Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst
Abstract
:1. Introduction
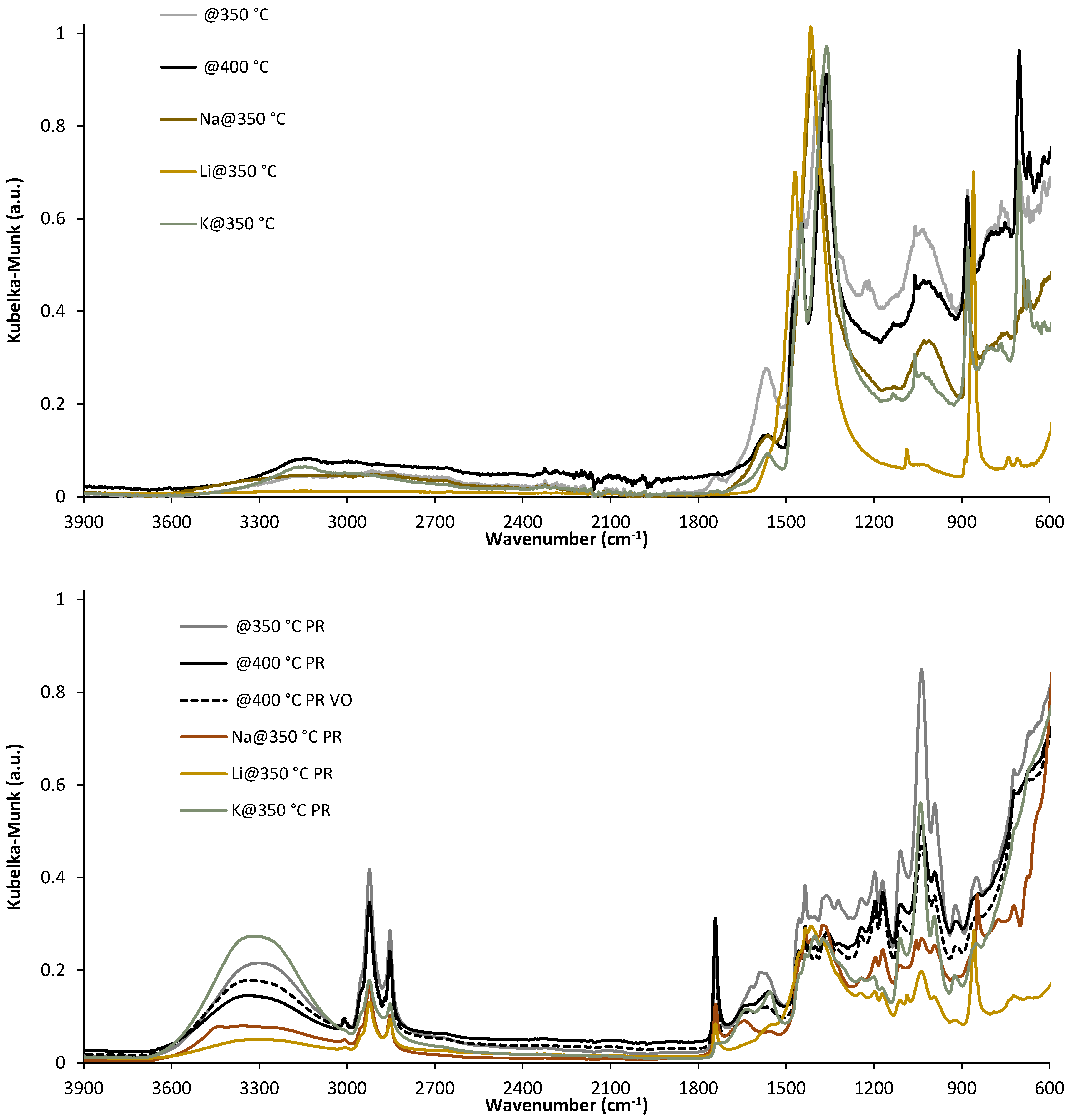
2. Results and Discussion
| Weight (%) | Ash Composition | Weight (%) | |
|---|---|---|---|
| Cellulose | 12.2 | K | 20–40 |
| Hemicellulose | 10.2 | Ca | 10 |
| Lignin (acid-detergent) | 2.9 | Mg | 3–5 |
| Sucrose | 15.6 | P | 1–2 |
| Glucose | 7.5 | Na | Trace |
| Fructose | 6.2 | Fe | |
| Protein | 5.1 | Zn | |
| Pectin | 15.9 | Mn | |
| Ash | 9.8 |
3. Experimental Section
3.1. Biochar Production and Characterization
3.2. Oil Methanolysis Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biodiesel and Diesel Prices, 2019 to April 2022—Charts—Data & Statistics—IEA, (n.d.). Available online: https://www.iea.org/data-and-statistics/charts/biodiesel-and-diesel-prices-2019-to-april-2022 (accessed on 31 July 2023).
