Water-Soluble Fe(III) Complex Catalyzed Coupling Aquathermolysis of Water-Heavy Oil-Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst
2.2. The Effect of Water on the Aquathermolysis
2.3. Influence of Water-Soluble Iron Complexes on Aquathermolysis of Heavy Oil
2.4. The Effect of Methanol on Hydroaquathermolysis of Heavy Oil
2.5. Elemental Analysis
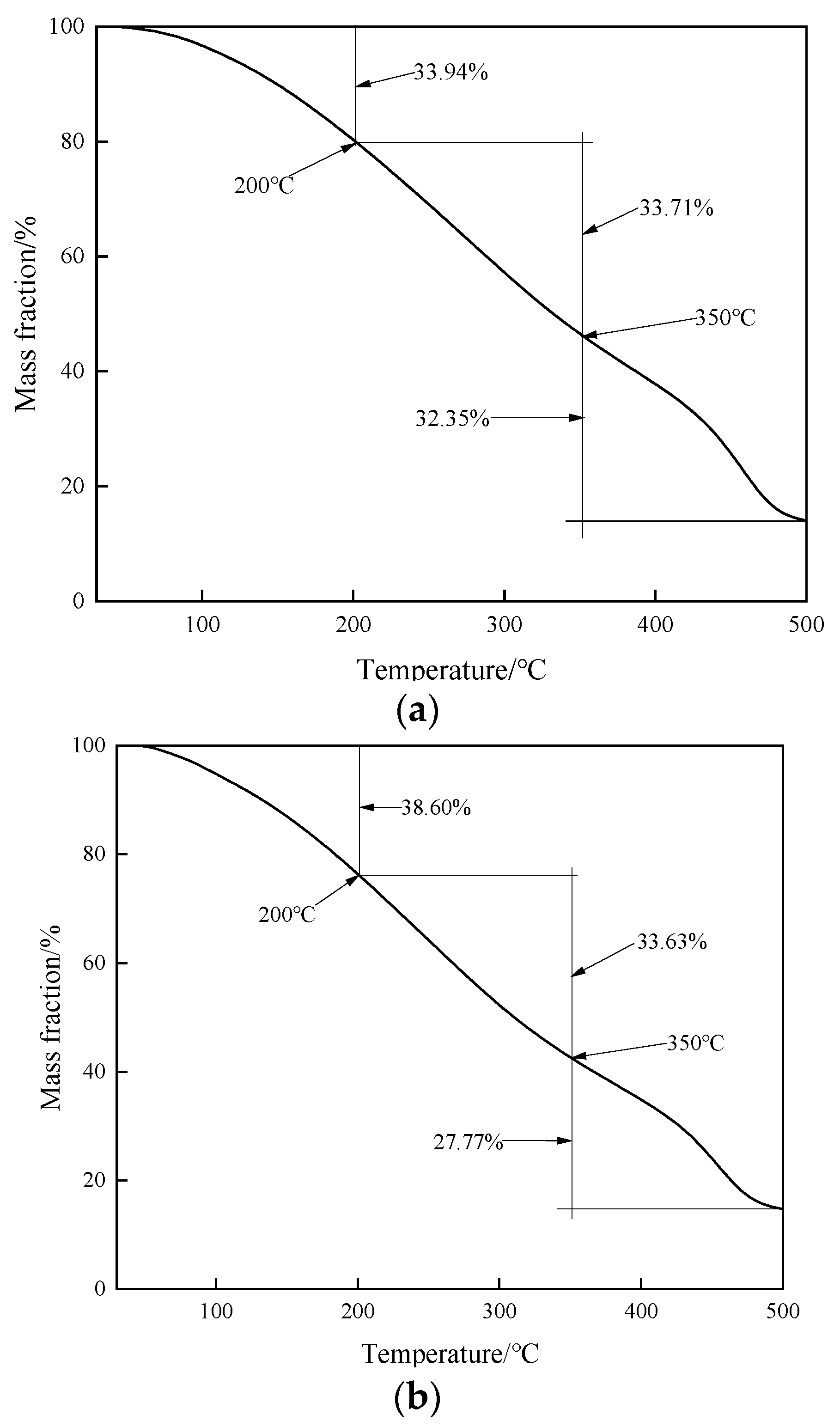
2.6. Thermogravimetric Analysis
2.7. Differential Scanning Calorimeter Analysis
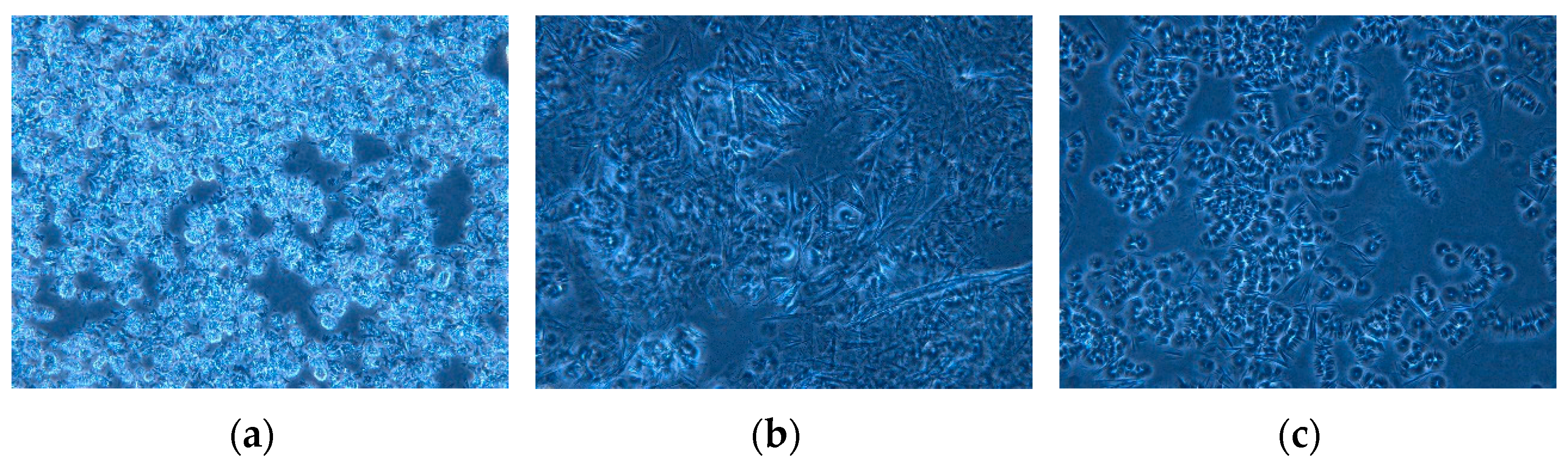
2.8. Paraffin Crystals
2.9. Mechanistic Analysis
3. Experimental
3.1. Materials
3.2. Synthesis of the Complexes
3.3. Characterization of the Complex
3.4. Catalysis of Complexes for Hydrothermal Decomposition of Heavy Oil
3.5. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaban, S.; Dessouky, S.; Badawi, A.E.F.; Sabagh, A.E.; Zahran, A.; Mousa, M. Upgrading and viscosity reduction of heavy oil by catalytic ionic liquid. Energy Fuels 2014, 28, 6545–6553. [Google Scholar] [CrossRef]
- Lv, S.Y.; Peng, S.; Zhang, R.J.; Guo, Z.; Du, W.C.; Zhang, J.; Chen, G. Viscosity reduction of heavy oil by ultrasonic. Pet. Chem. 2020, 60, 998–1002. [Google Scholar] [CrossRef]
- Eke, W.I.; Kyei, S.K.; Ajienka, J.; Akaranta, O. Effect of bio-based flow improver on the microscopic low-temperature flow properties of waxy crude oil. J. Pet. Explor. Prod. Technol. 2021, 11, 711–724. [Google Scholar] [CrossRef]
- Du, Y.N.; Huang, C.; Jiang, W.; Yan, Q.W.; Li, Y.F.; Chen, G. Preparation of surface modified nano-hydrotalcite and its applicaiton as a flow improver for crude oil. Fuel 2024, 357, 130005. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Ma, L.W.; Guo, R.; Dong, S.B.; Li, Y.F.; Slaný, M.; Chen, G. Study on synergistic catalysis of exogenous catalyst and in-situ clay in coupling aquathermolysis of water-heavy oil-ethanol at low temperature. Chem. Eng. J. 2023, 453, 139872. [Google Scholar] [CrossRef]
