Abstract
The extensive use of plastics has led to a significant environmental threat due to the generation of waste plastic, which has shown significant challenges during recycling. The catalytic hydrocracking route, however, is viewed as a key strategy to manage this fossil-fuel-derived waste into plastic-derived fuels with lower carbon emissions. Despite numerous efforts to identify an effective bi-functional catalyst, especially metal-loaded zeolites, the high-performing zeolite for hydrocracking plastics has yet to be synthesized. This is due to the microporous nature of zeolite, which results in the diffusional limitations of bulkier polymer molecules entering the structure and reducing the overall cracking of plastic and catalyst cycle time. These constraints can be overcome by developing hierarchical zeolites that feature shorter diffusion paths and larger pore sizes, facilitating the movement of bulky polymer molecules. However, if the hierarchical modification process of zeolites is not controlled, it can lead to the synthesis of hierarchical zeolites with compromised functionality or structural integrity, resulting in reduced conversion for the hydrocracking of plastics. Therefore, we provide an overview of various methods for synthesizing hierarchical zeolites, emphasizing significant advancements over the past two decades in developing innovative strategies to introduce additional pore systems. However, the objective of this review is to study the various synthesis approaches based on their effectiveness while developing a clear link between the optimized preparation methods and the structure-activity relationship of the resulting hierarchical zeolites used for the hydrocracking of plastics.
1. Introduction
Since the 1950s, plastics have been widely used and are an indispensable part of modern life. They have been marketed for their affordability, strength, and non-degradability [1]. However, the same qualities of plastics pose significant challenges to their recycling, and consequently, their accumulation has led to extensive waste generation with environmental damage [2,3]. For instance, global waste plastic generation reached 353 million tonnes in 2019 and is projected to increase to over 1 billion tonnes by 2060 [4]. Also, this growth is alarming when considering the cumulative production of plastic, which has surpassed the significant value of 8.3 billion tonnes since 1950. Despite the serious environmental pollution problems, the maximum percentage of plastic waste is managed through landfilling (55%) or incineration (8%) for energy recovery, and only a small fraction of plastic is recycled (500 Mt) through mechanical recycling. Despite this, the recycled plastic eventually ends up in either landfills or incinerators, with only 1.2% of the material being reused [4]. This is primarily due to the reliance on recycling, which is based on people’s goodwill, to segregate various waste plastics into different recycling bins. However, a large quantity of waste plastics winds up in household trash bags, making it unviable and economically unfeasible to segregate and/or recycle a mixture of waste plastics due to its complexity and the presence of various impurities [5]. In addition, pre-treatment processes are energy extensive and utilize a large quantity of electricity and other natural resources (i.e., water and natural gas). Moreover, recycled plastics usually experience a reduction in key attributes such as mechanical strength and longevity, which constrains their potential uses [6]. For instance, Merrild et al. [7] observed an average 10% degradation in plastic material along with 10% quality loss during the recycling process. These problems collectively pose significant challenges to the mechanical recycling of waste plastic mixtures.
Therefore, there is an urgent need to develop environmentally friendly and cost-effective methods for the management of waste plastics. Currently, chemical recycling of waste plastics is emerging as a promising solution that offers the potential to convert waste into value-added chemicals, such as naphtha and gasoline range hydrocarbons and aromatics compounds. Thermo-plastics such as polyolefins (i.e., PE, PP), PS and PET are subjected to thermal and catalytic methods [8,9,10] (i.e., pyrolysis, gasification, and hydrocracking, etc.), whereas thermosetting plastics, such as polyurethanes and polyamides, etc., are exposed to depolymerization reaction [11,12] (i.e., solvolysis, etc.). However, the primary focus of this study is to discuss the chemical recycling of thermo-plastics, as they are mostly used as single-use plastics and result in the maximum generation of waste plastics. Amongst all technologies for converting plastic into valuable fuels and/or chemicals, hydrocracking of plastics has shown promising results with the least impact on the environment. This process facilitates the cracking of bulkier polymeric chains into saturated lighter hydrocarbons, including gasoline and diesel range products, in the presence of hydrogen [13]. In comparison to thermal and catalytic cracking, it is probably the most feasible method for converting waste plastics into saturated gaseous and liquid hydrocarbons that are suitable for use as transportation fuels [14].
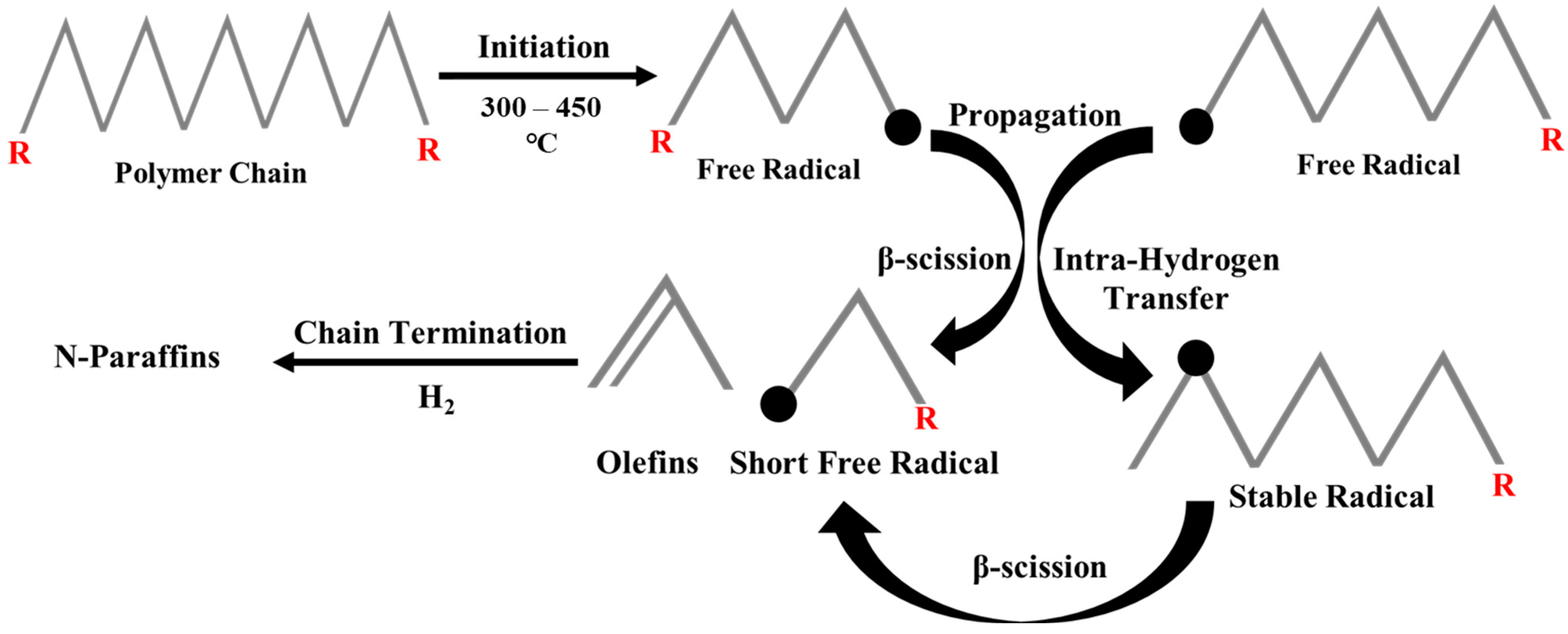
Hydrocracking experiments are generally carried out in continuous-flow tubular reactors and/or stirred-batch autoclaves with temperatures and cold H2 pressures ranging from ≥250 °C to 1–10 MPa, respectively [13,15]. High temperatures are required to provide the necessary heat for the reaction and to break down long-chain polymers, while cold H2 pressure is applied to prevent re-polymerization and dehydrogenation, which could otherwise lead to coke production [16]. Also, the introduction of hydrogen in the hydrocracking process facilitates the elimination of heteroatoms like chlorine, bromine, and fluorine found in waste plastics. Therefore, this technique not only converts environmentally harmful plastics (i.e., containing heteroatoms) into recyclable fuels, but it could also effectively reduce their potential negative impact on the environment [17,18]. Narksri et al. [19] recently studied the hydrocracking experiment (i.e., non-catalytic) of mixed plastics (PP, PS, PE, and PET) and achieved higher selectivity of lighter hydrocarbons (i.e., gases), as compared to thermal pyrolysis. Similarly, the presence of hydrogen in the reaction media suppresses the formation of unsaturated hydrocarbons (i.e., olefins and aromatics). This could be further explained based on the mechanistic pathway, known as the free radical mechanism, as shown in Figure 1. The cracking process involves extensive chain reactions that start with initiation, followed by propagation and termination. At elevated temperatures (300–450 °C), the chain initiation stage begins with random homolytic breakage of C-C bonds along the long polymeric chain, leading to the formation of free radicals. Subsequently, in the propagation step, the free radicals can either undergo β-scission, resulting in shorter free radicals and olefins, or experience intramolecular hydrogen transfer to form stable radicals, also known as secondary radicals (SRs). The further β-scission of these secondary radicals yields a pool of short free primary radicals and olefins. Finally, the formed short free radicals undergo hydrogenation in high partial pressure of hydrogen to form n-paraffins. The presence of high-pressure hydrogen may saturate the olefins and primary radicals before being further cracked [20]. Similarly, the recombination of these n-paraffins may also contribute to the production of iso-paraffins and cyclic hydrocarbons.
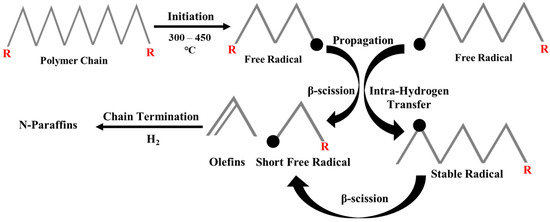
Figure 1.
Free radical mechanism of non-catalytic hydrocracking of plastics.
Despite the advantages of non-catalytic hydrocracking over pyrolysis (i.e., saturated products and low coke formation, etc.), the process insignificantly decreases the decomposition temperature of plastics. In detail, Iqbal et al. [21] compared the non-catalytic hydrocracking of PS to the pyrolysis process at different reaction temperatures (350–425 °C). Interestingly, both pyrolysis and hydrocracking showed similar conversions and product distribution (i.e., liquids and gases). Similarly, Narksri et al. [19] compared both processes based on the heating value (MJ/kg) of derived products and observed that pyrolysis and hydrocracking produced liquids with a similar heating value of 44 MJ/kg. Liu and Meuzelaar [22] also studied the effect of reactor gas on the decomposition temperature of commingled waste plastics in a high-pressure TG/GC-MS at 900 psig. Both reduve (H2) and inert atmosphere (He) showed a similar decomposition temperature (T95%). Therefore, non-catalytic hydrocracking seems to be an ineffective method to recycle waste plastics. However, the use of a catalyst may be a game-changer that can alter the overall reaction mechanism and help to significantly reduce the decomposition temperature of plastics (i.e., lower energy requirements), improve the reaction rates (i.e., high activity), enhance product selectivity (i.e., naphtha-based liquids) and reduce the formation of undesired products (i.e., solids and coke).
Therefore, to reduce the energy of activation, hydrocracking of plastics is generally performed using bi-functional catalysts (metal-loaded acid catalysts). Acidic support facilitates the cracking of long-chain polymers, whereas the metal functions in the hydrogenation-dehydrogenation reactions [23]. To date, researchers have investigated a range of catalysts with different metallic and acidic supports for hydrocracking reactions. Most commonly, noble metals, such as Ru, Pt, or Pd [24], and transition metals, such as Mo, Ni, and Co, are investigated over the acidic supports [25,26,27,28]. Similarly, for acidic supports, microporous zeolites, such as MFI, FAU, BEA [26,27,29,30], sulfated zirconia [31,32], and silica-alumina [33] have been utilized.
In spite of the high crystallinity and acid strength of sulfated zirconia, a significant loss of sulfur species during the reaction at high temperatures resulted in a decrease in activity and selectivity for the desired products [34]. In addition, it favors coke deposition on the active sites, which significantly reduces the acidity and stability of the catalyst [32,35]. Silica-alumina also did not receive much attention and industrial importance due to its low crystallinity, weak acidic characteristics compared to zeolites, and ordinary cracking activity [13]. Contrarily, crystalline zeolites as acid supports have shown promising results for the cracking of waste plastics into liquid fuels. These catalysts have been extensively utilized and reported because of their microporous structure, high specific surface area, crystallinity, concentration and strength of acid sites, long-term stability, exceptional activity, and selectivity [27,36,37,38]. Briefly, the acidic sites of crystalline zeolites can crack the C-C and C-H bonds in the long polymeric chains into shorter ones, whereas the pore structure of zeolites, known by their shape selectivity, leads to the formation of more value-added products, including lighter hydrocarbons.
However, practically, only up to 5% of the total known zeolite frameworks are utilized for industrial applications because of significant diffusional constraints [39]. The catalytic activity of microporous zeolites can be significantly improved by reducing the diffusion limitations in their micropores and channels or by synthesizing hierarchical zeolites that feature a combined combination of micro- and mesoporosity. Despite this, nano-structured zeolites often result in the loss of catalyst activity and stability. Contrary to this, hierarchically modified zeolites with comparable crystallinity present advantages as a result of the multi-level porosity [40], which facilitates the penetration of bulkier polymeric molecules into the zeolite structure and reduces the risk of catalyst deactivation due to the shorter residence time of molecules within the structure. Also, the incorporation of multi-level porosities reduces the diffusional limitations within the structure, thus enhancing the overall catalytic activity. However, the degree of hierarchy (also known as the hierarchy factor) directly influences the catalytic activity of modified hierarchical zeolite. A high degree of hierarchy may be advantageous in synthesizing hierarchical materials with enhanced porosity, but it leads to a decrease in the acidity of the zeolite. Similarly, a low degree of hierarchy could only retain the traditional properties of microporous materials. It is important to tune the structure of the zeolite, which essentially has a multi-level porosity, without greatly compromising its functionality.
Therefore, the present study provides a comprehensive review of the synthesis and modification of hierarchical zeolites through various methods, focusing on their practical use in the hydrocracking of plastics. Given the limited research on the application of hierarchical zeolites in plastic hydrocracking, this review also includes some studies on catalytic cracking that may be relevant for understanding potential modifications. Also, it discusses the synthesis protocols and the optimized parameters necessary for developing hierarchical zeolites that achieve high conversions and selectivity towards lighter hydrocarbons. Finally, the review addresses the challenges in designing efficient hierarchical structures and aims to guide researchers in this field by summarizing the latest advancements in the synthesis and applications of hierarchically porous zeolites.
2. Preparation of Hierarchical Zeolites
Globally, many researchers have been working to explore novel methodologies to fabricate hierarchical zeolites to broaden their applications [40,41]. In the past two decades, an incredible number of methodologies have been reported for modifying zeolites with secondary porosity. These methodologies are characterized as top-down and bottom-up approaches [42]. The detailed discussion and classification of top-down and bottom-up approaches with applications towards the hydrocracking of plastics is further explained below.
However, in this assessment, it is important to note that it is challenging to distinguish between the influences of textural and acidic properties of various hierarchical zeolites when comparing the hydrocracking of distinct plastics and/or mixtures of waste plastics, as hierarchical zeolites behave differently for different waste plastics streams. Additionally, some pathways for developing hierarchical zeolites have not yet been explored or studied in the context of plastic hydrocracking. Consequently, the discussion of these synthesis routes is based solely on the information available in the literature and the specific properties they impart to hierarchical zeolites. However, this discussion indirectly suggests potential directions for employing such routes in the development of hierarchical zeolites specifically for the hydrocracking of plastics. Similarly, rather than comparing specific values between different studies for different plastics, general trends will be analyzed, and the values will be primarily compared with those of their counterparts (i.e., microporous zeolites) investigated within the same study.
2.1. Desilication of Zeolites
Desilication is a top-down methodology for the production of hierarchical zeolites utilizing an alkaline medium (i.e., NaOH, KOH, or NH4OH) for selective removal of silica from the zeolite structure while generating secondary porosity in the framework. During alkaline treatment, silicon atoms are selectively extracted from the zeolite framework, while aluminum species accumulate on the external surface, playing a crucial role in pore direction [43]. The extracted silicate anions are then stabilized by alkali cations, resulting in the formation of voids. Notably, Na+ ions are the most effective leaching agents for silicon atoms due to their superior ability to stabilize silicate anions. Mesopores are initially generated on the outer surface or in defect areas within the crystalline structure, and desilication results in the formation of intra-crystalline porosity (i.e., within each crystal) while keeping the intra-crystalline porosity unchanged. However, a significant enhancement in the mesoporosity may potentially compromise the microporosity, as well as reduce the crystallinity and functionality of the zeolite [44]. For instance, Groen et al. [45] performed the desilication of MFI-based zeolite over 0.2 M NaOH and observed a decrease in the microporosity of the desilicated sample. Similar results were reported by Kots et al. [46], who modified a Mordenite (Si/Al = 10.5) zeolite through desilication, using NaOH (0.1–0.6 M) for 1–6 h at 85 °C, and studied the various desilicated zeolite samples for the hydrocracking of HDPE. Despite an increase in textural properties in the desilicated samples, the authors did not observe any significant increase in the activity and selectivity for the different desilicated zeolites due to a decrease in acidity. Therefore, it is important to critically analyze the available literature and to optimize the degree of desilication to create a hierarchical zeolite with mesoporosity without significantly sacrificing the intrinsic properties of zeolites (i.e., microporosity and acidity). To accomplish this, firstly, it is important to study the parameters affecting the desilication process. For instance, the degree of desilication is highly influenced by several parameters, namely the Si/Al ratio of the zeolite, the use of different alkaline media, and reaction conditions (temperature and time).
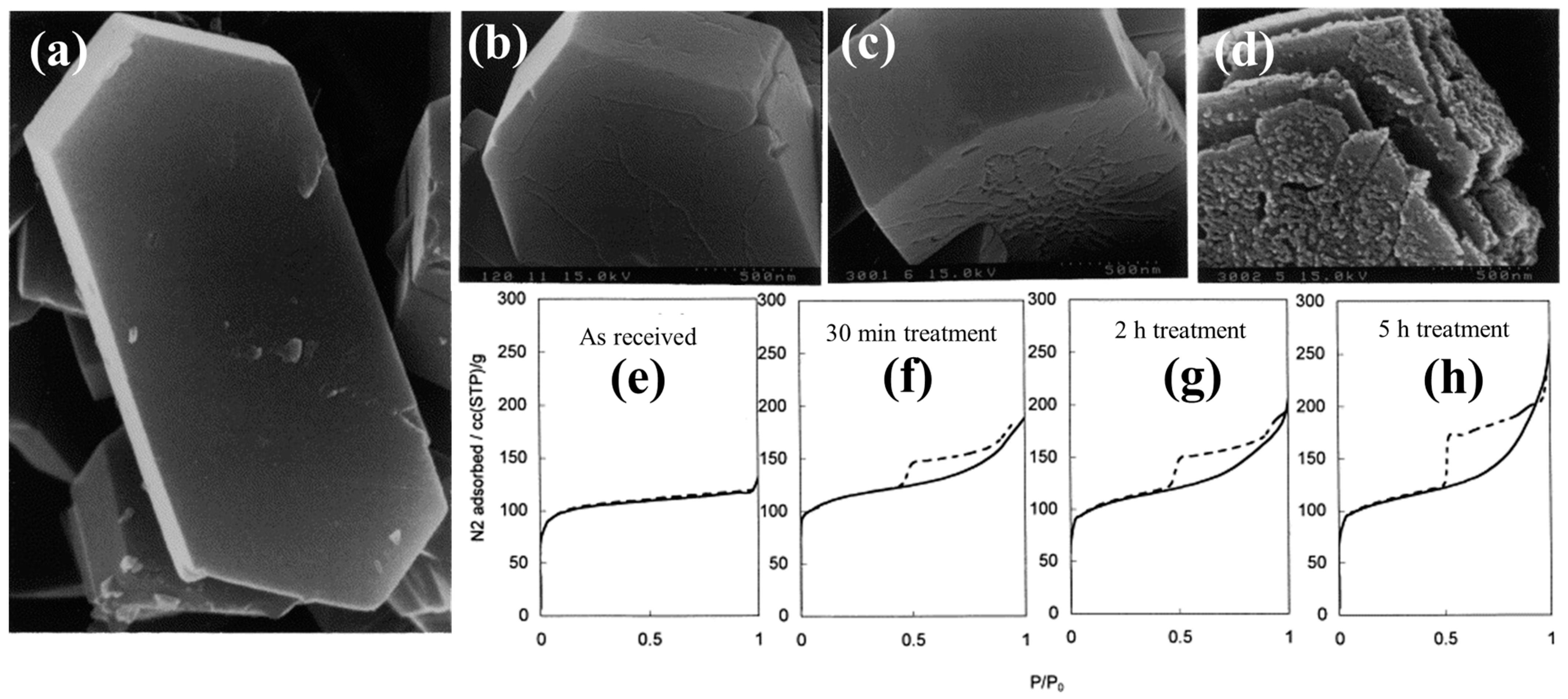
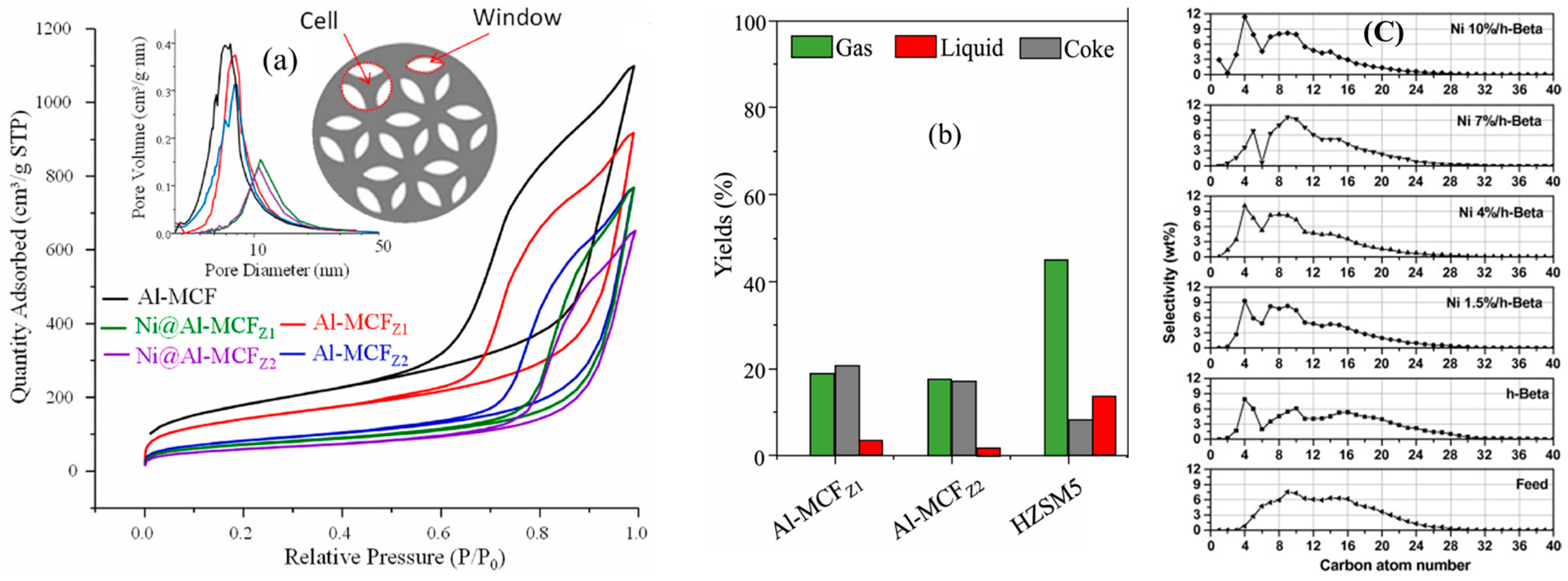
Ogura et al. [47] applied this technique and noticed that an alkaline medium (NaOH) was responsible for the enhancement of the mesoporous volume of a commercial HZSM-5 (Si/Al = 33) from 0.015 to 0.279 cm3/g, with a simultaneous reduction in the microporous volume by ~25%. Proceeding with their examination, these researchers studied the impact of different parameters, i.e., the effect of time and concentration of NaOH, on the desilication outcomes. They observed an increase in the degree of removal of silicon from the zeolite framework with time, while only a slight removal of aluminum (dealumination) took place, remaining constant with time. Moreover, the morphological modifications of the HZSM-5 generated by the desilication process under different concentrations of NaOH and time are illustrated in Figure 2. It was observed that desilication at 0.1 M NaOH for 120 min at 60 °C created some cracks on the external surface of the HZSM-5 crystal, while it almost doubled the mesopore volume and decreased the micropore volume by 25%. A further increase in the reaction time to 300 min resulted in the formation of deeper cracks in the zeolite structure (Figure 2c), which led to an increase in the mesopore volume by a factor of 3. On the other hand, it was found that a change in concentration of NaOH from 0.1 to 0.2 M at 80 °C significantly changed the mesopore volume of the zeolite (0.015 to 0.279 cm3/g) without further change in the microporosity (Figure 2d). However, under such severe reaction conditions, the zeolite crystal partly collapsed. Similarly, it resulted in a significant decrease in the acidity of the hierarchical zeolite from 0.589 mmol/g to 0.496 mmol/g.
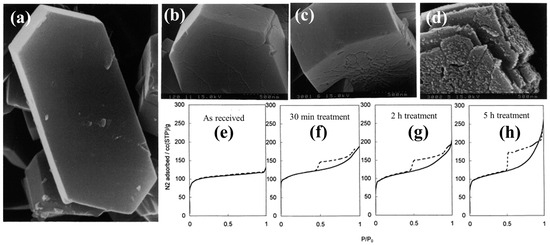
Figure 2.