- Esmaeili, H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process. Technol. 2022, 230, 107224. [Google Scholar] [CrossRef]
- Maleki, B.; Talesh, S.A.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Mater. Today Sustain. 2022, 18, 100157. [Google Scholar] [CrossRef]
- Riaz, I.; Shafiq, I.; Jamil, F.; Al-Muhtaseb, A.H.; Akhter, P.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on catalysts of biodiesel (methyl esters) production. Catal. Rev. 2022, 11, 1–53. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.; Wang, Y.; Shen, Z.; Huang, H.; Ge, Z.; Li, X.; He, J.; Wang, X.; Li, L.; et al. Recent advances in biodiesel production using functional carbon materials as acid/base catalysts. Fuel Process. Technol. 2022, 237, 107421. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, S.L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.H.; Kwon, E.E.; Bhatia, S.K.; Palai, A.K.; Kumar, A.; Bhatia, R.K.; Patel, A.K.; Thakur, V.K.; Yang, Y.H. Trends in renewable energy production employing biomass-based biochar. Renew. Sustain. Energy Rev. 2017, 77, 125644. [Google Scholar] [CrossRef]
- Parida, S.; Singh, M.; Pradhan, S. Biomass wastes: A potential catalyst source for biodiesel production. Bioresour. Technol. Rep. 2022, 18, 101081. [Google Scholar] [CrossRef]
- Garcia, B.; Alves, O.; Rijo, B.; Lourinho, G.; Nobre, C. Biochar: Production, Applications, and Market Prospects in Portugal. Environments 2022, 9, 95. [Google Scholar] [CrossRef]
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface Treatment of Biochar—Methods, Surface Analysis and Potential Applications: A Comprehensive Review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Yameen, M.Z.; AlMohamadi, H.; Naqvi, S.R.; Noor, T.; Chen, W.-H.; Amin, N.A.S. Advances in production & activation of marine macroalgae-derived biochar catalyst for sustainable biodiesel production. Fuel 2023, 337, 127215. [Google Scholar] [CrossRef]
