Catalytic Epoxidation Reaction
1. Introduction
- The epoxidation of gaseous molecules;
- The epoxidation of molecules in multiphase;
- The epoxidation of triglycerides;
- Enzymatic catalysis;
- Catalyst preparation and characterization
- The benefits of process intensification for epoxidation reaction;
- Kinetic modeling.
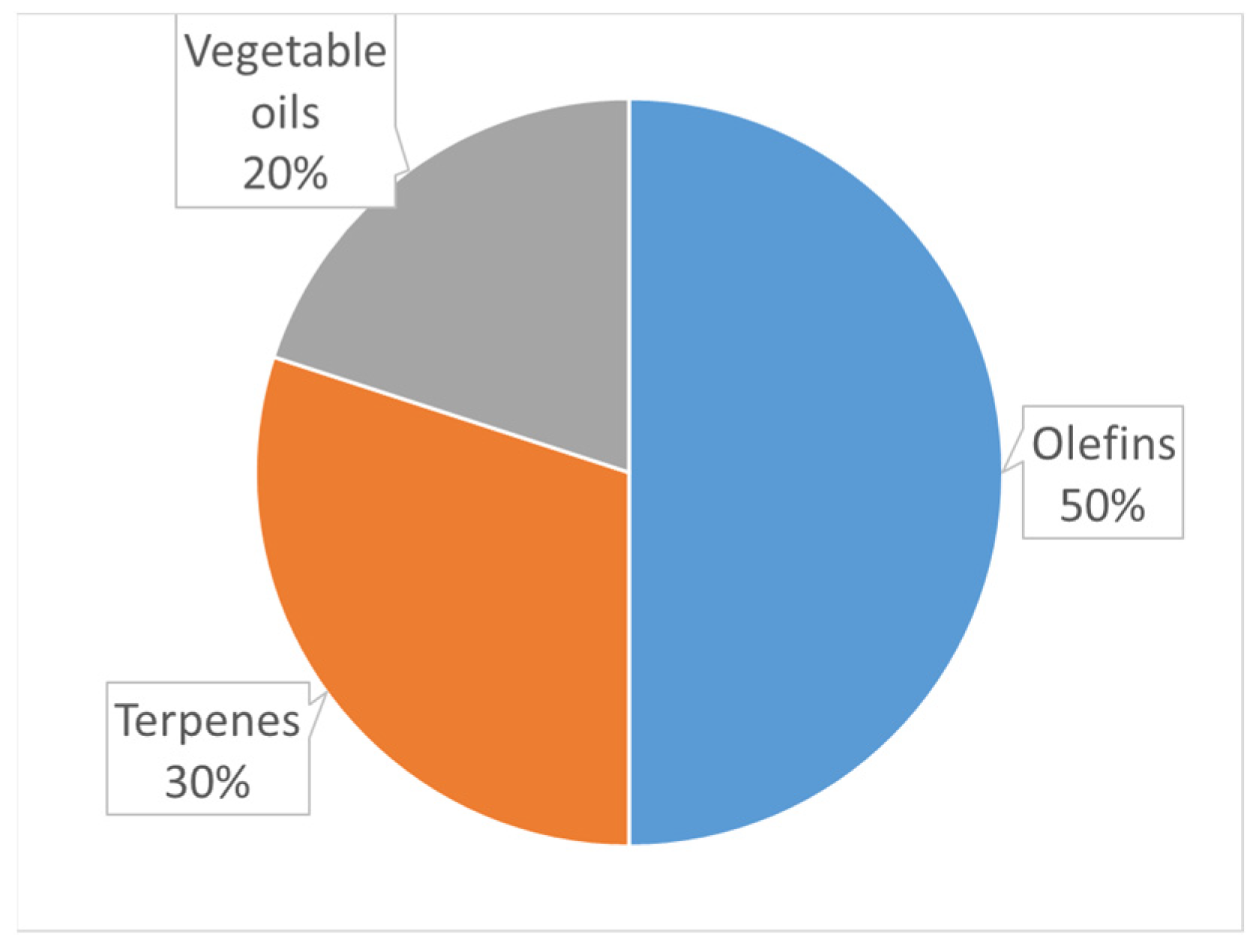
2. Epoxidation of Vegetable Oils
3. Epoxidation of Terpenes
4. Epoxidation of Olefins
5. Conclusions
Author Contributions
Conflicts of Interest
List of Contributions
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The Lord of the Chemical Rings: Catalytic Synthesis of Important Industrial Epoxide Compounds. Catalysts 2021, 11, 765. https://doi.org/10.3390/CATAL11070765.
