to-syngas processes [16,17,18]. In essence, advancements in CLPO technology are primarily driven by urgent demands for the economically efficient production of hydrogen and carbon monoxide with high purity [19].
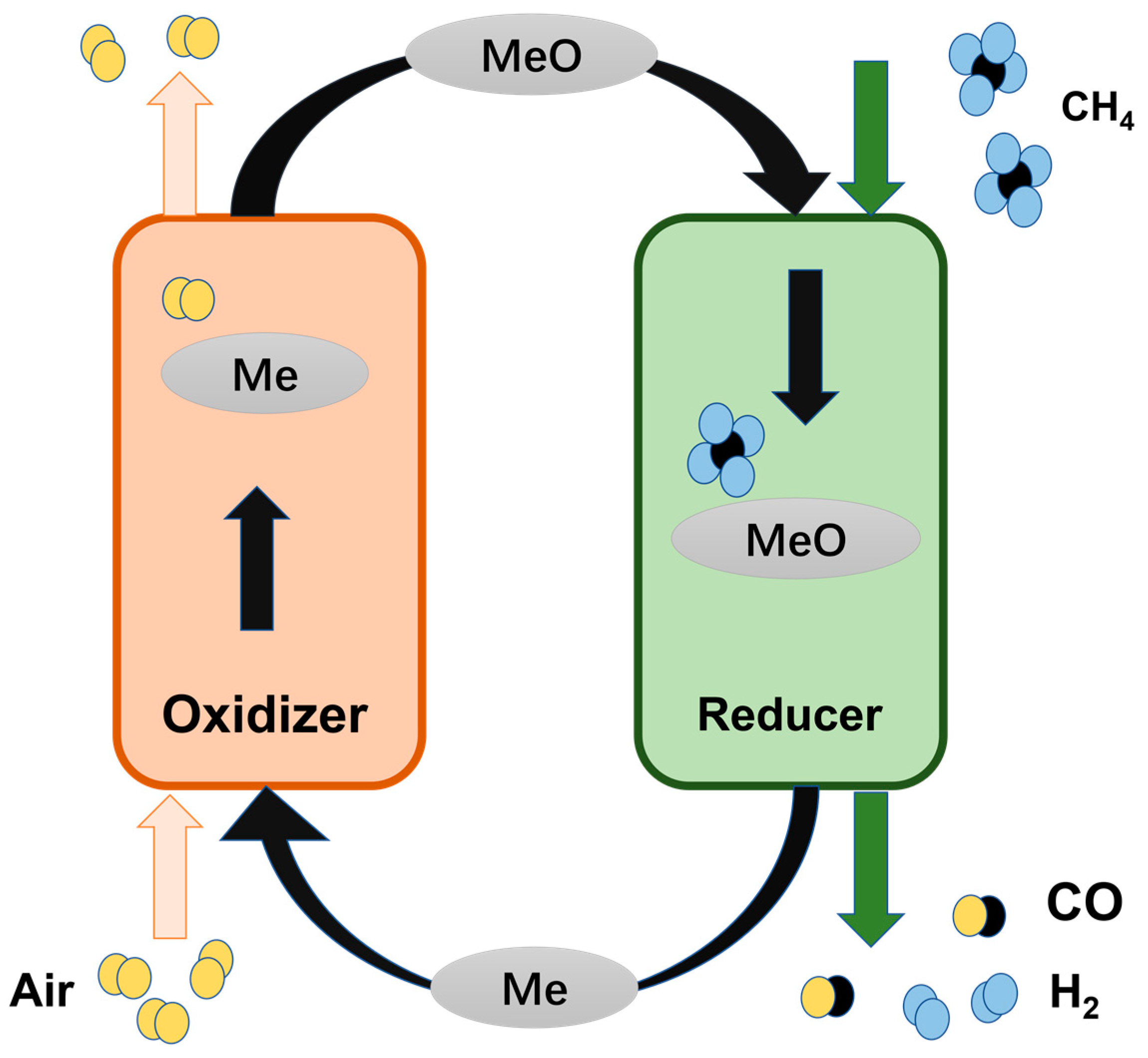
The CLPOM process involves oxidizing CH4 with the lattice oxygen from OCs to produce syngas, which is followed by re-oxidizing the reduced OCs with air to complete one cycle, as shown in Figure 1. This method circumvents direct contact between CH4 and oxygen, effectively minimizing the presence of N2 in the effluent by spatially dividing CH4 conversion into two steps. Additionally, this approach demonstrates the possibility of directly generating high-quality syngas with preferred H2/CO ratios [1], the process of which is shown in Figure 1. Zheng et al. investigated the production of H2 by a plasma-assisted chemical looping system that enabled the partial oxidation of CH4 to occur at moderate temperatures (573–773 K) [20]. Qin et al. demonstrated that iron-based OCs, with the addition of a low concentration of copper, can dramatically enhance the reactivity in the CLPO processes at low temperatures while maintaining the recyclability of these carriers. At seven hundred degrees, doped oxygen carriers can increase methane conversion by 470% compared to non-doped ones [21]. The core of the CLPO technology is OCs, and now we provide a review of OCs [8,22].
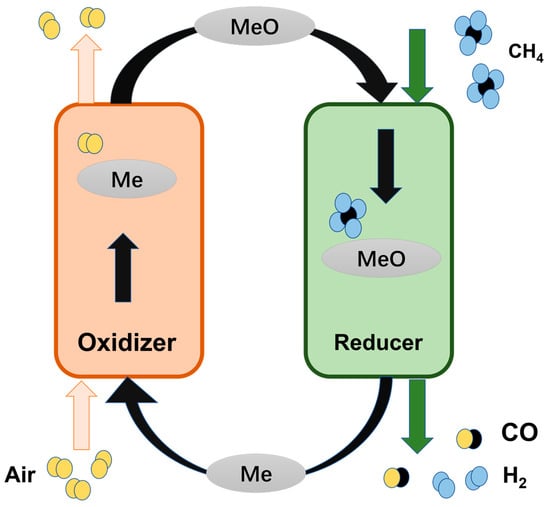
Figure 1.
The mechanism diagram of CLPOM.
3. Synthesis Methods of OCs
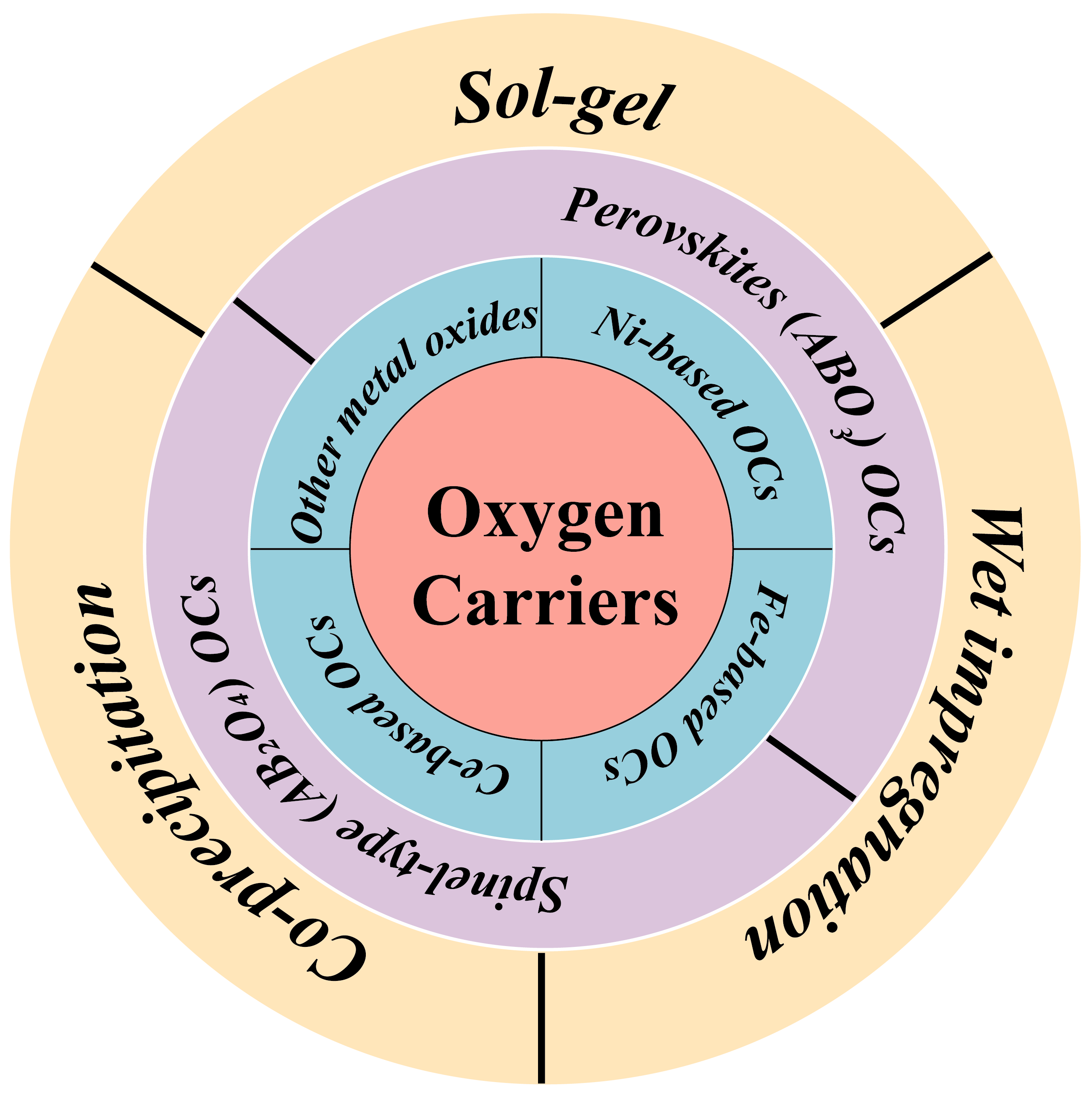
The synthesis method will affect the lattice oxygen distribution of the oxygen carriers, as well as the characteristics of the loaded doping, thereby leading to different catalytic activity and stability. Common oxygen carriers and methods of synthesis are summarized in Figure 2.
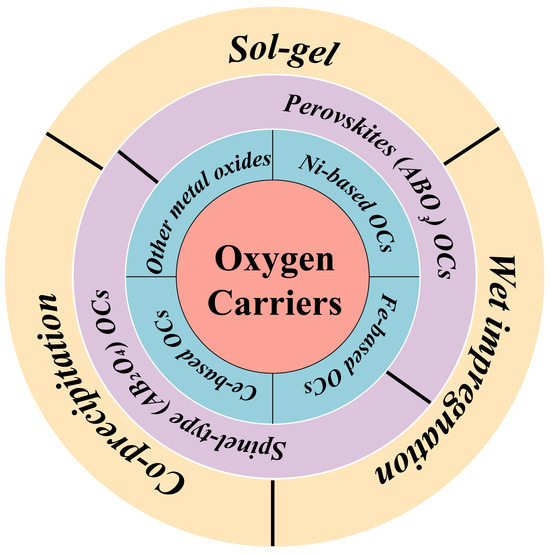
Figure 2.
Classification and preparation of OCs.
Liu et al. synthesized 5NiO/(xCeO2-Al2O3) oxygen carriers by two-step impregnation. The stable and highly active oxygen carrier was synthesized to minimize the formation of NiAl2O4 and the Ni2+ doping into the CeO2 lattice. The active Ni-O-Ce oxygen species can be devoted to partial oxidation performance, and the deactivation was found to be negligible for 5NiO/(40CeO2-Al2O3) over 20 cycles [23]. For stable monoatomic deposition, Carrillo et al. employed the exsolution technique to achieve consistent monoatomic deposition, thus resulting in evenly distributed Ru nanoparticles that were firmly attached to the cerium oxide framework. This ensures the enduring microstructural integrity and catalytic efficacy of the material throughout extensive cycling. Through this approach, the resilient Ru nanoparticles generated could enhance syngas generation via CLPOM combined with CO2 splitting, a process that is as of yet unexplored for ceria-based substances [24]. Li et al. synthesized a range of xwt% Ni/LaFeO3 samples via the wet impregnation technique. Their investigation revealed that the methane conversion and product selectivity are predominantly influenced by the nickel loading, with 5wt% Ni/LaFeO3 oxygen carriers exhibiting elevated CH4 conversion (95.2%) and CO selectivity (85.1%). These findings indicate the viability of enhancing the activation of lattice oxygen in LaFeO3 perovskite oxygen carriers through surface modification with NiO, thereby exerting a significant impact on their catalytic performance [25].