SEM images of the (a) parent HZSM-5 and post-treated HZSM-5 zeolites using 0.1 M NaOH at 65 °C for (b) 2 h, (c) 5 h, and (d) 0.2 M NaOH at 80 °C for 5 h, Nitrogen isotherms for (e) as-received and (e–h) alkali-treated ZSM-5 in a 0.2 M NaOH solution at 353 K for different treatment time. Adopted with permission from Ref. [47], Copyright 2001 Elsevier.
Groen et al. [48] observed a similar trend and discussed the role of temperature in the desilication process. It was observed that a mild temperature (65 °C) applied to a zeolite with a Si/Al ratio of 17 (CBV 3024E) produced a hierarchical structure with a slight increase in mesoporosity (0.10 to 0.16 cm3/g) and preserved the acidic properties, whereas an increase in reaction temperature to 85 °C resulted in a significant increase of uncontrolled mesoporosity (0.24 cm3/g). Therefore, based on these studies, it was concluded that temperature, time, and concentration of alkali medium play a key role in the desilication process. While lower temperature (≤60 °C), shorter reaction time, and lower concentration of NaOH generated controlled mesoporosity, an increase in temperature, prolonged reaction time, and high concentration of NaOH significantly increased the mesoporosity with severe structural defects and potential structure collapse.
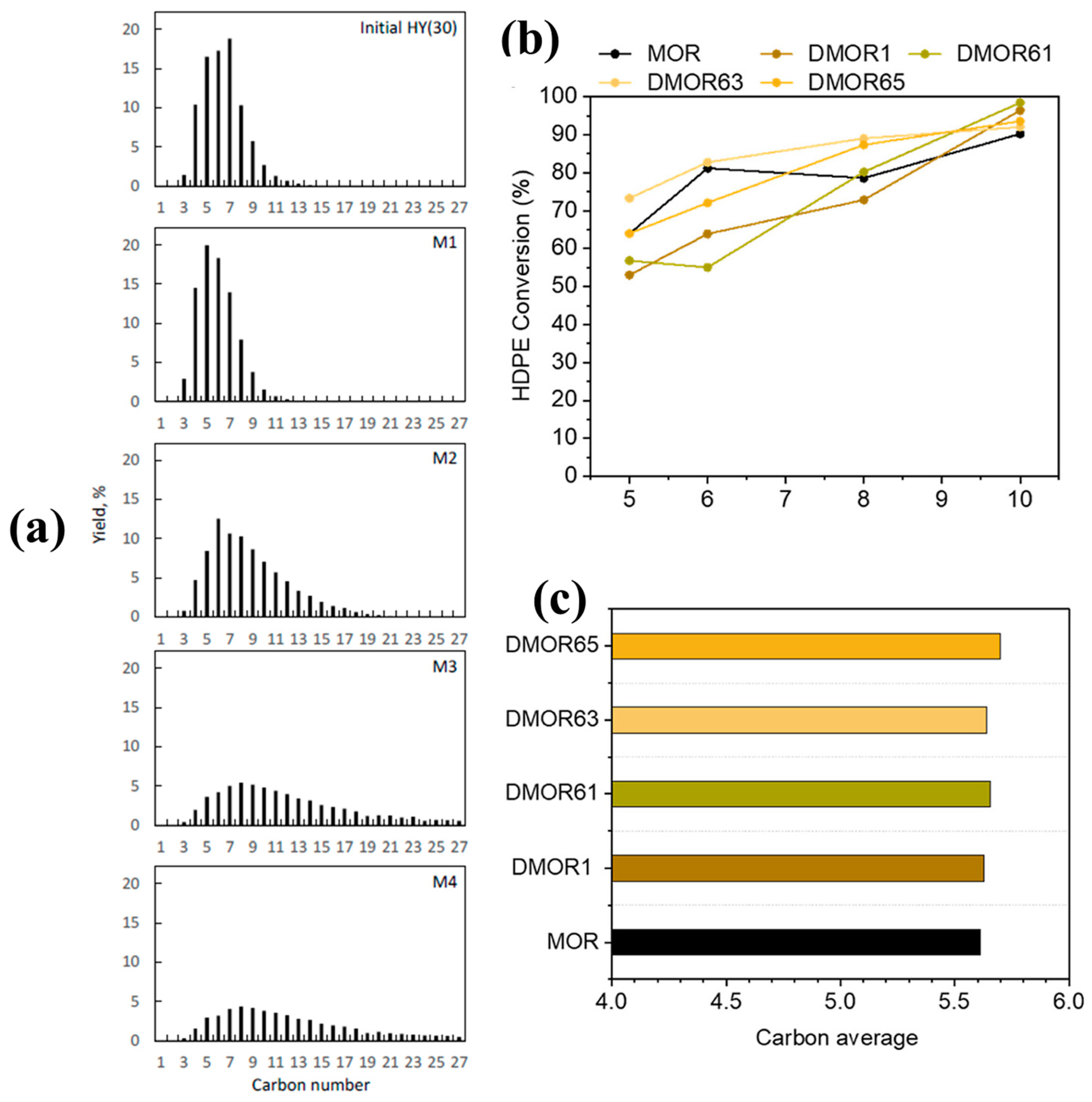
To further evaluate the effect of desilication on the catalytic properties of HY for the hydrocracking of LDPE, Liu et al. [24] performed the desilication of HY with different concentrations of NaOH solution, ranging from 0.1 M to 0.4 M for 30 min at 60 °C. The textural and acidic properties of parent and various desilicated samples are presented in Table 1. Engineering the porosity and acidic properties of HY significantly altered the activity and product distribution. The mild delicate zeolite M1 with enhanced mesoporosity showed similar activity in comparison with the parent HY but a higher selectivity for gasoline-range hydrocarbons. However, a further increase in the concentration of NaOH resulted in a decline in the activity and selectivity for gasoline-range fuels. This was attributed to the loss of relative crystallinity and concentration of acid sites with an increase in the concentration of NaOH. As clearly evident from the results in Table 1, both M3 and M4 almost completely lost the zeolite functionality, presenting almost zero microporosity and crystallinity. These amorphous alumino-silicates also resulted in a product shift towards higher hydrocarbons (C13–C25) at the expense of gasoline-range products (Figure 3a). Therefore, it can be concluded that an excessive increase in the degree of desilication may lead to a decrease in catalyst crystallinity and acidity, which results in a loss of catalyst activity and selectivity towards lighter hydrocarbons.

Table 1.
Role of desilication on the textural and acidic properties of HY zeolite. Data from [24].
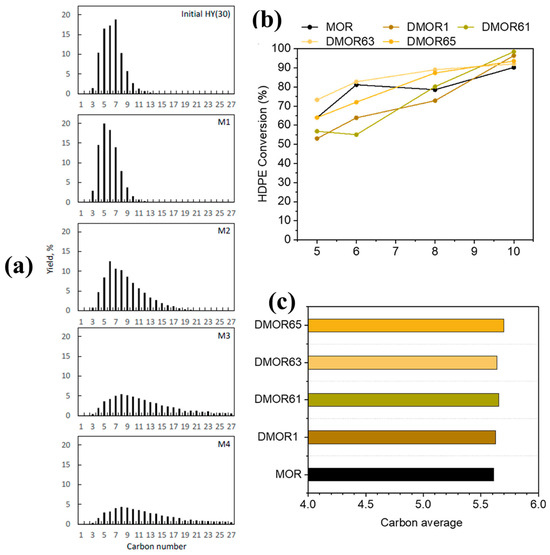
Figure 3.
(a) Product yield distribution by carbon number over pristine HY(30) and various desilicated zeolite samples, Reprinted from Ref. [24] under a Creative Commons Attribution Non-Commercial License 4.0 (CC BY-NC), Impact of degree of desilication on the catalytic activity of MOR for the hydrocracking of HDPE. Comparison of (b) conversion and (c) average carbon number °C. Reprinted with permission from Ref. [46], Copyright 2023 American Chemical Society.
However, Kots et al. [46] observed a different trend while studying the hydrocracking of HDPE over various desilicated Mordenite zeolites. In detail, the authors performed the desilication of MOR using 0.1–0.6 M NaOH and by varying the time from 1 to 6 h at 85 °C. The modification conditions and physiochemical properties of parent and various desilicated MOR samples are presented in Table 2. Overall, an interesting trend was observed where a mild degree of desilication (DMOR1) led to an increase in the overall porosity with a significant decrease in the Brønsted acidity of the sample. Although the authors did not explain the reason behind the observed trend, it might be due to the removal of some aluminum species during the course of desilication. Contrary to this, all other samples (i.e., under harsh conditions) exhibited comparable total acidity to that of the parent material, even though a decrease in the number of Brønsted acid sites with a consequent increase in the Lewis acid sites took place. This could be ascribed to the removal of framework aluminum species from the zeolite with consequent retention as extra-framework aluminum (EFAl).

Table 2.
Desilication conditions and physiochemical properties of MOR samples. Data from [46].
Despite the changes in the physiochemical properties of the desilicated zeolites when compared to the parent zeolite, desilicated MOR did not show significant effects on the hydrocracking of HDPE (Figure 3b) and average carbon numbers of the products (Figure 3c).
To further investigate the effect of textural and acidic properties of hierarchical zeolites on the reaction pathways, Marcos et al. [49] performed the hydrocracking of PS under kinetic control. The authors synthesized several desilicated HY samples by varying the concentrations of NaOH (i.e., 0.1 M, 0.2 M, 0.3 M). All the desilicated samples exhibited improved textural properties (i.e., surface area and porosity) with enhanced accessibility to the Brønsted acid sites. However, an increase in the degree of desilication led to a decrease in the overall acidity of the catalyst. These results are in accordance with the previously discussed studies of Ogura et al. [47] and Kots et al. [46]. Despite a decrease in acidity, all the catalysts showed almost similar conversion, however, with differences in the product distribution. A high degree of desilication resulted in a decrease in the selectivity for gasoline-range fuels. In addition, an increase in the concentration of NaOH from 0.1 to 0.2 and 0.3 M favored the formation of naphthenes, olefins, and paraffins at the expense of aromatics. Based on the kinetic modeling, the authors concluded that the presence of high surface area and weak acidity were associated with the high yield of gasoline range fuels. It was also observed that the selectivity of aromatics is a function of catalyst porosity and Lewis acidity. The desilicated sample with maximum porosity and least Lewis acidity showed minimum selectivity for aromatics.
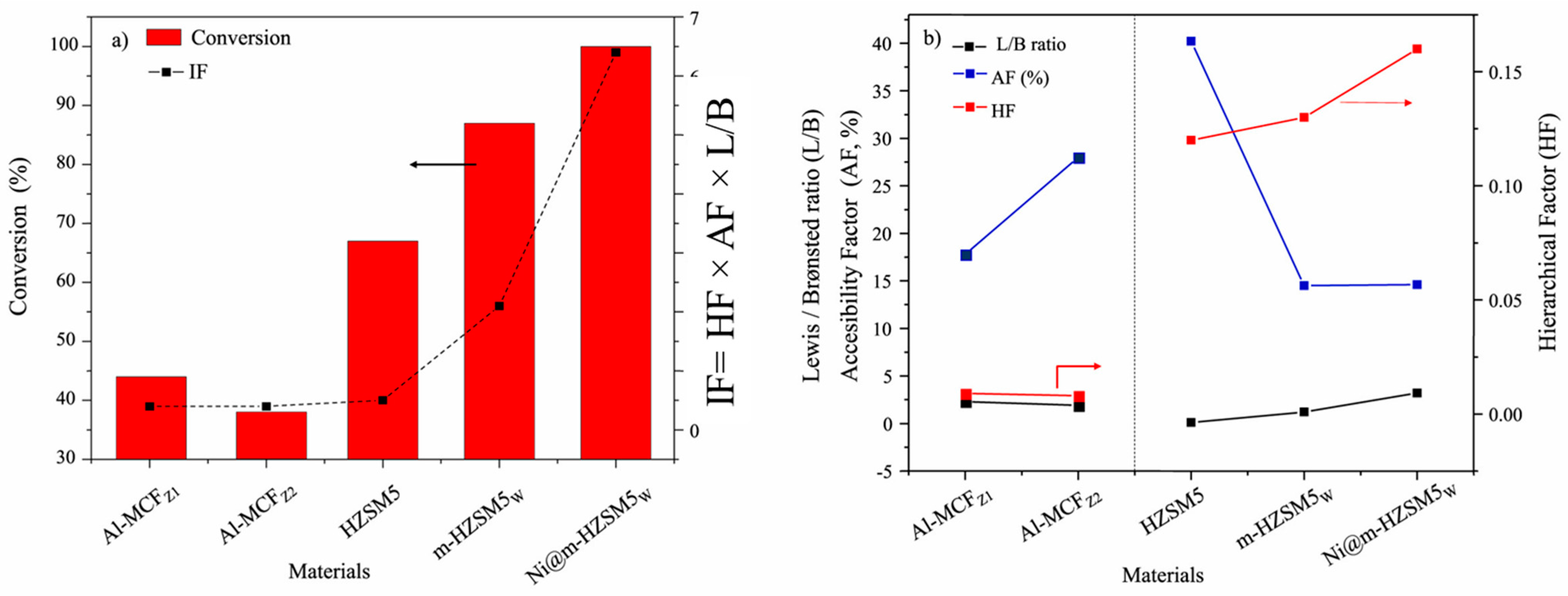
In another study by Armenise et al. [50], the authors analyzed the structure-activity relationship of desilicated zeolites and compared the results of parent and desilicated HZSM-5 for the hydrocracking of HDPE. The desilicated zeolite (m-HZSM5) was prepared by using 1M NaOH at 70 °C for 1 h. The bi-functional composites were synthesized by the addition of Ni, and their physiochemical properties are shown in Table 3. The desilication of HZSM-5 significantly increased the textural properties of the zeolite. Moreover, the authors developed an interplay factor (IF) based on the degree of hierarchy (HF), acidity, and textural properties of the zeolites, which directly correlate with the hydrocracking of HDPE. The interplay factor was expressed as IF = HF × AF × AS, where HF was the hierarchy factor and measured as a multiplication of Vmicro/Vtotal and Smeso/SBET, AF was the ratio between the sum of acid sites measured by pyridine at 150 °C and the concentration of n-propylamine measured by TPD (ACIPy/ACIpro) and AS was the ratio of Lewis to Brønsted acid sites determined by pyridine at 150 °C.

Table 3.
Textural and acidic properties of the parent and its desilicated zeolite samples with or without the addition of Ni. Data from [50].
For the hydrocracking of HDPE, the desilicated sample showed better cracking results due to the high accessibility of the pores (i.e., higher HF) and Lewis acidity, which even increased after the addition of Ni. The authors explained the results based on the empirical relation (IF) as expressed in Figure 4a. The results suggested a linear relation of the interplay factor with the conversion of HDPE.
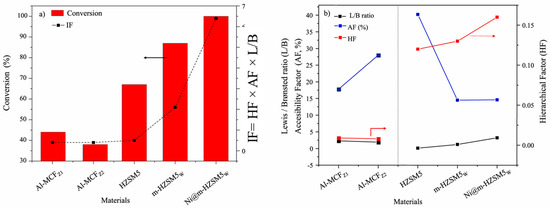
Figure 4.
(a) Correlation plot between the hydrocracking of HDPE and Interplay Factor (IF), where solid red bar showed the conversion and dotted lines represented the interplay factor (b) Evolution of different factors influencing the activity catalyst. Reprinted with permission from Ref. [50], Copyright 2023 Elsevier.
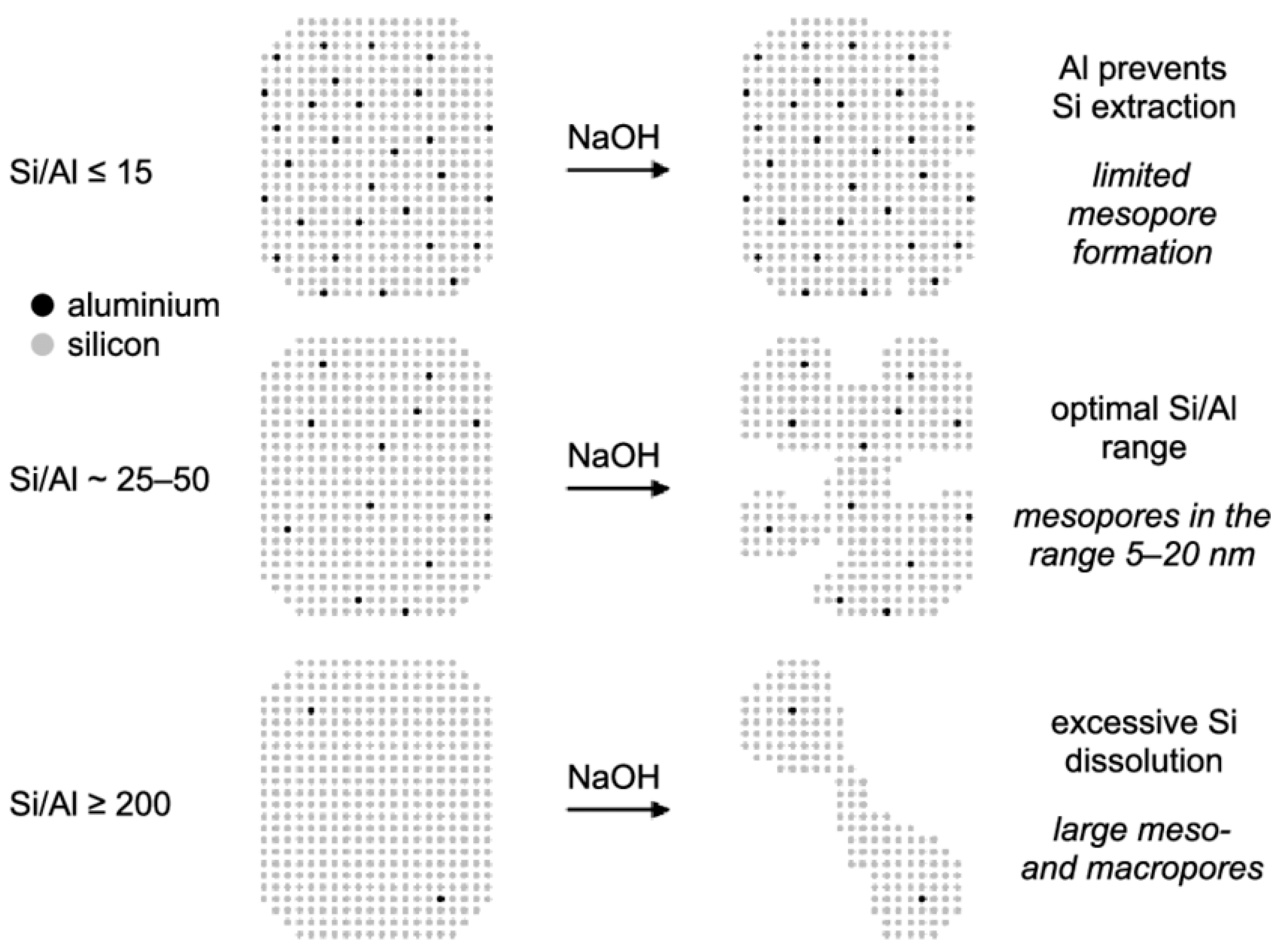
Moreover, another important parameter is the amount of aluminum in the parent zeolite structure, which plays a critical role in the degree of desilication. It was discovered that the process of desilication could be hindered by the existence of adjoining aluminum species in the structure. As reported in the literature, at a low Si/Al proportion of <15, the presence of high aluminum content in the zeolite structure restricts Si species from being separated and, therefore, prevents the generation of additional mesoporosity (Figure 5). Conversely, the pore size increased from ~10 nm to ~50 nm with the increase in Si/Al ratio [30]. Similarly, desilication of zeolites with Si/Al ratios between 25 and 50 leads to the prominent production of mesoporosity, whereas higher Si/Al (>200) fabricate materials with macropores along with reducing the surface of mesoporous, as shown in Figure 5 [48]. Therefore, the initial Si/Al ratio of the zeolite needs to be correctly selected in order to successfully direct the structural (Si-O-Si) bonds towards improved secondary porosity. In another study, Groen et al. [51] also observed similar results while performing the desilication of MFI zeolites. They examined various HZSM-5 zeolites with Si/Al ratio ranges from 15 to 1000 and concluded that the proportion of aluminum within the zeolite system performed a critical part in developing mesoporosity in MFI zeolite through alkaline treatment. In addition, they confirmed the expulsion of silicon from the zeolite framework after examining the filtrates, which contained a Si/Al over 1000.
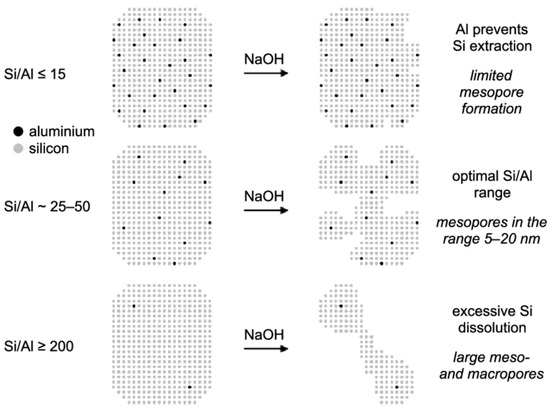
Figure 5.
Pictorial representation of the impact of Si/Al ratio on the desilication process of MFI zeolite in alkaline media. Reprinted with permission from Ref. [48], Copyright 2004 American Chemical Society.
Therefore, it is difficult to desilicate a zeolite with a lower Si/Al ratio (i.e., high aluminum content). However, partial removal of aluminum from the framework prior to the desilication may facilitate the process. In fact, Zhao et al. [52] performed the desilication of NaY (Si/Al = 2.25) using 0.1 M NaOH at 60 °C for 2 h. However, prior to desilication, the zeolite sample underwent a dealumination through high-temperature steaming and acid leaching (these methods will be discussed in detail in the next section). Interestingly, the as-synthesized desilicated HY showed an enhanced external surface area and mesopore volume. The authors further studied the hydrocracking of LDPE over the hierarchical HY zeolite promoted with Pt, with the catalysts exhibiting high conversion of LDPE (90.3%) at 280 °C under 20 bar cold H2 pressure. Similarly, the bi-functional hierarchical catalyst with its moderate acidity showed the maximum selectivity for gasoline range hydrocarbons (C5–C12 = 81.4%). However, no experiments were conducted with pristine Pt-loaded HY for comparison.
Furthermore, in an attempt to control the desilication of zeolites with high Si/Al ratios and without significantly destroying the framework, various organic bases (i.e., TPAOH, TBAOH) under mild conditions were used in the literature. Marek et al. [53] performed the desilication of BEA zeolite (Si/Al = 22) and employed it for the cracking of LDPE. The desilicated samples were prepared by varying the desilication time (i.e., 10 min and 30 min) at 65 °C using 0.2 M NaOH and TBAOH mixture (TBAOH/(NaOH + TBAOH) = 0.4) and named as a-βH (10 min) and b-βH (30 min). The nitrogen physisorption of the parent BEA zeolite showed type-I isotherm, confirming its microporous nature, whereas both desilicated zeolites exhibited type-IV isotherms at intermediate pressures, which corresponded to mesopores with diameters < 10 nm and a PSD pattern maximum at 3.5–4.5 nm. The desilication treatment also resulted in an increase in the mesoporous volume and external surface area at the expense of micropore volume. Similarly, the more pronounced desilication of b-βH led to the maximum accessibility of the acidic sites, which in turn resulted in a significant boost in the total acidity (980 μmol/g) of the sample. On the other hand, the mild desilication of a-βH resulted in a slight increase in the total acidity of the sample (670 μmol/g) as compared to parent BEA zeolite (615 μmol/g). However, the increase in the degree of desilication resulted in a decrease in acidic strength, which subsequently decreased the overall LDPE cracking as compared to parent BEA zeolite. The authors explained that despite the spacious structure of the desilicated samples, the lower acidic strength of the hierarchical zeolites led to a decrease in the cracking ability. This emphasizes the significance of a balance between acidic site strength and accessibility. Overall, enhancing mesoporosity by increasing accessible acidic sites can improve performance, but the efficiency heavily depends on maintaining high-strength Si(OH)Al groups. Overdevelopment of mesoporosity, which weakens acidic strength, leads to reduced efficiency, as strong acidity is critical for effective cracking. Therefore, it is necessary to control the desilication in a way that creates secondary porosity without significantly compromising the acidic strength of the catalyst.
To circumvent this problem, Baena et al. [54] performed the desilication of a USY (Si/Al = 31) using 0.2 M of NaOH + TBAOH (10% mol TBAOH) solution at room temperature for 30 min. The desilicated USY showed a controlled increase in textural properties, which significantly led to the reduction of the apparent activation energy required to crack 50% of LDPE (Ea = 76 kJ/mol) in comparison with parent USY (107 kJ/mol). Similarly, in another study by Abello et al. [55], the authors studied the desilication of ZSM-5 (Si/Al = 42) by utilizing various organic bases (TPAOH and TBAOH) and compared the results with an inorganic base (i.e., NaOH). Despite the similar alkalinity and reaction conditions, NaOH provided much better mesopore volume (0.48 cm3/g) and surface area (277 m2/g) development when compared to organic bases. The authors explained this behavior based on the total heat flow during the desilication process. Interestingly, desilication under NaOH released 12 times more heat (81 J/g) than TPAOH. This led to extensive removal of silicon during the first 30 min, whereas the use of TPAOH slowed desilication kinetics. However, during the benzene alkylation, the use of TPAOH enabled a controlled growth of mesopores, which was advantageous to enhance the yield of ethylbenzene as compared to the NaOH-treated desilicated zeolite. Also, the use of TPAOH produced the protonic form of zeolite upon calcination, eliminating the need for the typical ion exchange with NH4NO3 that follows the NaOH treatment.
