Nano-Magnetic Sugarcane Bagasse Cellulosic Composite as a Sustainable Photocatalyst for Textile Industrial Effluent Remediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cellulose Extraction from Sugarcane Bagasse Using Pretreatment Method
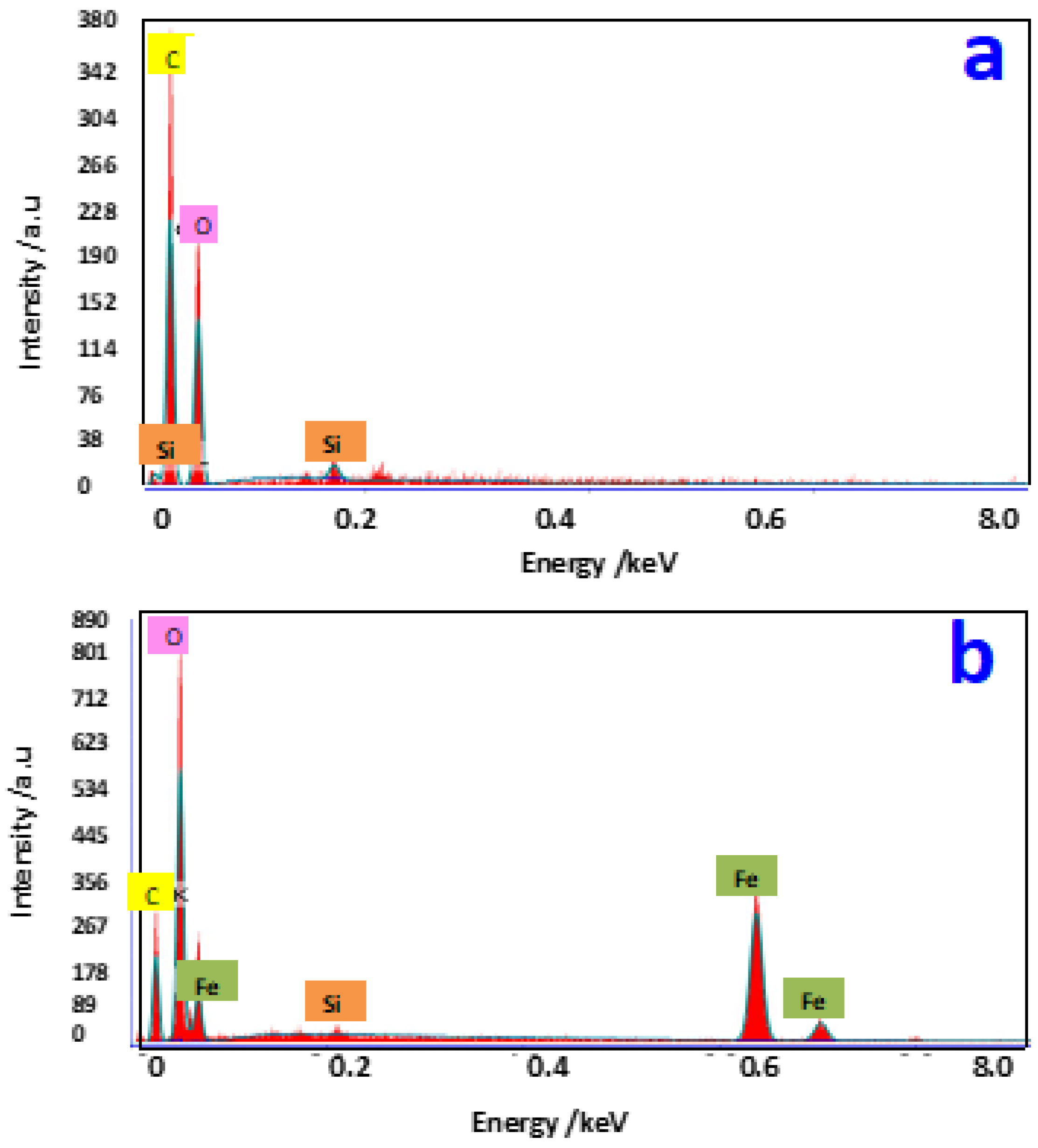
2.2. Structural and Morphological Characterization
2.3. Optical and Magnetic Properties
2.4. Photocatalytic Activity Comparison and Reaction Time Performances
2.5. Effect of Dye Loading
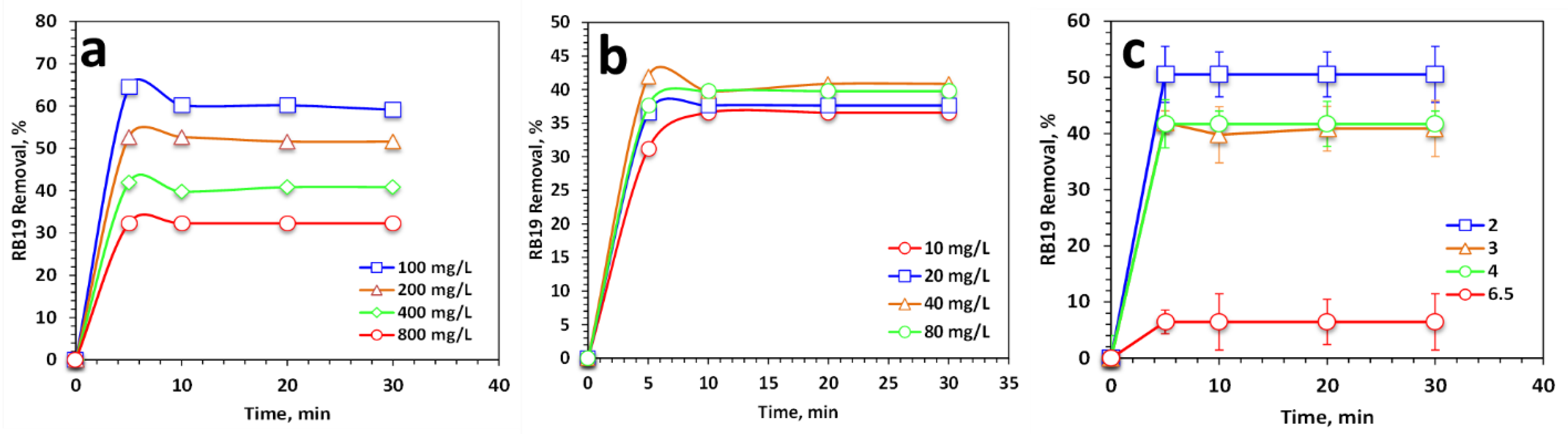
2.6. Multiple Operating Parameters Effect
2.7. Box/Behnken Model Design and Optimization
2.8. Comparison of Various Sugarcane Bagasse-Based Treatment Systems
2.9. Temperature Influence on Kinetic Parameters and Thermodynamic Behavior
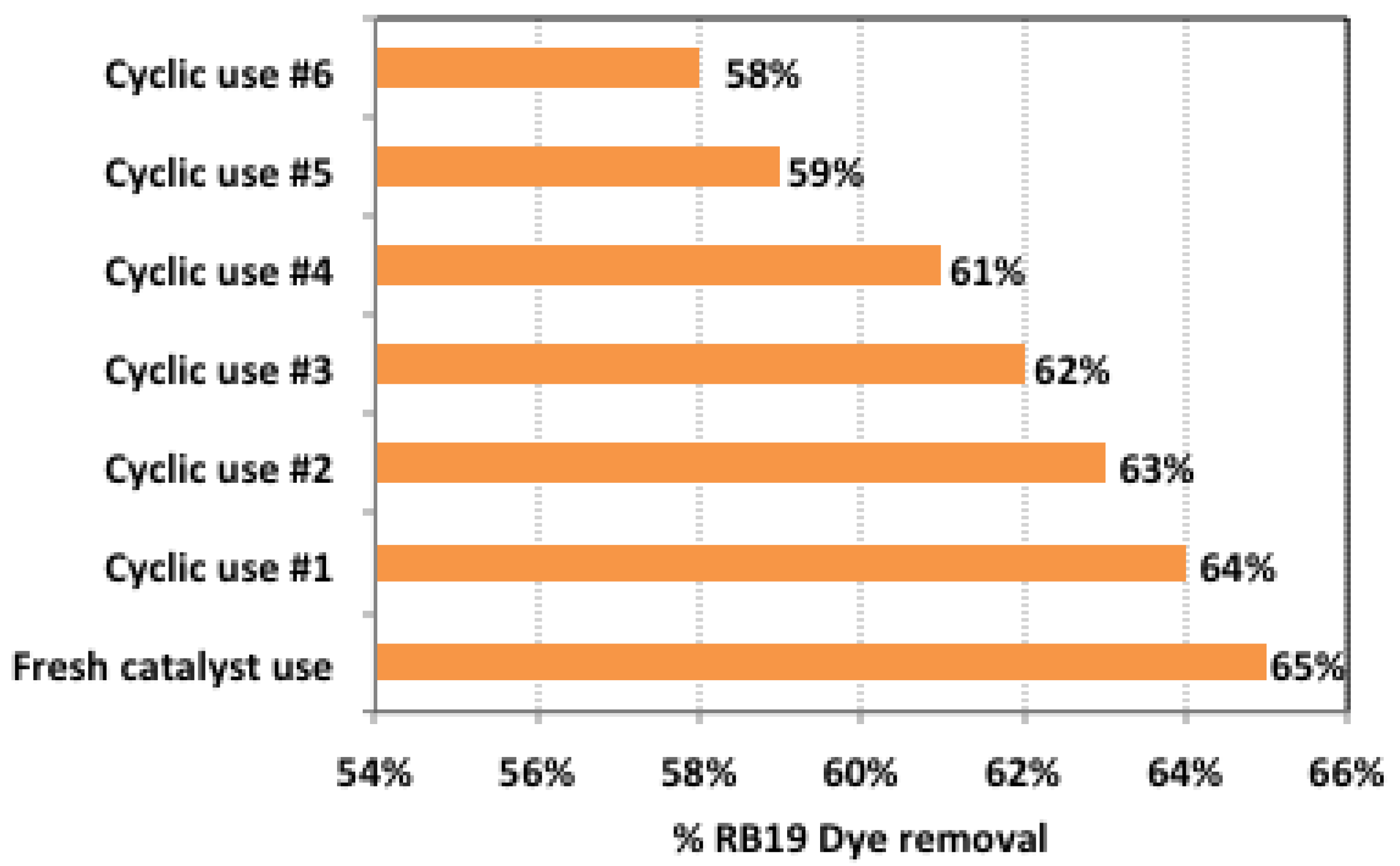
2.10. Recyclability
3. Materials and Methods
3.1. Materials
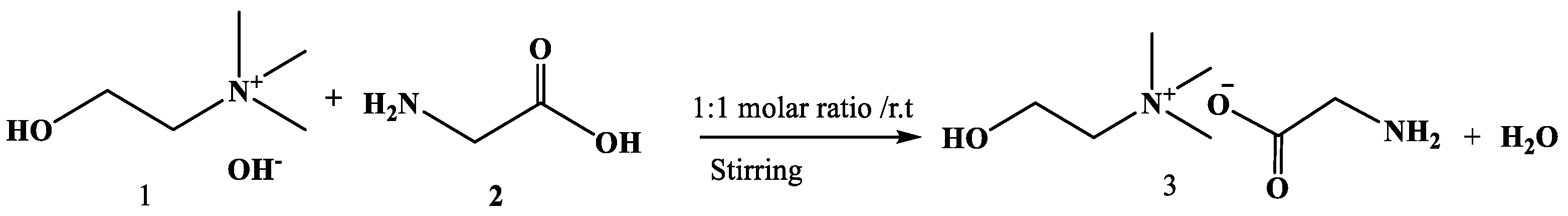
3.2. Synthesis of Cholinium/Glycinate Ionic Liquid [Ch][Gly]
3.3. Synthesis of Nanocomposite
3.4. Photocatalytic Oxidation Set-Up
3.5. Data Analysis and Analytical Procedures
3.6. Statistical Design for Modeling the Multiple Operating Parameters Effect
3.7. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mondal, M.I.H.; Haque, M.O.; Ahmed, F.; Pervez, M.N.; Naddeo, V.; Ahmed, M.B. Super-Adsorptive Biodegradable Hydrogel from Simply Treated Sugarcane Bagasse. Gels 2022, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Ahmed, H.B.; Bechtold, T. In-situ deposition of Cu2O micro-needles for biologically active textiles and their release properties. Carbohydr. Polym. 2017, 165, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.A.; Anannya, F.R. Sugarcane bagasse—A source of cellulosic fiber for diverse applications. Heliyon 2021, 7, e07771. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manag. 2021, 283, 111987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Oyekunle, I.P.; Oladunjoye, I.O.; Ibitogbe, E.M.; Olorunfemi, T.S. A Review on the Mitigation of Heavy Metals from Aqueous Solution using Sugarcane Bagasse. Sugar Tech 2022, 24, 1167–1185. [Google Scholar] [CrossRef]