- Huang, S.; Cao, M.; Huang, Q.; Liu, B.; Jiang, J. Study on reaction equations of heavy oil aquathermolysis with superheated steam. Int. J. Environ. Sci. Technol. 2019, 16, 5023–5032. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.F.; Zhao, W.; Qu, K.; Ning, Y.; Zhang, J. Investigation of cyclohexanone pentaerythritol ketal as a clean flow improver for crude oil. Fuel Process. Technol. 2015, 133, 64–68. [Google Scholar] [CrossRef]
- Glagoleva, O.F.; Kapustin, V.M.; Piskunov, I.V.; Usmanov, M.R. Controlling the aggregative stability of feedstock blends and petroleum products. Pet. Chem. 2020, 60, 971–978. [Google Scholar] [CrossRef]
- Zang, Y.L.; Liu, G.B.; Ji, W.Y.; Dong, S.B.; Li, Y.F.; Chen, G. Resource utilization of waste progesterone medicines as flow improver for crude oil. J. Environ. Manag. 2024, 349, 119524. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, Q.Y.; Wang, X.D.; Yu, L.G.; Yang, J.J. Aquathermolysis of heavy crude oil with ferric oleate catalyst. Pet. Sci. 2018, 15, 613–624. [Google Scholar] [CrossRef]
- Silva, A.M.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron (III) citrate speciation in aqueous solution. Dalton Trans. 2009, 40, 8616–8625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, L.; Wan, M.; Zheng, Y. Synthesis of porous manganese oxides bars via a hydrothermal-decomposition method. Mater. Chem. Phys. 2010, 124, 831–834. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Prakash, B.J. Bronsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- Bakore, G.V.; Bhardwaj, S.D. Spectrophotometric Study of Iron (III)-Lactate Complex and its Photo-reduction. Z. Phys. Chem. 1964, 227, 26–32. [Google Scholar] [CrossRef]
- Zhao, F.J.; Liu, Y.J.; Lu, N.; Xu, T.X.; Zhu, G.M.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- Ovalles, C.; Filgueiras, E.; Morales, A.; Scott, C.E.; Gonzalez-Gimenez, F.; Embaid, B.P. Use of a dispersed iron catalyst for upgrading extra-heavy crude oil using methane as source of hydrogen. Fuel 2003, 82, 887–892. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Dong, S.B.; Zhang, X.L.; Zhang, J.; Chen, G. Synthesis of multi-alkylpolyamine and performance in crude oil as flow improver. Tenside Surfactants Deterg. 2022, 59, 104–110. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.L.; Liu, H.C.; Wang, P.J.; Liu, F. Influences on the aquathermolysis of heavy oil catalyzed by two different catalytic ions: Cu2+ and Fe3+. Energy Fuels 2013, 27, 2555–2562. [Google Scholar] [CrossRef]