To conclude, the desilication method is particularly applicable to zeolites with medium-to-high Si/Al ratios and offers the advantage of generating incorporated mesopores with good pore connectivity within the zeolite structure. Additionally, this cost-effective method can be applied to a range of zeolites, including MFI, MOR, BEA, and FAU, with potential scalability for industrial applications. However, zeolites with varying Si/Al ratios exhibit different degrees of desilication under similar reaction conditions. Similarly, various operating parameters significantly influence the degree of desilication. If not properly controlled, desilication may alter the original Si/Al ratio of the zeolite, leading to the development of disordered mesopores and a reduction in both the crystallinity and acidity of the modified material. For instance, a higher concentration of NaOH accelerates the extraction of silicon, thereby resulting in uncontrolled mesoporosity with a loss of crystallinity and acidity. Also, elevated reaction temperature enhances the degree of desilication and may compromise the structural integrity of the parent zeolite. Finally, the extent of desilication is dependent on treatment time, and prolonged exposure can lead to excessive framework degradation with the loss of acidity and acidic strength. This eventually results in a decrease in the hydrocracking of waste plastics to lower hydrocarbons. The use of organic bases (i.e., TPABr) may counter this issue. However, it influences the nature of the mesopores formed, promoting the development of constricted mesopores while preserving some microporosity. Therefore, all these parameters must be carefully optimized to balance enhanced mesoporosity with the preservation of structural and acidic properties for effective hydrocracking of plastics. Although this modification process has not been significantly employed in the hydrocracking of waste plastics so far, the available literature underscores the importance of controlling the degree of desilication in zeolites to enhance activity and selectivity for value-added products during the hydrocracking of plastics.
2.1.1. Dealumination of Zeolites
Dealumination is another top-down and post-synthesis methodology for developing hierarchically zeolites utilizing various chemicals (e.g., hexafluoro silicate or silicon tetrachloride), acids (e.g., HCl, H2SO4, etc.), or steaming (i.e., heat treatment above 500 °C in the presence of steam), to hydrolyze Al-O-Si bonds to eliminate aluminum and generate mesoporosity in the zeolite framework [56,57,58,59]. To understand the mechanism of dealumination, Donk et al. [60] discussed the dealumination process based on the steaming of zeolite. In detail, steaming at high temperatures (>500 °C) removes the aluminum from the framework by breaking Al-O-Si bonds and building new defects (vacancies). This leads to the migration of less stable silicon to the vacancies, creating silanol-rich domains. The technique outlined can create several mesopores and heal a portion of the amorphous structure. Both the treatment time and steaming temperature are decisive parameters to determine the degree of Al-extraction and mesopores generation. The removed aluminum neutralizes lattice charges, stabilizes the remaining aluminum atoms in the framework, and prevents further dealumination. As a result, the degree of dealumination is limited in a single steaming cycle. In parallel, the removal of aluminum may result in the partial amorphization of the framework (i.e., loss of crystallinity) with the potential to block the formed mesopores with debris (i.e., extra-framework aluminum species). Therefore, an additional step of mild acid leaching is generally required to extract the extra-framework species from the pores and create significant mesoporosity in the zeolite structure [61]. For instance, Lohse et al. [62] observed the creation of mesopores in Y zeolite through steaming (pore size of 10 nm). However, the mesopores were significantly obstructed by residues of amorphous EFAl species. These blockages were subsequently cleared with HCl treatment, which enlarged the mesopore size to 20 nm. The reported method is widely used in producing ultra-stable zeolite Y (USY), a key component in fluid cracking catalysts for crude oil processing [30]. Therefore, this two-step method can yield hydrothermally stable zeolites with enhanced mesopore volume and a higher Si/Al ratio [63]. Similarly, the dealumination of zeolites could also be conducted by direct acid leaching, which involves the use of inorganic and or organic acids to remove aluminum from the framework. The removal of FAl species via acid leaching creates mesopores by hydrolyzing and breaking Si–O–Al bonds. This process forms Si–O– defects and leaves extra-framework alumina species.
Overall, the dealumination of zeolites results in an increase in the Si/Al ratio and alters the concentration and strength of the acidic sites in comparison to the parent material. The removal of Al species (i.e., increase in Si/Al ratio) and a decrease in acidity, thereby potentially impacting the overall conversion of plastics during the hydrocracking experiments. Therefore, before discussing the advancements in how to control the dealumination of zeolites, we will look at the role of the Si/Al ratio and the effect of acid sites as a result of dealumination on the hydrocracking of plastics. For instance, Sharratt et al. [64] developed zeolite samples with different Si/Al ratios (17.5 and >1000) and compared the catalytic activity for the cracking of HDPE at 360 °C. Although the synthesized zeolites are not the perfect example of dealumination, they clearly illustrate the impact of the Si/Al ratio and acidity of zeolites on the hydrocracking of plastics. The zeolite with no acidity (i.e., silicalite sample with no aluminum, Si/Al > 1000) hardly showed any conversion (5.6%) in 60 min, whereas the HZSM-5 sample with high acidity exhibited 91.3% conversion in just 15 min. Similarly, to analyze the effect of the acidity of dealuminated zeolites on the product selectivities, Jumah et al. [65] performed the hydrocracking of squalane (C30H62), which serves as a model hydrocarbon molecule to simulate the LDPE chain over Pt-loaded Beta zeolite of different Si/Al ratios of 12.5 and 175 at 275 °C and 20 bar initial H2 pressure. As expected, the silica-rich structure of Beta zeolite (Si/Al = 175) exhibited weak acidity (325 μmol/g) due to the removal of aluminum species and showed lower conversion with high yield of heavier hydrocarbons, whereas Pt-Beta (12.5) with significantly higher concentration of strong acid sites (342 μmol/g) resulted in 100% conversion of squalane with high yield of lighter hydrocarbons. Similar to what was observed before, Li et al. [66] recently compared the hydrocracking of LDPE over different samples of Pt@S-1 + Beta zeolite by varying the Si/Al of Beta zeolite from 25 to 200. A change in Si/Al from 25 to 40 changed the activity from ~99.5 to 40%, which even decreased to zero over Beta zeolite with Si/Al ratio ≥ 200. Similarly, increasing the Si/Al led to an increase in the selectivity of heavier hydrocarbons and a drastic decline in the selectivity of lighter naphtha. This decrease is attributed to the reduced acid strength and concentration of weak and medium acid sites and an increased Si/Al ratio, which decreased the polymer cracking. Overall, the results explained the collective importance of textural and acidic properties, which have a significant effect on the activity and selectivity of the synthesized dealuminated zeolites. Therefore, it is essential to dealuminate zeolites in a controlled manner, where an increase in mesopore volume is conducted without much compromising the crystallinity, micropore volume, Si/Al ratio, and acidity of the modified zeolite. Despite the challenges in performing the controlled dealumination, an optimization of dealumination parameters (i.e., time, steaming/acid leaching temperature, etc.) and/or mild dealumination (i.e., selective Al-extraction) can lead to the development of hierarchical zeolites that significantly preserve the functionality of the parent zeolite.
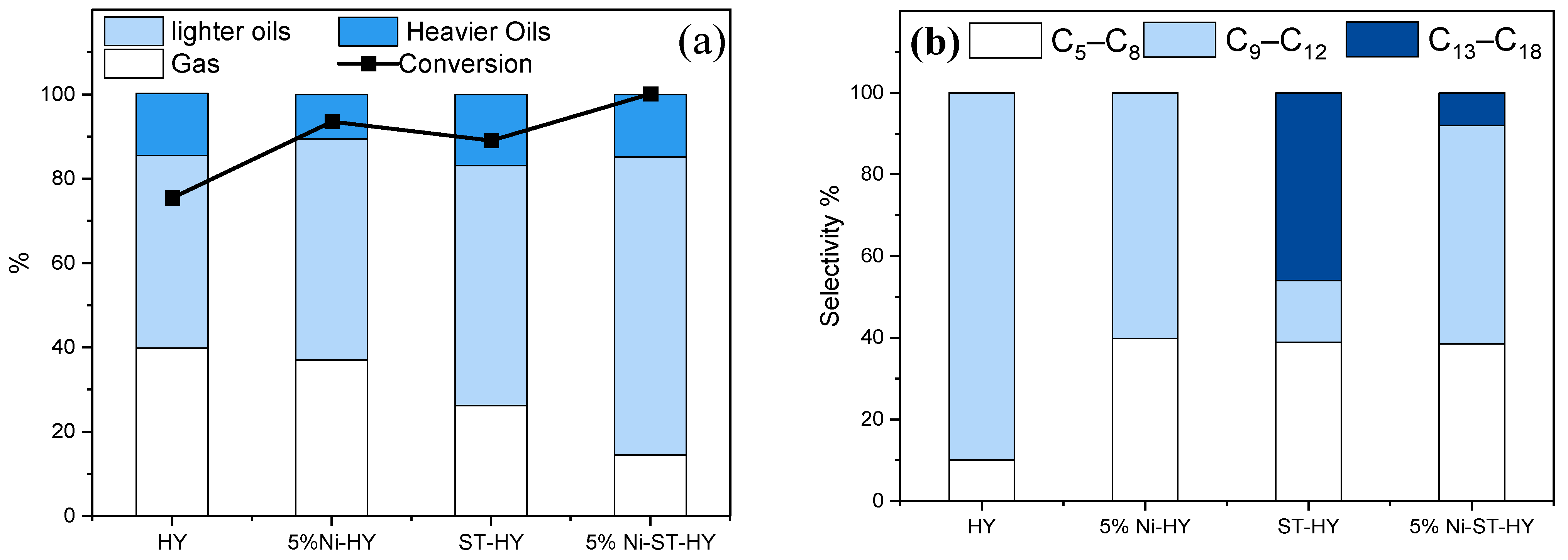
For instance, Azam et al. [67] utilized a mild steaming approach to modify a HY zeolite (Si/Al = 30) at 500 °C for 4 h. In comparison with the parent HY, dealuminated HY zeolite (ST-HY) showed notably enhanced porosity and external surface area by the removal of some FAl species. As a result, the dealuminated zeolite sample showed almost the same functionality as that of parent zeolite (i.e., crystallinity, Si/Al ratio, and micropore volume). Interestingly, it led to a slight increase in the concentration of Brønsted acid sites owing to better accessibility. However, the removal of extra-framework aluminum species resulted in a small decrease in the concentration of Lewis acid sites. The catalytic properties of the dealuminated HY zeolite were studied for the hydrocracking of polypropylene-based surgical face masks at 325 °C under 10 bar cold H2 pressure for 2 h. Based on the results, the hierarchical HY sample exhibited higher conversion (88.9%) than the parent material, which eventually increased to 100% with the addition of Ni (5% Ni-ST-HY), with maximum selectivity for lighter oils, as illustrated in Figure 6a. The authors attributed the improved conversion to an increase in the textural and acidic properties. However, a decrease in retention time inside the zeolite structure reduced the occurrence of chain cracking reaction, resulting in slightly lower selectivity for gasoline range hydrocarbons, as compared to parent and Ni-loaded HY zeolite (Figure 6b). Kung et al. [68], Masuda et al. [69] and Azam et al. [70] also reported the significance of mild steaming for the enhancement of the activity of zeolite for hydrocarbon cracking. Moreover, Pham et al. [71] observed that steaming at 480 °C for 4h enhanced the mobility of extra-framework aluminum to create synergistic sites that improved the activity of the zeolite by 1.75 times.
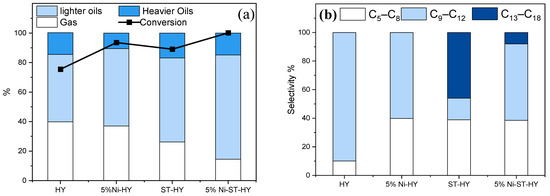
Figure 6.
(a) Product selectivity and conversion of surgical face masks through hydrocracking over zeolite Y with different loading of Ni and steamed zeolite Y with 5 wt.% Ni in a 100 mL autoclave reactor, 10 bar initial cold H2 pressure, feed to catalyst ratio of 10:1 (by weight), 325 °C for 120 min residence time. (b) Product selectivity is based on the carbon number of the n-heptane soluble liquids. Adopted with permission from Ref. [67], Copyright 2023 Elsevier.
Similarly, to optimize the concentration of acid used for the dealumination, Sarker et al. [72] studied the dealumination of HZSM-5 (Si/Al = 46) at four different molarities of H2SO4, i.e., 0.1, 0.3, 0.5, and 0.7 M for 30 min at room temperature. The as-synthesized zeolites were further investigated for the co-pyrolysis of sawdust and HDPE. All the dealuminated zeolites showed an increase in Brønsted and total acidity. This increased Brønsted acidity was responsible for an enhanced activity for all dealuminated zeolites. In addition, a higher production of olefinic, aromatic, and alkane hydrocarbons was observed, while the parent HZSM-5 showed a lower relative abundance of total hydrocarbons. Moreover, within various dealuminated zeolite samples, ZSM-5 (0.5 M) exhibited the best results due to the highest concentration of BAS (292 µmol/g). However, a further increase in the concentration of H2SO4 (i.e., 0.7 M) led to a reduction in the catalytic properties owing to a decrease in the acidity of ZSM-5 (0.7 M) (i.e., 277 µmol/g). This showed that the optimum concentration of H2SO4 to dealuminate HZSM-5 (Si/Al = 46) at room temperature is 0.5 M.
In another study, Wangsa et al. [73] performed the dealumination of natural zeolite (NZ) with 0, 1, 2, and 3 M of H2SO4 at 70 °C for 2 h and studied the catalytic properties for the consecutive pyrolysis-hydrocracking of glove wastes made of long-chain polymer (i.e., cis-1,4-polyisoprene). All the dealuminated zeolite samples showed an increase in the textural properties, with the maximum BET surface area and total pore volume being observed for the sample treated with 2 M H2SO4. A further increase in molarity of H2SO4 (3 M) resulted in a decrease in the textural properties and acidity of the sample due to the significant removal of aluminum species, which ultimately resulted in the structure collapse (i.e., loss of crystallinity). In terms of catalytic properties, SNZ2 (2 M H2SO4) showed the maximum selectivity for gasoline range products owing to its maximum acidity and porosity, whereas SNZ3 (3 M H2SO4) exhibited a lower selectivity for liquids and gasoline range hydrocarbons. Despite the several advantages, dealumination can cause some noticeable changes in the acidic properties of the hierarchical zeolite (i.e., change in the Brønsted/Lewis acidity and total acidity), which depends on the applied method of dealumination because of the adjustments in Si/Al proportion and crystallinity [29,74].
Another important parameter to consider is the treatment time, as an increase in reaction time results in a greater degree of dealumination. For example, Marcos et al. [49] studied the dealumination of Pt-modified BEA zeolite (Pt-HBETA) for the hydrocracking of polystyrene to liquid fuels. The zeolite was dealuminated at 30 °C by treating with a slightly higher concentration of HCl (1 M) for 20, 40, and 60 min and named as Pt-HBETA-20, Pt-HBETA-40, and HBETA-60, respectively. An increase in the dealumination time led to a slight decrease in the relative crystallinity (up to 3%), as well as the total acidity of the catalysts. However, the surface area, acid site accessibility, and pore volume increased. Compared to Pt-HBETA, both Pt-HBETA-40 and Pt-HBETA-60 exhibited enhanced gasoline selectivity with a wide range of products such as paraffin, iso-paraffins, and naphthene. The author suggested that the higher yield of gasoline fraction as compared to the parent BEA zeolite was because of the elevated surface area and accessibility of the Brønsted acid sites of the dealuminated catalysts.
The above results suggest that all the parameters, including the concentration of acid, treatment temperature, and time, collectively play a critical role in determining the properties of modified hierarchical zeolites. However, irrespective of the dealumination conditions, the degree of dealumination is also dependent on the zeolite framework, where different crystalline structures with similar Si/Al ratios can show distinct results. For instance, it is easier to dealuminate BEA zeolite than mordenite and HZSM-5 [30,75]. To observe this, Ajot et al. [76] compared the dealumination of two zeolites (BEA and mordenite) and discussed the effect of the initial Si/Al ratio on the degree of dealumination. An increase in Si/Al ratio above 10 only slightly dealuminated the mordenite zeolite, whereas the BEA zeolite showed a higher degree of dealumination. This was attributed to the difference in the initial crystallographic distribution of Al-species and the density of structural defects in the two zeolite frameworks. However, it was challenging to distinguish between intra- and inter-crystalline mesopores formed in BEA zeolite after the dealumination.
Additionally, another important parameter to consider is the Si/Al ratio of the parent zeolite. In zeolites with high Si/Al ratios, the low aluminum content throughout the framework makes it difficult to extract further Al species from the framework. For instance, Smirniotis and Zhang [77] compared different 12-membered ring pore zeolites and studied the effect of the initial Si/Al ratio on the degree of dealumination. Under similar treatment conditions, zeolites with lower and/or medium Si/Al ratios (up to 35) showed a higher degree of dealumination (increase in bulk Si/Al ratio) as compared to zeolite samples with higher Si/Al ratios (>50). This suggested that the dealumination of zeolite is more favorable at lower Si/Al ratios and that intense treatment may be required to remove aluminum from the zeolites at higher Si/Al ratios. However, it should be kept in mind that the zeolite framework may collapse under strong acids and if the Si/Al ratio is low [78].
Furthermore, to optimize the type of acid (i.e., HNO3, HCl, H2SO4) and to study their influences on the final porosity of the dealuminated sample, Yi et al. [79] studied the dealumination of BEA zeolite (Si/Al = 10.6) by varying the concentration of HNO3 (i.e., 1, 2.5 and 13.5 M) at 100 °C for 2 h. Interestingly, a relatively mild dealumination (1 and 2.5 M HNO3) led to an increase in the micropore volume, indicating the removal of debris (i.e., extra-framework aluminum species) from the pores of zeolite, in line with the results of FTIR-py (i.e., a decrease in Lewis acid sites) (Table 4). On the other hand, an intense acid treatment (13.5 M HNO3) resulted in a decrease in the micropore volume. The author suggested that this could be due to the transformation of small micropores into larger ones. This was in line with the observations by Wangsa et al. [73], who also observed a decrease in textural properties when using higher H2SO4 concentrations, as explained above. However, all the samples showed a drastic decrease in acidity. Zhang et al. [80] also observed a similar trend where dealuminated ZSM-5 zeolite by phosphoric acid showed a strong decrease in the strong acid sites as compared to the parent zeolite.

Table 4.
Textural and acidic properties of the parent and dealuminated BEA zeolite samples. Data from [79].
Therefore, the use of mild acids (i.e., oxalic acid or chromic acid, etc.) might be useful in preserving the framework properties. Recently, Babic et al. [81] reported the use of chromic acid to synthesize mildly dealuminated zeolites. The prepared dealuminated zeolites showed a slight increase in the porosity without much changing the Si/Al ratio, which was accompanied by an increase in the concentration of Brønsted and Lewis acid sites. To study the effect of mild dealumination of zeolites for the hydrocracking of plastics, Abdulridha et al. [82] performed the dealumination of Y zeolite using weak ethylenediaminetetraacetic acid (EDTA). In detail, a calculated amount of Y zeolite (CBV 300, Si/Al 2.6) was treated with 0.1 M 100 mL EDTA aqueous solution at 100 °C for 6 h under reflux, and the as-obtained dealuminated zeolite was named as HT-Y-0.1-360-100. In parallel, microwave-assisted (150 W) dealuminated zeolite samples were prepared at 100 °C by varying the time from 1 to 90 min in 0.1 M EDTA. Interestingly, the microwave-assisted dealumination surpassed the textural properties results of both parent and conventional heated dealuminated zeolite in only 1 min. A further increase in dealumination time to 90 min showed an insignificant increase (16%) in the mesopore volume. In terms of catalytic properties, the as-synthesized dealuminated zeolite samples were modified with Pt and studied for the hydrocracking of PP. However, both dealuminated zeolite samples exhibited similar conversions (~78%) while still significantly higher than that of the parent Y zeolite (39%). The authors concluded that the microwave-assisted process is a highly energy-efficient method to synthesize dealuminated zeolites with 21-fold lower energy requirements (~0.02 kWh/g), as compared to the conventional dealumination method (~0.42 kWh/g).
Although significant progress has been made in the field of plastic hydrocracking using a range of dealuminated zeolites, the majority of researchers continue to rely on commercially available dealuminated zeolites, whose specific modification methods often remain disclosed. Despite this lack of transparency, it remains crucial to explore the catalytic properties of these commercial zeolites to deepen our understanding of their effectiveness in the hydrocracking process. For example, Liu et al. [24] compared the hydrocracking of LDPE over commercial dealuminated Y zeolite with different SiO2/Al2O3 ratios of 30, 60, and 80, mixed with Pt/WO3/ZrO2. As expected, an increase in the Si/Al ratio (i.e., a high degree of dealumination) led to a decrease in Brønsted acid sites, which ultimately resulted in a decrease in the catalytic activity of the zeolite. However, it also triggered a significant change in the product distribution from gasoline-range hydrocarbons to diesel-range fuels. A change in SiO2/Al2O3 ratio from 30 to 60 shifted the yield of gasoline range products (C5–C12) from 72 to 32% with an increase in the yield of diesel range fuels (11 to 27%) and solids. Therefore, the authors suggested that zeolites with higher acidity notably influenced the product distribution by consuming the reaction intermediates over highly acidic sites to produce lower hydrocarbons. On the other hand, zeolites with higher Si/Al may proceed with a slow cracking of the initial polymer and ultimately generate larger molecules in the gasoline range products. Lee et al. [83] also observed a similar trend when comparing different dealuminated Y zeolites with different degrees of previous dealumination (i.e., different Si/Al ratios). During the hydrocracking of n-hexadecane (i.e., model substrate for PE) at 375 °C for 2 h under 45 bar cold hydrogen pressure, the use of zeolites with high degree of dealumination (i.e., increase in Si/Al ratio from 30 to 60) slightly decreased the overall conversion from 26.7 to 24.6%, whereas a further decrease in aluminum content (Si/Al = 80) significantly decreased the conversion to 14.9%. The authors explained that the increase in Si/Al ratios (SAR) resulted in a decrease in the catalyst surface acidity that led to the observed lower catalytic activity of Y zeolite with a high Si/Al ratio. Similarly, the Y zeolite with the highest Si/Al consumed the least amount of H2 (6%), whereas the sample with a Si/Al ratio of 30 consumed maximum hydrogen (11%), which indirectly justified the higher conversion achieved over zeolite Y (30). However, in another study by Costa et al. [18], the authors compared the hydrocracking of HDPE over two commercial H-USY zeolites with distinct Si/Al ratios of 2.9 and 15. Contrary to what was observed before, an increase in the degree of dealumination and Si/Al ratio from 2.9 to 15 resulted in an increase in the concentration of Brønsted acid sites with a significant decrease in the concentration of Lewis acid sites. The authors explained these unexpected results based on the 27Al MAS NMR results, where H-USY (2.9) showed three distinct peaks that corresponded to the presence of both framework aluminum (tetrahedrally coordinated) and extra-framework aluminum (tetrahedral coordination and octahedrally coordinated) species, whereas H-USY (15) only revealed a single peak confirming the removal of extra-framework aluminum species during treatment. This led to an increase in mesoporosity of the zeolite from 0.14 to 0.23 cm3/g, which could possibly have enhanced the accessibility to the acid sites and be at the origin of the observed behavior (i.e., an increase in Brønsted acidity). Similarly, H-USY (15) exhibited higher acidic strength (both Lewis and Brønsted acid sites), and collectively, this resulted in a high degradation of HDPE over H-USY (15) as compared to H-USY (2.9). Jumah et al. [65] reported a similar behavior when comparing 1% Pt-USY zeolites with Si/Al of 6 and 15 for the hydrocracking of squalane (C30H62), at 275 °C and at 20 bar initial H2 pressure. Despite the decrease in acidity of the catalyst with increasing Si/Al ratio, 1% Pt-USY (15) showed better activity with a slight increase in the selectivity for C6 products, as compared to the Pt-USY (6). This was because of the enhanced porosity of the 1% Pt-USY (15), which led to an increase in the accessibility of pores for better cracking of squalane to lower hydrocarbons. Similarly, the authors observed a high degree of coke formation with an increase in acidity of the catalyst (i.e., Pt–USY(6) > Pt–USY(15)). Thus, it suggested that both the acidity and pore structure of a zeolite must be considered in conjunction for better hydrocracking of plastics.
To conclude, dealumination is a promising way to add secondary porosity in zeolites. This cost-effective method is also widely employed to adjust the Si/Al ratio and enhance the hydrothermal stability of zeolites (i.e., formation of USY via steaming). However, it normally results in the formation of disordered mesopores with low pore connectivity, and an uncontrolled or severe dealumination is responsible for generating irregular and broad pore size vacancies due to the partial damage of the zeolite framework. Also, the dealumination of zeolite is applicable to Al-rich zeolites, and a high degree of dealumination, either through steaming or acid leaching, may result in the loss of micropore volume and acidity (i.e., the concentration of acid sites and their strength). Therefore, it is necessary to either perform mild or controlled dealumination to add regular mesoporosity to the zeolite structure [41] without compromising the functionality of the parent material. For instance, mild steaming can be performed at a temperature of ≤500 °C for up to 5 h. In the case of acid leaching using inorganic acids, several reaction parameters, including acid concentration, reaction temperature, and time, need to be optimized to enhance the textural properties and accessibility of the acid sites. Additionally, considering the distinct effects of acid leaching on various crystalline zeolite frameworks and on zeolites with different Si/Al ratios, it is crucial to critically optimize the treatment conditions separately for each zeolite structure. Controlled dealumination can also be achieved using mild inorganic or organic acids and preferably microwave-assisted treatment, which helps prevent structural damage. Finally, dealumination is an effective approach to developing secondary porosity in zeolites, which is essential for the hydrocracking of plastics. By considering the properties achieved during dealumination, one can easily tune the product distribution during the hydrocracking of plastics.