- Ahmat, Y.M.; Madadi, S.; Charbonneau, L.; Kaliaguine, S. Epoxidation of Terpenes. Catalysts 2021, 11, 847. https://doi.org/10.3390/CATAL11070847.
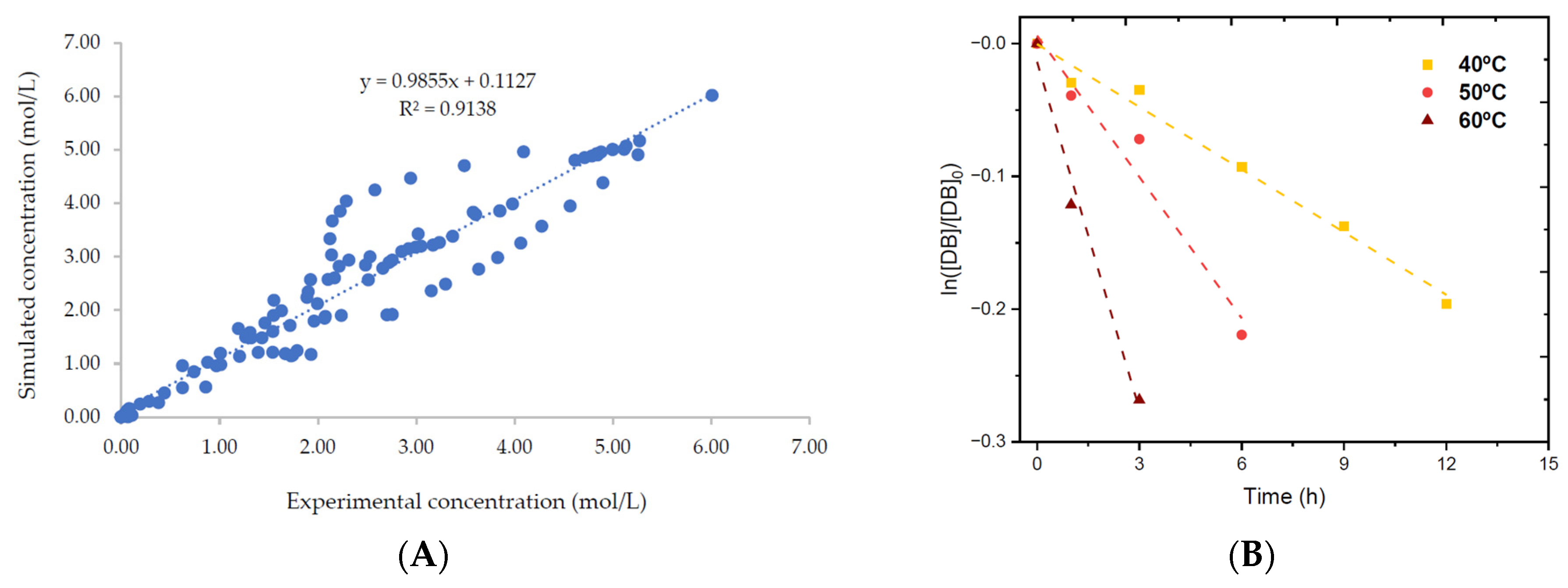
- Meng, Y.; Kebir, N.; Cai, X.; Leveneur, S. In-Depth Kinetic Modeling and Chemical Analysis for the Epoxidation of Vegetable Oils in a Liquid–Liquid–Solid System. Catalysts 2023, 13, 274. https://doi.org/10.3390/CATAL13020274.
- Catalá, J.; García-Vargas, J.M.; Ramos, M.J.; Rodríguez, J.F.; García, M.T. Kinetics of Grape Seed Oil Epoxidation in Supercritical CO2. Catalysts 2021, 11, 1490. https://doi.org/10.3390/CATAL11121490.