- Ali, N.; El-Harbawi, M.; Abo Jabal, A.; Yin, C.-Y. Characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: Oil removal suitability matrix. Environ. Technol. 2012, 33, 481–486. [Google Scholar] [CrossRef]
- Tajernia, H.; Ebadi, T.; Nasernejad, B.; Ghafori, M. Arsenic Removal from Water by Sugarcane Bagasse: An Application of Response Surface Methodology (RSM). Water Air Soil Pollut. 2014, 225, 2028. [Google Scholar] [CrossRef]
- Ullah, S.F.; Rong, A.; Nawshad, M. Editorial: Properties and Applications of Ionic Liquids in Energy and Environmental Science. Front. Chem. 2020, 8, 627213. [Google Scholar] [CrossRef] [PubMed]
- Gadilohar, B.L.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Tzani, A.; Karadendrou, M.-A.; Kalafateli, S.; Kakokefalou, V.; Detsi, A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals 2022, 12, 1776. [Google Scholar] [CrossRef]
- Gusrianto, P.; Zulharmita, H.R.; Rivai, H. Preparasi dan Karakterisasi Mikrokristalin Selulosa dari Limbah Serbuk Kayu Penggergajian. J. Sains dan Teknol. Farmasi 2011, 16, 180–188. [Google Scholar]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high—Value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Tony, M.A. An industrial ecology approach: Green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int. J. Environ. Anal. Chem. 2021, 101, 167–183. [Google Scholar] [CrossRef]
- Unal, B.O.; Bilici, Z.; Ugur, N.; Isik, Z.; Harputlu, E.; Dizge, N.; Ocakoglu, K. Adsorption and Fenton oxidation of azo dyes by magnetite nanoparticles deposited on a glass substrate. J. Water Process. Eng. 2019, 32, 11. [Google Scholar]
