Reaction Mechanisms and Production of Hydrogen and Acetic Acid from Aqueous Ethanol Using a Rn-Sn/TiO2 Catalyst in a Continuous Flow Reactor
Abstract
1. Introduction
2. Results and Discussion
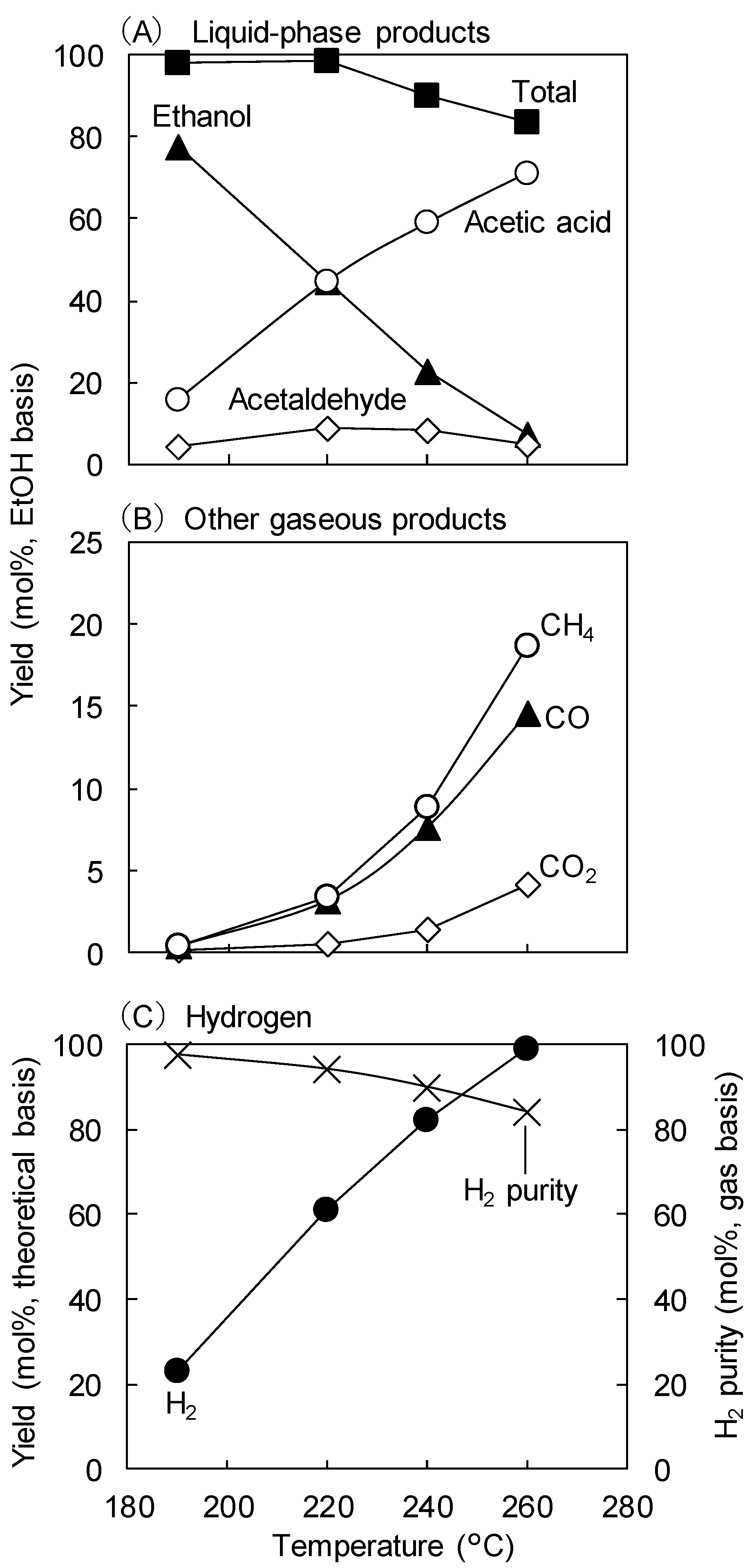
2.1. Effect of Temperature at 0.1 MPa
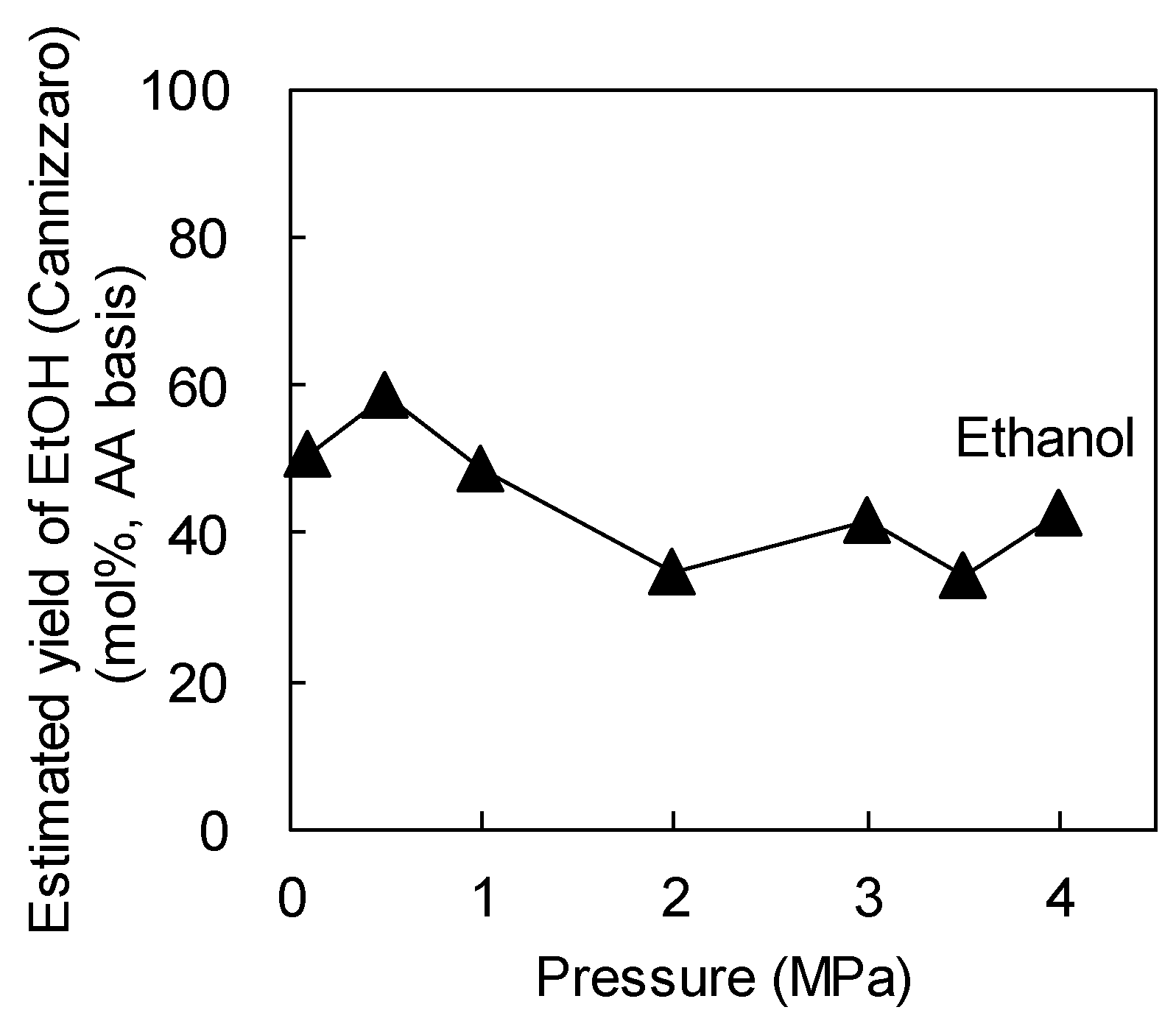
2.2. Effect of Pressure at 260 °C