The sol–gel method disperses raw materials into a solvent to form a low-viscosity solution to obtain homogeneity at the molecular level in a very short period of time; in the formation of the gel, the reactants are likely to be homogeneously mixed with each other at the molecular level. Thus, it has received a great deal of attention in the preparation of oxygen-carrying systems, especially for chalcogenide-type catalysts. Yao et al. synthesized the LaNixTi1−xO3−δ (x = 0, 0.25, 0.5, 0.75, and 1) perovskite oxides by a sol–gel method. The sol–gel process enabled precise control over their physical and chemical properties, thereby creating materials with high purity, excellent homogeneity, controllable morphology, and unique microstructures.. They used quasi in situ XPS to characterize the compounds, and they found that the relative concentration of partially oxidized nucleophilic lattice oxygen species increases with titanium substitution [26]. Zhu et al. synthesized a series of La1−xFeO3−δ oxygen carriers with A-site cation defects by sol–gel method. Tuned A-site cation defect engineering reduces the crystallite size of La1−xFeO3−δ perovskites, which effectively increases the oxygen vacancy concentration and thus improves the catalytic performance of CH4 conversion and CO2 splitting [27]. Zhao et al. synthesized La0.95Ce0.05NixFe1−xO3 by an improved sol–gel method (i.e., the Pechini method). The H2/CO molar ratio was maintained at the optimal value of 2.0 for a Fischer–Tropsch synthesis, which was conducted throughout the methane partial oxidation process. Afterward, near-100% hydrogen concentrations (i.e., higher than 99.6% and 99.5%) were observed on the La0.95Ce0.05Ni0.2Fe0.8O3 and La0.95Ce0.05Ni0.5Fe0.5O3 samples during the steam splitting process, thus indicating that an additional separation step for pure hydrogen acquisition can be omitted [27]. Ma et al. synthesized La-Fe-O perovskite-type oxygen carriers by the sol–gel auto-combustion method. They reported LF as the basic oxygen carrier and attempted to optimize the conventional gasification processes by introducing highly active molecular oxygen through the doping of the three most representative oxygen uncoupling elements (Cu, Mn, and Co) [28]. Lee et al. presented OCs with a surface enrichment of lanthanum on Co3O4. Their study identified that the optimal lanthanum doping concentration was 10wt%. The Co3O4-containing 10wt% lanthanum exhibited an average CO2 selectivity of 93.1% and a CH4 conversion of 95.4% at 800 °C over 50 cycles. This surface enrichment not only imparts resistance to sintering, but also enhances oxygen storage capacity, thus ensuring a prolonged cyclic chemical looping combustion performance [29].
For the same sample, different synthesis methods can have different effects on the loading of oxygen carriers. Ding et al. synthesized BaCoO3−δ OCs, which were supported by CeO2 with varying component distributions, using different methods such as dry mix, sol–gel, and co-sol–gel approaches. The dry mix sample exhibited a patchy and loosely attached interface between BaCoO3−δ and CeO2, thereby suppressing the synergistic effects between perovskite and CeO2, as well as impeding the mobility of oxygen species. Consequently, this led to a lower gas production of syngas and hydrogen. In contrast, the sol–gel sample featured a macroporous structure with dense contact between perovskite and CeO2, thereby enhancing the gas–solid contact and synergistic effects, as well as improving reaction selectivity and gas production. However, the co-sol–gel sample, while showing uniform component distribution, contained impurities like BaCeO3, BaCO3, and Co3O4, along with a lower fraction of perovskite on the surface, thus resulting in a relatively low reactivity in the partial oxidation of methane, as well as noticeable secondary methane decomposition reactions. In transitioning from the dry mix to the sol–gel and then to the co-sol–gel sample, the contact between BaCoO3−δ and CeO2 gradually intensified. The H2 selectivity of the co-sol–gel sample exceeded the ideal value of 1.0, reaching approximately 4.1, while the dry mix and sol–gel samples exhibited a H2 selectivity close to 1.0. These findings suggest that the co-sol–gel sample exhibited significant methane decomposition, yielding additional H2 and raising the H2/CO ratio above the ideal value [30].
4. Design Strategies of OCs
The CLPOM method is aimed at creating a closed loop, so identifying oxygen carriers that are capable of storing and releasing oxygen is crucial to the entire technology [31]. The predominant feature of OCs is their extensive surface area, which guarantees minimal resistance to transport between the external atmospheric air and the particle surface, so they typically consist of porous solids. Generally, OCs are composed of reducible MOx compounds, where they serve the following three primary functions: generating oxygen ions or vacancies and electrons or holes, facilitating their diffusion within the bulk phase, and providing active sites for surface reactions. In general, OCs consist of reducible MOx compounds with the following three main roles: generating oxygen ions and vacancies or electrons and holes, accelerating their transfer, and offering active sites for the reaction. In the reduction step, OC particles absorb thermal energy and acquire reactive oxygen species at high temperatures, and then oxygen anions migrate from the particle surface to the interior of the particles down the chemical potential gradient while the countercurrent flow of electrons is induced to maintain electroneutrality, which balances this migration. Subsequently, a redox reaction occurs on the particle surface and products are formed. Oxygen ions and electron holes undergo surface exchange and pass inward to combine with oxygen vacancies inside the particle [32]. Zhang et al. comprehensively reviewed the classification of oxygen carriers and the application scenarios of CLPO, and they proposed measures to enhance the reactivity and stability of oxygen carriers; for example, through metal–metal interactions, metal–support synergism, pathways of oxygen vacancy generation, de-activation resistance, etc. [3]. Liu et al. demonstrated that iron oxide nanoparticles embedded in a mesoporous silica matrix can effectively inhibit the complete oxidation of carbon in the reaction of CLPOM [33].
The OCs in the chemical looping processes need to meet the following requirements to achieve high activity and stability in practical applications. First, these carriers need to have stable redox properties and need to remain in two or more states. At the same time, the oxygen carriers also need to have an advantage in terms of reaction kinetics/thermodynamics, which is the key to increasing the reactivity. Mechanical and thermal stability must also be taken into account, and, of course, the cost is not to be ignored. Reactions involving oxygen carriers entail the generation and transfer of various oxygenic species within the bulk phase. Depending on the specific surface reaction pathway, these reactions can yield different products. Typically, nickel- and iron-based oxides are regarded as good oxygen carriers for CLPOM due to their good thermodynamic properties and high reactivity, and spinel- and tremolite-type metal oxides are also widely employed for CLPOM. Recent work on the utilization of OCs is summarized in Table 1.

Table 1.
Key parameters of the oxygen carriers used for syngas production via CLPOM.
The next section will focus on single-metal oxide materials and complex metal oxide materials to summarize the research results.