- Zang, Y.l.; Liu, H.Z.; Chen, D.; Zhang, S.; Li, S.J.; Chen, G. Synergistic catalysis of reservoir minerals and exogenous catalysts on aquathermolysis of heavy oil. Processes 2023, 11, 2635. [Google Scholar] [CrossRef]
- Boulet, P.; Greenwell, H.C.; Stackhouse, S.; Coveney, P.V. Recent advances in understanding the structure and reactivity of clays using electronic structure calculations. J. Mol. Struct. THEOCHEM 2005, 762, 33–48. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Zhang, W.Y.; Yu, T.; Li, Y.F.; Struhárová, A.; Matejdes, M.; Slaný, M.; Chen, G. The effect of sodium bentonite in the thermo-catalytic reduction of viscosity of heavy oils. Molecules 2023, 28, 2651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; He, J.; Wang, Y.Q.; Li, P. GC-MS used in study on the mechanism of the viscosity reduction of heavy oil through aquathermolysis catalyzed by aromatic sulfonic H3PMo12O40. Energy 2010, 35, 3454–3460. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.H.; Wu, Y.; Zhang, J.; Song, H.; Jeje, A.; Song, S.F.; Qu, C.T. Catalytic aquathermolysis of heavy oil by coordination complex at relatively low temperature. Pet. Chem. 2017, 57, 881–884. [Google Scholar] [CrossRef]
- Zhang, F.; Ouyang, J.; Feng, X.; Zhang, H.; Xu, L. Paraffin deposition mechanism and paraffin inhibition technology for high-carbon paraffin crude oil from the Kazakhstan PK Oilfield. Pet. Sci. Technol. 2014, 32, 488–496. [Google Scholar] [CrossRef]
- Ma, L.W.; Zhang, S.; Zhang, X.L.; Dong, S.B.; Yu, T.; Slaný, M.; Chen, G. Enhanced aquathermolysis of heavy oil catalyzed by bentonite supported Fe(III) complex in the present of ethanol. J. Chem. Technol. Biotechnol. 2022, 97, 1128–1137. [Google Scholar] [CrossRef]
- ASTM D97-2012; Standard Test Method for Pour Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2012.