- Hayashi, J.; Hasegawa, I.; Hagihara, T.; Takara, T. Production of activated carbon with a large specific surface area from banana peel and its methane adsorption ability. Carbon 2021, 176, 656. [Google Scholar] [CrossRef]
- Domingues, C.; Correia, M.J.N.; Carvalho, R.; Henriques, C.; Bordado, J.; Dias, A.P.S. Vanadium phosphate catalysts for biodiesel production from acid industrial by-products. J. Biotechnol. 2013, 164, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst. Renew. Energy 2021, 168, 1207–1216. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.A.; Carrillo, J.D.; Flórez-López, E.; Grande-Tovar, C.D. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 2021, 26, 5282. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J. Environ. Chem. Eng. 2021, 9, 106398. [Google Scholar] [CrossRef]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Ying, Z.; Zhang, J.; Sun, D.; Li, X. Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process. Saf. Environ. Prot. 2018, 122, 366–377. [Google Scholar] [CrossRef]
- Daimary, N.; Boruah, P.; Eldiehy, K.S.; Pegu, T.; Bardhan, P.; Bora, U.; Mandal, M.; Deka, D. Musa acuminata peel: A bioresource for bio-oil and by-product utilization as a sustainable source of renewable green catalyst for biodiesel production. Renew. Energy 2022, 187, 450–462. [Google Scholar] [CrossRef]
- Brahma, S.; Basumatary, B.; Basumatary, S.F.; Das, B.; Brahma, S.; Rokhum, S.L.; Basumatary, S. Biodiesel production from quinary oil mixture using highly efficient Musa chinensis based heterogeneous catalyst. Fuel 2023, 336, 127150. [Google Scholar] [CrossRef]