- Li, H.; Liu, L.; Cui, J.; Cui, J.; Wang, F.; Zhang, F. High-efficiency adsorption and regeneration of methylene blue and aniline onto activated carbon from waste edible fungus residue and its possible mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Soliman, E.M. Silica coated magnetic particles using microwave synthesis for removal of dyes from natural water samples: Synthesis, characterization, equilibrium, isotherm and kinetics studies. Appl. Surf. Sci. 2013, 284, 23–32. [Google Scholar] [CrossRef]
- Chen, R.; Pignatello, J.J. Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environ. Sci. Technol. 1997, 31, 2399–2406. [Google Scholar] [CrossRef]
- Thabet, R.H.; Tony, M.A.; El Sherbiny, S.A.; Ali, I.A.; Fouad, M.K. Catalytic oxidation over nanostructured heterogeneous process as an effective tool for environmental remediation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 975, 012004. [Google Scholar] [CrossRef]
- Nassar, H.N.; El-azab, W.I.M.; El-Gendy, N.S. Sustainable ecofriendly recruitment of bioethanol fermentation lignocellulosic spent waste biomass for the safe reuse and discharge of petroleum production produced water via biosorption and solid biofuel production. J. Hazard. Mater. 2022, 422, 126845. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, N.S.; Nassar, H.N. Study on the effectiveness of spent waste sugarcane bagasse for adsorption of different petroleum hydrocarbons water pollutants: Kinetic and equilibrium isotherm. Desalin. Water Treat. 2016, 57, 5514–5528. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Easwaran, S.N.; Mohanakrishnan, A.S.; Kalyanaraman, C.; Mahadevan, S. Biodegradation of tannery dye effluent using Fenton’s reagent and bacterial consortium: A biocalorimetric investigation. J. Environ. Manag. 2019, 242, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Maha, A.; Tony, M.M.E. End-of-life waste criteria: Synthesis and utilization of Mn-Zn ferrite nanoparticles as a super paramagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2021, 12, 21. [Google Scholar]