4.1. Single-Metal Oxide
4.1.1. Ni-Based OCs
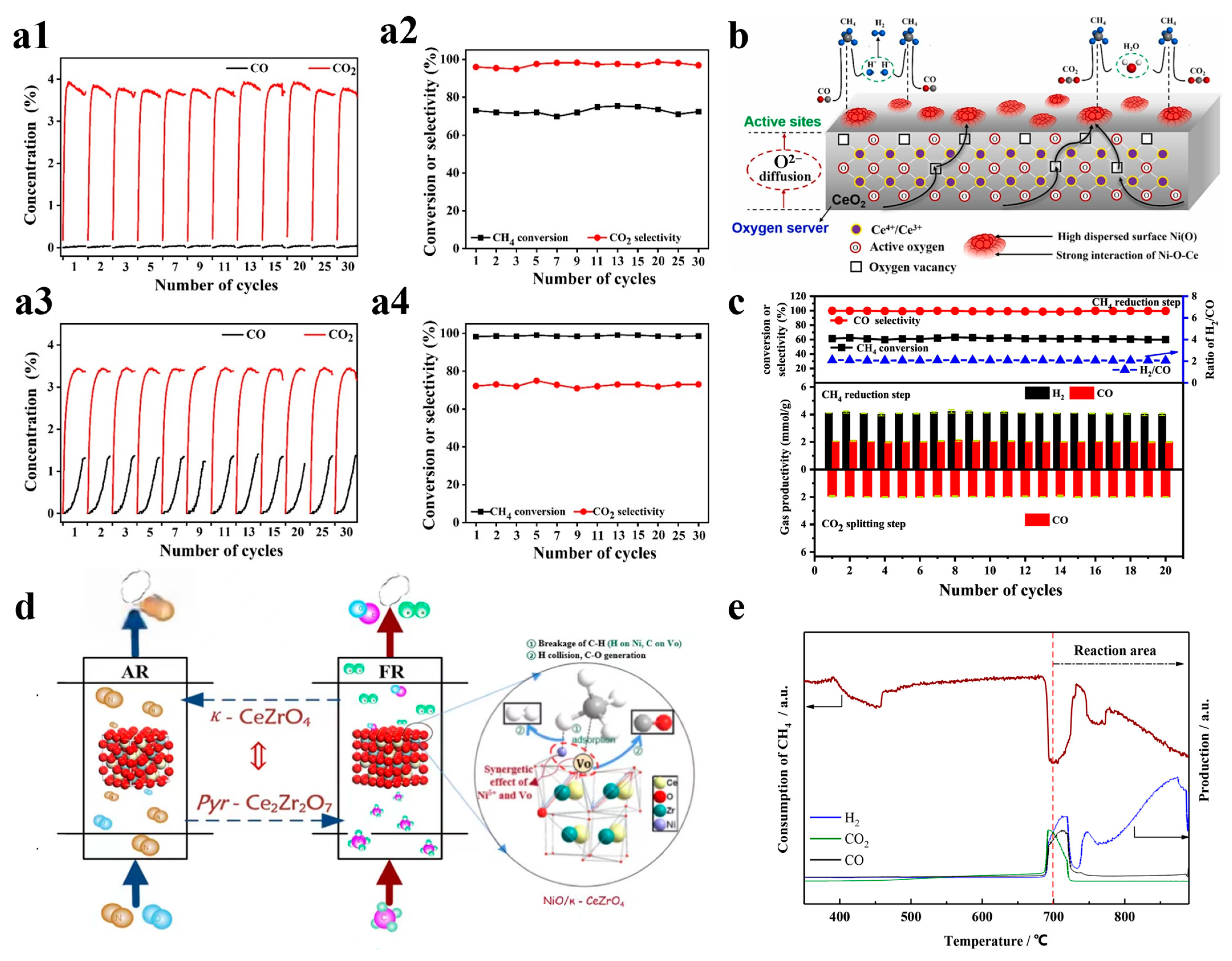
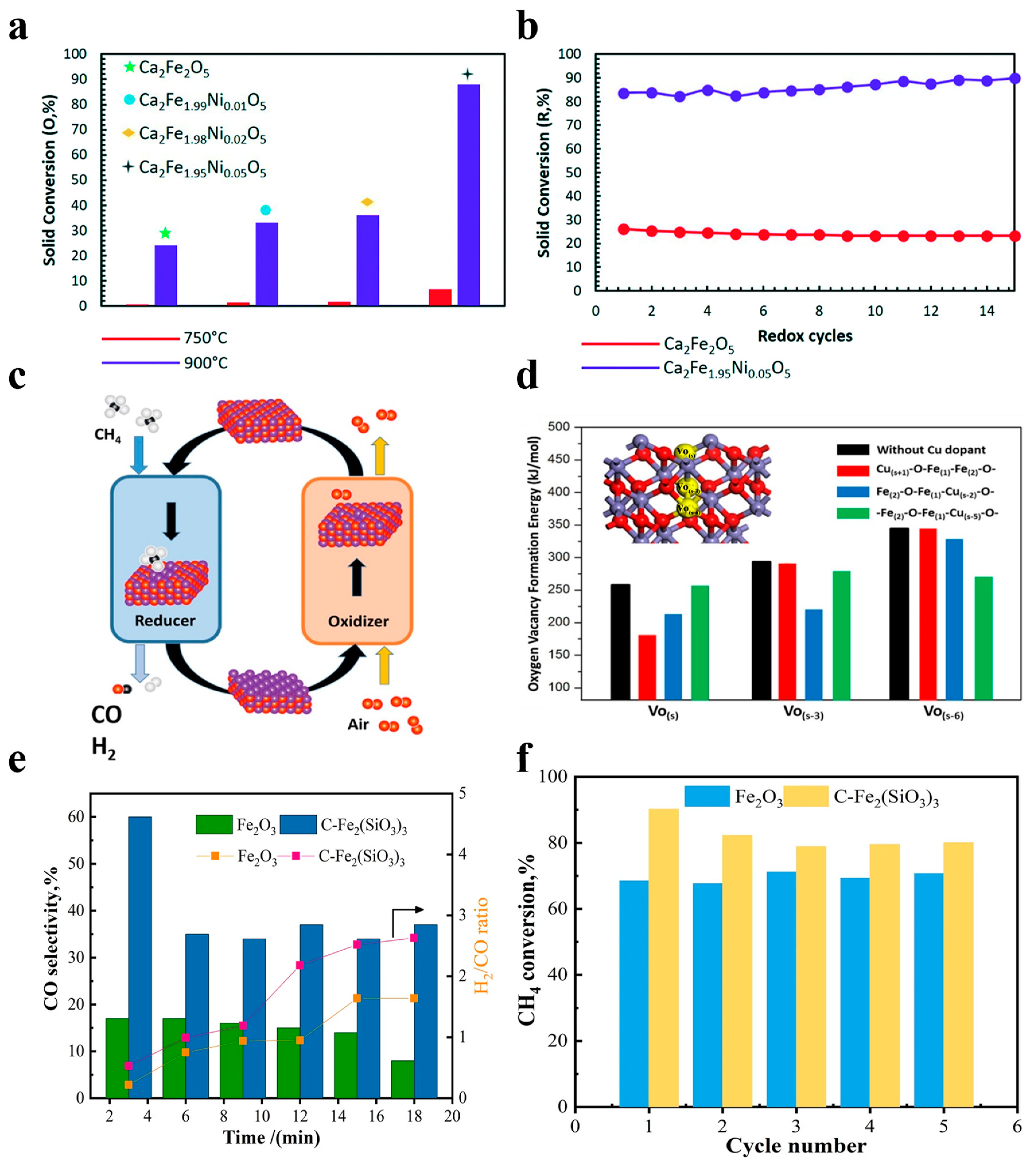
Nickel-based OCs, particularly NiO, have been widely employed in CLPOM because of their high potential in breaking C-C and C-H bonds during methane conversion and their reported ability to enhance ilmenite ore reactivity for syngas production. Long et al. prepared an OC consisting of two different parts, and they found that a reasonable design can fully utilize the advantages of the two parts where there is no carbon buildup, as shown in Figure 3(a1–a4). They also found that CuO/cordierite and NiO/cordierite have high conversion rates for both CO2 and CH4 in each cycle, with high selectivity for CO Regarding the CH4 conversion, the two fractions maintained a high conversion in the reaction, as well as a strong cyclic stability [48]. Liu et al. described the dynamic migration of the oxygen species on cerium oxide-based NiO OCs in CLPOM, and they found that the content of oxygen species was directly correlated and linearly related to the rate of CO generation; meanwhile, the consumed Ni-O-Ce oxygen species were supplemented by the lattice oxygen in CeO2, as shown in Figure 3b [23]. Li et al. prepared xNiO/(CeO2-MgO) OC and demonstrated that the activity of CH4 and CO2 conversion increased significantly with the addition of NiO, and the addition of 10wt% to the NiO increased the CH4 conversion from 15.62% to 59.64% at 800 °C. In addition, the stability was also greatly improved due to the formation of the CeO2-NiO and NiO-MgO double solid solutions. At the same time, due to the formation of a CeO2-NiO and NiO-MgO double-solid solution, the stability was greatly improved, as shown in Figure 3c [49]. Zhang et al. used NiO-doped κ-CeZrO4 for CLPOM, which reduces the energy compensation for methane conversion and takes place at low temperatures due to its excellent lattice oxygen transport properties and stable structure. It is worth noting that high methane conversion (52–72%) and syngas selectivity (88–92%) rates were obtained in a relatively low-temperature range (700–800 °C), which is competitive when compared to all the contemporary reports, as shown in Figure 3d,e [50]. Chein et al. utilized 15wt% NiO/Al2O3 as the OCs and achieved yields of H2 and CO approaching the theoretical amounts. The impact of OC loading and reaction temperature was then summarized [39]. Similarly, incorporating nickel as a doping element into other OC catalysts significantly enhances catalytic activity. Shah et al. prepared Ca2Fe2O5 OCs with different nickel doping concentrations as a recirculation medium for use in CLPOM, as shown in Figure 4a,b [51].
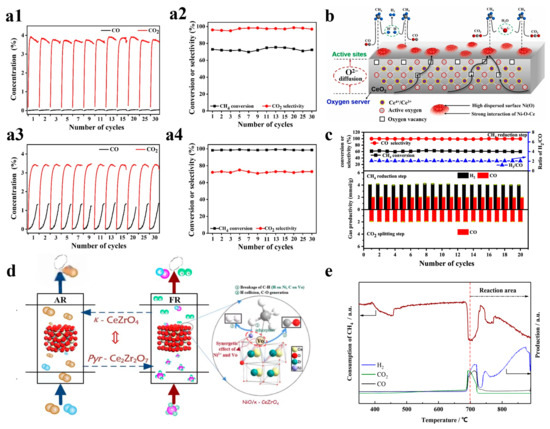
Figure 3.
(a1) CuO/cordierite monolithic oxygen carriers and (a2) CH4 conversion and CO2 selectivity for different cycles. (a3) CuO/cordierite monolithic oxygen carriers and (a4) NiO/cordierite monolithic oxygen carriers. Copyright 2021 Elsevier [48]. (b) Dynamic oxygen migration and reaction over ceria-supported nickel oxides in the chemical looping partial oxidation of methane. Copyright 2023 Elsevier [23]. (c) Redox cycle reactivity and a stability of 10% NiO/(CeO2-MgO) during multiple redox cycles. Copyright 2022 Elsevier [49]. (d) NiO/κ-CeZrO4 functional oxygen carriers with Niδ+ and oxygen vacancy synergy for the chemical looping partial oxidation reforming of methane. (e) Consumption of CH4 with temperature increase. Copyright 2021 Elsevier [50].
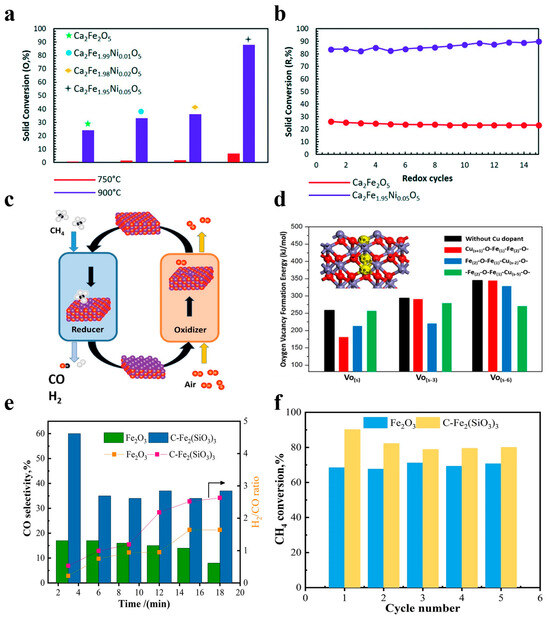
Figure 4.
(a) Solid conversion during the oxidation of the reduced sample with CO2 and (b) isothermal CH4–CO2 redox cycles at 900 °C and 1 atm. Copyright 2021 Royal Society of Chemistry [51]. (c,d) Enhanced methane conversion in chemical looping partial oxidation systems using a copper doping modification. Copyright 2018 Elsevier [21]. (e) CO selectivity and the H2/CO ratio. (f) CH4 conversion of Fe2O3 and C-Fe2(SiO3)3 during five cycles. Copyright 2023 Elsevier [34].
However, Ni is difficult to restore to its original state due to carbon accumulation and particle sintering at high temperatures after several cycles. Metallic nickel is also slightly insufficient for the reduction of H2O to produce H2, which also limits the application of Ni-based catalysts in CLPOM [52,53].
4.1.2. Fe-Based OCs
Fe-based OCs are widely used in practice because they are rich in lattice oxygen and a possess variety of oxidation states of iron [2,54,55,56]. In light of this issue, Fe2O3 is a widely developed and designed material for CLPOM, especially when coupled with a fitted reactor, where the advantages of its multiple oxidation state properties are strongly amplified [2]. Particularly, Fe2O3 is more effective than NiO for hydrogen generation under steam conditions [57].
In the realm of Fe-based OCs, previous studies have frequently incorporated other metals for various objectives. The lack of a deep reduction in Fe2O3 OCs is an important issue in the chemical cycle hydrogen production process, and it is an important area of concern for scientists. Feng et al. modified the reducibility of Fe2O3 OCs by doping them with different dopants. They evaluated 18 potential dopants and screened 8 ideal candidate dopants for modifying Fe2O3 OCs, and these dopants significantly enhanced the reduction process of Fe2O3 to Fe3O4 to FeO [58]. Meanwhile, Lang et al. demonstrated that a low concentration (1%) of copper dopant in Fe-based OCs can significantly improve the reactivity and recoverability of the CLPOM process at low temperatures, as shown in Figure 4c,d [21].
In heavy oil upgrading, the use of transition metals as oxidizing agents for partial oxidation has received widespread attention in recent years. Wang et al. conducted synthesized Fe2O3 samples doped with different metals, which they used in CLPO for the production of light fuels from heavy oil. Their results showed that the yield of light fuels is strongly influenced by the specific surface area and the concentration of oxygen species, and that Ca-doped iron-based oxygen carriers have higher yields [59]. Fang et al. prepared Ni/Cu/FeSO4, Ni/Cu/FeSiO3, Fe2(SO4)3, and Fe2(SiO3)3 for CLPO, and their results showed that the silicate of iron can significantly improve the conversion of methane compared to iron oxides. In addition, the optimal experimental conditions were found to be 800–850 °C when the aim is to maintain the pressure of one atmosphere. They found that the conversion of methane could reach 95%, which is an increase of 272%, as shown in Figure 4e,f [34].
In addition to doping metals into iron-based OCs, previous studies have explored replacing other metals with iron or modifying other OCs to enhance stability and reactivity. Zhu et al. used the Fe-substituted La-hexaaluminate (LF3A) in CLPO for syngas production, and they then compared it with conventional Fe-based oxygen carriers. They found that LF3A exhibited high reactivity and stability during the 50 cycles of the reaction process. The deep reduction did not destroy the hexaaluminate structure, and there was an internal charge compensation mechanism so that the activity toward CH4 and the utilization of CO2 could be improved. It was also found that the use of CO2 as the sole oxidant was more favorable to improve the selectivity than the use of oxygen, and their study provided better design ideas for the chemical state of the lattice oxygen of the oxygen carriers, the design of microstructures, and the conformational relationship between the CLPOM [60].