- Jitjamnong, J.; Thunyaratchatanon, C.; Luengnaruemitchai, A.; Kongrit, N.; Kasetsomboon, N.; Sopajarn, A.; Chuaykarn, N.; Khantikulanon, N. Response surface optimization of biodiesel synthesis over a novel biochar-based heterogeneous catalyst from cultivated (Musa sapientum) banana peels. Biomass Convers. Biorefinery 2020, 11, 2795–2811. [Google Scholar] [CrossRef]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Kumar, S.; Soomro, S.A.; Harijan, K.; Uqaili, M.A.; Kumar, L. Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review. Energies 2023, 16, 644. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Rego, F.; Fonseca, F.; Casquilho, M.; Rosa, F.; Rodrigues, A. Catalyzed pyrolysis of SRC poplar biomass. Alkaline carbonates and zeolites catalysts. Energy 2019, 183, 1114–1122. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kuramochi, H. Optimized production conditions and activation of biochar for effective promotion of long-chain fatty acid degradation in anaerobic digestion. Bioresour. Technol. 2022, 358, 127393. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Valorization of banana peel: A biorefinery approach. Rev. Chem. Eng. 2016, 32, 651–666. [Google Scholar] [CrossRef]
- Deokar, S.K.; Jadhav, A.R.; Pathak, P.D.; Mandavgane, S.A. Biochar from microwave pyrolysis of banana peel: Characterization and utilization for removal of benzoic and salicylic acid from aqueous solutions. Biomass Convers. Biorefinery 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Tarar, O.F.; Asghar, A.; Qayyum, S.A.; Kanwal, H.; Lateef, A.; Nazir, R.; Abidi, S.H.I.; Naeem, M.K.; Shahid, B. Synthesis and surface morphology of banana biochar-based nano-fertilizer and its effect on first stages of growth parameters of cucumber, broccoli, and red okra. J. Saudi Soc. Agric. Sci. 2023, 22, 535–545. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Sharrock, P. A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 2013, 58, 305–317. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Ding, Y.; Shao, Y.; Cai, L.; Jin, Z.-Y.; Liu, Z.; Zhao, J.; Li, F.; Pan, Z.; Li, X.; et al. Banana peel biochar with nanoflake-assembled structure for cross contamination treatment in water: Interaction behaviors between lead and tetracycline. Chem. Eng. J. 2021, 420, 129807. [Google Scholar] [CrossRef]
- Ercan, B.; Alper, K.; Ucar, S.; Karagoz, S. Comparative studies of hydrochars and biochars produced from lignocellulosic biomass via hydrothermal carbonization, torrefaction and pyrolysis. J. Energy Inst. 2023, 109, 101298. [Google Scholar] [CrossRef]
- Hussein, H.S.; Shaarawy, H.H.; Hussien, N.H.; Hawash, S.I. Preparation of nano-fertilizer blend from banana peels. Bull. Natl. Res. Cent. 2019, 43, 26. [Google Scholar] [CrossRef]
- Meriatna; Husin, H.; Riza, M.; Faisal, M.; Ahmadi; Sulastri. Biodiesel production using waste banana peel as renewable base catalyst. Mater. Today Proc. 2023, 87, 214–217. [Google Scholar] [CrossRef]
- Spataru, D.; Dias, A.P.S.; Ferreira, L.F.V. Acetylation of biodiesel glycerin using glycerin and glucose derived catalysts. J. Clean. Prod. 2021, 297, 126686. [Google Scholar] [CrossRef]
- Galvão, A.C.; Jiménez, Y.P.; Justel, F.J.; Robazza, W.S.; Donatti, F.S. Salting-out precipitation of NaCl, KCl and NH4Cl in mixtures of water and methanol described by the modified Pitzer model. J. Chem. Thermodyn. 2020, 150, 106202. [Google Scholar] [CrossRef]