2.1.2. Recrystallization of Zeolites
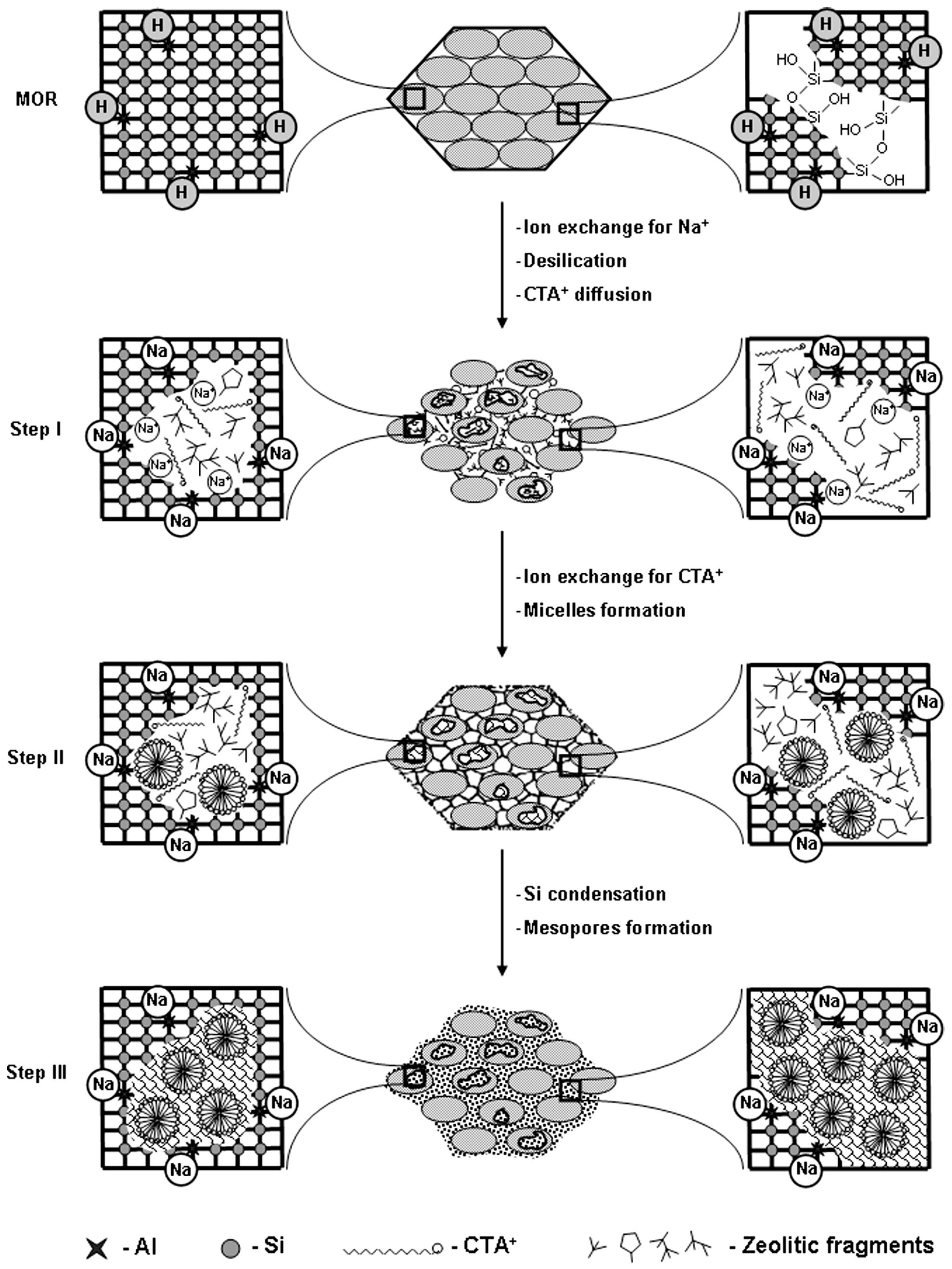
As mentioned above, both desilication and dealumination methods have been employed for decades to synthesize hierarchical zeolites due to their simplicity, scalability, and cost-effectiveness. However, most of the mesopores created through these methods are disordered, which does not significantly enhance mass transport. For instance, desilication has a limited ability to tailor mesoporosity and is heavily influenced by factors such as the Si/Al ratio leaching reaction parameters. Similarly, dealumination reduces the number of acid sites by extracting aluminum from the framework. Therefore, employing the recrystallization approach may address these challenges more effectively. The application of recrystallization in synthesizing hierarchical zeolites is an essential strategy for avoiding damage to the framework of the zeolite. In this technique, a controlled porosity can be effectively introduced into the structure with the simultaneous addition of cationic surfactants, including Cetyltrimethylammonium bromide (CTAB) and/or Cetyltrimethylammonium chloride (CTAC), etc. This two-step approach was first reported by Goto et al. [84] in 2002 and initially involved the dissolution of the zeolite structure under mild reaction conditions (i.e., desilication using dilute NaOH and low temperature) followed by the reassembling of dissolved species to form a mesoporous phase. To understand the mechanism of recrystallization, Ivanova et al. [85] highlighted the steps to develop hierarchical mordenite using a surfactant-assisted recrystallization route, as shown in Figure 7. Initially, the addition of NaOH destructed the Si-O-Si bonds and formed small intra- (inside the crystal) and large inter-crystallinity (between the crystallites) in the framework. It also resulted in the ion exchange of protonic zeolite to form Na+, whereas the negatively charged sites promoted the diffusion of CTA+ in the inter- and intra-crystalline spaces. Prolonged heating under autogenous pressure expedited the diffusion of CTA+ and facilitated the ion exchange process, where Na+ is replaced by CTA+ to create micelles. Also, it promoted the nucleation of a mesoporous phase both within the zeolitic mesopores and on the external surface of the crystallites. However, the relative amount of mesoporosity depended on the degree of dissolution. A mild dissolution of the framework may lead to a zeolite with a small change in mesoporosity. Conversely, under severe reaction conditions (i.e., high pH), the zeolite structure may collapse (i.e., no crystallinity and microporosity), leading to the formation of a pure mesoporous material after surfactant addition.
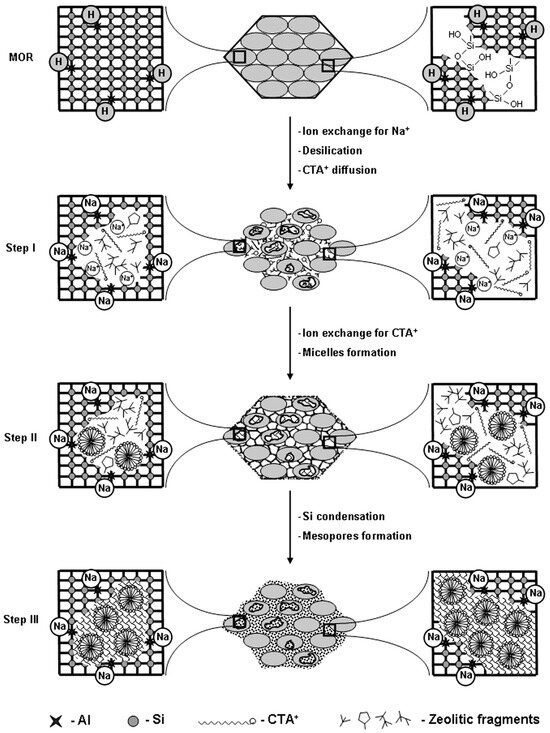
Figure 7.
Mechanism of mordenite recrystallization to micro/mesoporous structure. Reprinted with permission from Ref. [85], Copyright 2013 Elsevier.
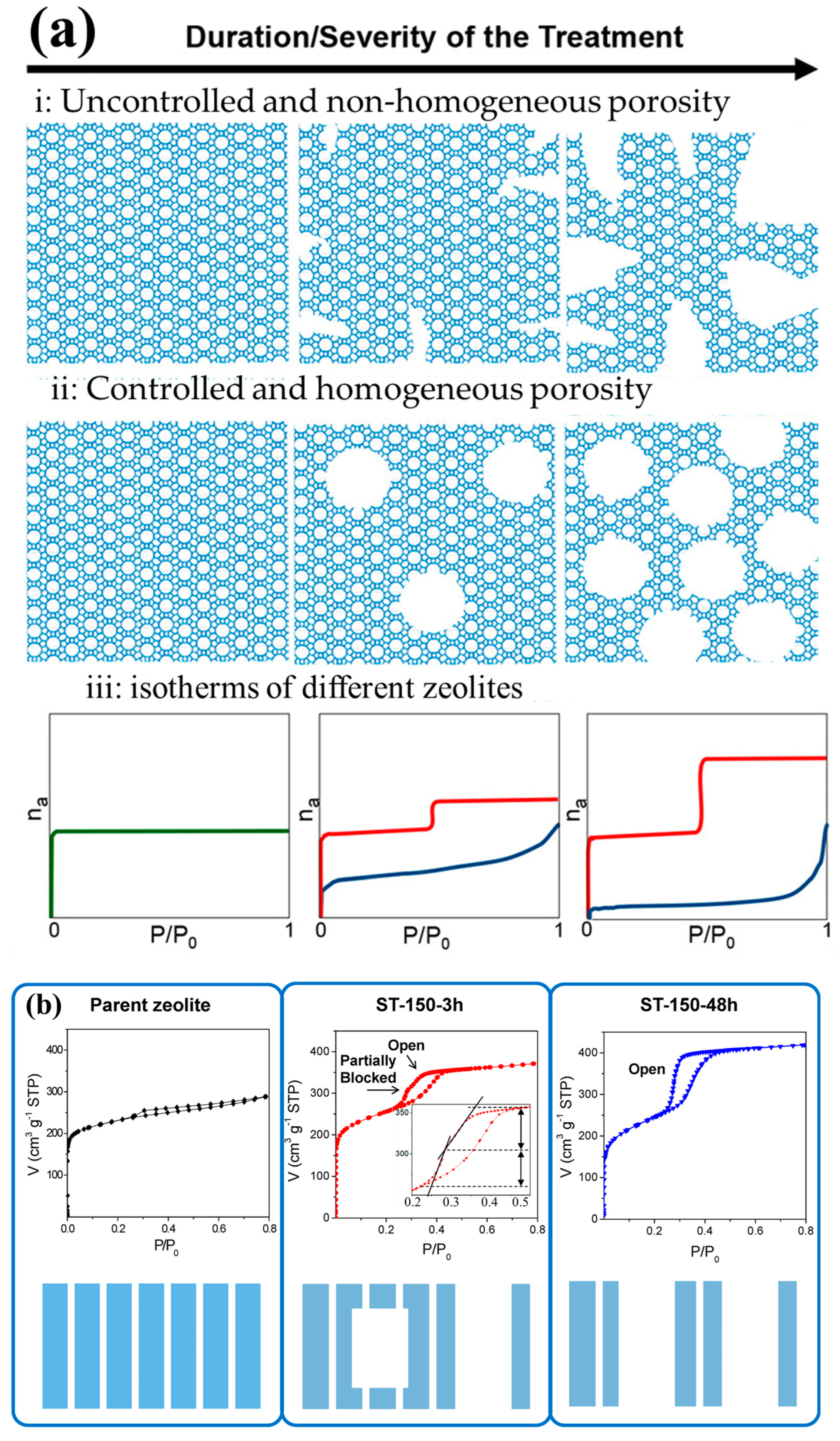
To overcome this problem, Garcia and co-workers [86,87] developed a new route based on surfactant-templated recrystallization, which involved the simultaneous addition of both template and alkaline medium to prepare intracrystalline mesoporous single crystals of zeolites. The idea of this one-step approach was to preserve the functionality of the zeolite while simultaneously adding mesoporosity to the framework without changing the Si/Al ratio. Compared to the traditional recrystallization process, this relatively new approach directed the meso-structuring of the zeolite structure rather than the desilication of the framework. A comparison between the two-step recrystallization and one-step surfactant-templating is shown in Figure 8a. It is clearly observed that the dissolution-reassembly introduced non-homogeneous and uncontrol porosity, which under harsh reaction conditions (i.e., prolonged reaction time, high temperature, etc.) caused severe structural damage with potential loss of crystallinity. Contrary to this, surfactant-templating exhibited a well-controlled and intra-crystalline mesoporosity.
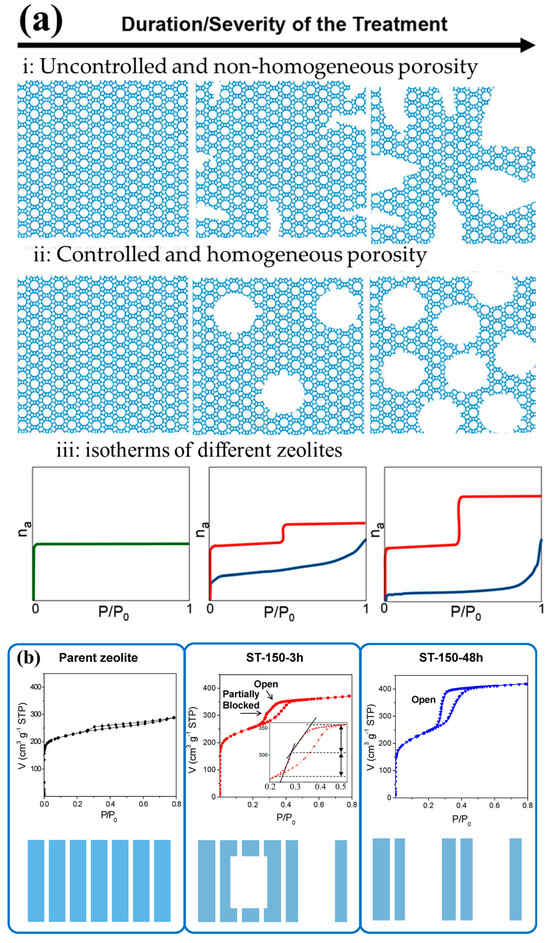
Figure 8.
(a) Schematic illustration and characteristic adsorption-desorption curves of a zeolite (green), a desilicated (blue), and a surfactant-templated zeolite (red) plotted against the time or the intensity of the applied treatment, (b) Isotherms for Ar adsorption and desorption with the corresponding schematic explanation for each type of zeolite: the original zeolite, ST-150 treated for 3 h, and ST-150 treated for 48 h. The diagrams at the bottom depict the nature of porosity for each case. Reprinted with permission from Ref. [86], Copyright 2017 American Chemical Society.
Moreover, a range of surfactants under mild basic conditions and over different temperature ranges (25–150 °C) were employed to introduce secondary porosity in the framework. Therefore, while discussing optimized reaction parameters and applicability of the recrystallization route on different zeolites and/or zeolites with different Si/Al ratios, it has been reported that both surfactant-templating and dissolution-reassembly approaches are applicable to various zeolites (i.e., zeolite Y, HZSM-5, BEA, and mordenite, etc.) over a wide range of Si/Al [30]. However, in the case of low Si/Al ratio, an additional step of acid pre-treatment (i.e., use of weak acids) is required before performing the surfactant-templating [86]. Yang et al. [88] compared the role of two different surfactants with distinct alkyl chain lengths to tailor the intra-crystalline mesoporous of BEA zeolite and studied the catalytic properties for ethanol-acetaldehyde conversion. Among all surfactants, CTAB-treated samples showed higher relative crystallinity, which showed that the CTAB template prevented the degradation of the zeolite framework. In terms of catalytic activity, the hierarchical zeolite increased the conversion by 10%. Similar results were reported by Sachse et al. [86], who compared CTAB with surfactants having bulkier hydrophilic headgroups (CTPAB and CTEAB). Both CTPAB and CTEAB barely generate any mesoporosity due to their incapability to diffuse into the pores of the zeolite. This suggested that a small headgroup surfactant is required to generate mesoporosity. Similarly, reaction temperature and time also play a critical role in developing mesoporosity inside the zeolite crystals. As discussed by Sachse et al. [86], a prolonged reaction time at higher temperature favors the formation of open and accessible mesoporosity on the external surface of zeolite, whereas an incomplete recrystallization (i.e., reaction for a shorter time) may generate mesoporosity embedded in the zeolite crystal with some open mesoporosity, as shown in Figure 8b. Despite the longer reaction time of 48 h, the catalyst still retained 70% of strong acidity. This confirmed the effect of surfactant in preventing the desilication of zeolite, as discussed above. In another study, Ordomsky et al. [89] evaluated the effects of the concentration of NaOH on the recrystallization of BEA zeolite. An increase in concentration of NaOH (3 M) significantly increased the mesoporosity of the material with a gradual decline in the micropore volume. Also, it led to severe structural damage with complete loss of crystallinity. Due to the severe reaction conditions, the solid catalyst almost completely lost its acidity. Contrary to this, an increase in the concentration of the basic medium during the surfactant-templating approach may preserve the acidity of the catalyst [86,87]. Gamba and Villa [90] also recrystallized zeolite Y (Si/Al = 2.6) using a surfactant-templating approach (NH4OH + CTAB) and studied the cracking of n-dodecane. The modified sample showed double the mesoporosity with 50% retention of acidity, which resulted in a higher conversion of n-dodecane on hierarchical zeolite as compared to the parent material.
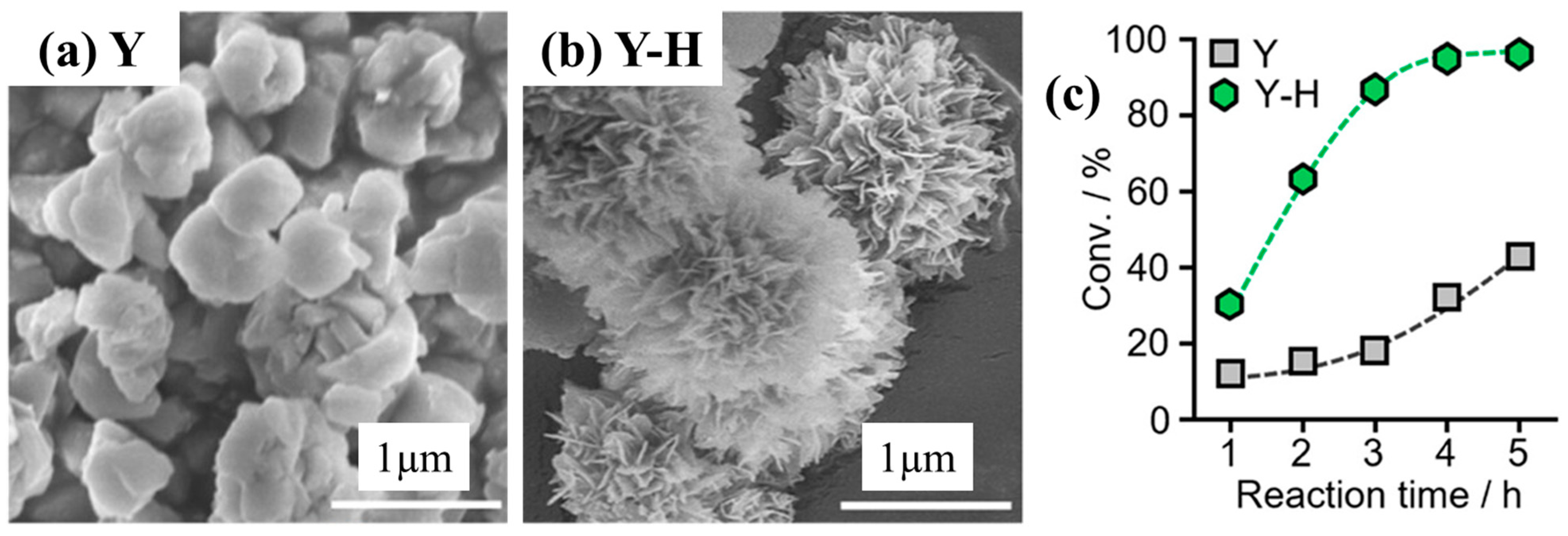
Kots et al. [46] also used this approach to synthesize hierarchical mordenite and studied it for the hydrocracking of HDPE. During the synthesis, 2 g of MOR was added to 40 mL DI water containing 0.32 mL NaOH (0.15 M) and 1.49 g CTAB. The sample was mixed and hydrothermally treated at 150 °C for 7 h. The as-synthesized catalyst was filtered, dried, calcined, and named HyMOR. In comparison with the parent zeolite, the recrystallized MOR showed an increase in the total pore volume, surface area, and concentration of acid sites due to the better accessibility of acid sites after the pores opened. Also, the HyMOR exhibited better conversion of HDPE at 250 °C under 30 bar cold H2 pressure with high selectivity for lower hydrocarbons owing to its better textural and acidic properties. Zhou et al. [91] also performed a similar modification technique to synthesize hierarchical Y zeolite (Si/Al = 17.5). During the recrystallization process, 1 g of HY and 0.7 g of CTAB were mixed in 64.0 mL of 0.37 M aqueous ammonia solution for 30 min. Subsequently, it was transferred to a Teflon-lined stainless-steel autoclave and heated at 170 °C for 25 h. The collected hierarchical zeolite was completely dried and calcined and denoted as Y-H. It resulted in a multilayered nanoflakes hierarchical zeolite with highly ordered micropores (Figure 9a,b). The modification process also led to improved textural properties (i.e., external surface area and porosity) with similar acidic properties to that of parent Y zeolite. As expected, in terms of catalytic properties for the hydrocracking of PE, the hierarchical Y-H zeolite showed better conversion (Figure 9c) due to its improved textural properties, with high selectivity for lower hydrocarbons with high iso-/n-paraffins ratio.

Figure 9.
(a,b) SEM images of commercial Y and Y-H zeolites after recrystallization and (c) effects of catalyst on the conversion and selectivity for hydrocracking PE at 280 °C under 3 MPa H2. Reprinted with permission from Ref. [91], Copyright 2023 American Chemical Society.
Recently, Tan et al. [92] modified an MFI zeolite (Si/Al = 40) using 0.2 M NaOH at 60–65 °C for 15, 30, or 60 min and compared the results with the surfactant-templated recrystallized MFI zeolite. In detail, the surfactant-templated sample was prepared using a similar procedure, with the exception that 0.2 M TPABr (surfactant) was added to the NaOH solution prior to desilication. As expected, an increase in the desilication time led to an improvement of the mesoporous volume and external surface area with a decrease in the microporosity (Table 5). However, the use of a surfactant-templated recrystallization approach resulted in a controlled increase in mesoporous volume without significantly destroying the functionality of the parent material (i.e., Vmicro). However, the interaction of TPABr with the catalyst surface led to the formation of constricted mesopores rather than open ones. Despite this, the as-modified desilicated zeolite (i.e., using NaOH + TPABr) showed the maximum density of strong acid sites. Furthermore, H-MFI-hier30-TPA showed the maximum conversion for the PE catalytic cracking, owing to its highest mesoporous surface area and number of strong acid sites in comparison with the parent and other desilicated MFI zeolites. This further confirms the importance of controlled desilication of zeolites to preserve the density of acid sites and achieve better-cracking results.

Table 5.
Textural Properties of Parent and Hierarchical MFI based zeolites. Data from [92].