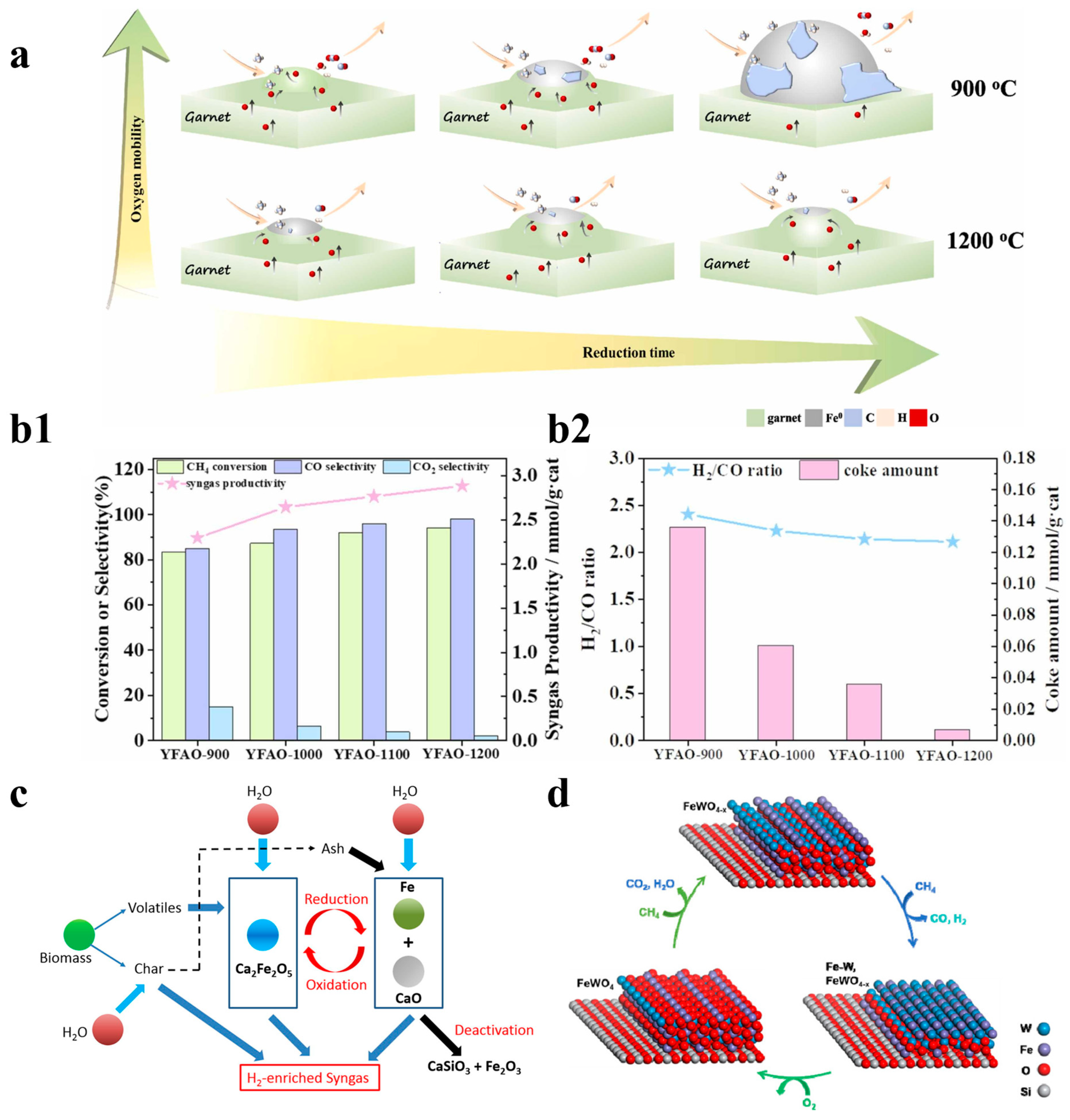
Previous studies have also explored composite methods involving iron combined with other metals. Wang et al. used Fe-Mn-mixed metal oxides for partial oxidation experiments of vacuum residue, and they found that the gasoline and diesel yields of Fe-Mn-mixed metal oxides increased; that study provided a new oxygen carrier setup idea for the production of lightweight fuels [59]. Zong et al. studied the influence of the encapsulation degree of Fe0 active sites on the performance of garnets for CLPOM. They found that oxygen mobility is closely related to temperature, where the number of surface oxygen vacancies increases when the temperature rises. Moreover, at higher temperatures, Fe0 is exposed less and less, thus resulting in the formation of large particles. The addition of garnet to iron includes not only the ability to introduce methane active sites, but also promotes the scavenging of the formed carbon, and their work demonstrated that oxygen mobility is modulated through strong metal–support interactions to improve the coke resistance of iron-based OCs, as shown in Figure 5a,b1,b2 [35].
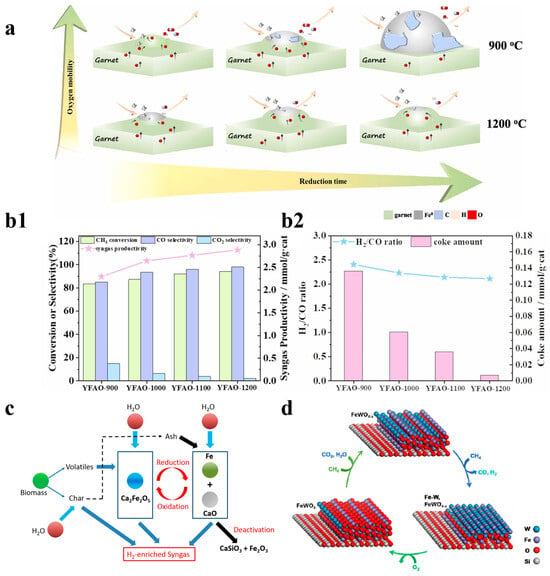
Figure 5.
(a) The conversion, selectivity, syngas productivity, and (b1) H2/CO ratio, as well as the amount of coke in the YFAO-T OCs. (b2) The conversion, selectivity, and syngas productivity. Copyright 2022 Elsevier [35]. (c) Chemical looping gasification of biomass, with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Copyright 2020 Elsevier [61]. (d) The mechanism of optimizing FeWOx/SiO2. Copyright 2020 Elsevier [62].
However, Fe-containing OCs can catalyze the side-reaction methane decomposition throughout the CLPO, which leads to carbon deposition on their surfaces and causes a variety of adverse effects. To solve this problem, Donat et al. doped cobalt into iron as a second redox active metal, thereby forming a bimetallic reduction structure with iron, which not only changed the activation path of the methane decomposition, but also controlled the extent of reduction and reduced the formation of carbon accumulation. In addition to the carbon deposition, coke was also found to be a tricky problem in iron-based catalysts because, during the activation of methane, the temperature is usually very high and site clashes of Fe at high temperatures can lead to coke production [63]. Hu et al. doped the oxides of Ca in iron-based oxygen carriers and investigated their catalytic effect in the CLPO of rice straw; the results showed that the highest hydrogen production of the 23.07 mmol/g of biomass was achieved when Ca was added in equal proportions, which suggests that the presence of Ca facilitates the further reduction and oxidation of the iron-based oxygen carrier, which ultimately achieves hydrogen production, as shown in Figure 5c [62]. Regarding this issue, Liu et al. designed FeWOx-based OCs for CLPOM, which greatly improved the catalytic activity of CLPO because of its stable structure. It was shown that the catalyst could significantly increase the total methane conversion and CO selectivity compared to unmodified WO3/SiO2. These performances can be attributed to its high lattice oxygen content, its high catalytic activity at the bimetallic interface, etc., as shown in Figure 5d [63]. In addition, it was found that the deactivation of pure Fe2O3 still exists at high temperatures, mostly due to the sintering and agglomeration effects that occur at high temperatures, and that more deactivation-resistant, iron-based oxygen carriers are needed [64,65,66,67].
4.1.3. Ce-Based OCs
With the popularization and application of renewable energy, solar energy as an energy source for producing syngas has become a promising production technology. Coupling solar energy with CLPO technology relies on the stability and catalytic activity of the intermediates of metal compounds, and, cerium, as a multivalent metal element, has attracted attention because of its outstanding optical properties and its ability to promote the separation of carbon dioxide.
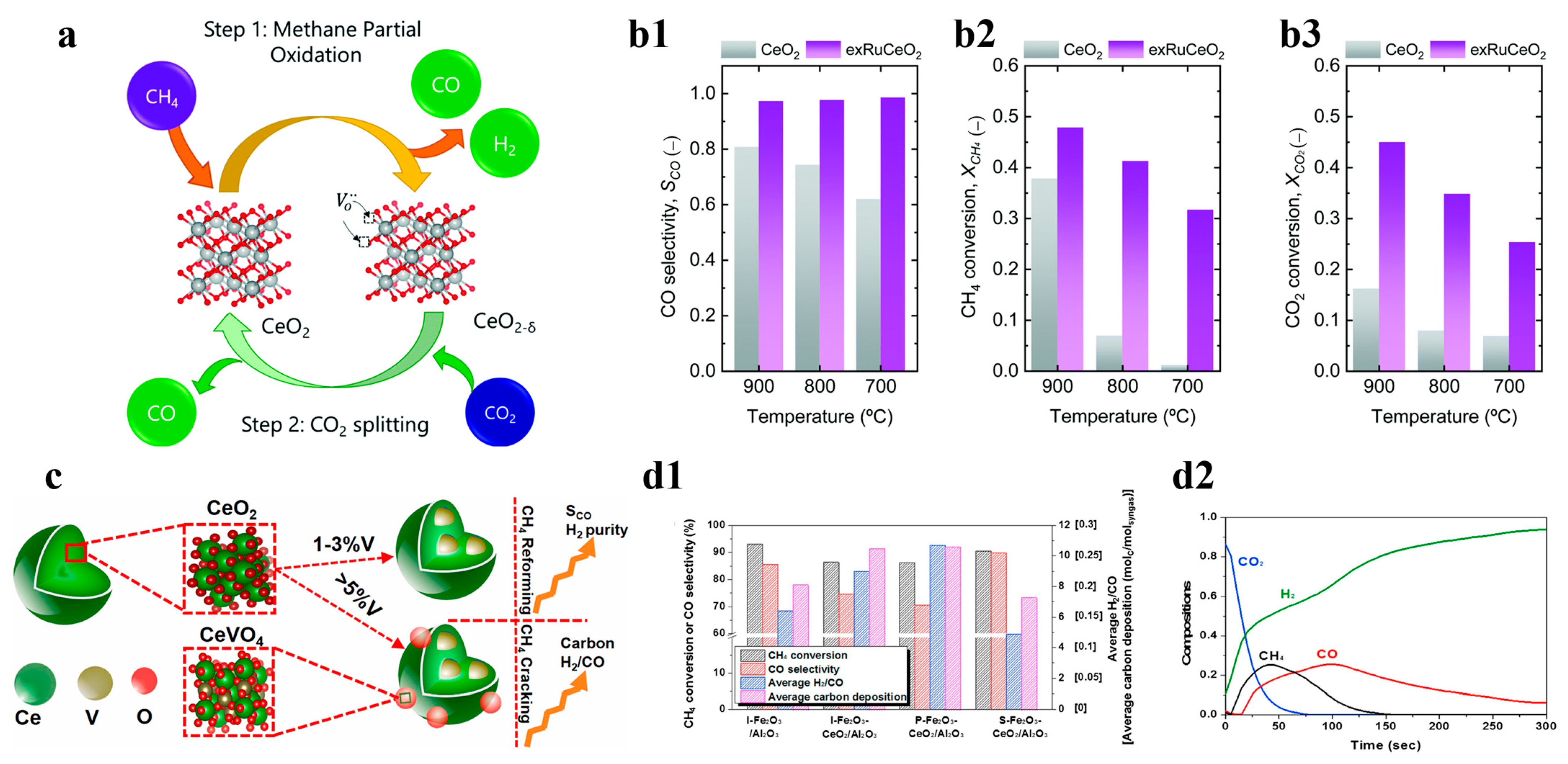
Compared to other oxides, cerium is characterized by low oxygen exchange or supply capacity leading to its slow CLPO reaction kinetics, and these problems can be solved by doping with other reactive cations, as well as by surface modification [68,69]. To address this challenge, Carrillo et al. immobilized Ru nanoparticles on the cerium oxide skeleton using an exsolution method to ensure its cyclic catalytic activity and structural stability. The catalysts designed in this scheme could greatly improve CO selectivity and H2 generation rate, which doubled when compared with a single CeO2 at 700 °C, whereas the selectivity of carbon monoxide at a higher temperature of 900 °C was somewhat reduced to 80%, as shown in Figure 6a,b1–b3 [24]. Asim et al. used vanadium- and cerium-doped materials to separate the CO2 and H2O in CLPO in order to prepare syngas. They found that, as vanadium doping increased, the lattice inhalation of cerium increased and saturated to form CeVO4, which led to vanadium atoms migrating to the surface of the powder to cause methane cracking; meanwhile, the lattice vanadium atoms provided an active site for the redox reaction to promote cycling, which was achieved over 200 cycles of operation. The catalyst was stable for 200 cycles and yielded up to 4.5 mmol−1/cycle, as shown in Figure 6c [70]. Ceria is also frequently employed as a carrier to improve the utilization of OC feedstock. Kang et al. added a small amount of CeO2 to iron oxide carriers and used it in CLPO to produce syngas. Due to the addition of CeO2, the selectivity of the syngas was improved, and high-purity syngas production with H2/CO equal to two was realized. Moreover, it was found that the ideal ratio of syngas could be obtained by controlling the feed ratio in the production, as shown in Figure 6(d1,d2) [71].