- Fayoud, N.; Tahiri, S.; Younssi, S.A.; Albizane, A.; Gallart-Mateu, D.; Cervera, M.L.; De la Guardia, M. Kinetic, isotherm and thermodynamic studies of the adsorption of methylene blue dye onto agro-based cellulosic materials. Desalin. Water Treat. 2016, 57, 16611–16625. [Google Scholar] [CrossRef]
- El-Geundi, M.S.; Nassar, M.M.; Farrag, T.E.; Ahmed, M.H. Methomyl adsorption onto Cotton Stalks Activated Carbon (CSAC): Equilibrium and process design. Procedia Environ. Sci. 2013, 17, 630–639. [Google Scholar] [CrossRef]
- El Naeem, G.A.; Abd-Elhamid, A.I.; Farahat, O.O.; El-Bardan, A.A.; Soliman, H.M.; Nayl, A.A. Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: Characterization, kinetic and isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar]
- Buthiyappan, A.; Jayaprina, G.; Abdul Aziz, A.R. Synthesis of iron oxides impregnated green adsorbent from sugarcane bagasse: Characterization and evaluation of adsorption efficiency. J. Environ. Manag. 2019, 249, 109323. [Google Scholar] [CrossRef] [PubMed]
- Dehvari, M.; Behzad, J.; Sahand, J.; Sudabeh, P.; Zahra, S. Cadmium removal from aqueous solution using cellulose nanofibers obtained from waste sugarcane bagasse (SCB): Isotherm, kinetic, and thermodynamic studies. Desalin. Water Treat. 2021, 221, 218–228. [Google Scholar] [CrossRef]
- Praipipat, P.; Pimploy, N.; Amornrat, S. Modification of sugarcane bagasse with iron (III) oxide-hydroxide to improve its adsorption property for removing lead (II) ions. Sci. Rep. 2023, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Moghaddam, L.; O’Hara, I.M.; Doherty, W.O.S. Congo Red adsorption by ball-milled sugarcane bagasse. Chem. Eng. J. 2011, 178, 122–128. [Google Scholar] [CrossRef]
- Parker, H.L.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Use of starbon for the adsorption and desorption of phenols. ACS Sustain. Chem. Eng. 2013, 1, 1311–1318. [Google Scholar] [CrossRef]
- Al, M.F.; Moayyad, S.; Ahmad, S.; Mohammad, A.S. Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds. J. Environ. Sci. 2008, 20, 675–682. [Google Scholar]