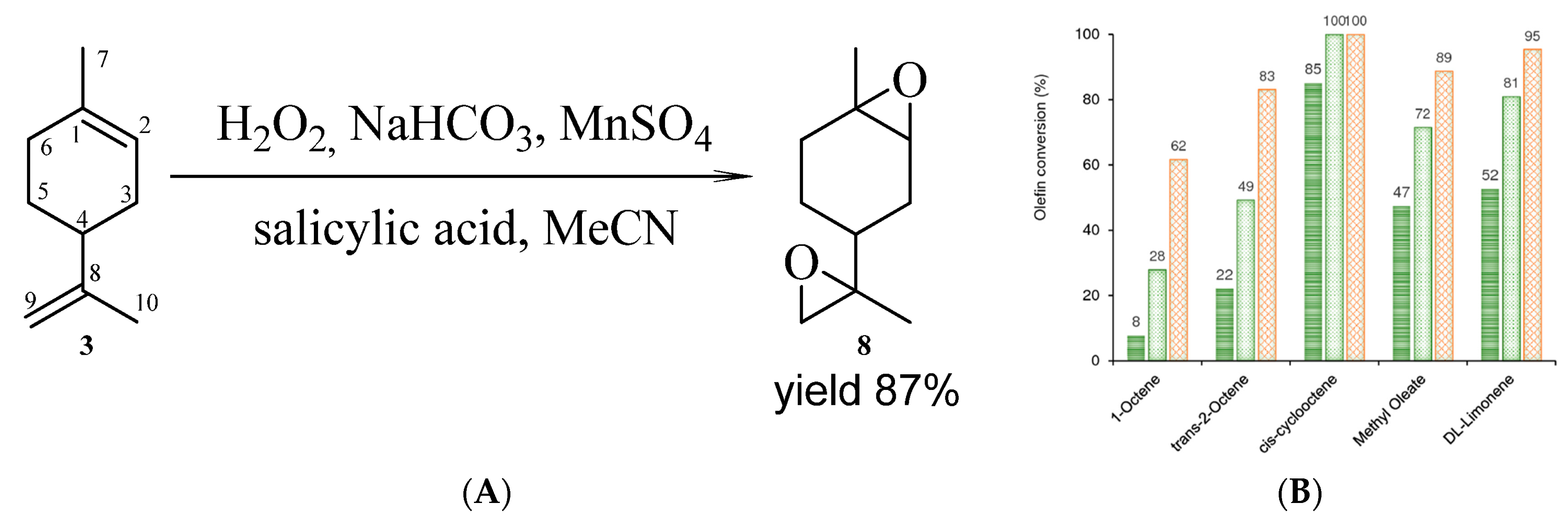
- Fomenko, V. V.; Laev, S.S.; Salakhutdinov, N.F. Catalytic Epoxidation of 3-Carene and Limonene with Aqueous Hydrogen Peroxide, and Selective Synthesis of α-Pinene Epoxide from Turpentine. Catalysts 2021, 11, 436. https://doi.org/10.3390/CATAL11040436/S1.
- Gomes, D.M.; Neves, P.; Antunes, M.M.; Fernandes, A.J.S.; Pillinger, M.; Valente, A.A. Post-Synthesis Strategies to Prepare Mesostructured and Hierarchical Silicates for Liquid Phase Catalytic Epoxidation. Catalysts 2022, 12, 1513. https://doi.org/10.3390/CATAL12121513/S1.
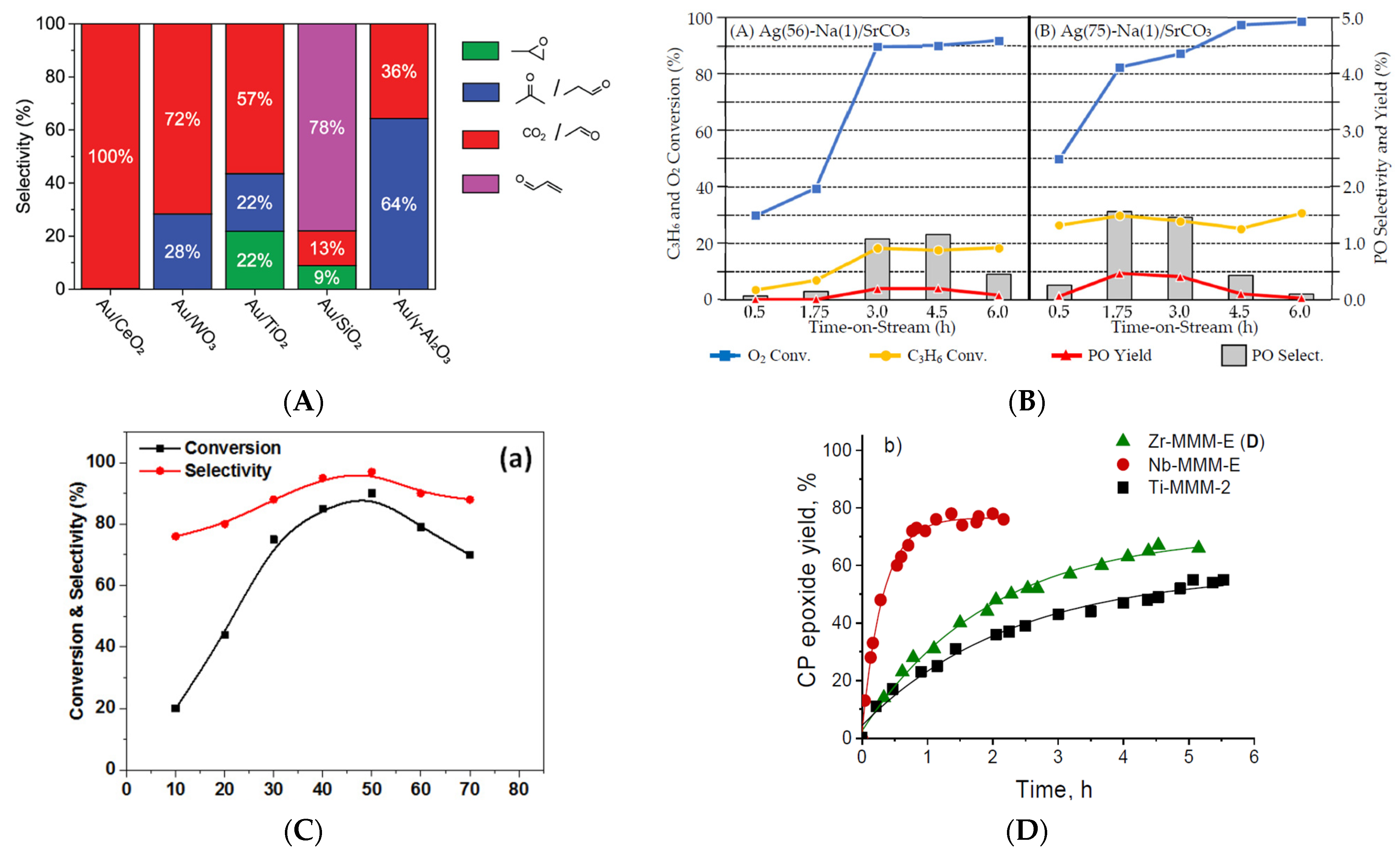
- de Boed, E.J.J.; Folmer, B.J.; Tang, M.; Donoeva, B.; de Jongh, P.E. Influence of the Support on Propene Oxidation over Gold Catalysts. Catalysts 2022, 12, 327. https://doi.org/10.3390/CATAL12030327/S1.
- Sugiyama, S.; Okitsu, I.; Hashimoto, K.; Maki, Y.; Shimoda, N.; Furube, A.; Kato, Y.; Ninomiya, W. Improvement of Propylene Epoxidation Caused by Silver Plasmon Excitation by UV-LED Irradiation on a Sodium-Modified Silver Catalyst Supported on Strontium Carbonate. Catalysts 2021, 11, 398. https://doi.org/10.3390/CATAL11030398.
- Alhumaimess, M.S. Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin. Catalysts 2022, 12, 1246. https://doi.org/10.3390/catal12101246.