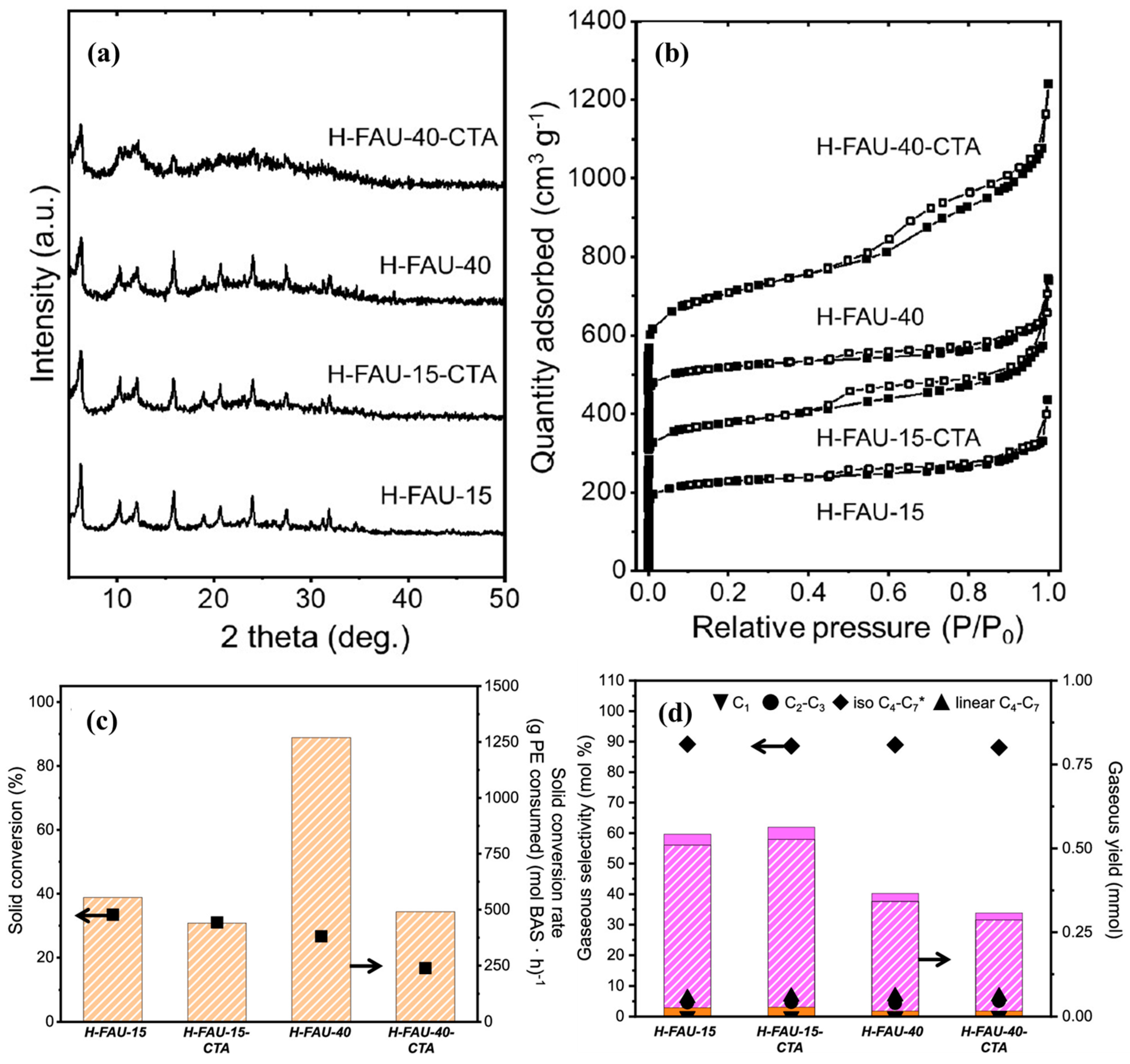
Furthermore, to evaluate the effect of starting Si/Al ratio of zeolite on the developed hierarchical zeolites, Tan et al. [92] performed the recrystallization of two FAUs with distinct Si/Al ratios (15 and 40). Briefly, surfactant templating was conducted by adding 0.2 M CTABr (surfactant) in a 500 mL solution of 0.2 M NaOH in an HDPE bottle and performing the recrystallization at 65 °C for 30 min. The textural and acidic properties of parent and as-prepared recrystallized hierarchical zeolites are shown in Figure 10 and Table 6. Interestingly, the diffraction peaks of H-FAU-15-CTA are very similar to those of its parent counterpart, whereas H-FAU-40-CTA exhibited a significant loss of crystallinity (Figure 10a). As suggested by the authors, the loss of crystallinity is attributed to the low aluminum content in the parent zeolite (Table 6), which was previously proven to protect against substantial dissolution of Si–O–Si bonds. Furthermore, both the hierarchical zeolite samples showed a combined type I–IV isotherm, representing the formation of mesopores owing to the recrystallization (Figure 10b). However, the isotherm of H-FAU-40-CTA exhibited significantly higher N2 uptake across a P/P° range of 0.2–1.0 in comparison to other zeolite samples, indicating the formation of interconnected and ordered mesoporous structure.
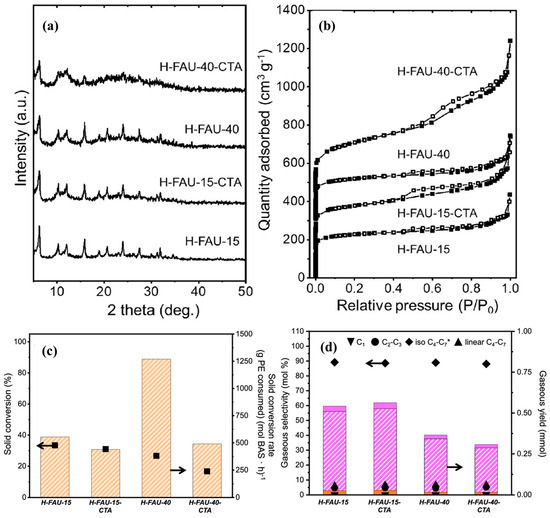
Figure 10.
(a) X-ray diffractograms of parent and hierarchical FAU catalysts. (b) N2 physisorption isotherms of parent and hierarchical FAU materials, (c) Solid conversion and solid conversion rate, and (d) gaseous product selectivity and yield for PE catalytic cracking on parent and hierarchical FAU catalysts. Orange and purple solid bars indicate C2–C3 and C4–C7 linear alkanes, respectively, while orange and purple striped bass indicate C1 and C4–C7 isoalkanes, respectively. * Indicates that C4–C7 alkenes are also present and included under this value. Reprinted with permission from Ref. [92], Copyright 2024 American Chemical Society.

Table 6.
Textural Properties of Parent and surfactant-templated FAU based zeolites. Data from [92].
Similarly, both hierarchical FAU zeolites showed a significant increase in the mesopore volume and external surface area but with a decrease in the micropore volume. The difference was more prominent over H-FAU-40-CTA due to the excessive extraction of Si and Al species as a result of recrystallization. This was in accordance with the results of XRD, where H-FAU-40-CTA showed a notable decrease in crystallinity (Figure 10a). Despite this, both hierarchical zeolite samples showed an increase in the acid site density (i.e., both weak and strong) and BAS. This was due to the better accessibility of the acid sites owing to the significant increase in the mesopore volume. Furthermore, to observe the catalytic properties of hierarchical zeolite samples, hydrocracking of PE was performed at 200 °C for 5 h under 10 bar cold H2 pressure. As shown in Figure 10c, no significant difference in the conversion was observed over H-FAU-15-CTA (31%) in comparison to H-FAU-15 (33%), whereas H-FAU-40-CTA showed a decrease in the overall conversion (17%), which could be due to the significant loss in the crystallinity of the sample. Moreover, all the samples exhibited similar selectivities of gaseous products (Figure 10d). This suggested that the large pore diameter (~0.74 nm) and significant mesopore volume of the parent FAU zeolite were already enough for the initial diffusion of bulkier PE molecules into the pores of zeolite, where further cracking takes place. Therefore, the selection of zeolite should always consider the diameter of the plastic oligomer. If the diameter of the zeolite is already larger than that of the plastic oligomer, it becomes challenging to assess the impact of hierarchical modification on the catalytic properties of the zeolite.
To further study the activity of a hierarchical zeolite formed by the above-mentioned approaches, García et al. [93] recrystallized the structure of HZSM-5 using cetyltrimethylammonium bromide (CTABr) and named it a hybrid zeolitic–mesoporous material. The authors also studied the effect of crystallization time (typically in days) on the textural and acidic properties of hybrid zeolitic–mesoporous material. A prolonged crystalline time (6 days) exhibited a progressive decrease in the surface area and interparticle porosity. Despite this, it resulted in a shift of the ammonia deposition peak towards higher temperatures (i.e., an increase in the overall acidity of the zeolite). García et al. [93] further compared the catalytic activity of hierarchical and parent zeolites for the cracking of HDPE. CTAB-modified zeolites showed better conversions and selectivity for gasoline range fuels, as compared to parent HZSM-5, due to the better accessibility of the plastic molecules to the acid sites. Moreover, Munir and Usman [94] synthesized and compared the activity and selectivity of two mesoporous materials with their corresponding hierarchical zeolites for the hydrocracking of model municipal waste plastic mixture (i.e., 40 wt.% of HDPE, 30 wt.% of PP, 10 wt.% of LDPE and 20 wt.% of PS). The authors employed P-123 and F-127 as mesoporogens for the recrystallization of a desilicated commercial USY in the presence of different TEOS to USY ratios. Based on the results, the authors observed that an increase in the TEOS/USY ratio resulted in an increase in the Si/Al ratio and mesoporosity at the expense of microporosity and BET surface area. All the hierarchical zeolites showed better activity and selectivity for lighter oils than parent USY. This was because of the enhanced accessibility of the reactant molecules to the active sites of the zeolites. Similarly, the hierarchical zeolite with the lowest TEOS/USY ratio showed better activity at a lower reaction temperature (375 °C), whereas the composite with a higher TEOS/USY ratio (UC1.2) exhibited better activity and selectivity of lighter hydrocarbons at higher temperature. The high mesoporosity of UC1.2 and low diffusion limitations were at the origin of the observed behavior. Also, compared to P-123, the hierarchical zeolite modified by mesoporogens F-127 showed better activity and selectivity for lower hydrocarbons, which was assigned to the more favorable diffusion in the 3D cubic mesoporous arrangement of F-123 as compared to P-123 (2D hexagonal pores).
Moreover, in a follow-up research study, Munir et al. [95] fabricated various BEA zeolite composites with different TEOS/BEA ratios and modified the zeolite structure with the addition of a mixture of F127 and P123 mesoporogens during the recrystallization process. During synthesis, the authors also tailored the composite structure with the initial desilication step and found this step resulted in a distortion of the zeolite structure. However, composites without desilication presented uniform-shaped discrete structures with some unevenly shaped mesoporous content. Moreover, for the hydrocracking of model plastics (i.e., a mixture of HDPE, LDPE, PP, and PS), these composites (i.e., without desilication) showed improved activity with high selectivity for lighter oils (i.e., n-heptane solubles) because of the preserved microporous volume and crystallinity. This contradiction with the above study suggests the re-evaluation of the recrystallization process with and without the initial desilication approaches to study the impact of recrystallization on the physio-chemical and catalytic properties of hierarchical zeolites.
To conclude, recrystallization is a better approach to synthesizing hierarchical zeolites than desilication and dealumination. This approach allows the preparation of hierarchical zeolites with a controlled degree of demetallation while enhancing the mesopore volume. Moreover, recrystallization can be performed by two different routes (single-step and two-step), and interestingly, both showed possibilities for enhancing the textural properties of the zeolite. However, compared to the dissolution-reassembling route, the surfactant-templating method receives much more attention due to the formation of well-controlled and intra-crystalline mesoporosity. Similarly, the degree of hierarchy can be precisely controlled by adjusting the pH of the treatment or its duration without causing the formation of disordered mesoporosity or loss of crystallinity. Additionally, in comparison to surfactants with bulkier hydrophilic headgroups, a small headgroup surfactant (i.e., CTABr) is better at generating intra-crystalline mesoporosity in the zeolite. Although expensive, this route is applicable to various zeolites over a wide range of Si/Al without much changing the Si/Al ratio of the parent zeolite and with the development of homogeneous mesoporosity. Overall, a range of parameters could potentially affect the formation of mesoporosity in different zeolites and zeolites with distinct Si/Al ratios. Therefore, one needs to optimize these parameters to prepare a zeolite with tailored porosity and enhanced acidity.
2.1.3. Hard Templating Approach
Hard templating is a bottom-up methodology for the preparation of hierarchical zeolites utilizing strong and rigid template materials [96]. Hard templating has been widely used to generate hierarchical zeolites with different structures, including BEA, TS-1, ZSM-11, ZSM-5, TS-2, ZSM-12, silicalite-2, Y zeolites, etc. [97,98,99,100,101,102]. Generally, carbonaceous materials, which include carbon aerogels, carbon nanotubes, carbon nanoparticles [97,98], carbon nanofibers [103], and mesoporous carbon [104,105], have been commonly investigated and employed as hard templates because of their easy accessibility and versatility. However, recently, a range of other materials, such as calcium carbonate [106,107], polymeric materials (mostly polystyrene) [108], and carbonized rice husk [101], have been studied for tailoring the structure of zeolites to prepare a range of hierarchical zeolites. The primary thing to keep in mind is that the hard template used for creating additional mesoporosity during zeolite crystallization should be low-cost and stable under an alkaline medium, as it serves as a sacrificial material during the hydrothermal synthesis of zeolites. Therefore, selecting an economical and alkaline-resistant template not only minimizes production costs but also makes the process more sustainable and scalable for industrial applications.
This approach to fabricating hierarchical zeolites follows three basic steps: (I) the template material is introduced into the zeolite precursor gel, followed by the aging at a temperature range of 25–100 °C for a certain time, (II) the mother liquor obtained is crystallized by hydrothermal treatment, and (III) the template material is removed either by calcination or through dissolution treatment [41]. Figure 11a demonstrates the pictorial illustration of the different steps involved in the synthesis of hierarchical zeolites by using CNT-based materials. However, this route to prepare hierarchical zeolite is not as easy as it may seem at first glance. For example, it is challenging to control the crystallization process within the carbon template due to the inherent incompatibility between the hydrophobic carbon template and the aqueous zeolitic precursor gel. This may result in the crystallization of precursor gel without any template, as reported by Chou et al. [100]. To prevent phase separation between zeolite crystals and carbon materials during zeolite crystallization, carbons are typically pretreated either with a mixture of nitric and sulfuric acids [30,109] and/or sodium hypochlorite [110]. This treatment creates surface oxygen species with hydrophilic characteristics. The synthesis procedure is illustrated in Figure 11c. Despite this, some researchers have suggested that the hydrophobic nature of hard templates, such as graphene oxide sheets, offers an opportunity to confine zeolite growth to smaller crystals. Without this confinement, larger zeolite crystals would otherwise form [111].
Figure 11.
(a) Carbon-based hard template materials for the synthesis of hierarchical zeolites Adopted with permission from Ref. [112], Copyright 2006 Elsevier (b) confined space synthesis of zeolite. Reprinted with permission from Ref. [102] Copyright 2006 Elsevier, (c) Hydrophilic carbon templated synthesis of mesoporous ZSM-5. Reprinted with permission from Ref. [109] Copyright 2016 Elsevier.
Further, Schmidt et al. [102] developed a new technique and named it “confined space synthesis”, where dry-gel conversion was used. In detail, the initially formed zeolite precursors were transformed into a dry powder, followed by the impregnation of the carbon template using the incipient wetness method. After aging the mixture at room temperature for several hours, the mixture was hydrothermally treated at 110 °C in a water-vapor-rich atmosphere for 72 h. This method greatly reduced the mobility within the reaction mixture, facilitating the subsequent crystallization of zeolite gel inside the mesopores of carbon. The removal of the embedded template at high-temperature calcination resulted in a single zeolite crystal featuring intracrystalline mesopores. Also, the ultimate crystalline size of the zeolites was determined by the mesopore size of carbon nanoparticles, as shown in Figure 11b. However, generally confined space synthesis led to the formation of embedded mesoporosity in the crystal, which was not connected to the external surface of the zeolite. They were exclusively accessible through the micropores, offering some mass transfer limitations [30]. This was addressed by using long nano-template materials that penetrate the whole zeolite single crystal. For instance, Schmidt et al. [113] used carbon nanotubes (12 nm diameter and several mm in length) to produce hierarchical silicalite-1 with uniform and straight intracrystalline mesoporous channels (12–30 nm wide) and showed the penetration of CNTs in a single crystal of zeolite. Similarly, Tang et al. [112] developed hierarchical nanosized ZSM-5 and NaY zeolites inside the mesopores of carbon nanotubes (CNTs), and the as-synthesized zeolites showed crystal size related to the diameter of CNTs.
However, Abdulridha et al. [110] observed an opposite trend where the use of CNTs was not an effective way to create mesoporosity in Y zeolite. Therefore, this approach appeared to be quite dependent on the specific experimental conditions and mesoporous material. For instance, Abdulridha et al. [110] prepared mesoporous Y zeolites using two different templates (multiwall carbon nanotubes and cellulose nanocrystals) and observed an insignificant increase in the mesoporosity of CNTs-modified zeolite, whereas CNCs showed an enhancement in mesoporosity and external surface area. This was attributed to the low mesoporosity of the CNTs (0.09 cm3/g) as compared to the CNCs (0.52 cm3/g). Similarly, with the better textural properties of the CNCs-Y, this zeolite sample provided more accessibility to the acidic sites (i.e., high acidity), which led to superior catalytic cracking of 1,3,5-triisopropylbenzene (TiPBz). Similarly, Schmidt et al. [102] utilized different carbon matrices (i.e., BP700 and BP2000) with distinct pore volumes and sizes for the synthesis of HZSM-5 and BEA zeolites. BP2000 with a slightly larger pore diameter resulted in bigger crystal sizes. Similar results were reported by Madsen et al. [114], who successfully synthesized hierarchical HZSM-5 using different carbon black as a hard template. The modified zeolite showed an increased external surface area with highly crystalline nanostructured ZSM-5. Moreover, varying the amount of hard template material significantly influences the porosity of the synthesized zeolite.
Therefore, to assess the impact of template loading, Deng et al. [115] used different amounts of CNTs to synthesize hierarchical HZSM-5 zeolites. A change of CNTs amount from 0.4 to 0.8 g in the gel doubled the total porosity of the material with enhanced intercrystalline mesoporosity. Wei and Smirniotis [97] also discussed the same process based on the template/Si ratio and prepared various mesoporous ZSM-12 with distinct Si/Al ratios using carbon particles. Zeolites with higher Si/Al ratios (i.e., high template/Si ratio) favored the generation of mesoporosity as compared to zeolites with lower Si/Al ratios. Similarly, a prolonged hydrothermal treatment of 10 days helped to achieve high crystallinity of the material. Moreover, in terms of the catalytic activity of hierarchical zeolite samples, hierarchical zeolites exhibited better cracking ability of tridecane and 1,3-dimethylcyclohexane due to the accessibility of pores and decreased intracrystalline mass transfer limitations.
Furthermore, to study the catalytic cracking of polyolefins over hierarchical HZSM-5, Yu et al. [116] synthesized several HZSM-5 samples with different Si/Al ratios (Si/Al = 27, 80, and 150) with/without the addition of microcrystalline cellulose (MCC) as co-template during the hydrothermal synthesis. The physiochemical properties of various synthesized samples are illustrated in Table 7. Irrespective of the Si/Al ratio, the addition of cellulose led to an increase in the external surface area and mesoporosity of the zeolite with a slight decrease in the micropore volume. It also resulted in a decrease in the total acidity of the sample, where a low Si/Al ratio HZ sample showed a higher acidity than the zeolite sample with a higher Si/Al ratio. However, the reduction in acidity was only pronounced in the weak acid region of MCC-based samples with the same Si/Al ratio (i.e., based on NH3-TPD curves in the region between 100 and 230 °C). An increase in the strong acid sites was also observed, suggesting the exposure of acid sites during the crystallization process.

Table 7.
Physicochemical properties of parent and hierarchical modified HZSM-5 samples. Data from [116].
During the catalytic cracking of HDPE, all the hierarchical zeolite samples showed a slightly higher yield of liquids and better light aromatics (BTEX) as compared to their respective parent zeolite (Figure 12). Overall, HZ-27-MCC exhibited the highest yield of lighter aromatics owing to its high total acidity and maximum mesopore volume, whereas HZ-150-MCC led to the production of a high yield of light olefins due to its moderate acidity and micropore volume. Similarly, the moderate acidity and a higher micropore volume in MZ-150-MCC were revealed to be beneficial for limiting coke formation and enhancing the stability of the catalyst. The results suggested that both the acidic density and pore structure of hierarchical zeolites significantly affected the overall conversion and product distribution.
Figure 12.
(a) Product yield and product distribution for the cracking of HDPE over the prepared catalysts. Adopted from Ref. [116]. (b) Schematic of the formation of 3DOm-i zeolite confined in the pore space of 3DOm carbon. Reprinted with permission from Ref. [117] Copyright 2011 American Chemical Society.
In addition, the structure of the zeolite can be modified by tuning the synthesis conditions of mesoporous carbons. For instance, Yang et al. [118] discussed the hierarchical modification of ZSM-5 with supermicropores templated by mesoporous ordered carbon, resulting in a bi-modal or tri-modal pore distribution. Also, the size of the large mesopores can be tailored by altering the nature of the mesoporous carbon templates. Therefore, ordered mesoporous carbons have been used as hard templates for the synthesis of hierarchical zeolites. Such materials not only result in ordered mesoporous with highly ordered networks but also produce hierarchical zeolites with controlled mesoporous volume and narrower pore size distributions compared to those made from commercial carbon blacks [119,120]. Fang et al. [121] used this method to synthesize mesoporous zeolites with crystalline wall structures, known as OMZ-1. During the synthesis, CMK-5 serves dual roles, acting as a hard template and kinetically controlling the crystallization process. Chen et al. [117] also employed the same approach to synthesize various hierarchical zeolites. For instance, the authors used three-dimensionally ordered mesoporous-imprinted (3DOm-i) to develop single-crystal zeolite, as shown in Figure 12b. Interestingly, secondary porosity can be readily adjusted by altering the mesopore size of 3DOm carbon (i.e., 10, 20, or 40 nm) and the mesoporous structure of the carbon template. Additionally, a diverse range of crystal morphologies can be obtained by varying the nucleation and crystal growth rates. However, this approach of using ordered mesoporous carbon has significant drawbacks, including being time-consuming and costly. As a result, this approach is not recommended to synthesize hierarchical zeolites for commercial applications.
In conclusion, the hard templating route offers several advantages in producing hierarchical zeolites with high zeolite character and versatility. Unlike top-down synthesis approaches, this method is applicable for generating organized, interconnected secondary porosity without much changing the Si/Al ratio. Also, this method is applicable to a wide range of zeolites and Si/Al ratios. However, it may result in the generation of highly isolated mesopores, depending on the alignment and organization of the templates during the modification process of the zeolites. To counter this, various ordered mesoporous carbon-based templates can be used, which can easily tune the mesopore volume of the hierarchical zeolite. It also results in the generation of ordered and interconnected mesopores. Therefore, it is important to select the appropriate reaction conditions and compatible template materials to enhance the mesoporosity of the zeolites. Finally, to the best of our knowledge, this approach has not been employed to synthesize hierarchical zeolites intended for use in hydrocracking plastics, possibly due to environmental concerns. Specifically, the calcination of templates contributes to air pollution and poses sustainability issues. Despite these concerns, given its potential to synthesize hierarchical zeolites with micro-mesoporous structures, this route shows promise for applications in the synthesis of ordered hierarchical zeolites utilized for the hydrocracking of plastics.
2.1.4. Soft Templating Approach
The soft templating technique has become prevalent in the synthesis of hierarchical zeolites with ordered mesoporosity after the discovery of highly ordered mesoporous molecular sieves (M41S) [122]. As compared to the hard templating route to synthesize hierarchical zeolite, this method provides significant flexibility to tune the porosity of zeolites by providing physical support and a tendency to interact directly with the silica species [30,123,124]. Kresge et al. [122] reported the first endeavor to incorporate ordered mesoporous molecular sieves with a surfactant. The silicate material was proposed to construct inorganic walls with ordered surfactant micelles, resulting in the formation of mesopores after calcination. The catalyst synthesized by this technique was not considered truly zeolitic since it exhibited low-angle diffraction patterns with amorphous pores structure. Nevertheless, the authors gave a new direction to researchers, and after this, a range of soft templates, including cationic organosilanes [125,126], hydrophilic cationic polymers [127,128], block co-polymers [29,129], surfactants [130,131], silylated polymers [132], silanized zeolitic seeds [133,134] have been widely used and studied to synthesize hierarchical zeolites either with inter- or intracrystalline mesopores. Therefore, it is important to choose the right template material to design the appropriate meso-structure of zeolite. Keeping in mind the synthesis of zeolites at higher reaction temperatures (100–200 °C) for prolonged time (1–10 days) and under alkaline media (pH = 9–12), a soft template material needs to be stable under such reaction conditions. Also, a soft template should be of low cost, possess a positive charge to establish a strong interaction with negatively charged silica species, and have an appropriate structure (mesoscale) to control the mesoporosity of zeolite [30]. However, it is generally possible to control the structure of a soft template material either by modifying its functional groups, which help to increase its stability in alkaline medium and tailor surface properties or by adjusting the packing parameter, which influences the self-assembly of surfactants. As a result, this method provides a flexible means to customize the mesoporosity (i.e., either inter- or intracrystalline mesopores) in zeolites. Generally, there are two methods: a one-step method, which introduces all components (including the surfactant) into the synthesis system at the start, or a two- [135] step method, in which all components are added first, followed by the addition of surfactant in the seed gel solution.
Based on the two-step synthesis, Serrano et al. [136] synthesized true hierarchical zeolites. In detail, HZSM-5 and BEA zeolite precursors were pre-crystallized to produce zeolite nano seeds, and later, these seeds were functionalized with organosilane (i.e., phenylaminopropyltrimethoxysilane) followed by complete crystallization at 170 °C for 5 days. The as-prepared crystals were further calcined at high temperatures to remove the organosilane. This two-step synthesis approach was responsible for producing true hierarchical zeolites with enhanced surface area, mesoporosity, and crystallinity. Moreover, Wang and Pinnavaia [132] utilized a single-step method to synthesize intracrystalline mesoporosity (2–3 nm) in HZSM-5 zeolite using a silylated polymer as a mesoporogen. They discovered that the repeating units of -SiO3 in polymer enabled its grafting onto the zeolite surface through the formation of covalent Si–O–Si linkages (Figure 13a). As the zeolite crystals developed, the integrated polymers segregated from the matrix, leading to the formation of an intracrystalline polymer network that was covalently bonded to the zeolite structure. Mesoporous MFI zeolites with uniform intracrystalline mesopores were obtained after the template was calcinated.
Furthermore, Ryoo et al. [137] synthesized single-unit-cell MFI nanosheets by using a di-quaternary ammonium-type surfactant consisting of a long alkyl chain (C22) featuring di-quaternary ammonium groups separated by a C6 alkyl linkage. The zeolite structure was directed by the two quaternary ammonium moieties, whereas the hydrophobic tail effectively controlled the growth of the zeolite crystal size in the b-direction. Mesopores were formed by the stacking of nanosheets, and the resultant hierarchical zeolite with a high concentration of acid sites on the external surface exhibited enhanced and stable activity for the cracking of HDPE (85%), which otherwise showed a lower cracking of 27% over conventional MFI zeolite. However, it is not appealing to use expensive surfactants (i.e., di-quaternary ammonium) to synthesize hierarchical zeolites. Therefore, Meng et al. [138] reported a dual-templating method to synthesize hierarchical zeolite, which showed similar results to those obtained by nanosheets developed by using di-quaternary ammonium surfactant. The authors combined a cheap mesoporogen (C16MP) and a different structure-directing agent (diethylamine) to prepare hierarchical HZSM-5 zeolites and studied them for the methanol-to-hydrocarbons reaction. The as-prepared hierarchical zeolites exhibited high mesoporosity and crystallinity and showed better results for the conversion of methanol to hydrocarbons.