- Semikhina, L.P.; Nelyubov, D.V.; Vazhenin, D.A. Effect of the structure of pour-point depressant additives on the deposition of solid petroleum hydrocarbons. Pet. Chem. 2015, 55, 575–577. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Slaný, M.; Kuzielová, E.; Zhang, W.Y.; Ma, L.W.; Dong, S.B.; Zhang, J.; Chen, G. Influence of reservoir minerals and ethanol on catalytic aquathermolysis of heavy oil. Fuel 2022, 307, 121871. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- SY/T 5119-2016; Ananlysis Mothed for Family Composition of Rock Extracts and Crude Oil. China National Petroleum Corporation: Beijing, China, 2016.
- Hazrati, N.; Abdouss, M.; Miran-Beigi, A.A.; Pasban, A.A. Long chain alkylated ionic liquids as pour point depressant and rheology improver for crude oil. Pet. Chem. 2021, 61, 206–213. [Google Scholar] [CrossRef]
- SY/T 0545-2012; Determination of Thermal Property Parameters of the Wax Precipitation in Crude Oil. Test Method by Differential Scanning Calorimetry. China National Petroleum Corporation: Beijing, China, 2012.
- Behbahani, T.J.; Miranbeigi, A.A.; Sharifi, K. A new experimental investigation on upgrading of waxy crude oils by methacrylate polymers. Pet. Chem. 2017, 57, 874–880. [Google Scholar] [CrossRef]
- Hu, J.Q.; Gan, J.H.; Li, J.P.; Luo, Y.; Wang, G.K.; Wu, L.; Gong, Y.M. Extraction of crude oil from petrochemical sludge: Characterization of products using thermogravimetric analysis. Fuel 2017, 188, 166–172. [Google Scholar] [CrossRef]











| Oil Sample | Element Content, % | ||||
|---|---|---|---|---|---|
| C | H | N | S | Other | |
| Blank | 79.83 | 11.10 | 1.75 | 2.50 | 4.82 |
| After reaction with water | 81.12 | 11.21 | 1.70 | 2.30 | 3.67 |
| After reaction with Fe−1 | 84.21 | 11.19 | 1.63 | 1.86 | 1.11 |
| After reaction with Fe−1 and MeOH | 84.72 | 11.70 | 1.42 | 1.43 | 0.73 |
| Pour Point, °C | Viscosity, mPa·s (15 °C) | Saturates, % | Aromatics, % | Resins, % | Asphaltenes, % |
|---|---|---|---|---|---|
| 14.4 | 1,174,500 | 42.13 | 19.70 | 21.83 | 13.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, S.; Feng, J.; Long, X.; Hu, T.; Chen, G. Water-Soluble Fe(III) Complex Catalyzed Coupling Aquathermolysis of Water-Heavy Oil-Methanol. Catalysts 2024, 14, 353. https://doi.org/10.3390/catal14060353
Chen S, Zhang S, Feng J, Long X, Hu T, Chen G. Water-Soluble Fe(III) Complex Catalyzed Coupling Aquathermolysis of Water-Heavy Oil-Methanol. Catalysts. 2024; 14(6):353. https://doi.org/10.3390/catal14060353
Chicago/Turabian StyleChen, Shijun, Shu Zhang, Jinchao Feng, Xiaolong Long, Tianbao Hu, and Gang Chen. 2024. "Water-Soluble Fe(III) Complex Catalyzed Coupling Aquathermolysis of Water-Heavy Oil-Methanol" Catalysts 14, no. 6: 353. https://doi.org/10.3390/catal14060353