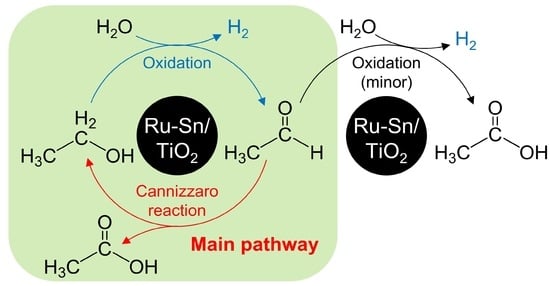
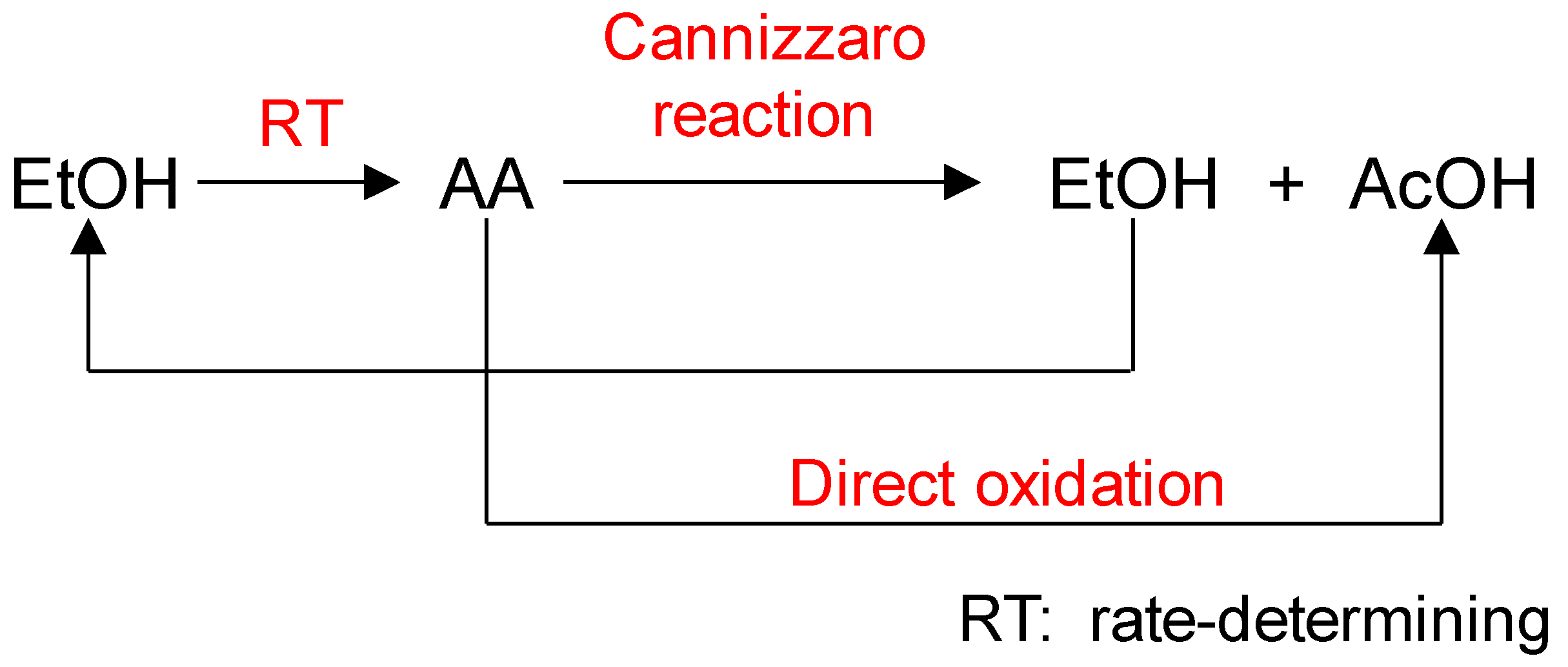
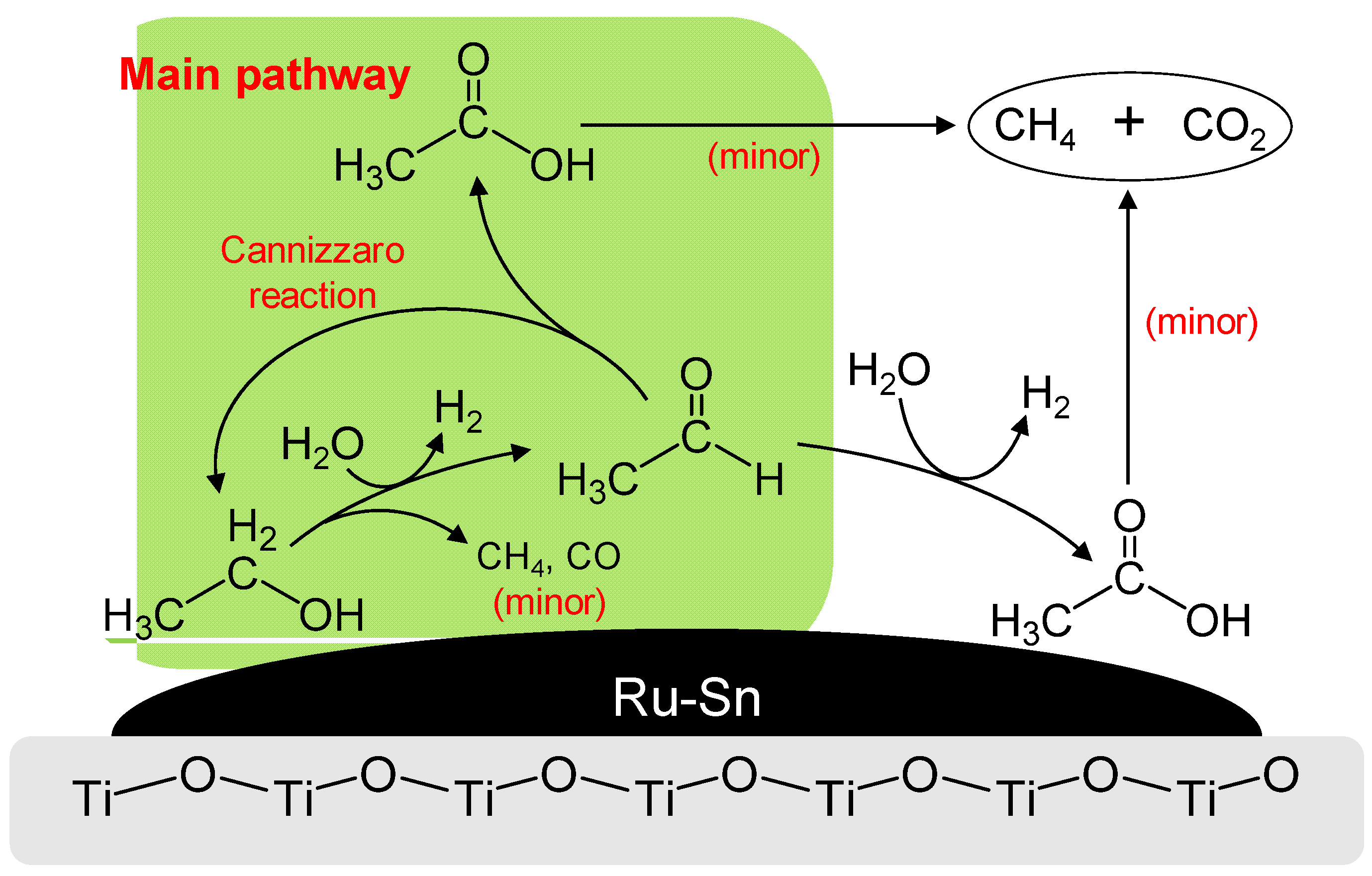
2.3. Cannizzaro Reaction