A few examples in the literature were found where hierarchical zeolites produced by soft templating were used in the cracking of plastics of analogous processes. Song et al. [139] employed the single-step process to synthesize hierarchical HZSM-5 using a mesoporogen soft template (C24H57O13NSi3). During the high-temperature hydrothermal crystallization process, the soft template formed stable covalent –Si–O–Si– bonds with the MFI frameworks and generated intracrystalline mesoporosity with enhanced external surface area and accessibility of the acid sites. Also, for the catalytic cracking of LDPE, the as-synthesized hierarchical zeolite (MZ-3) exhibited better cracking ability in comparison with parent (TZ) and thermal run (Figure 13b). This enhanced performance was attributed to the better textural properties and abundance of surface acid sites in MZ-3. These characteristics facilitated the improved diffusion of bulkier polymer molecules, allowing for the cracking of LDPE at lower reaction temperatures.
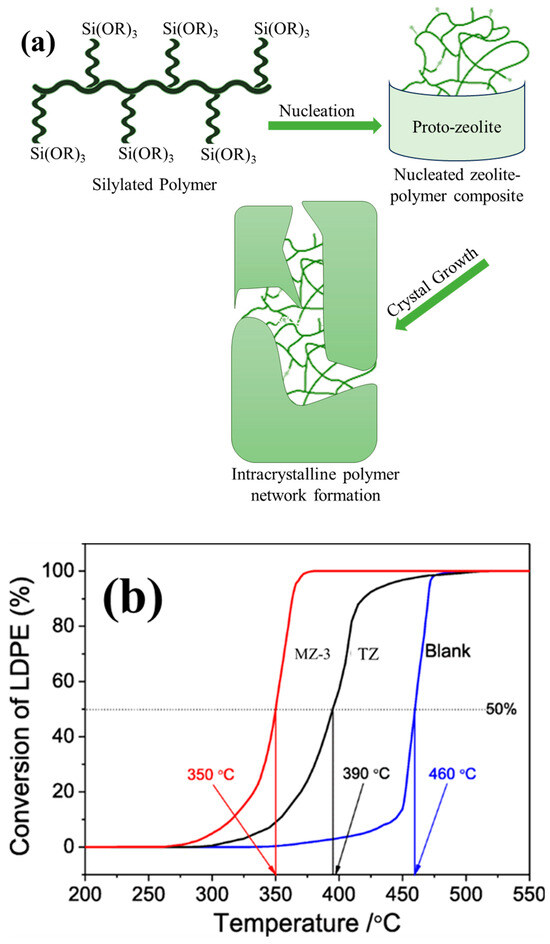
Figure 13.
(a) Conceptional approach to the synthesis of a zeolite with intracrystalline mesopores using a silylated polymer as the mesoporogen, Adopted from Ref. [132], (b) TG curves of LDPE thermal cracking (blank) and catalytic over TZ and MZ-3. Reprint from Ref. [139] under the terms of the CC 4.0, Copyright 2018 Springer.
Figure 13.
(a) Conceptional approach to the synthesis of a zeolite with intracrystalline mesopores using a silylated polymer as the mesoporogen, Adopted from Ref. [132], (b) TG curves of LDPE thermal cracking (blank) and catalytic over TZ and MZ-3. Reprint from Ref. [139] under the terms of the CC 4.0, Copyright 2018 Springer.
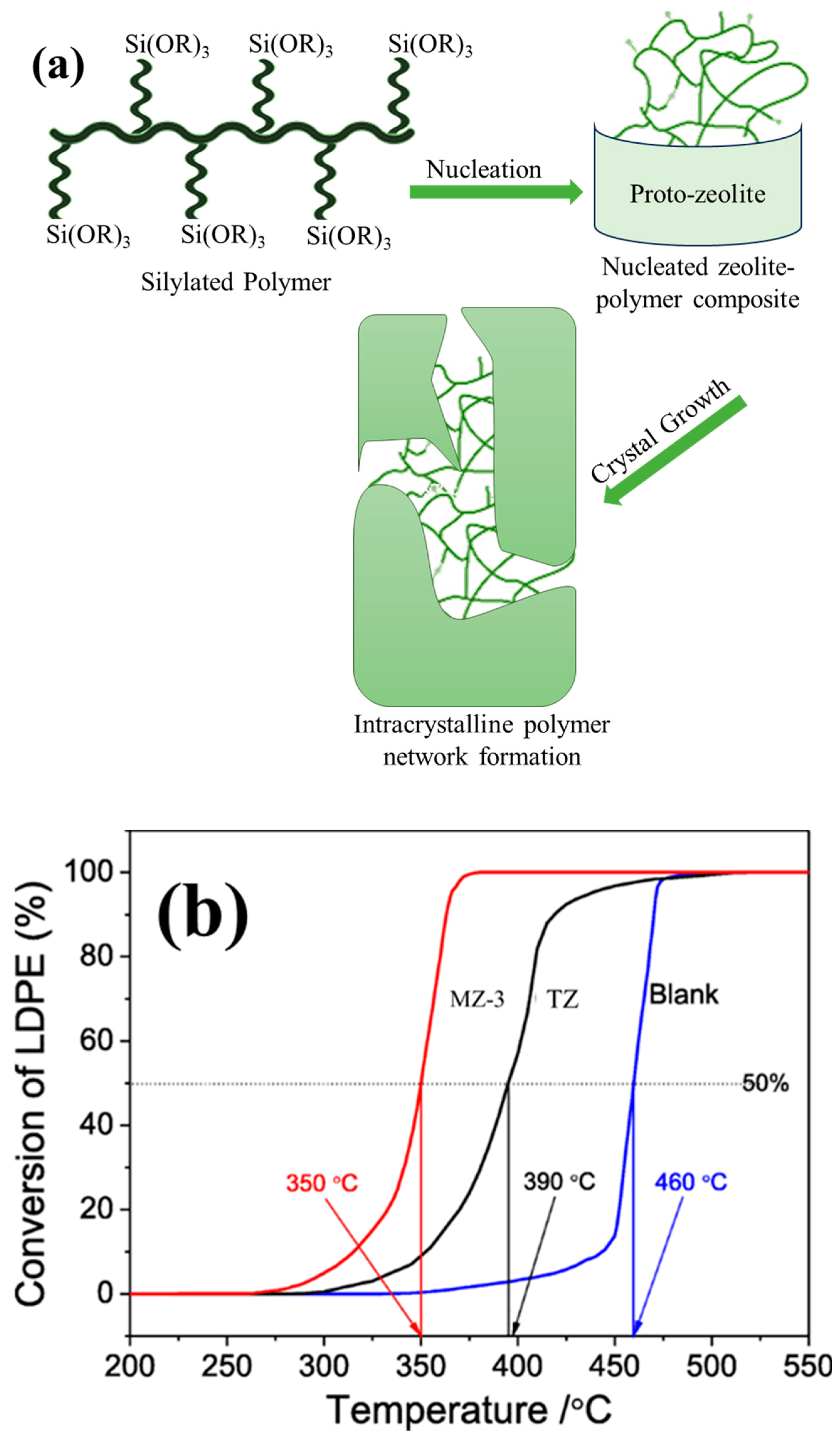
Furthermore, different organosilanes and under different reaction conditions can produce hierarchical zeolites with varying properties. This is primarily because of their different molecular sizes and functional groups, which influence how they interact with the zeolite framework during hydrothermal synthesis. To study that, Aguado et al. [134] prepared a hierarchical BEA zeolite from organofunctionalized seeds using the procedure reported by Serrano et al. [136]. Two different organosilanes (i.e., isobutyltriethoxysilane: IBTES and phenylaminopropyltrimethoxysilane: PHAPTMS) were used under different reaction conditions to prepare distinct hierarchical BEA zeolite sample. Interestingly, the use of silanized seeds resulted in a relatively higher synthesis yield, as illustrated in Table 8. Similarly, an increase in pre-crystallization time helped to achieve hierarchical zeolite with enhanced external surface area and microporosity. In addition, the difference in external surface area and microporosity of the two different organosilanes showed that PHAPTMS with higher molecular weight exhibited a higher degree of hierarchical change and resulted in a zeolite with higher Si/Al ratio, as compared to IBTES. However, it resulted in a slight decrease in the total acidity of the sample (Table 8). The enhanced textural properties of both IBTES-3 and PHAPTMS-3 assisted in the catalytic cracking of LDPE with higher TOF values as compared to parent material (Beta (0)), whereas the decrease in acidity led to a product shift from lower hydrocarbons to higher hydrocarbons. In another study by Serrano et al. [140], the authors compared four different silanization agents and observed the most remarkable results with PHAPTMS with the generation of ordered mesoporosity, whereas ODTMS remained ineffective in generating secondary porosity due to its poor incorporation ability.

Table 8.
Textural and acidic properties of parent and various hierarchically modified BEA zeolites. Data from [134].
Therefore, to enhance the incorporation of silanization agents, Serrano et al. [141] in another study, discussed the role of adding alcohols with silanization agents to enhance the textural properties of hierarchical zeolites. In detail, the addition of alcohols led to a decrease in the gel viscosity, resulting in an increased grafting of silanization agents with protozeolitic units. It also initiated alkoxylation reactions on the external surface of protozeolitic units, as shown in Figure 14. The as-obtained hierarchical zeolite showed higher mesoporosity, which enhanced the cracking of LDPE and gave it better selectivity for gasoline-range fuels.
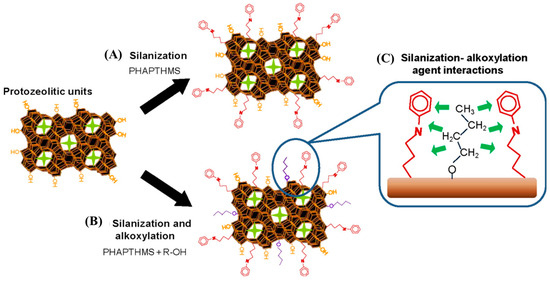
Figure 14.
Schematic diagram showing the distribution of PHAPTMS on the surface of protozeolitic units after the silanization step (A), the incorporation of alkoxy moieties by alkoxylation when the silanization proceeds in the presence of alcohols (B), and the interaction between PHAPTMS and n-butoxy grafted species in close vicinity on the surface of protozeolitic units (C). Reprinted with permission from Ref. [141], Copyright 2011 Elsevier.
Recently, Ordónez et al. [44] also studied the hydrocracking of polystyrene over Pt-supported hierarchical (Pt/h-HZSM-5) and its corresponding parent zeolite (Pt-HZSM-5). The hierarchical zeolite was synthesized using the same approach as discussed by Serrano et al. [136] using 2-propanol and an organosilane. The as-prepared zeolite showed increased Brønsted acidity. Moreover, the hierarchical zeolite exhibited enhanced external surface area and mesoporosity while maintaining the microporosity of the parent zeolite. The chemical composition and textural properties of the parent and modified hierarchical zeolite are shown in Table 9. Based on the results, the hierarchical zeolite showed much higher activity in comparison with the parent material. The improved activity was directly associated with the increased textural of the material, which provided high accessibility to the active sites favoring cracking. Similarly, the accessibility of the active sites led to an increase in the yield of iso-paraffins and naphthene at the expense of n-paraffins and aromatics, as shown in Figure 15.

Table 9.
Physiochemical and textural properties of hierarchical zeolite (Pt-h-HZSM-5) and its corresponding microporous zeolite (Pt-HZSM-5). Data from [44].
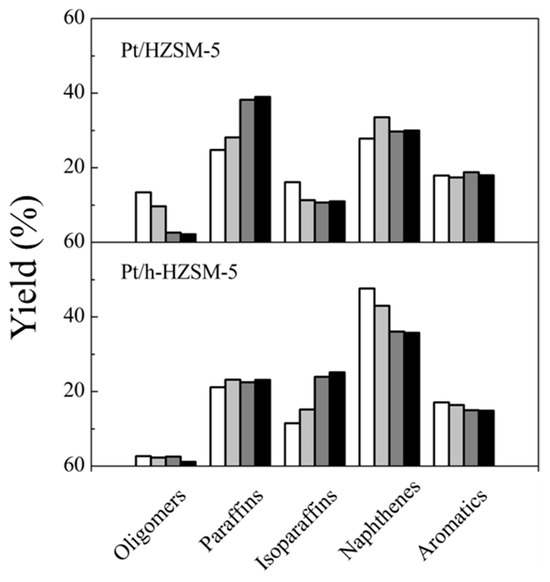
Figure 15.
Selectivity in PS hydrocracking: ( ) 573 K, (
) 573 K, ( ) 598 K, (
) 598 K, ( ) 623 K, (
) 623 K, ( ) 648 K. Reprinted with permission from Ref. [44], Copyright 2014 Elsevier.
) 648 K. Reprinted with permission from Ref. [44], Copyright 2014 Elsevier.
 ) 573 K, (
) 573 K, ( ) 598 K, (
) 598 K, ( ) 623 K, (
) 623 K, ( ) 648 K. Reprinted with permission from Ref. [44], Copyright 2014 Elsevier.
) 648 K. Reprinted with permission from Ref. [44], Copyright 2014 Elsevier.
Furthermore, to study the effect of templating content (wt.%) on the properties of hierarchical zeolites, Hensen and co-workers [142] synthesized FAU zeolites by varying the amount of amphiphilic organosilane (TPOAC) in the synthesis gel. They observed an increase in mesopore volume of hierarchical zeolite with the increase in % of TPOAC. To further understand the role of template content on the catalytic properties of zeolites, Reiprich et al. [143] synthesized layers-like Y zeolite by the addition of organosilane (TPOAC), which acted as a soft template. Interestingly, the crystallization was performed at a relatively lower temperature (75 °C) and in the presence of sulfuric acid (i.e., low pH). Similarly, the authors used different molar ratios of TPOAC/Al2O3 to synthesize different samples, including LY-0.144 and LY-0.255, where the specific numbers represented the TPOAC/Al2O3 molar ratios. As a result, a high silica layer like Y zeolite was synthesized and characterized by an enhanced textural and acidic property. For instance, the LY-0.144 and LY-0.225 exhibited a mesopore volume of 0.11 cm3/g and 0.16 cm3/g, respectively. These enhancements reduce the diffusion limitations and increase the accessibility of active sites. In terms of acidic properties, both samples showed a high total acidity (~1600 µmol/g). Furthermore, the catalytic properties of synthesized hierarchical zeolite samples were tested for the cracking of PE, where it was revealed that the layer-like zeolite Y, particularly the LY-0.225-H sample, exhibits superior catalytic activity. It achieves higher conversion rates and produces more value-added C3–C4 gases and C5–C6 liquid fractions compared to conventional zeolites (Figure 16). Overall, LY-0.225 exhibited superior catalytic properties owing to its higher textural properties.
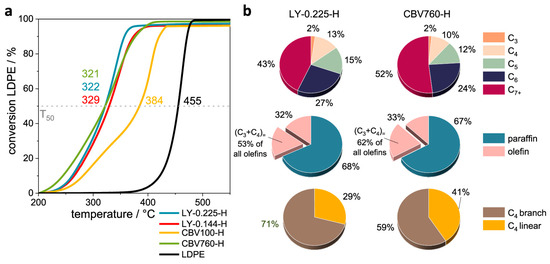
Figure 16.
(a) Conversion in a LDPE-cracking reaction for layer-like zeolite Y samples (LY-0.144-H and LY-0.225-H) and commercial zeolite Y samples (CBV100-H and CBV760-H) as catalysts and the conversion in the absence of any catalyst (LDPE). (b) Distribution of the cracking products: selectivity (%) (upper); the share (%) of the paraffin and olefin fractions (middle); and the ratio between the branched and linear compounds in the C4 fraction (lower). Reprinted with permission from Ref. [143] under licensed CC-BY 4.0, Copyright 2022 American Chemical Society.
In conclusion, a wide range of soft templates have been used in the literature, and they have shown potential to enhance the mesoporosity of the zeolites with good pore connectivity. This route leads to the generation of intracrystalline mesoporosity and is applicable to a wide range of zeolites (MFI, BEA, FAU, AFI, etc.) with low-high Si/Al ratios. Overall, the specific properties of hierarchical zeolites synthesized using different soft templates are influenced by the molecular structure, functional groups, templating effect, concentration, synthesis conditions, and interactions with other components. Similarly, the addition of different alcohols leads to an increased grafting of silanization agents with protozeolitic units, resulting in an enhancement in the textural properties of hierarchical zeolites. These factors collectively determine the textural, structural, and catalytic properties of the resulting zeolites. Despite the potential use of hierarchical zeolites for hydrocracking plastics, their use remains limited, primarily due to the challenges in finding commercially viable and environmentally friendly templates. Also, the use of soft templates, specifically organosilanes, can introduce complexity in the synthesis procedure, requiring precise control over conditions such as pH, temperature, and concentration to achieve the desired properties. However, studies on the catalytic cracking of plastics suggest that the development of cost-effective and sustainable templates could significantly advance the application of hierarchical zeolites in plastic hydrocracking.
2.1.5. Zeolitization of Materials
Zeolitization of materials is the process of converting mesoporous structures with amorphous walls (e.g., mesoporous silica) into crystalline zeolites. The idea behind this technique is to control the size, shape, structural configuration, dispersion, and position of mesopores, which is purely dependent on the type of template used [144]. However, during conventional hydrothermal treatment, both zeolite and mesoporogen are simultaneously crystalline, which leads to the formation of a physical mixture made up of two components. Therefore, Xu et al. [145] developed a novel path based on the crystallization of dry aluminosilicate gel in the presence of amine vapors and water to synthesize HZSM-5. Later on, this technique was broadly employed with some modifications. Generally, the zeolitization of materials could be performed by two key methods [146]: vapor phase transport (VPT), in which the solid precursor gel of zeolite is crystallized with the vaporized mixture of water and structure directing agent, and steam-assisted conversion (SAC), which involves the mixing of structure directing agents with dry gel of zeolite and only water in vaporized form is used to crystalline it. Compared with the conventional hydrothermal synthesis, this technique significantly shows an identical composition of mesoporous zeolites with greater transportation rates and uses a minimal amount of structure-directing agent. In addition, the waste products generated may not require any treatment as it only contains water vapors.
Lysenko and Yue used this method to transform MCM-41 and SBA 15 into mesoporous MFI and BEA zeolite, respectively [147,148]. During the synthesis of MFI, the author impregnated the structure directing agent (SDA, i.e., TPAOH) into the mesoporous material (MCM-41 or AlMCM-41) and later converted the amorphous silica into zeolite crystal via dry-gel conversion under steam. The process involved two steps, which started with the introduction of TPAOH to the as-prepared siliceous or aluminum-containing MCM-41, followed by using dry-gel conversion to transform the amorphous silica wall into crystalline zeolite. In the second step, TPAOH, mesoporogen, and micelles were removed by calcination to produce mesoporous MFI zeolite. Similarly, steaming time influences the transformation of amorphous silica to zeolite. This process partially crystallizes the walls, resulting in a zeolite structure and creating intercrystalline mesopores, whereas prolonged steaming (12–24 h) enhances the crystallinity of the zeolite.
Moreover, the crystallization time also depends on the quantity of water being used during the SAC process. Therefore, to optimize this parameter, Möller et al. [149] synthesized hierarchical BEA zeolite using nano-crystallites and by varying the amount of water based on a steam-saturated atmosphere. A higher saturation value led to complete crystallization in a short span of time with high crystallinity and textural properties. The preparation scheme is depicted in Figure 17, where the synthesis started with the nuclei formation in the concentrated precursor gel, followed by the crystallization of the nuclei to nano-crystallites of BEA, and finally, the nano-crystallites aggregation, giving rise to a mesoporous structure during the SAC treatment.
Figure 17.
Formation of (c) hierarchical BEA zeolite from (a) a dense precursor gel; (b) contraction (densification) and partial conversion of the gel into nanozeolites after short SAC treatment; at this stage, filtration yields a colloidal solution of zeolite beta. Reprinted with permission from Ref. [149], Copyright 2011 American Chemical Society.
During zeolitization, various types of solid precursors, for example, amorphous hierarchical solids, silica-based nanoparticles, or dry gels [146] have been tested to tailor the amorphous pore walls and structure to micro-mesoporous hierarchical zeolites. Zhou et al. [146] introduced a steam-assisted conversion whereby the precursor TS-1 was crystallized with triethanolamine (TEA) and tetra propylammonium hydroxide (TPAOH) with water vapors. The calcined as-obtained zeolite showed mesopores in the size of about 11.2 nm. This mesoporous zeolite showed higher selective oxidation of 2,3,6-trimethylphenol in cyclic runs. Moreover, to study the effect of the zeolitization process on zeolites with different Si/Al ratios, Li et al. [150] crystallized amorphous SiO2 or Al-SiO2 colloidal nanoparticles to synthesize nano-zeolite with intercrystalline mesopores using SAC method. The obtained catalyst showed a higher surface area (230 m2/g) and an average pore size of 3.2 nm. Also, the authors prepared zeolites with different Si/Al ratios, and all the samples showed hierarchical porosity, suggesting the applicability of steam assistant crystallization for zeolites with different aluminum content. Similar results were reported by Möller et al. [149], who synthesized the hierarchical structure of BEA zeolite in a few hours at 170–180 °C over a range of Si/Al (10–44).
Recently, Armenise et al. [50] synthesized hierarchical support using mesocellular alumino-silica foam (Al-MCF). Initially, Al-MCF was synthesized using a microwave technique followed by the zeolitization of Al-MCF with fluorides and tetra propylammonium bromide, adjusting the pH (i.e., 0.1 M NaOH) to promote zeolite formation on the alumina-silica surface under hydrothermal treatment. Finally, the ion-exchanged and calcined hierarchical zeolite was synthesized and named Al-MCFZ1 (180 °C, 500 W for 4.5 h) or Al-MCFZ2 (200 °C, 1200 W for 4.5 h). The textural and acidic properties of parent HZSM-5 and Al-MCF-modified zeolites with and without the addition of Ni are illustrated in Table 10. Based on the N2 sorption isotherms (Figure 18a), the Al-MCF exhibited wide hysteresis loops (0.5 < P/P° < 1.0). Interestingly, zeolitization at 180 °C and 500 W barely altered the textural properties of Al-MCF, whereas synthesis at 200 °C and 1200 W led to a significant reduction in surface area. The decrease was even more pronounced after the addition of Ni. According to the authors, the loss of micropore volume after the zeolitization process was due to pore blockage. Also, both Al-MCF-modified zeolites showed a significant reduction in the Brønsted acidity as compared to the baseline catalyst (HZSM-5). This showed the partial (i.e., incomplete) conversion of Al-MCF into HZSM-5 deposits. Moreover, the hydrocracking results exhibited a sharp decrease in the conversion of HDPE over hierarchical zeolite owing to the loss of acidity and micropore volume. On the other hand, HZSM-5 showed better conversion with a high yield of gases (Figure 18b). Both Ni-loaded Al-MCF samples did not exhibit any activity, possibly due to their low concentration of Brønsted acid sites. This suggests that the hierarchical zeolite samples synthesized using mesocellular foam and its derivatives are not suitable to synthesize true hierarchical zeolites (i.e., multi-level porosity) for the hydrocracking of plastics owing to the loss of their acidity.

Table 10.
Physiochemical properties of HZSM-5, Al-MCF and hierarchical derivatives of Al-MCF with or without the addition of Ni. Data from [50].
Figure 18.
(a) N2 adsorption-desorption isotherms of hierarchical materials with or without Ni, as well as their parents, are used for zeolitization approaches. Insets in the graph display the pore size distribution obtained from desorption branches (BJH model), The average values for the cell (Dcell) and window (Dwin) diameters, estimated by the BJH method applied to the adsorption and desorption branches, were Dcell = 20 and Dwin = 9 nm, respectively. (b) HDPE hydrocracking over Al-MCF (Z1 and Z2) and HZSM5 (Conditions: 260 °C for 60 min under 20 bar of H2, using 20 wt.% of catalyst). Reprinted with permission from Ref. [50], Copyright 2023 Elsevier, (c) Selectivity by carbon atom number of the feed and the hydro-reforming products. Reprinted with permission from Ref. [151], Copyright 2014 Elsevier.
To avoid such issues during the synthesis process, Escola et al. [151] synthesized BEA zeolite in three stages. Initially, silicon and aluminum precursors were aged at room temperature for 20 h in the presence of SDA (TEAOH) and DI water, followed by pre-crystallization at 135 °C for 3 days. To prevent the growth of protozeolitic units into large, less accessible crystals, 8 mol% of phenylaminopropyltrimethoxysilane (PHAPTMS) was introduced to the gel and kept at 90 °C under continuous stirring. The organosilane bonds with surface hydroxyls on the protozeolitic units formed an organic moiety that limited their aggregation into larger crystals. Finally, the crystallization took place at 135 °C for 7 days, followed by washing, drying, and calcination of the sample to obtain the hierarchical zeolite sample. Interestingly, the hierarchical BEA zeolite sample exhibited high surface area (SBET = 654 m2/g), micropore volume (Vmicro = 0.279 cm3/g) and total acidity (0.385 meq NH3/g). This confirmed the formation of highly crystalline hierarchical BEA zeolite using a three-stage modification process. Moreover, the catalytic hydro-reforming of LDPE oil showed high selectivity for gasoline and diesel range hydrocarbons, suggesting the efficient cracking over acidic hierarchical Beta zeolite. The overall results were further improved after the addition of Ni (Figure 18c). In an alternative method, zeolitized porous materials were synthesized by carefully depositing pre-synthesized nano zeolite seeds onto an ordered/non-ordered macro- or mesoporous support [152]. For instance, in the presence of zeolite seeds, Wang et al. [153] synthesized hierarchical zeolites by converting macroporous diatomites into crystalline zeolites by the use of the vapor-phase transport (VPT) method. Initially, nanosized crystals of silicalite-1 (80 nm) and β zeolites (45 nm) were synthesized via hydrothermal methods followed by layer-by-layer and electrostatically deposited with diatomites. First, a single layer of a cationic polyelectrolyte, such as poly(diallyl dimethylammonium chloride) (PDDA, Mr < 200,000), was deposited onto the pretreated diatomites. This was performed repeatedly over the course of several cycles until uniform multilayers of PDDA and nano zeolite seeds were formed. Finally, the seeded diatomites underwent VPT treatment, resulting in zeolitized diatomites. The study demonstrated that colloidal zeolite seeds facilitated the zeolitization of diatomites, whereas diatomaceous silica without seeds was insufficiently active for direct transformation into zeolites.