- Pintor, A.M.A.; Vilar, V.J.P.; Boaventura, R.A.R. Decontamination of cork wastewaters by solar-photo-Fenton process using cork bleaching wastewater as H2O2 source. Sol. Energy 2011, 85, 579–587. [Google Scholar] [CrossRef]
- Ahmadi, M.; Behin, J.; Mahnam, A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of Reactive Yellow 84. J. Saudi Chem. Soc. 2016, 20, 644–650. [Google Scholar] [CrossRef]
- Pourali, P.; Behzad, M.; Arfaeinia, H.; Ahmadfazeli, A.; Afshin, S.; Poureshgh, Y.; Rashtbari, Y. Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep. Sci. Technol. 2021, 56, 3079–3091. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, S.P.; Fan, M.H.; Guo, H.Q.; Qiao, L.P.; Sun, R.X. A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J. Hazard. Mater. 2007, 148, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Tony, M.A.; Lin, L.-S. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int. J. Environ. Res. 2021, 15, 191–201. [Google Scholar] [CrossRef]
- Tony, M.A. Central composite design optimization of Bismarck Dye oxidation from textile effluent with Fenton’s reagent. Appl. Water Sci. 2020, 10, 108. [Google Scholar] [CrossRef]
- Pinheiro, D.R.; Neves, R.d.F.; Paz, S.P.A. A sequential Box-Behnken Design (BBD) and Response Surface Methodology (RSM) to optimize SAPO-34 synthesis from kaolin waste. Microporous Mesoporous Mater. 2021, 323, 111250. [Google Scholar] [CrossRef]







| Experimental Parameter | Representation | Codified Levels | |||
|---|---|---|---|---|---|
| Minimum | Medium | Maximum | |||
| Un-Coded | Coded | −1 | 0 | 1 | |
| H2O2 (mg/L) | 50 | 100 | 150 | ||
| SCB: M Catalyst (mg/L) | 30 | 40 | 50 | ||
| pH | 2.0 | 2.5 | 3.0 | ||
| Case No. | Coded Values | Un-Coded Values | Response (%, Removal) | |||||
|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||||
| 1 | −1 | −1 | 0 | 50 | 40 | 2.5 | 80.05 | 73.88 |
| 2 | −1 | 1 | 0 | 50 | 40 | 2.5 | 81.48 | 72.59 |