2.4. Oxidation of High-Concentration Aqueous EtOH Solutions
3. Materials and Methods
3.1. Materials and Catalyst Preparation
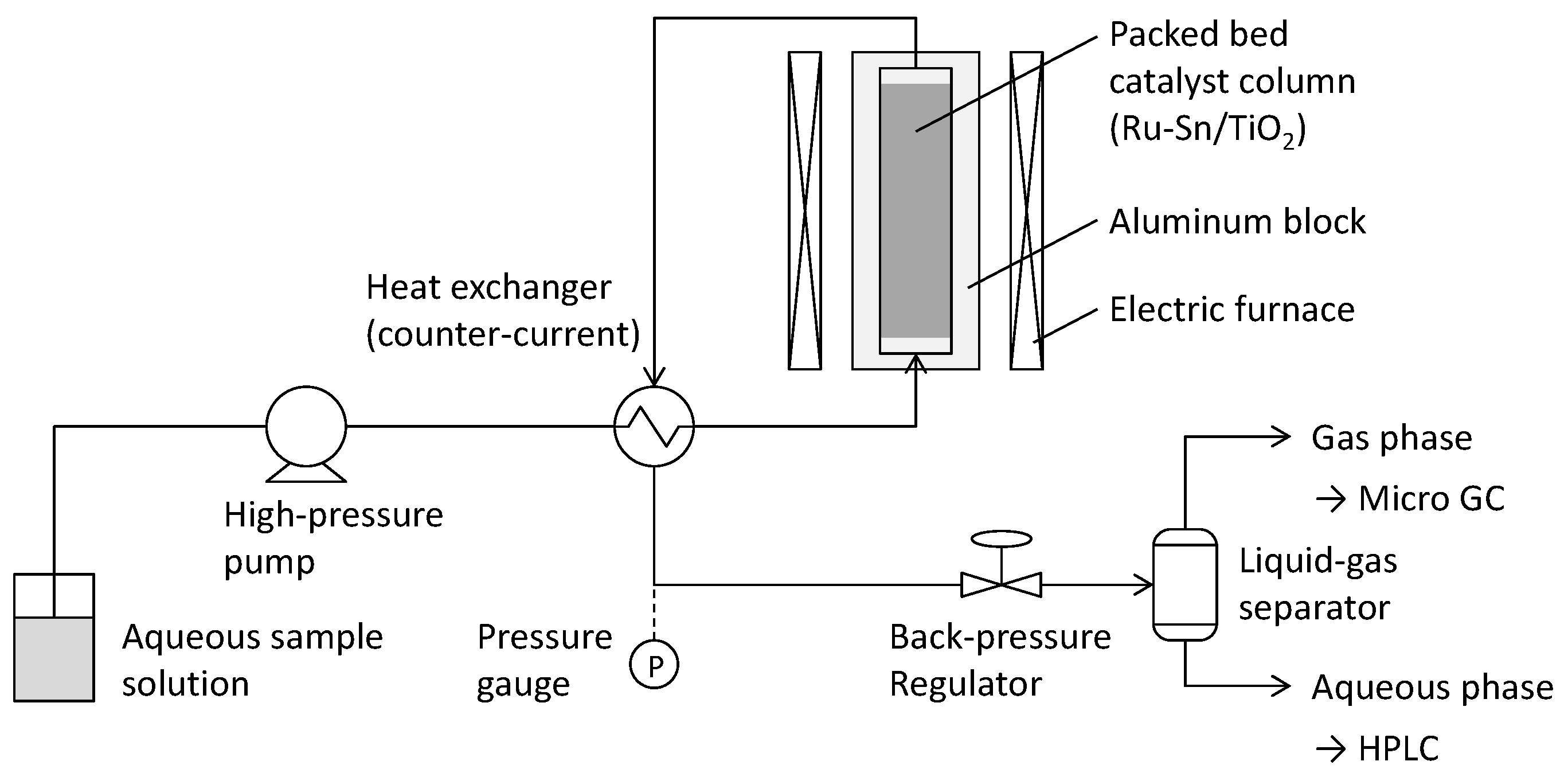
3.2. Catalytic Conversion and Product Analysis
4. Conclusions
- The conversion of aqueous EtOH to AcOH and H2 over 4 wt%Ru-4 wt%Sn/TiO2 was more efficient in the gas phase than in the liquid aqueous phase.