- Rijo, B.; Fernando, E.; Ramos, M.; Dias, A.P.S. Biodiesel production over sodium carbonate and bicarbonate catalysts. Fuel 2022, 323, 124383. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Lin, J.-H.; Huang, S.-T.; Lee, W.-L.W.; Hsieh, C.-H.; Chen, F.-H.; Chen, C.-C. Natural soil and lithium carbonate as economical solid-base catalysts for biodiesel production. Energy Rep. 2020, 6, 2743–2750. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process. Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Karadendrou, K.; Ioannou, D.; Karadendrou, M.-A.; Detsi, A.; Kalderis, D.; Massas, I.; Gasparatos, D. Effects of Biochars Derived from Sewage Sludge and Olive Tree Prunings on Cu Fractionation and Mobility in Vineyard Soils over Time. Land 2023, 12, 416. [Google Scholar] [CrossRef]
- Catarino, M.; Ferreira, E.; Dias, A.P.S.; Gomes, J. Dry washing biodiesel purification using fumed silica sorbent. Chem. Eng. J. 2019, 386, 123930. [Google Scholar] [CrossRef]
- Jayaraju, R.M.; Gaddam, K.; Ravindiran, G.; Palani, S.; Paulraj, M.P.; Achuthan, A.; Saravanan, P.; Muniasamy, S.K. Biochar from waste bio-mass as a biocatalyst for biodiesel production: An overview. Appl. Nanosci. 2022, 12, 3665–3676. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhang, X.; Ling, P.; Xu, K.; He, L.; Su, S.; Wang, Y.; Hu, S.; Xiang, J. Chemical imaging of coal in micro-scale with Raman mapping technology. Fuel 2020, 264, 116826. [Google Scholar] [CrossRef]
- Kapteijn, F.; Abbel, G.; Moulijn, J.A. CO2 gasification of carbon catalysed by alkali metals: Reactivity and mechanism. Fuel 1984, 63, 1036–1042. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Puna, J.; Gomes, J.; Correia, M.J.N.; Bordado, J.; Joana, M.; Correia, N.; Paula, A.; Dias, S.; Puna, J. Biodiesel production over lime. Catalytic contributions of bulk phases and surface Ca species formed during reaction. Renew. Energy 2016, 99, 622–630. [Google Scholar] [CrossRef]