To conclude, the zeolitization approach to incorporate nano zeolite seeds into mesoporous structures has shown potential to prepare hierarchical zeolite with high crystallinity and controlled porosity. Wet impregnation using a clear solution yields better results compared to mechanically mixing the powders before calcination. However, the deposition of zeolite particles on the external surfaces of mesoporous materials may cause pore blockage [154]. Also, the distinct nature of the zeolites at the nanoscale may differ significantly from those of a bulk crystalline zeolite. Similarly, partial crystallization of mesoporous silica may result in the synthesis of amorphous hierarchical zeolite with a significant loss of acidity. Therefore, these points need to be addressed carefully before synthesizing hierarchical zeolites using the zeolitization of mesoporous materials.
3. Conclusions and Future Trends
Over the past few decades, various chemical recycling approaches have been developed to convert plastic waste into value-added chemicals. However, the sustainability analysis through LCA showed the advantages of the catalytic hydrocracking route in converting this waste resource into saturated hydrocarbons (i.e., gasoline and diesel range products) with the least impact on the environment [155,156]. Also, catalysts, especially zeolites and zeo-based bi-functional materials (i.e., metal-loaded zeolites), have been extensively used in prior research for the hydrocracking of plastics. This is because of their high acidity, crystallinity, tunable porosity, and shape selectivity, all of which contribute to their enhanced activity during the hydrocracking of plastics into value-added hydrocarbons. In addition, zeolite-based catalysts have demonstrated resistance to plastics containing heteroatoms (i.e., PVC, PA6, and ABS) and have notably produced high-value-added hydrocarbons through hydrocracking [18]. Despite the advantages, the microporous nature of zeolites led to several mass and diffusional constraints towards polymers entering the pores of zeolites, which resulted in poor activity and, ultimately, catalyst deactivation. As a result, the development of hierarchical zeolites has significantly expanded, owing to their need to overcome the diffusion limitations presented by microporous zeolites during the hydrocracking of plastics. However, a controlled and/or well-optimized synthesis of hierarchical zeolites is required to capitalize on the benefits of mesoporosity while simultaneously preserving the acidity and crystallinity of the zeolite. Therefore, many top-down and bottom-up approaches are discussed in terms of the post-modification and/or in-situ synthesis of zeolites, respectively.
Interestingly, the majority of research in the literature predominantly discussed the top-down approaches to synthesizing hierarchical zeolites, specifically through desilication and dealumination pathways. However, a persistent challenge remains in the formation of mesopores that are accessible from the external surface area of zeolite. Therefore, in comparison to dealumination, desilication is considered a better route to develop hierarchical zeolites with better pore-connectivity accessible from the external surface of the zeolite. Similarly, for the hydrocracking of plastics, desilication under mild conditions has been shown to enhance the textural properties by selective extraction of Si without much changing the acidity of the zeolite, which in turn facilitates the diffusion of bulkier polymeric molecules and improves access to active sites. On the other hand, dealumination may result in a loss in the acidity of the zeolite due to the removal of aluminum species. Therefore, for the effective hydrocracking of plastics using dealuminated zeolites, compensation for the loss of acidity is essential, which can be achieved either by mild dealumination (i.e., controlled) by selectively removing FAl and/or EFAl species without much compromising the acidity of the parent material [67] and/or by the introduction of additional acidic component (i.e., metals for Lewis acidity or formation of tandem catalyst [66]). Despite this, both desilication and dealumination routes may alter the functionality of the zeolite (i.e., a change in micropore volume and Si/Al ratio), with the generation of disordered mesoporosity and are only applicable to zeolites within a specific Si/Al range.
Therefore, a two-step recrystallization approach, using the desilication of zeolite followed by reassembly in the presence of surfactants, may counter the aforementioned issues and has proven to synthesize highly crystalline hierarchical zeolites while causing relatively minimal structural damage to zeolites compared to demetallization techniques alone. However, synthesizing hierarchical zeolites with an interconnected pore structure continues to pose significant challenges. To address this, a single-step surfactant-templating method has been successful in producing zeolites with intracrystalline-ordered mesoporosity, exhibiting enhanced textural and acidic properties. This method effectively prevents excessive desilication and maintains the crystallinity of zeolites. In addition, it can be applied to zeolites with various Si/Al ratios, ranging from high Si/Al ratio zeolites (e.g., USY, MOR, BEA, and MFI) to low Si/Al ratio zeolites (e.g., NaY). Similarly, the degree of hierarchy can be tuned by adjusting the pH or the duration of the treatment, ensuring that disordered mesoporosity is avoided and crystallinity is maintained. Additionally, compared to surfactants with larger hydrophilic headgroups, a surfactant with a smaller headgroup, such as CTABr, is more effective in creating intracrystalline mesoporosity in zeolites. However, the use of various expensive cationic surfactants with relatively prolonged reaction times significantly reduces the sustainability of the developed hierarchical zeolites. Despite this, the application of a surfactant-templating recrystallization approach is highly advisable and should be further developed to optimize the hydrocracking of plastics.
Overall, considering the properties of the developed hierarchical zeolites (i.e., ordered mesoporosity, enhanced textural properties with the retention of crystallinity and acidity), the recrystallization route exhibited the most promising results followed by desilication, whereas dealuminated zeolites showed the least preference. Nevertheless, the dealumination route seems to present the least environmental impact and potentially lower cost of preparation, as compared to other top-down approaches. Moreover, for the hydrocracking of plastics, controlled dealumination of zeolites tends to selectively produce diesel-range hydrocarbons, whereas desilicated and recrystallized zeolites exhibit a greater affinity for gasoline-range products. However, the exact range of products is dependent on the type of zeolite and the degree of hierarchical modification. Each method is, thus, responsible for modulating the properties of zeolites differently, which has implications for the range of products obtained. Therefore, the choice of modification method should be based on the type of zeolite, desired physicochemical properties, environmental impact, and cost of preparation to achieve the targeted hydrocracked plastic products.
Moreover, in the bottom-up approach, which involves the in-situ synthesis of hierarchical zeolites, various direct templates (i.e., hard or soft templates) and/or indirect templates (i.e., mesoporous structures) are used. A major advantage of the bottom-up approach is the ability to synthesize mesoporous zeolites with chemical compositions identical or similar to conventional zeolites. However, an optimum interaction between templates and aluminosilicate species is required to effectively synthesize true hierarchical zeolites. In addition, the employed synthesis route (i.e., hard or soft templating and/or zeolitization) and template used (i.e., size of the template) are responsible for defining the mesoporosity (i.e., inter- or intracrystalline mesoporosity) and functionality (i.e., intrinsic microporosity) of the final hierarchical zeolite. Despite this, little progress has been made in the synthesis of hierarchical zeolites using bottom-up approaches, particularly in the hydrocracking of plastics. This is primarily due to the challenges associated with the synthesis of hierarchical structures with control over their final physiochemical properties (i.e., both textural and acidic). Similarly, it is difficult to scale these methods due to the energy-intensive synthesis conditions and requirements of high costs and environmentally unfriendly templates. Specifically, the challenges associated with the removal of templates, long reaction time, cost, and potential environmental issues during calcination may result in researchers’ lack of interest in developing hierarchical zeolites using hard and/or soft templating methods. However, several promising alternatives, including carbon materials derived from sugars (i.e., sucrose and glucose) and/or cheap mesoporogens, ordered mesoporous carbon-based templates, and structure-directing agents, can be used. Also, the use of microwave heating may help reduce reaction time and decrease utility costs.
As seen in this review, the application of hierarchical zeolites to the hydrocracking of waste plastics is only at its early stages of development. However, trends show that regardless of the synthesis approach taken, hierarchical zeolites with mild acidity and controlled mesoporosity seem to be better suited for this application to allow for good activity without compromising the selectivity for the more valuable liquid products. In fact, while very high acidity may be more advantageous in terms of overall activity, it mostly leads to the production of gases, resulting in smaller economic benefits. Similarly, although very high mesoporosity generation is known to have beneficial effects on the mass transfer mechanisms and on the accessibility of the active sites, this results in the reduction of the retention time of reactant molecules in the structure, leading to an increase in heavier, not so desirable, products. Another important textural property to consider while developing hierarchical zeolites for the hydrocracking of waste plastics is the external surface area. Given the size of the plastic molecules, it is not expected that they will immediately enter the pore structure of the zeolite. Instead, the primary cracking of these molecules will most likely take place on the surface of the catalyst before the molecules can diffuse to the pores. Based on this, designing hierarchical zeolites with enhanced external surface areas that are populated by a good number of acid sites will also be crucial to reaching good levels of performance for the hydrocracking of plastics. Thus, additional research work is required to fabricate hierarchical zeolites that are on-purposely designed for this process. This can only be achieved via the systematic understanding of the impact of synthesis conditions on the physicochemical properties of the materials and their implications on the catalytic performance. Therefore, it is utterly needed that activity-selectivity-properties relationships are further developed in the literature to determine optimal synthesis conditions to obtain crystalline hierarchical zeolites with controlled porosity and acidic properties while reducing the severity of the in-situ synthesis or post-synthesis treatments. This advancement will require innovative strategies to refine pore structure control and reduce dependency on complex templates.
While doing this, it will be key to bear in mind that the reproducibility, cost, scalability, and environmental impact of the proposed synthesis routes will be of major importance to turn catalyst development into commercially viable applications, as these have been major drawbacks of hierarchical zeolites commercial implementation. Therefore, efforts should be made to develop easy-to-follow synthesis strategies that involve benign chemicals or are based on other organic wastes, minimize waste production, or use more sustainable heating sources, such as microwaves or ultrasounds, and that follow green chemistry principles. In addition, alongside catalyst development, a detailed life cycle assessment contemplating steps involved during hierarchical zeolite synthesis and cost evaluation must be carried out to assess the feasibility of the proposed methods. Moreover, present research efforts have been mostly focused on the use of LDPE and HDPE in combination with hierarchical zeolites. Thus, further studies on the hydrocracking of other plastics, such as PP or PS, or mixtures of waste plastics mimicking typical waste plastic composition would be extremely encouraged, as different plastic molecules will certainly have different reactivities when exposed to a catalyst with given properties. Finally, efforts should also be put into the assessment of the catalytic performance of hierarchical zeolites when exposed to real waste plastic feedstocks containing heteroatoms and other impurities (heavy metals, dyes, plastic additives, etc.), as the presence of these elements can have a negative impact on the catalytic performance, and, so, synthesis strategies should also take this into account.
Hence, this review has shown the great potential of hierarchical zeolites, with combined micro- and mesopores, for the hydrocracking of waste plastics into value-added fuels and chemicals. The field is now open to fully exploring these benefits and developing on-purpose hierarchical zeolites for application in waste plastic upcycling that follow green chemistry principles and contribute to the overall sustainability of these processes.
Author Contributions
Conceptualization, M.U.A. and I.G.; writing—original draft preparation, M.U.A.; writing—review and editing, W.A. and I.G.; supervision, W.A. and I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by The LEVERHULME TRUST (Grant DS-2017-073). Muhammad Usman Azam, a Leverhulme Trust Doctoral Scholar, was part of the 15 PhD scholarships of the “Leverhulme Centre for Doctoral Training in Sustainable Production of Chemicals and Materials” at the University of Aberdeen (Scotland, United Kingdom).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- García, J.M. Catalyst: Design Challenges for the Future of Plastics Recycling. Chem. 2016, 1, 813–815. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The Future of Plastics Recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure-Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Brandoni, C.; Jaffar, M.; Hewitt, N.J.; Dunlop, P.; Zhang, K.; Huang, Y. An Experimental Investigation of Hydrogen Production through Biomass Electrolysis. Processes 2024, 12, 112. [Google Scholar] [CrossRef]
- Azam, M.U.; Vete, A.; Afzal, W. Process Simulation and Life Cycle Assessment of Waste Plastics: A Comparison of Pyrolysis and Hydrocracking. Molecules 2022, 27, 8084. [Google Scholar] [CrossRef]
- Merrild, H.; Larsen, A.W.; Christensen, T.H. Assessing Recycling versus Incineration of Key Materials in Municipal Waste: The Importance of Efficient Energy Recovery and Transport Distances. Waste Manag. 2012, 32, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A Review on Catalytic Pyrolysis of Plastic Wastes to High-Value Products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Kots, P.A.; Vance, B.C.; Vlachos, D.G. Polyolefin Plastic Waste Hydroconversion to Fuels, Lubricants, and Waxes: A Comparative Study. React. Chem. Eng. 2021, 7, 41–54. [Google Scholar] [CrossRef]
- Qin, L.; Xu, Z.; Zhao, B.; Zou, C.; Chen, W.; Han, J. Kinetic Study on High-Temperature Gasification of Medical Plastic Waste Coupled with Hydrogen-Rich Syngas Production Catalyzed by Steel-Converter Ash. J. Energy Inst. 2022, 102, 14–21. [Google Scholar] [CrossRef]
- Saha, N.; Banivaheb, S.; Toufiq Reza, M. Towards Solvothermal Upcycling of Mixed Plastic Wastes: Depolymerization Pathways of Waste Plastics in Sub- and Supercritical Toluene. Energy Convers. Manag. X 2022, 13, 100158. [Google Scholar] [CrossRef]
- Kobylarski, M.; Berthet, J.C.; Cantat, T. Reductive Depolymerization of Polyesters and Polycarbonates with Hydroboranes by Using a Lanthanum(Iii) Tris(Amide) Catalyst. Chem. Commun. 2022, 58, 2830–2833. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of Virgin and Waste Plastics: A Detailed Review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Mosio-Mosiewski, J.; Warzala, M.; Morawski, I.; Dobrzanski, T. High-Pressure Catalytic and Thermal Cracking of Polyethylene. Fuel Process. Technol. 2007, 88, 359–364. [Google Scholar] [CrossRef]
- Ostroumova, V.A.; Severina, V.A.; Maksimov, A.L. Application of Ni-W Sulfide Catalysts Prepared In Situ from Embryonic and Highly Crystalline ZSM-5 Zeolites in Hydrocracking Reaction of 1-Methylnaphthalene. Pet. Chem. 2021, 61, 341–349. [Google Scholar] [CrossRef]
- Scherzer, J.; Gruia, A.J. Hydrocracking Science and Technology; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Klimov, O.V.; Nadeina, K.A.; Potapenko, O.V.; Vatutina, Y.V.; Saiko, A.V.; Koveza, V.A.; Mukhacheva, P.P.; Krestyaninova, V.S.; Yurtaeva, A.S.; Bogomolova, T.S.; et al. Refining of Chlorine-Containing Plastic Wastes by Traditional Hydrotreating and Catalytic Cracking Processes. Fuel 2023, 349, 128651. [Google Scholar] [CrossRef]
- Qiu, Z.; Lin, S.; Chen, Z.; Chen, A.; Zhou, Y.; Cao, X.; Wang, Y.; Lin, B.L. A Reusable, Impurity-Tolerant and Noble Metal-Free Catalyst for Hydrocracking of Waste Polyolefins. Sci. Adv. 2023, 9, eadg5332. [Google Scholar] [CrossRef] [PubMed]
- Narksri, P.; Angnanon, S.; Guntasub, J.; Wijitrattanatri, K.; Kingputtapong, S.; Phumpradit, S.; Hinchiranan, N. Production of Alternative Liquid Fuels from Catalytic Hydrocracking of Plastics over Ni/SBA-15 Catalyst. Mater. Today Proc. 2022, 57, 1040–1047. [Google Scholar] [CrossRef]
- Ding, W.; Liang, J.; Anderson, L.L. Hydrocracking and Hydroisomerization of High-Density Polyethylene and Waste Plastic over Zeolite and Silica–Alumina-Supported Ni and Ni-Mo Sulfides. Energy Fuels 1997, 11, 1219–1223. [Google Scholar] [CrossRef]
- Iqbal, F.; Shafeeq, A.; Aslam, R.; Rauf, A. Comparative Analysis of Catalytic Cracking and Hydrocracking of Waste Plastic. Chem. Eng. Technol. 2023, 46, 723–730. [Google Scholar] [CrossRef]
- Liu, K.; Meuzelaar, H.L.C. Catalytic Reactions in Waste Plastics, HDPE and Coal Studied by High-Pressure Thermogravimetry with on-Line GC/MS. Fuel Process. Technol. 1996, 49, 1–15. [Google Scholar] [CrossRef]
- Jumah, A.B.; Tedstone, A.A.; Garforth, A.A. Hydrocracking of Virgin and Post-Consumer Polymers. Microporous Mesoporous Mater. 2021, 315, 110912. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic Waste to Fuels by Hydrocracking at Mild Conditions. Sci. Adv. 2021, 7, 8283–8304. [Google Scholar] [CrossRef] [PubMed]
- Vela, F.J.; Palos, R.; Trueba, D.; Bilbao, J.; Arandes, J.M.; Gutiérrez, A. Different Approaches to Convert Waste Polyolefins into Automotive Fuels via Hydrocracking with a NiW/HY Catalyst. Fuel Process. Technol. 2021, 220, 106891. [Google Scholar] [CrossRef]
- Costa, C.S.; Muñoz, M.; Ribeiro, M.R.; Silva, J.M. A Thermogravimetric Study of HDPE Conversion under a Reductive Atmosphere. Catal. Today 2021, 379, 192–204. [Google Scholar] [CrossRef]
- Costa, C.S.; Muñoz, M.; Ribeiro, M.R.; Silva, J.M. H-USY and H-ZSM-5 Zeolites as Catalysts for HDPE Conversion under a Hydrogen Reductive Atmosphere. Sustain. Energy Fuels 2021, 5, 1134–1147. [Google Scholar] [CrossRef]
- Muñoz, M.; Morales, I.; Costa, C.S.; Multigner, M.; de la Presa, P.; Alonso, J.M.; Silva, J.M.; Ribeiro, M.D.R.; Torres, B.; Rams, J. Local Induction Heating Capabilities of Zeolites Charged with Metal and Oxide MNPs for Application in HDPE Hydrocracking: A Proof of Concept. Materials 2021, 14, 1029. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A. Hierarchical Zeolites: Synthesis and Catalytic Properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Chen, L.H.; Sun, M.H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Wijaya, K.; Kurniawan, M.A.; Saputri, W.D.; Trisunaryanti, W.; Mirzan, M.; Hariani, P.L.; Tikoalu, A.D. Synthesis of Nickel Catalyst Supported on ZrO2/SO4pillared Bentonite and Its Application for Conversion of Coconut Oil into Gasoline via Hydrocracking Process. J. Environ. Chem. Eng. 2021, 9, 105399. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-Promoted Sulfated Zirconia as Catalyst for Hydrocracking of LDPE Plastic Waste into Liquid Fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Uemichi, Y.; Hattori, M.; Itoh, T.; Nakamura, J.; Sugioka, M. Deactivation Behaviors of Zeolite and Silica-Alumina Catalysts in the Degradation of Polyethylene. Ind. Eng. Chem. Res. 1998, 37, 867–872. [Google Scholar] [CrossRef]
- Venkatesh, K.; Hu, J.; Tierney, J.; Wender, I.; Papers, I.W.-P. Hydrocracking of Polyolefins to Liquid Fuels over Strong Solid Acid Catalysts; American Chemical Society, Division of Fuel Chemistry: Chicago, IL, USA, 1995. Available online: https://www.osti.gov/biblio/462530 (accessed on 4 July 2024).