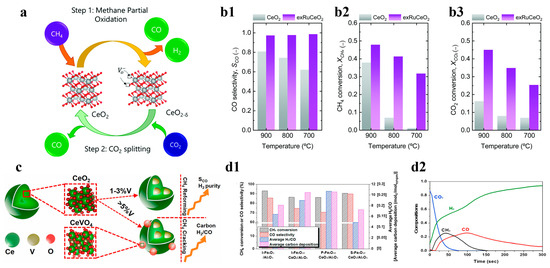
Figure 6.
(a) Schematic of the chemical looping reforming of the methane process. Comparison of (b1) selectivity, (b2) CH4 conversion during the MPO step, and (b3) CO2 conversion during the CO2 splitting step for the two materials tested. Copyright 2021 Royal Society of Chemistry [24]. (c) The mechanism of optimizing the vanadium (V5+) doping to the CeO2 lattice. Copyright 2021 Elsevier [70]. (d1) Comparison of the results of CLPD (CO2/CH4 = 0.28) on the oxygen carriers. (d2) compositions of the gaseous product streams at the 20th cycle of CLPD on the S-Fe2O3-CeO2/Al2O3 under the fixed CO2/CH4 ratio. Copyright 2018 Elsevier [71].
4.1.4. Other Metal Oxide-Based OCs
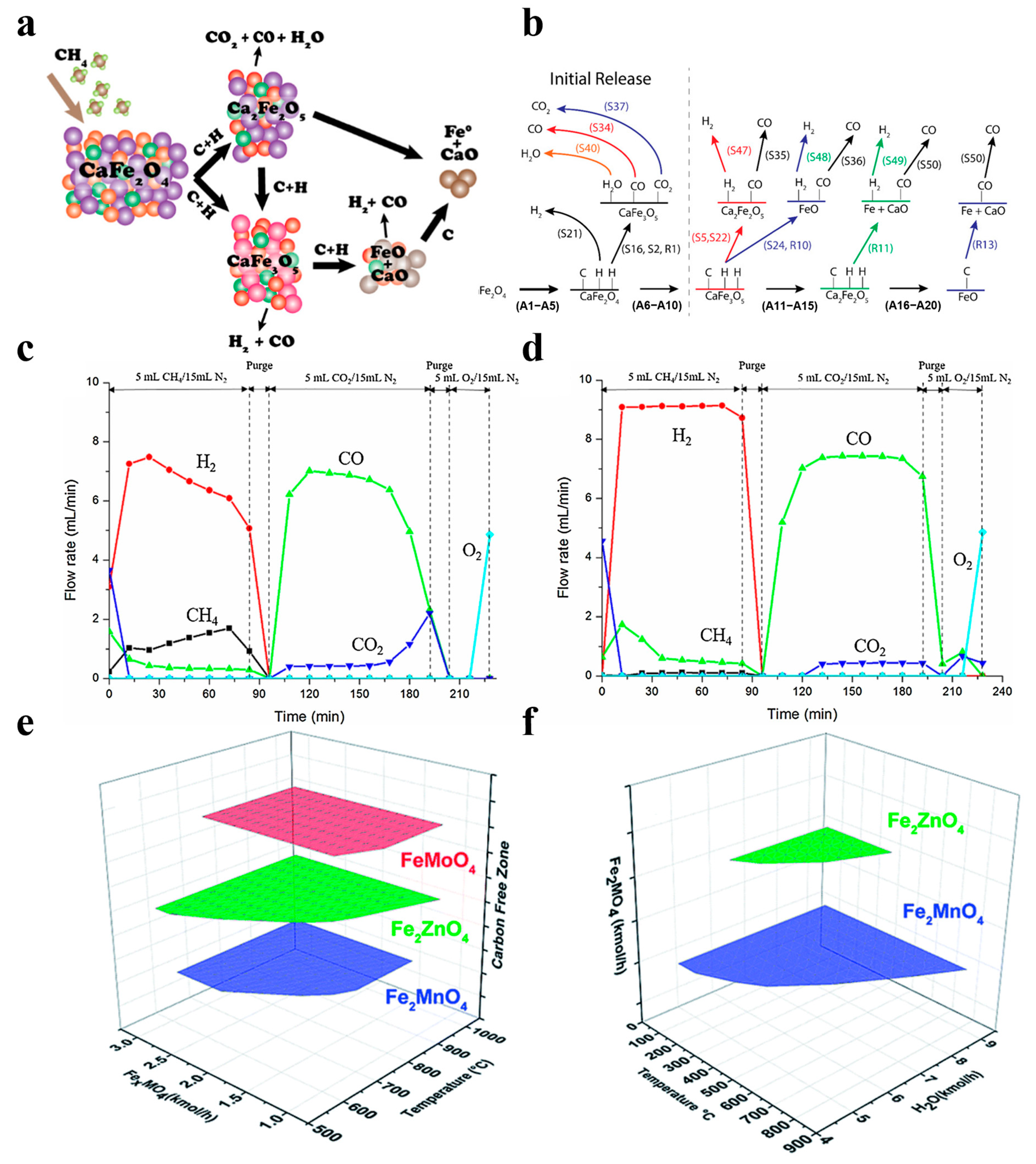
In addition to these three extensively researched metal oxides, the selection of oxygen carriers for CLPO also includes several other metal oxides with excellent properties. Chen et al. designed WO3-based oxygen carriers, which have strong high-temperature resistance and a sufficient lattice oxygen source, and they also introduced nickel oxide clusters to further improve the availability of lattice oxygen and the activation ability of methane, which was found to increase the methane conversion by about 2.7 times [72]. Miller et al. found that calcium ferrate can deeply reduce methane and selectively undergo carbon oxidation, thus benefiting the production of syngas in desired proportions, and they used thermodynamic analysis and DFT to give a reaction mechanism for the overall reaction, as shown in Figure 7a,b [73].
4.2. Complex Metal Oxide
Complex metal oxides can be designed with a greater surface area, higher thermal stability, and strength than mono-metallic substances, thus ensuring their superior heat and mass transfer, especially in the context of oxygen transfer. As a result, they have excellent catalytic, syngas-selective, and redox properties [2,74].
4.2.1. Spinel-Type (AB2O4) OCs
The spinel structure has a special structure of A-O tetrahedra and B-O octahedra, which promotes synergistic interactions between the constituent metals and results in composite metal oxides with excellent redox properties [75,76,77]. Hyun et al. synthesized Fe2O3-NiO/La0.8Sr0.2FeO3 spinel particles and used them for a CLPO of methane, and they found that the reduced particles were converted to CO after oxidation of CO2 in 80 min, which saw the conversion maintained at 93%. The formation of NiFe2O4 increased the oxygen-carrying capacity and improved the activity and stability during the cycle. CO reduction in the two OC samples was conducted, and the results are shown in Figure 7c,d [78]. Zhang et al. prepared different structures for the CaFe2O4, Ca2Fe2O5, and FeAl2O4 metal oxides as oxygen carriers for CLPO. CaFe2O4 is considered to be a more promising oxygen carrier due to its special structure, fast reaction rate, high oxygen-carrying property, and carbon monoxide selectivity, which has gained wide attention in practical applications [79]. Virginia et al. synthesized the spinel structures of compounds such as FeMoO4, Fe2ZnO4, and Fe2MnO4, and then used them in a redox chemical chain cycle of CH4-H2O. They found that the thermodynamic and sensitive process simulations of Fe2MnO4 allowed for the highest syngas yields and high-purity hydrogen production in addition to the advantage of complete regeneration with the assistance of steam, as shown in Figure 7e,f [80].
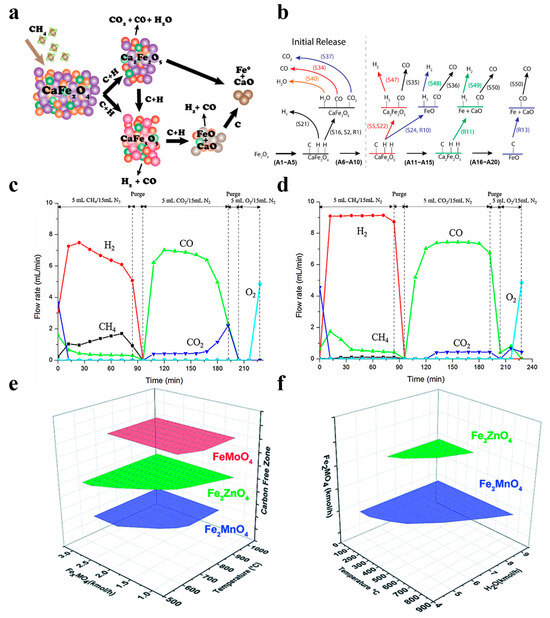
Figure 7.
(a,b) Interaction of methane with calcium ferrite in the chemical looping partial oxidation application. Copyright 2019 American Chemical Society [73]. (c) Gas evolution profiles of the O2–CLRD process with a fresh particle. (d) The 10-cycle-spent particle. Copyright 2018 Elsevier [78]. (e) Fuel reactor carbon-free operating conditions. (f) Regeneration reactor full-oxygen carrier steam oxidation conditions. Copyright 2021 Royal Society of Chemistry [80].
Figure 7.
(a,b) Interaction of methane with calcium ferrite in the chemical looping partial oxidation application. Copyright 2019 American Chemical Society [73]. (c) Gas evolution profiles of the O2–CLRD process with a fresh particle. (d) The 10-cycle-spent particle. Copyright 2018 Elsevier [78]. (e) Fuel reactor carbon-free operating conditions. (f) Regeneration reactor full-oxygen carrier steam oxidation conditions. Copyright 2021 Royal Society of Chemistry [80].
4.2.2. Perovskite (ABO3) OCs
Perovskite materials have attracted a great deal of attention in several fields, and researchers have made several attempts at the methane CLPO process [2]. In these substances, examples of lanthanide or alkaline earth metals fill the A sites as supporting agents for the skeleton, while transition elements occupy the B sites as a centering agent for the architecture [81,82,83]. Transition metals generally have good redox properties, syngas selectivity, and high oxygen transport properties, so they can be used as oxygen carriers in the activation of hydrocarbons and they have a wide range of uses in hydrogenation, the complete oxidation of carbon, and catalytic combustion.