- The selectivities for AcOH and H2 were generally high.
- Methane, CO, and CO2 were produced as gaseous by-products via the fragmentation of AA to give CO and CH4 and of AcOH to give CO2 and CH4, thus reducing the H2 purity.
- At a fixed reaction temperature of 260 °C in the gas phase, the EtOH conversion decreased with increasing pressure, whereas the H2 purity increased because of the less-efficient reaction of AA to generate CO and CH4 at the higher pressure.
- The fragmentation of AcOH to CO2 and CH4 was independent of the reaction pressure.
- The first reaction, EtOH → AA, was the rate-determining step in this process and AA was converted to AcOH and EtOH by the Cannizzaro reaction rather than undergoing direct oxidation to AcOH.
- The fragmentation of AA occurred during the conversion of EtOH on the surface of the 4 wt%Ru-4 wt%Sn/TiO2 catalyst prior to the removal from the surface.
- Highly concentrated aqueous EtOH solutions (100 and 500 g/L) could also be employed with this process to selectively produce H2 and AcOH.
- The reaction pressure should be slightly less than the saturation vapor pressure of water to selectively obtain AcOH and H2.
- The use of the 4 wt%Ru-4 wt%Sn/TiO2 catalyst in a flow reactor was found to be an effective method for the aqueous-phase reforming of EtOH to AcOH and H2 with high purity. This provides insight into the industrial production of green hydrogen and AcOH.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin-Espejo, J.L.; Gandara-Loe, J.; Odriozola, J.A.; Reina, T.R.; Pastor-Perez, L. Sustainable routes for acetic acid production: Traditional processes vs. a low-carbon, biogas-based strategy. Sci. Total Environ. 2022, 840, 156663. [Google Scholar] [CrossRef] [PubMed]
- Sponholz, P.; Mellmann, D.; Cordes, C.; Alsabeh, P.G.; Li, B.; Li, Y.; Nielsen, M.; Junge, H.; Dixneuf, P.; Beller, M. Efficient and selective hydrogen generation from bioethanol using ruthenium pincer-type complexes. ChemSusChem 2014, 7, 2419–2422. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Nishioka, M.; Yoshida, M.; Fujita, K. A Sustainable method for the synthesis of acetic acid based on dehydrogenation of an ethanol-water solution catalyzed by an iridium complex bearing a functional bipyridonate ligand. ChemCatChem 2018, 10, 3636–3640. [Google Scholar] [CrossRef]
- Brei, V.V.; Sharanda, M.E.; Prudius, S.V.; Bondarenko, E.A. Synthesis of acetic acid from ethanol-water mixture over Cu/ZnO-ZrO2-Al2O3 catalyst. Appl. Catal. A-Gen 2013, 458, 196–200. [Google Scholar] [CrossRef]
- Xiang, N.; Xu, P.; Ran, N.; Ye, T. Production of acetic acid from ethanol over CuCr catalysts via dehydrogenation-(aldehyde-water shift) reaction. RSC Adv. 2017, 7, 38586–38593. [Google Scholar] [CrossRef]
- Nozawa, T.; Mizukoshi, Y.; Yoshida, A.; Naito, S. Aqueous phase reforming of ethanol and acetic acid over TiO2 supported Ru catalysts. Appl. Catal. B 2014, 146, 221–226. [Google Scholar] [CrossRef]