- Aboul-Gheit, A.K.; Gad, F.K.; Abdel-Aleem, G.M.; El-Desouki, D.S.; Abdel-Hamid, S.M.; Ghoneim, S.A.; Ibrahim, A.H. Pt, Re and Pt–Re Incorporation in Sulfated Zirconia as Catalysts for n-Pentane Isomerization. Egypt. J. Pet. 2014, 23, 303–314. [Google Scholar] [CrossRef]
- Figueiredo, A.L.; Araujo, A.S.; Linares, M.; Peral, Á.; García, R.A.; Serrano, D.P.; Fernandes, V.J. Catalytic Cracking of LDPE over Nanocrystalline HZSM-5 Zeolite Prepared by Seed-Assisted Synthesis from an Organic-Template-Free System. J. Anal. Appl. Pyrolysis 2016, 117, 132–140. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef] [PubMed]
- Baile, P.; Fernández, E.; Vidal, L.; Canals, A. Analyst CRITICAL REVIEW Zeolites and Zeolite-Based Materials in Extraction and Microextraction Techniques. Analyst 2019, 144, 366. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored Crystalline Microporous Materials by Post-Synthesis Modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef]
- Mitchell, S.; Pinar, A.B.; Kenvin, J.; Crivelli, P.; Kärger, J.; Pérez-Ramírez, J. Structural Analysis of Hierarchically Organized Zeolites. Nat. Commun. 2015, 6, 8633. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C.K. Modern Synthesis Strategies for Hierarchical Zeolites: Bottom-up versus Top-down Strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Cho, H.J.; Fan, W.; Valla, J.A. On the Effectiveness of Tailored Mesoporous MFI Zeolites for Biomass Catalytic Fast Pyrolysis. Appl. Catal. A Gen. 2016, 522, 109–119. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Desilication Mechanism Revisited: Highly Mesoporous All-Silica Zeolites Enabled Through Pore-Directing Agents. Chem.—Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ordóñez, E.G.; Salbidegoitia, J.A.; Ayastuy, J.L.; Gutiérrez-Ortiz, M.A.; González-Marcos, M.P.; González-Velasco, J.R. High External Surface Pt/Zeolite Catalysts for Improving Polystyrene Hydrocracking. Catal. Today 2014, 227, 163–170. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramírez, J. Mechanism of Hierarchical Porosity Development in MFI Zeolites by Desilication: The Role of Aluminium as a Pore-Directing Agent. Chem.—Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Kots, P.A.; Doika, P.A.; Vance, B.C.; Najmi, S.; Vlachos, D.G. Tuning High-Density Polyethylene Hydrocracking through Mordenite Zeolite Crystal Engineering. ACS Sustain. Chem. Eng. 2023, 11, 9000–9009. [Google Scholar] [CrossRef]
- Ogura, M.; Shinomiya, S.-Y.; Tateno, J.; Nara, Y.; Nomura, M.; Kikuchi, E.; Matsukata, M. Alkali-Treatment Technique-New Method for Modification of Structural and Acid-Catalytic Properties of ZSM-5 Zeolites. Appl. Catal. A Gen. 2001, 219, 33–43. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Optimal Aluminum-Assisted Mesoporosity Development in MFI Zeolites by Desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- González-Marcos, M.P.; Fuentes-Ordóñez, E.G.; Salbidegoitia, J.A.; González-Velasco, J.R. Optimization of Supports in Bifunctional Supported Pt Catalysts for Polystyrene Hydrocracking to Liquid Fuels. Top. Catal. 2021, 64, 224–242. [Google Scholar] [CrossRef]
- Armenise, S.; Costa, C.S.; Luing, W.S.; Ribeiro, M.R.; Silva, J.M.; Onfroy, T.; Valentin, L.; Casale, S.; Muñoz, M.; Launay, F. Evaluation of Two Approaches for the Synthesis of Hierarchical Micro-/Mesoporous Catalysts for HDPE Hydrocracking. Microporous Mesoporous Mater. 2023, 356, 112605. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Desilication: On the Controlled Generation of Mesoporosity in MFI Zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, W.; Gui, Z.; Jiang, J.; Zhu, Z.; Li, J.J.; Zhao, L.; Zhou, J.; Xi, Z. Selective Hydrocracking of Waste Polyolefins toward Gasoline-Range Liquid Fuels via Tandem Catalysis over a Cerium-Promoted Pt/HY Catalyst. ACS Sustain. Chem. Eng. 2024, 12, 5738–5752. [Google Scholar] [CrossRef]
- Kinga Góra-Marek, S.; Pyra, K.; Tarach, K.A.; Majda, D.; Góra-Marek, K. As Featured in: Desilicated Zeolite BEA for the Catalytic Cracking of LDPE: The Interplay between Acidic Sites’ Strength and Accessibility. Catal. Sci. Technol. 2019, 9, 1794. [Google Scholar] [CrossRef]
- Mingorance-Baena, A.; Siles-Quesada, S.; Tarach, K.; Morales, M.V.; Gora-Marek, K.; Melián-Cabrera, I. Improved Catalytic Technology for Waste Plastic Processing: Toward Novel Remediation and Emission Control Measures. ACS Sustain. Chem. Eng. 2019, 7, 129–133. [Google Scholar] [CrossRef]
- Abelló, S.; Bonilla, A.; Pérez-Ramírez, J. Mesoporous ZSM-5 Zeolite Catalysts Prepared by Desilication with Organic Hydroxides and Comparison with NaOH Leaching. Appl. Catal. A Gen. 2009, 364, 191–198. [Google Scholar] [CrossRef]
- Najar, H.; Zina, M.S.; Ghorbel, A. Study of the Effect of the Acid Dealumination on the Physico-Chemical Properties of Y Zeolite. React. Kinet. Mech. Catal. 2010, 100, 385–398. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, H.; Liao, W.; Tang, P.; Yang, K.; Su, T.; Ren, W.; Lü, H. Insight into Tri-Coordinated Aluminum Dependent Catalytic Properties of Dealuminated Y Zeolites in Oxidative Desulfurization. Appl. Catal. B 2021, 288, 120022. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; De Jong, K.P.; Zečević, J. Tailoring and Visualizing the Pore Architecture of Hierarchical Zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef]
- Beyer, H.K.; Belenykaja, I. A New Method for the Dealumination of Faujasite-Type Zeolites. Stud. Surf. Sci. Catal. 1980, 5, 203–210. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; De Jong, K.P. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Janssen, A.H.; Koster, A.J.; De Jong, K.P. On the Shape of the Mesopores in Zeolite Y: A Three-Dimensional Transmission Electron Microscopy Study Combined with Texture Analysis. J. Phys. Chem. B 2002, 106, 11905–11909. [Google Scholar] [CrossRef]
- Lutz, W. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef]
- Beyer, H.K. Dealumination Techniques for Zeolites. In Post-Synthesis Modification I; Springer: Berlin/Heidelberg, Germany, 2002; pp. 203–255. [Google Scholar] [CrossRef]
- Sharratt, P.N.; Lin, Y.-H.; Garforth, A.A.; Dwyer, J. Investigation of the Catalytic Pyrolysis of High-Density Polyethylene over a HZSM-5 Catalyst in a Laboratory Fluidized-Bed Reactor. Ind. Eng. Chem. Res. 1997, 36, 5118–5124. [Google Scholar] [CrossRef]
- Bin Jumah, A.; Anbumuthu, V.; Tedstone, A.A.; Garforth, A.A. Catalyzing the Hydrocracking of Low Density Polyethylene. Ind. Eng. Chem. Res. 2019, 58, 20601–20609. [Google Scholar] [CrossRef]
- Li, L.; Luo, H.; Shao, Z.; Zhou, H.; Lu, J.; Chen, J.; Huang, C.; Zhang, S.; Liu, X.; Xia, L.; et al. Converting Plastic Wastes to Naphtha for Closing the Plastic Loop. J. Am. Chem. Soc. 2023, 145, 1847–1854. [Google Scholar] [CrossRef]
- Usman Azam, M.; Fernandes, A.; Graça, I.; Afzal, W. Hydrocracking of Surgical Face Masks over Y Zeolites: Catalyst Development, Process Design and Life Cycle Assessment. Fuel 2023, 349, 128704. [Google Scholar] [CrossRef]
- Kung, H.H.; Williams, B.A.; Babitz, S.M.; Miller, J.T.; Haag, W.O.; Snurr, R.Q. Enhanced Hydrocarbon Cracking Activity of Y Zeolites. Top. Catal. 2000, 10, 59–64. [Google Scholar] [CrossRef]
- Masuda, T.; Fujikata, Y.; Mukai, S.R.; Hashimoto, K. Changes in Catalytic Activity of MFI-Type Zeolites Caused by Dealumination in a Steam Atmosphere. Appl. Catal. A Gen. 1998, 172, 73–83. [Google Scholar] [CrossRef]
- Azam, M.U.; Afzal, W.; Fernandes, A.; Graça, I. Insights into the Development of Greener Mild Zeolite Dealumination Routes Applied to the Hydrocracking of Waste Plastics. Appl. Catal. A Gen. 2024, 119873, in press, journal pre-proof. [Google Scholar] [CrossRef]
- Pham, T.N.; Nguyen, V.; Wang, B.; White, J.L.; Crossley, S. Quantifying the Influence of Water on the Mobility of Aluminum Species and Their Effects on Alkane Cracking in Zeolites. ACS Catal. 2021, 11, 6982–6994. [Google Scholar] [CrossRef]
- Sarker, M.; Liu, R.; Rahman, M.M.; Li, C.; Chai, M.; Nishu; He, Y. Impact of Acid-Modified ZSM-5 on Hydrocarbon Yield of Catalytic Co-Pyrolysis of Poplar Wood Sawdust and High-Density Polyethylene by Py-GC/MS Analysis. J. Energy Inst. 2020, 93, 2435–2443. [Google Scholar] [CrossRef]
- Wangsa, W.; Aldino; Saviola, J.; Wijaya, K.; Bhagaskara, A.; Hauli, L.; Dita; Saputra, A. Utilization of Laboratory Glove Waste for Fuel Production through Pyrolysis-Hydrocracking Consecutive Process Catalyzed by Sulfated Indonesian Natural Zeolite. React. Kinet. Mech. Catal. 2024, 137, 1495–1514. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and Hierarchical Zeolites: A Short Review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Giudici, R.; Kouwenhoven, H.W.; Prins, R. Comparison of Nitric and Oxalic Acid in the Dealumination of Mordenite. Appl. Catal. A Gen. 2000, 203, 101–110. [Google Scholar] [CrossRef]
- Ajot, H.; Joly, J.F.; Lynch, J.; Raatz, F.; Caullet, P. Formation of Secondary Pores in Zeolites During Dealumination: Influence of TheCrystallographic Structure and Of the Si/Al RATIO. Stud. Surf. Sci. Catal. 1991, 62, 583–590. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Zhang, W. Effect of the Si/Al Ratio and of the Zeolite Structure on the Performance of Dealuminated Zeolites for the Reforming of Hydrocarbon Mixtures. Ind. Eng. Chem. Res. 1996, 35, 3055–3066. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al Ratios and Al Distributions of Zeolites and Their Impact on Properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef]
- Yi, F.; Chen, H.; Huang, L.; Hu, C.; Wang, J.; Li, T.; Wang, H.; Tao, Z.; Yang, Y.; Li, Y. Effects of the Acidity and Shape Selectivity of Dealuminated Zeolite Beta on Butene Transformations. Fuel 2021, 300, 120694. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, S.; Luo, M.; Xiao, R. The Comparison of Chemical Liquid Deposition and Acid Dealumination Modified ZSM-5 for Catalytic Pyrolysis of Pinewood Using Pyrolysis-Gas Chromatography/Mass Spectrometry. Bioresour. Technol. 2017, 244, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Babić, V.; Koneti, S.; Moldovan, S.; Debost, M.; Gilson, J.P.; Valtchev, V. Chromic Acid Dealumination of Zeolites. Microporous Mesoporous Mater. 2022, 329, 111513. [Google Scholar] [CrossRef]
- Abdulridha, S.; Zhang, R.; Xu, S.; Tedstone, A.; Ou, X.; Gong, J.; Mao, B.; Frogley, M.; Bawn, C.; Zhou, Z.; et al. An Efficient Microwave-Assisted Chelation (MWAC) Post-Synthetic Modification Method to Produce Hierarchical Y Zeolites. Microporous Mesoporous Mater. 2021, 311, 110715. [Google Scholar] [CrossRef]
- Lee, W.T.; van Muyden, A.; Bobbink, F.D.; Mensi, M.D.; Carullo, J.R.; Dyson, P.J. Mechanistic Classification and Benchmarking of Polyolefin Depolymerization over Silica-Alumina-Based Catalysts. Nat. Commun. 2022, 13, 4850. [Google Scholar] [CrossRef]
- He, J.; Duan, X.; Evans, D.G.; Howe, R.F. Mesoporous Material from Zeolite. J. Porous Mater. 2002, 9, 43–48. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kasyanov, I.A.; Maerle, A.A.; Zaikovskii, V.I. Mechanistic Study of Zeolites Recrystallization into Micro-Mesoporous Materials. Microporous Mesoporous Mater. 2014, 189, 163–172. [Google Scholar] [CrossRef]
- Sachse, A.; Grau-Atienza, A.; Jardim, E.O.; Linares, N.; Thommes, M.; García-Martínez, J. Development of Intracrystalline Mesoporosity in Zeolites through Surfactant-Templating. Cryst. Growth Des. 2017, 17, 4289–4305. [Google Scholar] [CrossRef]
- Garcıá-Martı´nezmartı´nez, J.; Johnson, M.; Valla, J.; Li, K.; Ying, J.Y. Mesostructured Zeolite Y-High Hydrothermal Stability and Superior FCC Catalytic Performancew. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Jiang, H. Preparation of β Zeolite with Intracrystalline Mesoporosity via Surfactant -Templating Strategy and Its Application in Ethanol-Acetaldehyde to Butadiene. Microporous Mesoporous Mater. 2021, 316, 110949. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Murzin, V.Y.; Monakhova, Y.V.; Zubavichus, Y.V.; Knyazeva, E.E.; Nesterenko, N.S.; Ivanova, I.I. Nature, Strength and Accessibility of Acid Sites in Micro/Mesoporous Catalysts Obtained by Recrystallization of Zeolite BEA. Microporous Mesoporous Mater. 2007, 105, 101–110. [Google Scholar] [CrossRef]
- Imbachi-Gamba, C.F.; Villa, A.L. Statistical Analysis of the Influence of Synthesis Conditions on the Properties of Hierarchical Zeolite Y. Mater. Today Chem. 2021, 20, 100442. [Google Scholar] [CrossRef]
- Zhou, X.; Han, X.; Qu, Z.; Zhang, J.; Zeng, F.; Tang, Z.; Chen, R. Hierarchical FAU Zeolites Boosting the Hydrocracking of Polyolefin Waste into Liquid Fuels. ACS Sustain. Chem. Eng. 2024, 12, 6013–6022. [Google Scholar] [CrossRef]
- Tan, J.Z.; Ortega, M.; Miller, S.A.; Hullfish, C.W.; Kim, H.; Kim, S.; Hu, W.; Hu, J.Z.; Lercher, J.A.; Koel, B.E.; et al. Catalytic Consequences of Hierarchical Pore Architectures within MFI and FAU Zeolites for Polyethylene Conversion. ACS Catal. 2024, 14, 7536–7552. [Google Scholar] [CrossRef]
- García, R.A.; Serrano, D.P.; Otero, D. Catalytic Cracking of HDPE over Hybrid Zeolitic–Mesoporous Materials. J. Anal. Appl. Pyrolysis 2005, 74, 379–386. [Google Scholar] [CrossRef]
- Munir, D.; Usman, M.R. Catalytic Hydropyrolysis of a Model Municipal Waste Plastic Mixture over Composite USY/SBA-16 Catalysts. J. Anal. Appl. Pyrolysis 2018, 135, 44–53. [Google Scholar] [CrossRef]
- Munir, D.; Amer, H.; Aslam, R.; Bououdina, M.; Usman, M.R. Composite Zeolite Beta Catalysts for Catalytic Hydrocracking of Plastic Waste to Liquid Fuels. Mater. Renew. Sustain. Energy 2020, 9, 9. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating Hierarchical Pores in Zeolite Catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Wei, X.; Smirniotis, P.G. Synthesis and Characterization of Mesoporous ZSM-12 by Using Carbon Particles. Microporous Mesoporous Mater. 2006, 89, 170–178. [Google Scholar] [CrossRef]
- Kustova, M.Y.; Hasselriis, P.; Christensen, C.H. Mesoporous MEL—Type Zeolite Single Crystal Catalysts. Catal. Letters 2004, 96, 205–211. [Google Scholar] [CrossRef]
- Pavlačková, Z.; Košová, G.; Silková, N.; Zukal, A.; Čejka, J. Formation of Mesopores in ZSM-5 by Carbon Templating. Stud. Surf. Sci. Catal. 2006, 162, 905–912. [Google Scholar] [CrossRef]
- Chou, Y.H.; Cundy, C.S.; Garforth, A.A.; Zholobenko, V.L. Mesoporous ZSM-5 Catalysts: Preparation, Characterisation and Catalytic Properties. Part I: Comparison of Different Synthesis Routes. Microporous Mesoporous Mater. 2006, 89, 78–87. [Google Scholar] [CrossRef]
- Katsuki, H.; Furuta, S.; Watari, T.; Komarneni, S. ZSM-5 Zeolite/Porous Carbon Composite: Conventional- and Microwave-Hydrothermal Synthesis from Carbonized Rice Husk. Microporous Mesoporous Mater. 2005, 86, 145–151. [Google Scholar] [CrossRef]
- Schmidt, I.; Madsen, C.; Jacobsen, C.J.H. Confined Space Synthesis. A Novel Route to Nanosized Zeolites. Inorg. Chem. 2000, 39, 2279–2283. [Google Scholar] [CrossRef]
- Janssen, A.H.; Schmidt, I.; Jacobsen, C.J.H.; Koster, A.J.; de Jong, K.P. Exploratory Study of Mesopore Templating with Carbon during Zeolite Synthesis. Microporous Mesoporous Mater. 2003, 65, 59–75. [Google Scholar] [CrossRef]
- Nandan, D.; Saxena, S.K.; Viswanadham, N. Synthesis of Hierarchical ZSM-5 Using Glucose as a Templating Precursor. J. Mater. Chem. A Mater. 2013, 2, 1054–1059. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.S. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.S. Solvent-Free Synthesis of Zeolites from Solid Raw Materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Wang, Y.; Kong, D.; Yuan, X.; Xie, Z. Nanosized CaCO3 as Hard Template for Creation of Intracrystal Pores within Silicalite-1 Crystal. Chem. Mater. 2008, 20, 1134–1139. [Google Scholar] [CrossRef]
- Valtchev, V. Silicalite-1 Hollow Spheres and Bodies with a Regular System of Macrocavities. Chemistry of Materials 2002, 14, 4371–4377. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Meng, L.; Jiang, N. Synthesis of Uniform Mesoporous ZSM-5 Using Hydrophilic Carbon as a Hard Template. Mater. Chem. Phys. 2016, 177, 112–117. [Google Scholar] [CrossRef]
- Abdulridha, S.; Jiang, J.; Xu, S.; Zhou, Z.; Liang, H.; Mao, B.; Zhou, Y.; Garforth, A.A.; Jiao, Y.; Fan, X. Cellulose Nanocrystals (CNCs) as Hard Templates for Preparing Mesoporous Zeolite Y Assemblies with High Catalytic Activity. Green. Chemistry 2020, 22, 5115–5122. [Google Scholar] [CrossRef]
- Ren, Z.; Kim, E.; Pattinson, S.W.; Subrahmanyam, K.S.; Rao, C.N.R.; Cheetham, A.K.; Eder, D. Hybridizing Photoactive Zeolites with Graphene: A Powerful Strategy towards Superior Photocatalytic Properties. Chem. Sci. 2011, 3, 209–216. [Google Scholar] [CrossRef]
- Tang, K.; Wang, Y.G.; Song, L.J.; Duan, L.H.; Zhang, X.T.; Sun, Z.L. Carbon Nanotube Templated Growth of Nano-Crystalline ZSM-5 and NaY Zeolites. Mater. Lett. 2006, 60, 2158–2160. [Google Scholar] [CrossRef]
- Schmidt, I.; Boisen, A.; Gustavsson, E.; Ståhl, K.; Pehrson, S.; Dahl, S.; Carlsson, A.; Jacobsen, C.J.H. Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Madsen, C.; Jacobsen, C.J.H. Nanosized Zeolite Crystals—Convenient Control of Crystal Size Distribution by Confined Space Synthesis. Chem. Commun. 1999, 8, 673–674. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Y.; Zhu, K.; Qian, G.; Zhou, X. Carbon Nanotubes as Transient Inhibitors in Steam-Assisted Crystal-Lization of Hierarchical ZSM-5 Zeolites. Mater. Lett. 2015, 159, 466–469. [Google Scholar] [CrossRef]
- Yu, H.; Li, F.; He, W.; Song, C.; Zhang, Y.; Li, Z.; Lin, H. Synthesis of Micro–Mesoporous ZSM-5 Zeolite with Microcrystalline Cellulose as Co-Template and Catalytic Cracking of Polyolefin Plastics. RSC Adv. 2020, 10, 22126–22136. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal Synthesis of Zeolites with Three-Dimensionally Ordered Mesoporous-Imprinted Structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.R.; Reitzel, N.; Hassenkam, T.; Kjaer, K.; Howes, P.B.; Larsen, N.B.; Bùgelund, J.; Jayaraman, M.; Ewbank, P.C.; McCullough, R.D.; et al. Zeolite ZSM-5 with Unique Supermicropores Synthesized Using Mesoporous Carbon as a Template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Sakthivel, A.; Huang, S.J.; Chen, W.H.; Lan, Z.H.; Chen, K.H.; Kim, T.W.; Ryoo, R.; Chiang, A.S.T.; Liu, S. Bin Replication of Mesoporous Aluminosilicate Molecular Sieves (RMMs) with Zeolite Framework from Mesoporous Carbons (CMKs). Chem. Mater. 2004, 16, 3168–3175. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.S.; Yoo, W.C.; McCormick, A.V.; Lee Penn, R.; Stein, A.; Tsapatsis, M. Hierarchical Nanofabrication of Microporous Crystals with Ordered Mesoporosity. Nat. Mater. 2008, 7, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, H. An Ordered Mesoporous Aluminosilicate with Completely Crystalline Zeolite Wall Structure. J. Am. Chem. Soc. 2006, 128, 10636–10637. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Bolshakov, A.; Van Diepen, M.; Van Hoof, A.J.F.; Romero Hidalgo, D.E.; Kosinov, N.; Hensen, E.J.M. Hierarchically Porous (Alumino)Silicates Prepared by an Imidazole-Based Surfactant and Their Application in Acid-Catalyzed Reactions. ACS Appl. Mater. Interfaces 2019, 11, 40151–40162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Dong, L.; Chen, C.; Wang, Y.; Zhang, J.; Qian, W.; Hong, M. Direct Synthesis of Core–Shell MFI Zeolites with Spatially Tapered Trimodal Mesopores via Controlled Orthogonal Self-Assembly. Nanoscale 2019, 11, 16667–16676. [Google Scholar] [CrossRef]
- Cho, K.; Cho, H.S.; De Ménorval, L.C.; Ryoo, R. Generation of Mesoporosity in LTA Zeolites by Organosilane Surfactant for Rapid Molecular Transport in Catalytic Application. Chem. Mater. 2009, 21, 5664–5673. [Google Scholar] [CrossRef]
- Chmelka, B.F. Large Molecules Welcome. Nat. Mater. 2006, 5, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Yilmaz, B.; Müller, U.; Bein, T. Hierarchical Zeolite Beta via Nanoparticle Assembly with a Cationic Polymer. Chem. Mater. 2011, 23, 4301–4310. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; Van Der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y. Highly Mesoporous Single-Crystalline Zeolite Beta Synthesized Using a Nonsurfactant Cationic Polymer as a Dual-Function Template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Gody, G.; Zhao, W.; Perrier, S.; Peng, X.; Ducati, C.; Zhao, D.; Cheetham, A.K. Hierarchical Bicontinuous Porosity in Metal–Organic Frameworks Templated from Functional Block Co-Oligomer Micelles. Chem. Sci. 2013, 4, 3573–3577. [Google Scholar] [CrossRef]
- Shen, X.; enting Mao, W.; anhang Ma, Y.; ongdong Xu, D.; Wu, P.; samu Terasaki, O.; Han, L.; Che, S.; Shen, X.; Mao, W.; et al. A Hierarchical MFI Zeolite with a Two-Dimensional Square Mesostructure. Angew. Chem. 2018, 130, 732–736. [Google Scholar] [CrossRef]
- Jo, C.; Jung, J.; Shin, H.S.; Kim, J.; Ryoo, R. Capping with Multivalent Surfactants for Zeolite Nanocrystal Synthesis. Angew. Chem. Int. Ed. 2013, 52, 10014–10017. [Google Scholar] [CrossRef]
- Wang, H.; Pinnavaia, T.J.; Wang, H.; Pinnavaia, T.J. MFI Zeolite with Small and Uniform Intracrystal Mesopores. Angew. Chem. 2006, 118, 7765–7768. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Morales, G.; Rodríguez, J.M.; Peral, A.; Thommes, M.; Epping, J.P.; Chmelka, B.F. Molecular and Meso- and Macroscopic Properties of Hierarchical Nanocrystalline ZSM-5 Zeolite Prepared by Seed Silanization. Chem. Mater. 2009, 21, 641–654. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Rodríguez, J.M. Zeolite Beta with Hierarchical Porosity Prepared from Organofunctionalized Seeds. Microporous Mesoporous Mater. 2008, 115, 504–513. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis Strategies in the Search for Hierarchical Zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M.; Rodríguez, J.M.; Peral, A. Hierarchical Zeolites with Enhanced Textural and Catalytic Properties Synthesized from Organofunctionalized Seeds. Chem. Mater. 2006, 18, 2462–2464. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable Single-Unit-Cell Nanosheets of Zeolite MFI as Active and Long-Lived Catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhu, X.; Wannapakdee, W.; Pestman, R.; Goesten, M.G.; Gao, L.; van Hoof, A.J.F.; Hensen, E.J.M. A Dual-Templating Synthesis Strategy to Hierarchical ZSM-5 Zeolites as Efficient Catalysts for the Methanol-to-Hydrocarbons Reaction. J. Catal. 2018, 361, 135–142. [Google Scholar] [CrossRef]
- Song, G.; Chen, W.; Dang, P.; Yang, S.; Zhang, Y.; Wang, Y.; Xiao, R.; Ma, R.; Li, F. Synthesis and Characterization of Hierarchical ZSM-5 Zeolites with Outstanding Mesoporosity and Excellent Catalytic Properties. Nanoscale Res. Lett. 2018, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.P.; Aguado, J.; Escola, J.M.; Rodriguez, J.M.; Peral, A. Effect of the Organic Moiety Nature on the Synthesis of Hierarchical ZSM-5 from Silanized Protozeolitic Units. J. Mater. Chem. 2008, 18, 4210–4218. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M.; Peral, A.; Morales, G.; Abella, E. Synthesis of Hierarchical ZSM-5 by Silanization and Alkoxylation of Protozeolitic Units. Catal. Today 2011, 168, 86–95. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Zhu, X.; Gudun, K.; Mezari, B.; Shen, B.; Hensen, E.J.M. Texture, Acidity and Fluid Catalytic Cracking Performance of Hierarchical Faujasite Zeolite Prepared by an Amphiphilic Organosilane. Fuel Process. Technol. 2015, 139, 248–258. [Google Scholar] [CrossRef]
- Reiprich, B.; Tarach, K.A.; Pyra, K.; Grzybek, G.; Góra-Marek, K. High-Silica Layer-like Zeolites y from Seeding-Free Synthesis and Their Catalytic Performance in Low-Density Polyethylene Cracking. ACS Appl. Mater. Interfaces 2022, 14, 6667–6679. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the Commercial Potential of Hierarchical Zeolites: New Opportunities in Catalytic Cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Xu, W.; Dong, J.; Li, J.; Li, J.; Wu, F. A Novel Method for the Preparation of Zeolite ZSM-5. J. Chem. Soc. Chem. Commun. 1990, 755–756. [Google Scholar] [CrossRef]
- Zhou, J.; Hua, Z.; Cui, X.; Ye, Z.; Cui, F.; Shi, J. Hierarchical Mesoporous TS-1 Zeolite: A Highly Active and Extraordinarily Stable Catalyst for the Selective Oxidation of 2,3,6-Trimethylphenol. Chem. Commun. 2010, 46, 4994–4996. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.B.; Sun, L.B.; Zhuang, T.T.; Dong, X.; Chun, Y.; Zhu, J.H. Directly Transforming As-Synthesized MCM-41 to Mesoporous MFI Zeolite. J. Mater. Chem. 2008, 18, 2044–2050. [Google Scholar] [CrossRef]
- Lysenko, N.D.; Il’in, V.G.; Yaremov, P.S. Structural and Sorption Characteristics of the Products from Zeolitization of Sba-15 in the Presence of Tetraalkylammonium Hydroxides. Theor. Exp. Chem. 2011, 47, 257–263. [Google Scholar] [CrossRef]
- Möller, K.; Yilmaz, B.; Jacubinas, R.M.; Müller, U.; Bein, T. One-Step Synthesis of Hierarchical Zeolite Beta via Network Formation of Uniform Nanocrystals. J. Am. Chem. Soc. 2011, 133, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Shi, B.; Ren, J.; Liu, X.; Wang, Y.; Guo, Y.; Guo, Y.; Lu, G. Synthesis of Hierarchical MFI Zeolite Microspheres with Stacking Nanocrystals. Microporous Mesoporous Mater. 2009, 117, 104–110. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; Briones, L. Transportation Fuel Production by Combination of LDPE Thermal Cracking and Catalytic Hydroreforming. Waste Manag. 2014, 34, 2176–2184. [Google Scholar] [CrossRef]
- Jia, Y.; Han, W.; Xiong, G.; Yang, W. A Method for Diatomite Zeolitization through Steam-Assisted Crystallization with in-Situ Seeding. Mater. Lett. 2008, 62, 2400–2403. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Dong, A.; Wang, X.; Ren, N.; Gao, Z. Zeolitization of Diatomite to Prepare Hierarchical Porous Zeolite Materials through a Vapor-Phase Transport Process. J. Mater. Chem. 2002, 12, 1812–1818. [Google Scholar] [CrossRef]
- Mumtaz, F.; Irfan, M.F.; Usman, M.R. Synthesis Methods and Recent Advances in Hierarchical Zeolites: A Brief Review. J. Iran. Chem. Soc. 2021, 18, 2215–2229. [Google Scholar] [CrossRef]
- Cappello, V.; Sun, P.; Zang, G.; Kumar, S.; Hackler, R.; Delgado, H.E.; Elgowainy, A.; Delferro, M.; Krause, T. Conversion of Plastic Waste into High-Value Lubricants: Techno-Economic Analysis and Life Cycle Assessment. Green. Chem. 2022, 24, 6306–6318. [Google Scholar] [CrossRef]
- Hernández, B.; Kots, P.; Selvam, E.; Vlachos, D.G.; Ierapetritou, M.G. Techno-Economic and Life Cycle Analyses of Thermochemical Upcycling Technologies of Low-Density Polyethylene Waste. ACS Sustain. Chem. Eng. 2023, 11, 7170–7181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).