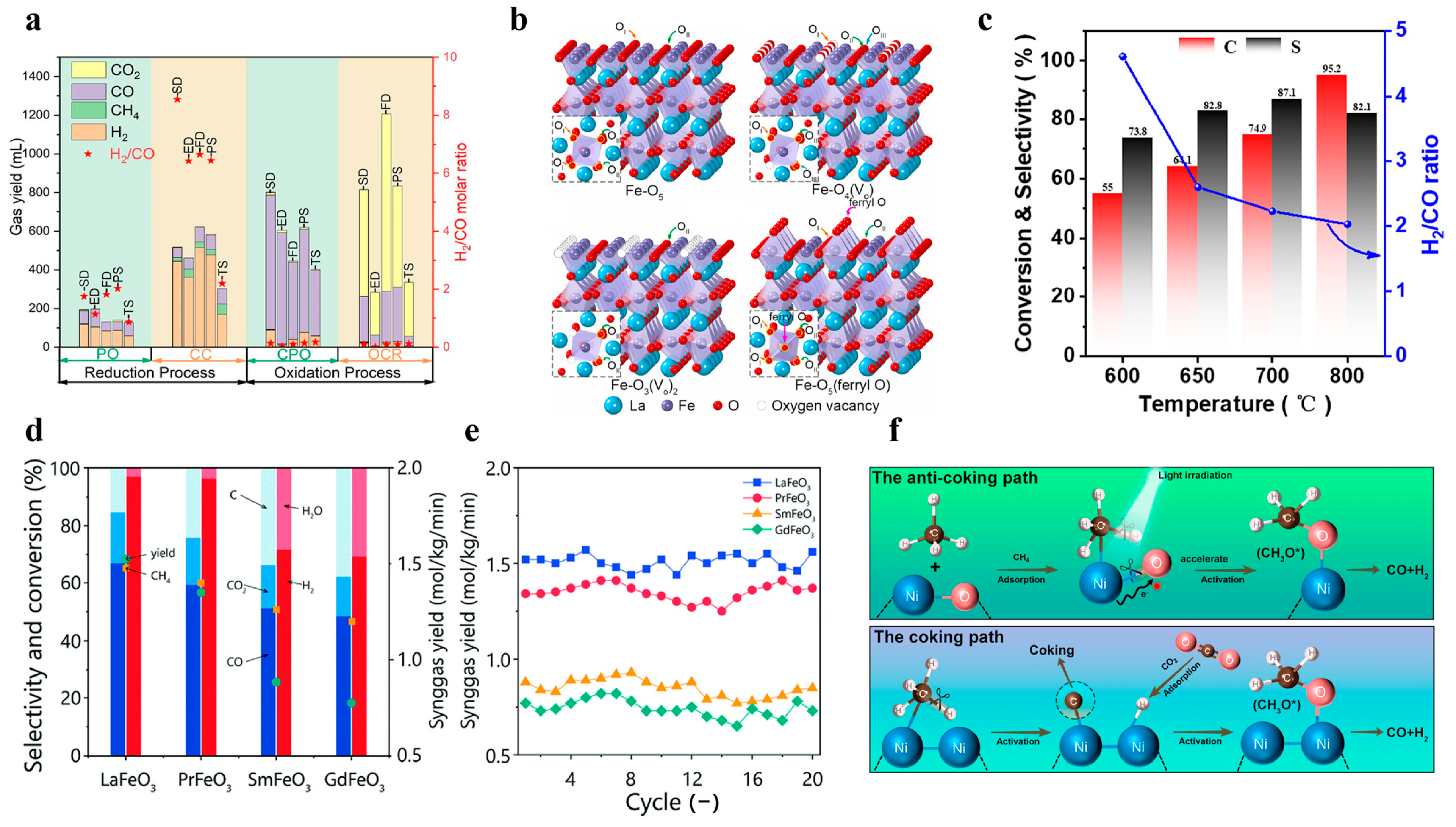
Lanthanide perovskite-type metal oxides demonstrate exceptional reactivity, selectivity, and thermal stability when employed as oxygen carriers in CLPO [3]. Lv et al. used different methods to synthesize a LaFeO3 chalcogenide structure as a carrier for toluene CLPO, and they tested its performance. The conversion of toluene is mainly divided into two phases, the first is partial oxidation, and the second is the cracking reaction of the carrier, in which the chalcogenide carrier occupies a great role. The results also showed that the structure can efficiently realize the conversion of toluene to syngas, as shown in Figure 8a [44].
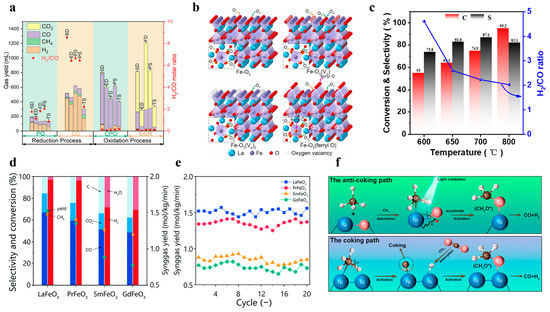
Figure 8.
(a) The columns are the simulative gas yield in each stage, and the star is the H2/CO ratio in each stage. Copyright 2023 Elsevier [44]. (b) Schematic representations of the local structures around Fe on LaFeO3 (001). Copyright 2022 Elsevier [38]. (c) CH4 conversion, CO selectivity, and the hydrogen-to-carbon ratio in the partial oxidation of methane with 5wt% Ni/LaFeO3 oxygen carriers at different temperatures. Copyright 2022 Elsevier [25]. (d) Redox test performance, including product selectivity, methane conversion, CO/CO2 ratio, and syngas yields, for the CLPO of methane at 850 °C. (e) The 20-cycle stability test performance of four perovskites at 850 °C. Copyright 2021 Royal Society of Chemistry [84]. (f) Light-reinforced key intermediates for anticaking to boost highly durable methane dry reforming over a single atom in the active sites on CeO2. (*: the tracked anticoking paths during the DRM process.). Copyright 2023 American Chemical Society [31].
In perovskite structures, the A-site metal affects the coordination environment and the iron–oxygen bonding, which, in turn, affects the concentrations of the lattice oxygen and the synthesis gas yield. Yang et al. investigated the distribution of active sites on La-Fe-based catalysts and analyzed the migration during the chemical chain process, as shown in Figure 8b [38]. Their results showed that the selectivity of the catalyst is closely related to the oxygen vacancies and the coordination environment of the core metal. Li et al. focused on improving the lattice oxygen activity of oxygen carriers, and they prepared a LaFeO3 oxygen carrier for the CLPO process that was modified by NiO. During this period, they found that the relationship between temperature and yield was extremely close. Moreover, they conducted methane conversion experiments at different temperatures, and they finally concluded that Ni doping can increase methane conversion by about 25%, as shown in Figure 8c [25].
Introducing simple metal oxides to modify perovskites is another effective method that can enhance oxygen activity and reactivity. Jiang et al. found that the lanthanide LnFeO3 could influence the syngas yield by controlling the chemical interaction between Fe and oxygen through a combination of theoretical and experimental methods, as shown in Figure 8d,e [84]. Zhang et al. focused on the activity of CLPO at low temperatures, and they proposed a CuO-modified La0.7Sr0.3FeO3. It was shown that the CuO-modified methane conversion was increased by more than a factor of two, and that the obtained syngas had an H2/CO molar ratio of about 2.5 [85].
The B-site doping of perovskites can also be applied to other metals, but their sum has to be kept the same. Yao et al. have investigated the constitutive relationship between the oxygen and catalytic properties of LaNixTi1-xO3-δ perovskites structures in CLPO with different Ti doping degrees, and they showed that the higher the substitution degree of Ti, the more the main reaction of CLPO tends to be partially oxidized. Such work also demonstrates the feasibility of tailoring the oxygen carriers according to the specific needs of the CLPO, as shown in Figure 8f [31]. Nalbandian et al. prepared (La1-xSrx)MnO3±δ perovskite structures as oxygen carriers for use in CLPO, and it was pointed out that the conversion of CH4 with increasing Sr has a close relationship with the oxygen vacancies δ, whereas the oxygen transport capacity with CO2 as the oxidant decreases with increasing Sr. According to this defect model, Mn4+ may be reduced to Mn3+ and produce oxygen vacancies; meanwhile, the overall oxygen vacancies increase with the increase in Sr, but these oxygen vacancies will not participate in the CO2 separation reaction [86]. Zhang et al. introduced tin as a promoter into the perovskites’ BaFe1-xSnxO3-δ structure, which significantly improved the coking resistance, and the syngas yield was 2.9 times higher than that of the doped case, reaching 19.2 mmol/g or more [87]. Donat et al. developed a La0.85Sr0.15Fe0.95Al0.05O3-δ perovskite structure with a high lattice oxygen contribution of up to 9wt%, which can be synthesized at high temperatures of 900 °C with a higher than 99% selectivity to synthesize syngas in the right proportions [88].
4.3. Function of Supports
Metal–support synergism involves phenomena such as charge transfer, contact interface range, nanoparticle morphology, chemical composition of the material, and strong metal–carrier interactions (SMSI). It is an effective strategy for the construction of oxygen vacancies, and the enhancement of inactivation resistance to achieve a superior reactivity and stability in OCs [89,90]. Chein et al. prepared the NiO/Al2O3 oxygen carrier. Spherical Al2O3 particles with an average diameter of 0.5–1.2 mm were used as the support. They found that the specific area of Al2O3 was larger than NiO/Al2O3, and the average pore size of Al2O3 was smaller than NiO/Al2O3 [39]. Fang et al. found that Fe2(SiO3)3 could enhance reactivity in the CLPOM process. Ni/Cu/FeSO4, Ni/Cu/FeSiO3, Fe2(SO4)3, and Fe2(SiO3)3 were, respectively, calculated to find that Fe2(SiO3)3 was a promising candidate. It could increase CH4 conversion by 272% [34]. CeO2 can greatly enhance the oxygen transfer performance of oxygen carriers due to its large number of oxygen vacancy properties as support. Ding et al. used CeO2 as a support to synthesize Ba0.3Sr0.7CoO3−δ/CeO2. The catalysts showed high syngas and H2 productivity in the CLPOM, which was ascribed to the formation of BaCeO3 and SrCeO3 from CeO2 and Ba/Sr, as evidenced by X-ray diffraction measurements. Zhu et al. designed sandwich- and core-shell-structured 5% Ni-SiO2@Ce0.8M0.2O2-δ (M = Fe, Co, Ni) catalysts for moderate temperature CLPOM. They found the transition metal doping of Ni-phyllosilicate@CeO2-δ increased the oxygen donation ability [69]. Wang et al. prepared Co3O4/CeO2 (where the Co/Ce ratios = 7/3), and showed a better performance with a CH4 conversion above 70%. The highest hydrogen yield, and a CH4 conversion of up to 55.73 mmol/g and 94.09% were obtained at 700 °C. The enhancement in the performance of Co3O4/CeO2 during hydrogen production was attributed to the interaction between Co3O4 and CeO2 particles at the interfaces, which could cause charge redistribution [32].
5. Conclusions
We provide an overview of the characteristics and fundamental concepts of CLPOM, thereby emphasizing the significance of OCs in this process. With the decline in carbon-containing fuel availability, chemical looping technologies offer advantages such as low environmental impact and high energy efficiency, and they serve as alternatives to conventional processes. Compared to conventional methods, CLPO processes mitigate the shortcomings inherent in traditional approaches. In CLPO, fuels undergo partial oxidation by the OCs in the reactor, thereby yielding high-purity syngas. Given the critical role of OCs, a significant amount of research has focused on the design and development of highly efficient OCs with high levels of oxygen-carrying capacity and syngas selectivity. While many of the OCs used for CLPOM are single-metallic oxygens, such as NiO and Fe2O3, complex metallic oxides such as spinel-type and tremolite-type OCs are also being investigated. We noticed that, at atmospheric pressure, within the range of 700 °C to 900 °C, the methane conversion rate can achieve 95%, and the selectivity of syngas can reach 99% for twenty or more cycles. We also discussed the influence of different preparation methods on the OCs and found that the selectivity of the sample prepared by sol–gel method was four times that of the dry mix method. Nevertheless, these materials still have room for improvement in terms of durability, cost, and sustainability. Although various approaches, including doping and modification, enhance the creation of oxygen vacancies and resistance to deactivation, further improving OC reactivity and stability remains a significant challenge.