- Xiong, H.; DeLaRiva, A.; Wang, Y.; Datye, A.K. Low-temperature aqueous-phase reforming of ethanol on bimetallic PdZn catalysts. Catal. Sci. Technol. 2015, 5, 254–263. [Google Scholar] [CrossRef]
- Nozawa, T.; Yoshida, A.; Hikichi, S.; Naito, S. Effects of Re addition upon aqueous phase reforming of ethanol over TiO2 supported Rh and Ir catalysts. Int. J. Hydrog. Energy 2015, 40, 4129–4140. [Google Scholar] [CrossRef]
- Ito, Y.; Kawamoto, H.; Saka, S. Efficient and selective hydrogenation of aqueous acetic acid on Ru-Sn/TiO2 for bioethanol production from lignocellulosics. Fuel 2016, 178, 118–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Konishi, K.; Minami, E.; Saka, S.; Kawamoto, H. Hydrogenation of aqueous acetic acid over Ru-Sn/TiO2 catalyst in a flow-type reactor, governed by reverse reaction. Catalysts 2020, 10, 1270. [Google Scholar] [CrossRef]
- Haffad, D.; Kameswari, U.; Bettahar, M.M.; Chambellan, A.; Lavalley, J.C. Reduction of benzaldehyde on metal oxides. J. Catal. 1997, 172, 85–92. [Google Scholar] [CrossRef]
- Saadi, A.; Rassoul, Z.; Bettahar, M.M. Reduction of benzaldehyde on alkaline earth metal oxides. J. Mol. Catal. A-Chem 2006, 258, 59–67. [Google Scholar] [CrossRef]


| Step | Preferred Condition |
|---|---|
| EtOH → AA (rate-determining) | Lower pressure (0.1–2 MPa) |
| AA → AcOH | Higher pressure (2–4 MPa) |
| AA → CH4 + CO | Lower pressure (0.1–2 MPa) |
| AcOH → CH4 + CO2 | No effect |
| Temp. | Press. | Conc. | EtOH Conversion (%) | Yield (mol%) * | H2 Purity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (MPa) | (g/L) | AcOH | AA | CH4 | CO | CO2 | H2 | (%) | |
| 260 | 4 | 10 | 76.2 | 71.9 | 0.4 | 7.75 | 0.06 | 4.99 | 88.4 | 93.2 |
| 100 | 26.7 | 21.4 | 1.0 | 0.66 | 0.07 | 0.38 | 30.1 | 98.2 | ||
| 320 | 10 | 100 | 62.8 | 50.7 | 2.4 | 10.89 | 1.18 | 7.23 | 72.3 | 88.1 |
| 500 | 13.1 | 10.4 | 1.5 | 2.81 | 1.39 | 1.36 | 29.7 | 90.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, T.; Zhao, Y.; Minami, E.; Kawamoto, H. Reaction Mechanisms and Production of Hydrogen and Acetic Acid from Aqueous Ethanol Using a Rn-Sn/TiO2 Catalyst in a Continuous Flow Reactor. Catalysts 2024, 14, 249. https://doi.org/10.3390/catal14040249
Nomura T, Zhao Y, Minami E, Kawamoto H. Reaction Mechanisms and Production of Hydrogen and Acetic Acid from Aqueous Ethanol Using a Rn-Sn/TiO2 Catalyst in a Continuous Flow Reactor. Catalysts. 2024; 14(4):249. https://doi.org/10.3390/catal14040249
Chicago/Turabian StyleNomura, Takashi, Yuanyuan Zhao, Eiji Minami, and Haruo Kawamoto. 2024. "Reaction Mechanisms and Production of Hydrogen and Acetic Acid from Aqueous Ethanol Using a Rn-Sn/TiO2 Catalyst in a Continuous Flow Reactor" Catalysts 14, no. 4: 249. https://doi.org/10.3390/catal14040249
APA StyleNomura, T., Zhao, Y., Minami, E., & Kawamoto, H. (2024). Reaction Mechanisms and Production of Hydrogen and Acetic Acid from Aqueous Ethanol Using a Rn-Sn/TiO2 Catalyst in a Continuous Flow Reactor. Catalysts, 14(4), 249. https://doi.org/10.3390/catal14040249