- Catarino, M.; Martins, S.; Dias, A.P.S.; Pereira, M.F.C.; Gomes, J. Calcium diglyceroxide as a catalyst for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103099. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Carvalho, J.P.S. Recovery of carbon fibers from aviation epoxy composites by acid solvolysis. Sustain. Mater. Technol. 2023, 35, 14–16. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Zhu, H.; Chen, X.; Yu, G. In-situ study on structure evolution and gasification reactivity of biomass char with K and Ca catalysts at carbon dioxide atmosphere. Carbon Resour. Convers. 2023, 6, 27–33. [Google Scholar] [CrossRef]



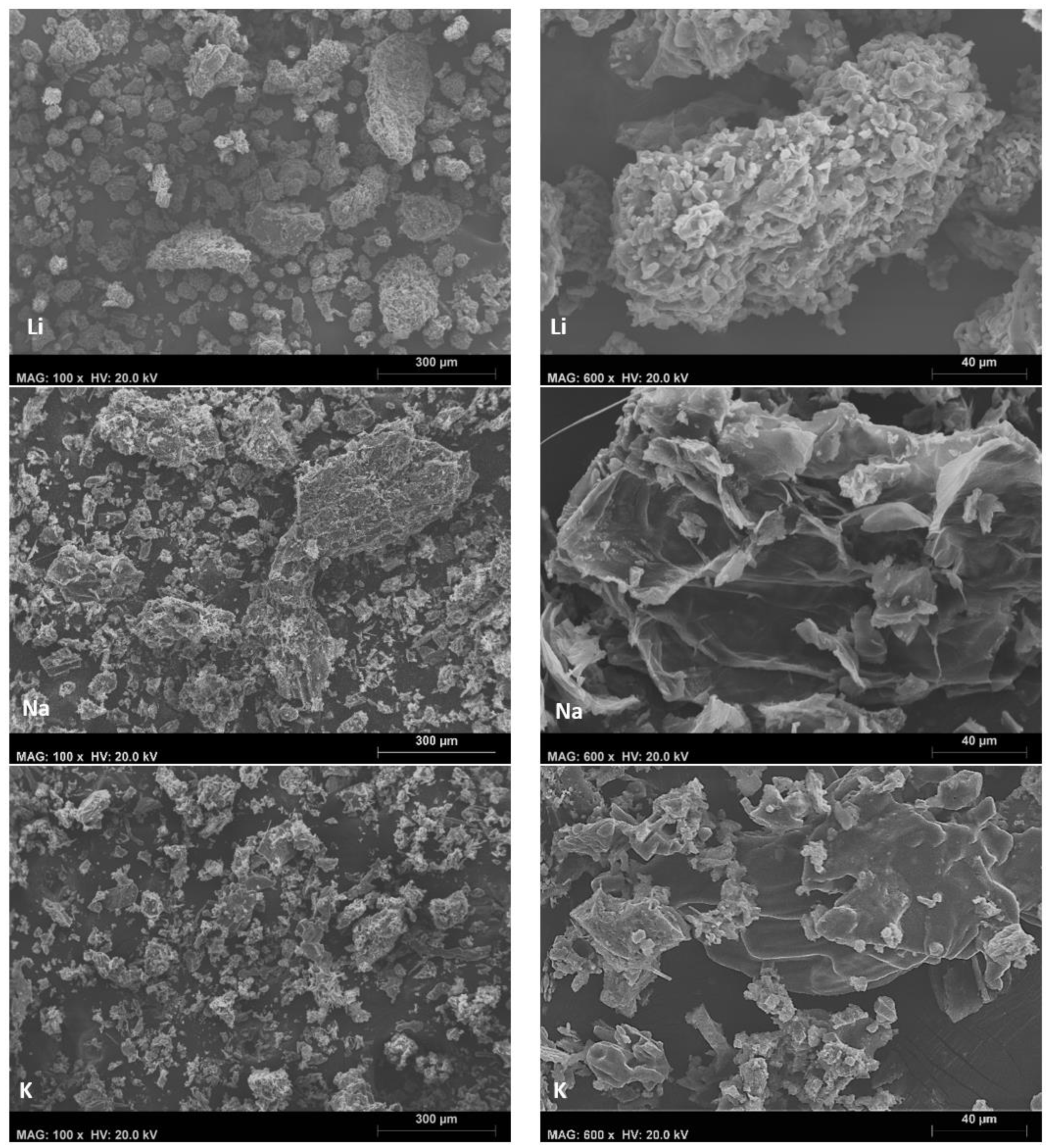
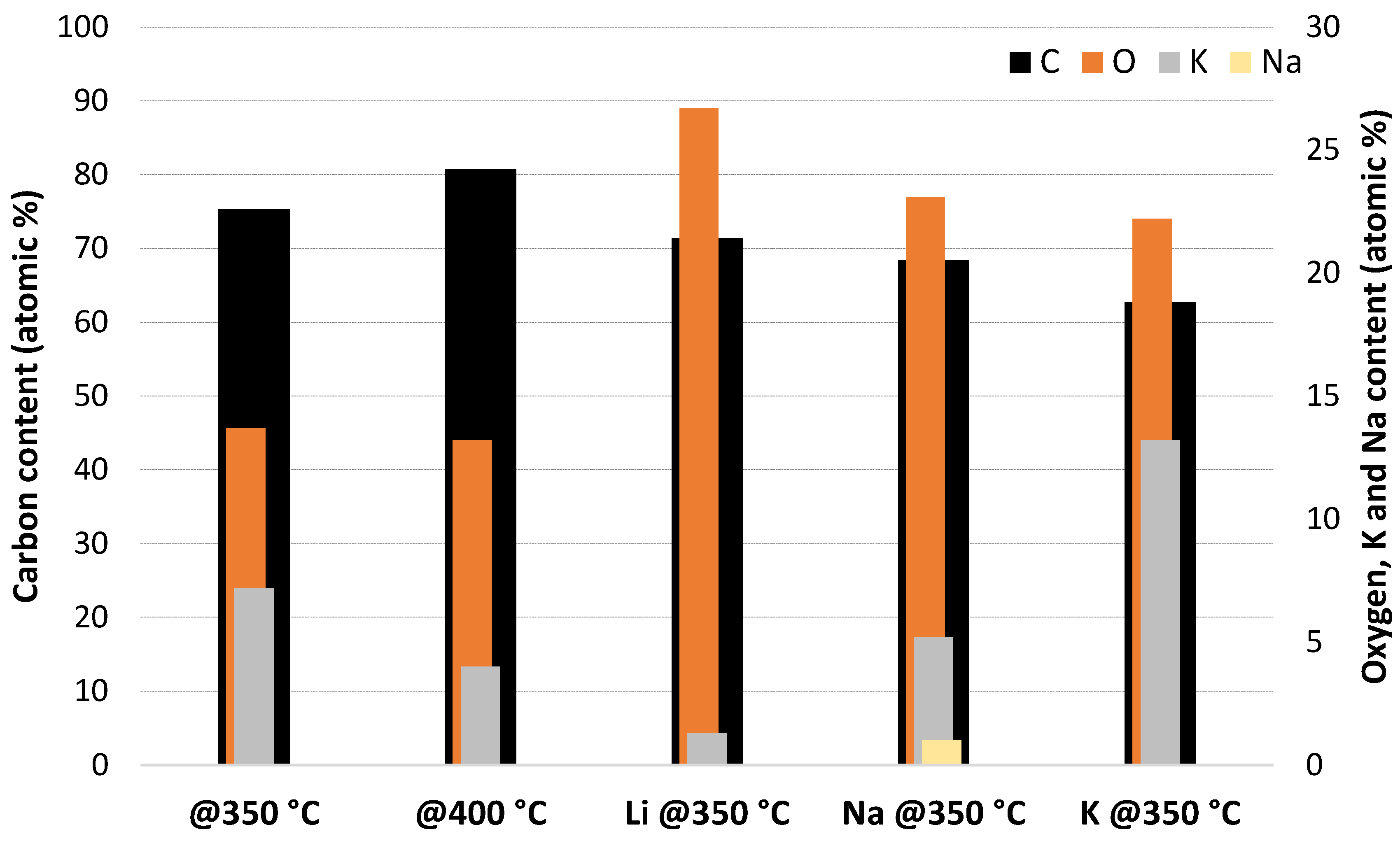

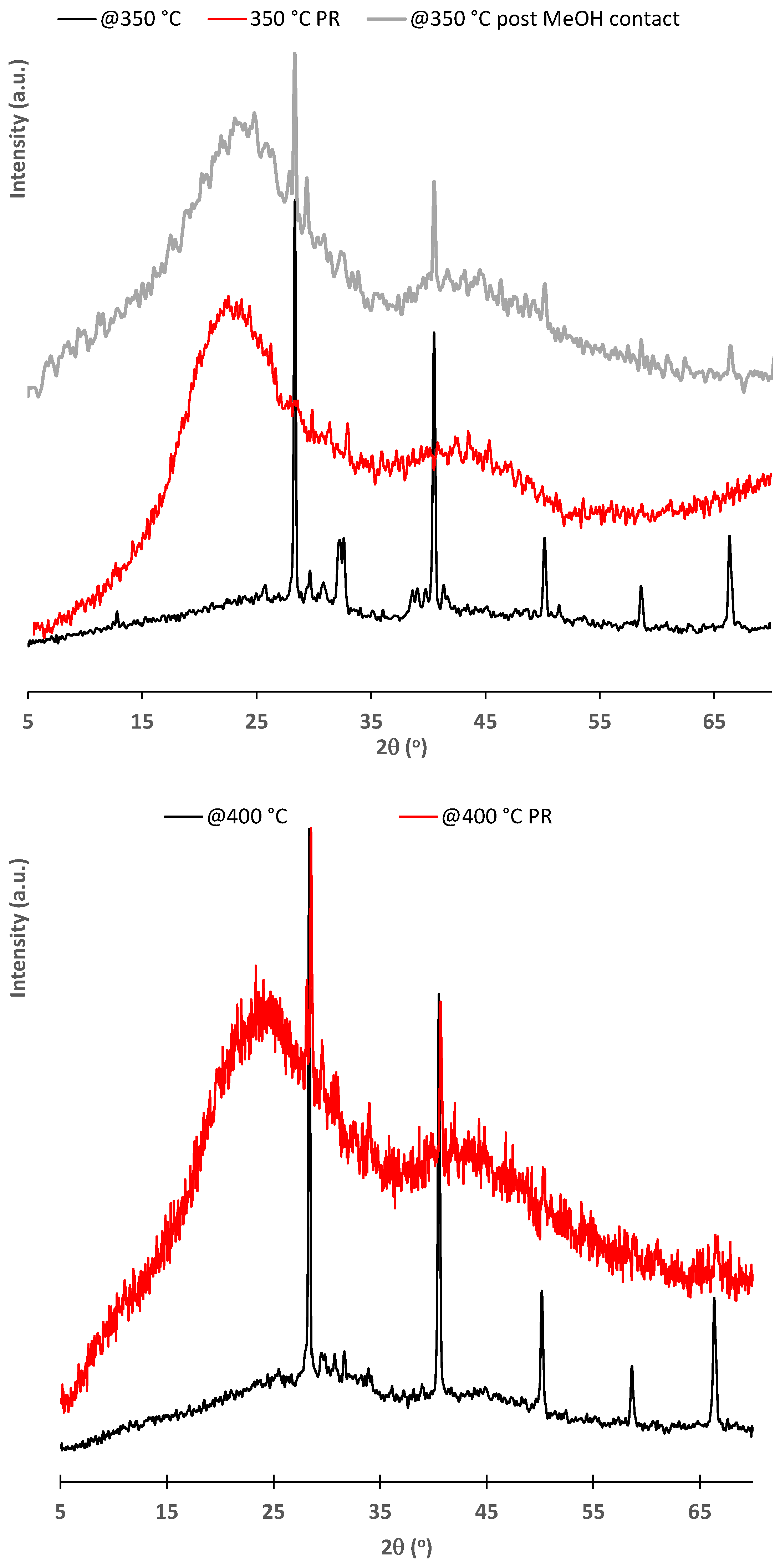




| Char | @350 °C | @400 °C | Li@350 °C | Na@350 °C | K@350 °C |
|---|---|---|---|---|---|
| ID/IG | 1.737 | 1.643 | 1.212 | 1.165 | 1.360 |
| IG/Iall | 0.298 | 0.264 | 0.267 | 0.253 | 0.290 |
| Wcat/Wfat (wt. %) | MeOH/WFO (molar) | FAME (wt. %) | |
|---|---|---|---|
| @400 °C WFO; 180 min | 5 | 15 | 95.8 |
| 12 | 91.0 | ||
| 9 | 69.1 | ||
| 7 | 12 | 91.8 | |
| 15 | 92.9 | ||
| 9 | 92.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares Dias, A.P.; Pedra, I.; Salvador, É.; Rijo, B.; Costa Pereira, M.F.; Serralha, F.; Nogueira, I. Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst. Catalysts 2024, 14, 266. https://doi.org/10.3390/catal14040266
Soares Dias AP, Pedra I, Salvador É, Rijo B, Costa Pereira MF, Serralha F, Nogueira I. Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst. Catalysts. 2024; 14(4):266. https://doi.org/10.3390/catal14040266
Chicago/Turabian StyleSoares Dias, Ana Paula, Igor Pedra, Érica Salvador, Bruna Rijo, Manuel Francisco Costa Pereira, Fátima Serralha, and Isabel Nogueira. 2024. "Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst" Catalysts 14, no. 4: 266. https://doi.org/10.3390/catal14040266