There are still several topics related to the development of oxygen carriers that require further research: (i) Techniques to characterize lattice oxygen mobility, in particular, to quantify oxygen diffusion rates and surface exchange kinetics, should be further developed. (ii) The development of new OCs suitable for use within pressurized reactor systems to improve reactor integration. (iii) A design of low-cost oxygen carrier catalysts and a design of efficient catalysts from widely available feedstocks to accelerate industrialization. (vi) The latest preparation methods for special-structured OCs have been thoroughly discussed, thereby providing new options for designing functionalized OCs materials. (v) Machine learning-based, high-throughput material screening technologies have attracted widespread attention in fields such as energy and the environment, but they are less commonly applied in chemical looping technologies.
Author Contributions
Validation, Y.Z., Y.G. and P.W.; formal analysis and investigation, Q.Z.; resources, Q.Z.; data curation, J.Z.; writing—original draft preparation, J.Z and Y.C.; writing—review and editing, W.S.-m. and J.Z., visualization, Q.Z.; project administration, W.S.-m.; funding acquisition, J.Z. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was found by the Science and Technology Innovation Fundation of Dalian (grant no. 2023JJ12WZ038), the National Natural Science Foundation of China (grant no. 52106078), the Key Laboratory of Ocean Energy Utilization and Energy Conservation of Ministry of Education Fund (grant no. LOEC-202203), and the China Postdoctoral Science Foundation (grant no. 2023M740480).
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, F.; Chen, S.; Duan, L.; Xiang, W. Carbon Dioxide Capture and Hydrogen Production with a Chemical Looping Concept: A Review on Oxygen Carrier and Reactor. Energy Fuels 2023, 37, 16245–16266. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, L.; Fan, L.-S. Chemical Looping Technology: Oxygen Carrier Characteristics. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, F.; Song, Z.; Lin, L.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Review of chemical looping process for carbonaceous feedstock Conversion: Rational design of oxygen carriers. Fuel 2022, 325, 124964. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, M.; Wang, K.; Yue, X.; Chen, X.; Dai, W.; Fu, X. Visible light-assisted thermal catalytic reverse water gas reaction over Cu-CeO2: The synergistic of hot electrons and oxygen vacancies induced by LSPR effect. Fuel 2022, 315, 123186. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Jiang, L.; Tian, D.; Li, K. Perovskites as oxygen storage materials for chemical looping partial oxidation and reforming of methane. Phys. Chem. Chem. Phys. 2024, 26, 1516–1540. [Google Scholar] [CrossRef] [PubMed]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Sunny, A.A.; Meng, Q.; Kumar, S.; Joshi, R.; Fan, L.-S. Nanoscaled Oxygen Carrier-Driven Chemical Looping for Carbon Neutrality: Opportunities and Challenges. Acc. Chem. Res. 2023, 56, 3404–3416. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion—A perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Otsuka, K.; Wang, Y.; Sunada, E.; Yamanaka, I. Direct partial oxidation of methane to synthesis gas by cerium oxide. J. Catal. 1998, 175, 152–160. [Google Scholar] [CrossRef]
- Steinfeld, A.; Kuhn, P.; Karni, J. High-temperature solar thermochemistry: Production of iron and synthesis gas by Fe3O4-reduction with methane. Energy 1993, 18, 239–249. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Tian, D.; Wang, Y.; Zhu, X.; Wei, Y.; Zheng, M.; Luo, Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl. Catal. B Environ. 2017, 202, 51–63. [Google Scholar] [CrossRef]
- Zhao, K.; Zheng, A.; Li, H.; He, F.; Huang, Z.; Wei, G.; Shen, Y.; Zhao, Z. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl. Catal. B Environ. 2017, 219, 672–682. [Google Scholar] [CrossRef]
- Kulkarni, P.; Guan, J.; Subia, R.; Cui, Z.; Manke, J.; Frydman, A.; Wei, W.; Shisler, R.; Ayala, R.; Rizeq, G. Fuel-Flexible Gasification-Combustion Technology for Production of H2 and Sequestration-Ready CO2; GE Energy & Environmental Research Corporation: Irvine, CA, USA, 2008. [Google Scholar]
- Jia, Z.; Li, H.; He, B.; Xu, B.; Shao, L.; Chen, H.; Li, K.; Zeng, L. Design Basis of 1 MWth Calcium Looping Gasification Pilot Unit. In Proceedings of the International Pittsburgh Coal Conference, Zuzhou, China, 15–18 October 2018. [Google Scholar]
- Chung, C.; Qin, L.; Shah, V.; Fan, L.-S. Chemically and physically robust, commercially-viable iron-based composite oxygen carriers sustainable over 3000 redox cycles at high temperatures for chemical looping applications. Energy Environ. Sci. 2017, 10, 2318–2323. [Google Scholar] [CrossRef]
- Fan, L.-S.; Zeng, L.; Wang, W.; Luo, S. Chemical looping processes for CO2 capture and carbonaceous fuel conversion–prospect and opportunity. Energy Environ. Sci. 2012, 5, 7254–7280. [Google Scholar] [CrossRef]
- Zeng, L.; Kathe, M.V.; Chung, E.Y.; Fan, L.-S. Some remarks on direct solid fuel combustion using chemical looping processes. Curr. Opin. Chem. Eng. 2012, 1, 290–295. [Google Scholar] [CrossRef]
- Qin, L.; Cheng, Z.; Fan, J.A.; Kopechek, D.; Xu, D.; Deshpande, N.; Fan, L.-S. Nanostructure formation mechanism and ion diffusion in iron–titanium composite materials with chemical looping redox reactions. J. Mater. Chem. A 2015, 3, 11302–11312. [Google Scholar] [CrossRef]
- Sun, Z.; Russell, C.K.; Whitty, K.J.; Eddings, E.G.; Dai, J.; Zhang, Y.; Fan, M.; Sun, Z. Chemical looping-based energy transformation via lattice oxygen modulated selective oxidation. Prog. Energy Combust. Sci. 2023, 96, 101045. [Google Scholar] [CrossRef]
- Zheng, Y.; Marek, E.J.; Scott, S.A. H2 production from a plasma-assisted chemical looping system from the partial oxidation of CH4 at mild temperatures. Chem. Eng. J. 2020, 379, 122197. [Google Scholar] [CrossRef]
- Qin, L.; Guo, M.; Liu, Y.; Cheng, Z.; Fan, J.A.; Fan, L.-S. Enhanced methane conversion in chemical looping partial oxidation systems using a copper doping modification. Appl. Catal. B Environ. 2018, 235, 143–149. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, Z.; Fan, J.A.; Fan, L.-S.; Gong, J. Metal oxide redox chemistry for chemical looping processes. Nat. Rev. Chem. 2018, 2, 349–364. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Liu, T.; Yao, X.; Zhao, Z.; Pei, C.; Gong, J. Dynamic oxygen migration and reaction over ceria-supported nickel oxides in chemical looping partial oxidation of methane. Appl. Catal. B Environ. 2023, 328, 122478. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Navarrete, L.; Laqdiem, M.; Balaguer, M.; Serra, J.M. Boosting methane partial oxidation on ceria through exsolution of robust Ru nanoparticles. Mater. Adv. 2021, 2, 2924–2934. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Lu, C.; Li, D.; Li, Z.; Gao, J.; Wei, J.; Li, K. Enhanced performance of LaFeO3 oxygen carriers by NiO for chemical looping partial oxidation of methane. Fuel Process. Technol. 2022, 236, 107396. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, X.; Liu, R.; Pei, C.; Zhao, Z.J.; Gong, J. Oxygen activity regulation over LaNiO3 perovskites by Ti substitution for chemical looping partial oxidation of methane. Chem. Eng. Sci. 2023, 278, 118911. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, R.; Gao, Y.; Lin, Y.; Liu, A.; Wang, X.; Zheng, A.; Huang, Z.; Zhao, Z. High syngas selectivity and near pure hydrogen production in perovskite oxygen carriers for chemical looping steam methane reforming. Fuel Process. Technol. 2022, 236, 107398. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Liao, Y.; Liang, S.; Ma, X. Oxygen uncoupling chemical looping gasification of biomass over heterogeneously doped La-Fe-O perovskite-type oxygen carriers. Fuel Process. Technol. 2023, 250, 107883. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.; Lim, H.S.; Kim, H.; Lee, J.W. Surface enrichment of lanthanum on Co3O4 for stable chemical looping combustion. J. CO2 Util. 2023, 73, 102532. [Google Scholar] [CrossRef]
- Ding, H.; Jin, Y.; Hawkins, S.C.; Zhang, L.; Luo, C. Development and performance of CeO2 supported BaCoO3−δ perovskite for chemical looping steam methane reforming. Fuel Process. Technol. 2023, 239, 1075463. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, K.; Cao, Y.; Feng, Y.; Huang, Z.; Chen, Y.; Wei, S.; Liu, L.; Gong, Z.; Cui, Y.; et al. Light-Reinforced Key Intermediate for Anticoking To Boost Highly Durable Methane Dry Reforming over Single Atom Ni Active Sites on CeO2. J. Am. Chem. Soc. 2023, 145, 24625–24635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Song, Z.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Co3O4-CeO2 for enhanced syngas by low-temperature methane conversion with CO2 utilization via a catalytic chemical looping process. Fuel Process. Technol. 2023, 245, 107741. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, L.; Cheng, Z.; Goetze, J.W.; Kong, F.; Fan, J.A.; Fan, L.-S. Near 100% CO selectivity in nanoscaled iron-based oxygen carriers for chemical looping methane partial oxidation. Nat. Commun. 2019, 10, 5503. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, Z.; Wang, C.; Duan, H.; Li, Y.; Zhang, G. Enhanced Methane Conversion in Chemical Looping Partial Oxidation of Iron-Based Oxygen Carriers. JOM 2023, 75, 1530–1539. [Google Scholar] [CrossRef]
- Zong, T.; Li, L.; Han, Y.; Wang, C.; Kang, Y.; Tian, M.; Huang, C.; Wang, X. Influence of the encapsulation degree of Fe0 active sites on performance of garnets for chemical looping partial oxidation of CH4. Appl. Catal. B Environ. 2022, 312, 121421. [Google Scholar] [CrossRef]
- Cabello, A.; Mendiara, T.; Abad, A.; Izquierdo, M.T.; García-Labiano, F. Production of hydrogen by chemical looping reforming of methane and biogas using a reactive and durable Cu-based oxygen carrier. Fuel 2022, 322, 124250. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Y.; Wang, Z.; Long, M.; Chen, D.; Duan, H.; Li, Y.; Zhang, G. Performance and mechanism study of Ce2(SO4)3 for methane chemical looping partial oxidation. Fuel 2023, 334, 126817. [Google Scholar] [CrossRef]
- Yang, J.; Bjørgum, E.; Chang, H.; Zhu, K.-K.; Sui, Z.-J.; Zhou, X.-G.; Holmen, A.; Zhu, Y.-A.; Chen, D. On the ensemble requirement of fully selective chemical looping methane partial oxidation over La-Fe-based perovskites. Appl. Catal. B Environ. 2022, 301, 120788. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Lu, C.-Y.; Chen, W.-H. Syngas production via chemical looping reforming using methane-based feed and NiO/Al2O3 oxygen carrier. Energy 2022, 250, 123815. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Zhao, W.; Li, F.; Chen, X. Ni-Fe bimetallic hexaaluminate for efficient reduction of O2-containing CO2 via chemical looping. Chem. Eng. J. 2022, 441, 136071. [Google Scholar] [CrossRef]
- Zheng, Y.; Sukma, M.S.; Scott, S.A. The exploration of NiO/Ca2Fe2O5/CaO in chemical looping methane conversion for syngas and H2 production. Chem. Eng. J. 2023, 465, 142779. [Google Scholar] [CrossRef]