- Ivanchikova, I.D.; Zalomaeva, O.V.; Maksimchuk, N.V.; Stonkus, O.A.; Glazneva, T.S.; Chesalov, Y.A.; Shmakov, A.N.; Guidotti, M.; Kholdeeva, O.A. Alkene Epoxidation and Thioether Oxidation with Hydrogen Peroxide Catalyzed by Mesoporous Zirconium-Silicates. Catalysts 2022, 12, 742. https://doi.org/10.3390/CATAL12070742.
- Freindorf, M.; Kraka, E. Mechanistic Details of the Sharpless Epoxidation of Allylic Alcohols—A Combined URVA and Local Mode Study. Catalysts 2022, 12, 789. https://doi.org/10.3390/CATAL12070789/S1.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leveneur, S.; Tolvanen, P.; Russo, V. Catalytic Epoxidation Reaction. Catalysts 2024, 14, 285. https://doi.org/10.3390/catal14050285
Leveneur S, Tolvanen P, Russo V. Catalytic Epoxidation Reaction. Catalysts. 2024; 14(5):285. https://doi.org/10.3390/catal14050285
Chicago/Turabian StyleLeveneur, Sébastien, Pasi Tolvanen, and Vincenzo Russo. 2024. "Catalytic Epoxidation Reaction" Catalysts 14, no. 5: 285. https://doi.org/10.3390/catal14050285
APA StyleLeveneur, S., Tolvanen, P., & Russo, V. (2024). Catalytic Epoxidation Reaction. Catalysts, 14(5), 285. https://doi.org/10.3390/catal14050285