- Song, H.; Wang, W.; Sun, J.; Wang, X.; Zhang, X.; Chen, S.; Pei, C.; Zhao, Z.-J. Chemical looping oxidative propane dehydrogenation controlled by oxygen bulk diffusion over FeVO4 oxygen carrier pellets. Chin. J. Chem. Eng. 2023, 53, 409–420. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Zhang, J.; Yang, T.; Rao, Q.; Gai, Z.; Wang, X.; Pan, Y.; Jin, H. Mid-temperature chemical looping methane reforming for hydrogen production via iron-based oxygen carrier particles. Fuel Process. Technol. 2024, 253, 108026. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, B.; Yang, H.; Cui, X.; Zhao, M. Chemical looping partial oxidation (CLPO) of toluene on LaFeO3 perovskites for tunable syngas production. Chem. Eng. J. 2023, 451, 138968. [Google Scholar] [CrossRef]
- Oh, D.; Colombo, F.; Nodari, L.; Kim, J.H.; Kim, J.K.; Lee, S.; Kim, S.; Kim, S.; Lim, D.-K.; Seo, J.; et al. Rocking chair-like movement of ex-solved nanoparticles on the Ni-Co doped La0.6Ca0.4FeO3-δ oxygen carrier during chemical looping reforming coupled with CO2 splitting. Appl. Catal. B Environ. 2023, 332, 122745. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Wang, J.; Li, D.; Li, K.; Zhu, X. Oxygen vacancies enriched Ni-Co/SiO2@CeO2 redox catalyst for cycling methane partial oxidation and CO2 splitting. Chin. J. Chem. Eng. 2023, 63, 235–245. [Google Scholar] [CrossRef]
- Khani, H.; Khandan, N.; Eikani, M.H.; Eliassi, A. Investigation of synthesized Fe2O3 and CuO–Fe2O3 for pure hydrogen production by chemical-loop reformng of methanol in a micro-channel reactor. Int. J. Hydrogen Energy 2023, 48, 6436–6450. [Google Scholar] [CrossRef]
- Long, Y.; Gu, Z.; Lin, S.; Yang, K.; Zhu, X.; Wei, Y.; Wang, H.; Li, K. NiO and CuO coated monolithic oxygen carriers for chemical looping combustion of methane. J. Energy Inst. 2021, 94, 199–209. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Gu, Z.; Lu, C.; Li, D.; Zhu, X.; Jiang, L.; Deng, G.; Li, K. Enhanced performance of the CeO2MgO oxygen carrier by NiO for chemical looping CO2 splitting. Fuel Process. Technol. 2022, 225, 107045. [Google Scholar] [CrossRef]
- Zhang, Y.-k.; Zhao, Y.-j.; Yi, Q.; Wei, G.-q.; Shi, L.-j.; Zhou, H. NiO/κ-CeZrO4 functional oxygen carriers with Niδ+ and oxygen vacancy synergy for chemical looping partial oxidation reforming of methane. Fuel Process. Technol. 2021, 219, 106875. [Google Scholar] [CrossRef]
- Shah, V.; Cheng, Z.; Mohapatra, P.; Fan, L.-S. Enhanced methane conversion using Ni-doped calcium ferrite oxygen carriers in chemical looping partial oxidation systems with CO2 utilization. React. Chem. Eng. 2021, 6, 1928–1939. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Jiang, B.; Wang, K.; Tang, D.; Dou, B. An intelligent oxygen carrier of La2−xSrxNiO4−λ for hydrogen production by chemical looping reforming of ethanol. Int. J. Hydrogen Energy 2017, 42, 17102–17111. [Google Scholar] [CrossRef]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B Environ. 2015, 172–173, 116–128. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; Wei, G.; He, F.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Exploration of Reaction Mechanisms on Hydrogen Production through Chemical Looping Steam Reforming Using NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 11621–11632. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Yang, Y.; Liu, B. High-Performance Ni–Fe Redox Catalysts for Selective CH4 to Syngas Conversion via Chemical Looping. ACS Catal. 2018, 8, 1748–1756. [Google Scholar] [CrossRef]
- Wang, D.; Jin, L.; Li, Y.; Wei, B.; Yao, D.; Hu, H. Upgrading of vacuum residue with chemical looping partial oxidation over Fe-Mn mixed metal oxides. Fuel 2019, 239, 764–773. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, N.; Guo, X.; Zhang, S. Dopant screening of modified Fe2O3 oxygen carriers in chemical looping hydrogen production. Fuel 2020, 262, 116489. [Google Scholar] [CrossRef]
- Wang, D.; Jin, L.; Li, Y.; Hu, H. Upgrading of Heavy Oil with Chemical Looping Partial Oxidation over M2+ Doped Fe2O3. Energy Fuels 2018, 33, 257–265. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Sun, X.; Ma, X.; Kang, Y.; Wang, X.; Wang, J. La-hexaaluminate for synthesis gas generation by Chemical Looping Partial Oxidation of Methane Using CO2 as Sole Oxidant. AIChE J. 2017, 64, 550–563. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- Liu, R.; Pei, C.; Zhang, X.; Chen, S.; Li, H.; Zeng, L.; Mu, R.; Gong, J. Chemical looping partial oxidation over FeWO/SiO2 catalysts. Chin. J. Catal. 2020, 41, 1140–1151. [Google Scholar] [CrossRef]
- Donat, F.; Kierzkowska, A.; Müller, C.R. Chemical Looping Partial Oxidation of Methane: Reducing Carbon Deposition through Alloying. Energy Fuels 2022, 36, 9780–9784. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G.; Xia, C. Reaction schemes of barium ferrite in biomass chemical looping gasification for hydrogen-enriched syngas generation via an outer-inner looping redox reaction mechanism. Energy Convers. Manag. 2019, 189, 81–90. [Google Scholar] [CrossRef]
- Kang, Y.; Tian, M.; Huang, C.; Lin, J.; Hou, B.; Pan, X.; Li, L.; Rykov, A.I.; Wang, J.; Wang, X. Improving Syngas Selectivity of Fe2O3/Al2O3 with Yttrium Modification in Chemical Looping Methane Conversion. ACS Catal. 2019, 9, 8373–8382. [Google Scholar] [CrossRef]
- Zeng, D.; Qiu, Y.; Peng, S.; Chen, C.; Zeng, J.; Zhang, S.; Xiao, R. Enhanced hydrogen production performance through controllable redox exsolution within CoFeAlOx spinel oxygen carrier materials. J. Mater. Chem. A 2018, 6, 11306–11316. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Ma, S.; Xiang, W. Carbon formation on iron-based oxygen carriers during CH4 reduction period in Chemical Looping Hydrogen Generation process. Chem. Eng. J. 2017, 325, 322–331. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-Looping Conversion of Methane: A Review. Energy Technol. 2019, 8, 1900925. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Wang, H.; Li, Z.; Zhu, X. Sandwich Ni-phyllosilicate@doped-ceria for moderate-temperature chemical looping dry reforming of methane. Fuel Process. Technol. 2022, 232, 107268. [Google Scholar] [CrossRef]
- Riaz, A.; Kremer, F.; Kim, T.; Sattayaporn, S.; Tsuzuki, T.; Lipiński, W.; Lowe, A. Experimental demonstration of vanadium-doped nanostructured ceria for enhanced solar thermochemical syngas production. Nano Energy 2021, 81, 105639. [Google Scholar] [CrossRef]
- Kang, D.; Lee, M.; Lim, H.S.; Lee, J.W. Chemical looping partial oxidation of methane with CO2 utilization on the ceria-enhanced mesoporous Fe2O3 oxygen carrier. Fuel 2018, 215, 787–798. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, L.; Tian, H.; Li, X.; Gong, J. Enhanced Lattice Oxygen Reactivity over Ni-Modified WO3-Based Redox Catalysts for Chemical Looping Partial Oxidation of Methane. ACS Catal. 2017, 7, 3548–3559. [Google Scholar] [CrossRef]
- Miller, D.D.; Riley, J.; Siriwardane, R. Interaction of Methane with Calcium Ferrite in the Chemical Looping Partial Oxidation Application: Experimental and DFT Study. Energy Fuels 2019, 34, 2193–2204. [Google Scholar] [CrossRef]
- Jiang, S.; Shen, L.; Wu, J.; Yan, J.; Song, T. The investigations of hematite-CuO oxygen carrier in chemical looping combustion. Chem. Eng. J. 2017, 317, 132–142. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Feng, Y.; Chen, T.; Chen, D.; Zheng, A.; Wei, G.; He, F.; Zhao, Z.; Wu, J.; et al. Exploring the Conversion Mechanisms of Toluene as a Biomass Tar Model Compound on NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 16539–16548. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; He, F.; Chen, D.; Wei, G.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Reactivity investigation on chemical looping gasification of biomass char using nickel ferrite oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 14458–14470. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Li, D.; Li, K.; Wang, H.; Zhu, T.; Zhu, X. Enhanced performance of La1-xFeO3-δ oxygen carrier via A-site cation defect engineering for chemical looping dry reforming of methane. Fuel Process. Technol. 2023, 248, 107820. [Google Scholar] [CrossRef]
- Lim, H.S.; Kang, D.; Lee, J.W. Phase transition of Fe2O3–NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion. Appl. Catal. B Environ. 2017, 202, 175–183. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Wang, Z.; Zhu, M.; Zhang, K.; Li, B.; Wu, J. The search of proper oxygen carriers for chemical looping partial oxidation of carbon. Appl. Energy 2017, 190, 1119–1125. [Google Scholar] [CrossRef]
- Collins-Martinez, V.H.; Cazares-Marroquin, J.F.; Salinas-Gutierrez, J.M.; Pantoja-Espinoza, J.C.; Lopez-Ortiz, A.; Melendez-Zaragoza, M.J. The thermodynamic evaluation and process simulation of the chemical looping steam methane reforming of mixed iron oxides. RSC Adv. 2020, 11, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, Y.; Wu, Y.; Wang, J.; Jiang, E.; Xu, X.; Su, J.; Jia, Z. The coupling of CH4 partial oxidation and CO2 splitting for syngas production via double perovskite-type oxides LaFexCo1−xO3. Fuel 2020, 268, 117381. [Google Scholar] [CrossRef]
- Ksepko, E. Perovskite-type Sr(Mn1−xNix)O3 materials and their chemical-looping oxygen transfer properties. Int. J. Hydrogen Energy 2014, 39, 8126–8137. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Hosseini, D.; Müller, C.R. Review of Oxygen Carriers for Chemical Looping with Oxygen Uncoupling (CLOU): Thermodynamics, Material Development, and Synthesis. Energy Technol. 2013, 1, 633–647. [Google Scholar] [CrossRef]
- Jiang, B.; Li, L.; Zhang, Q.; Ma, J.; Zhang, H.; Yu, K.; Bian, Z.; Zhang, X.; Ma, X.; Tang, D. Iron–oxygen covalency in perovskites to dominate syngas yield in chemical looping partial oxidation. J. Mater. Chem. A 2021, 9, 13008–13018. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, Y.; Li, H.; Zhao, Z.; Zhao, K.; Jiang, L. The role of CuO modified La0·7Sr0·3FeO3 perovskite on intermediate-temperature partial oxidation of methane via chemical looping scheme. Int. J. Hydrogen Energy 2020, 45, 4073–4083. [Google Scholar] [CrossRef]
- Nalbandian, L.; Evdou, A.; Matsouka, C.; Zaspalis, V. Assessment of (La1-xSrx)MnO3±δ perovskites as oxygen- carrier materials in chemical-looping processes. Fuel Process. Technol. 2022, 226, 107086. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Xu, W.; Huang, C.; Su, Y.; Tian, M.; Zhu, Y.; Gong, H.; Wang, X. Anti-coke BaFe1–xSnxO3−δ Oxygen Carriers for Enhanced Syngas Production via Chemical Looping Partial Oxidation of Methane. Energy Fuels 2020, 34, 6991–6998. [Google Scholar] [CrossRef]
- Donat, F.; Müller, C.R. CO2-free conversion of CH4 to syngas using chemical looping. Appl. Catal. B Environ. 2020, 278, 119328. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X.; Chen, L. Characteristics of microalgae gasification through chemical looping in the presence of steam. Int. J. Hydrogen Energy 2017, 42, 22730–22742. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).