| 3 | 1 | −1 | 0 | 150 | 40 | 2.5 | 50.92 | 59.80 |
| 4 | 1 | 1 | 0 | 150 | 40 | 2.5 | 45.37 | 51.53 |
| 5 | 0 | −1 | −1 | 100 | 30 | 2.0 | 56.07 | 48.87 |
| 6 | 0 | −1 | 1 | 100 | 30 | 3.0 | 42.06 | 46.53 |
| 7 | 0 | 1 | −1 | 100 | 50 | 2.0 | 41.48 | 37.18 |
| 8 | 0 | 1 | 1 | 100 | 50 | 3.0 | 41.66 | 48.67 |
| 9 | −1 | 0 | −1 | 50 | 30 | 2.0 | 43.33 | 46.21 |
| 10 | 1 | 0 | −1 | 150 | 30 | 2.0 | 36.29 | 24.12 |
| 11 | −1 | 0 | 1 | 50 | 40 | 3.0 | 34.11 | 46.27 |
| 12 | 1 | 0 | 1 | 150 | 40 | 3.0 | 36.11 | 33.22 |
| 13 | 0 | 0 | 0 | 100 | 40 | 2.5 | 86.21 | 86.19 |
| 14 | 0 | 0 | 0 | 100 | 40 | 2.5 | 86.23 | 86.19 |
| 15 | 0 | 0 | 0 | 100 | 40 | 2.5 | 86.15 | 86.19 |
| Source | DF * | SS * | MS * | F-Value * | p-Value * |
|---|---|---|---|---|---|
| Model (Y) | |||||
| Regression | 9 | 10.58333 | 1.175926 | 7.839506 | 0.017729 |
| Linear | 3 | 730.51292 | 730.512920 | 7.767593 | 1.003254 |
| Quadratic | 3 | 841.32800 | 841.328000 | 8.945898 | 1.426933 |
| Cross Product | 3 | 4480.80562 | 4480.805620 | 47.644713 | 0.725736 |
| Error | 5 | 470.23120 | 94.046240 | ||
| Total | 14 | 6132.77700 | |||
| R2 | 93.00% | ||||
| Adj-R2 | 89.00% | ||||
| Treatment System | Aqueous Stream Pollutant | Operating Conditions | Removal % | Ref. | |||
|---|---|---|---|---|---|---|---|
| Catalyst | pH | Temp. °C | Time min | ||||
| Oxid./Ads. | Reactive blue 19 dye | SCB:M (3:1) (39 mg/L) | 2.5 | 30 °C | 5 | 92.5 | This work |
| Ads. | Methylene blue | TSCB | 7 | 25 °C | 30 | 96 | [29] |
| Ads. | Basic Crystal violet dye | TSCB | 7 | 25 °C | 30 | 96 | [29] |
| Ads. | Methylene blue | ISCB | 8.4 | 25 °C | 360 | 94 | [30] |
| Ads. | Cadmium | (CNF)-ISCB | 5 | 30 °C | 70 | 99 | [31] |
| Ads. | Pb(II) | (SBFB) | 5 | 25 °C | 120 | 99 | [32] |
| Kinetic Model | Linearized Eq. | Parameters | Values | |||
|---|---|---|---|---|---|---|
| T, °C | ||||||
| 30 °C | 40 °C | 50 °C | 60 °C | |||
| Zero-order | KZ (min−1) | 0.1805 | 0.1257 | 0.046 | 0.0134 | |
| t0.5 | 28.85 | 41.42 | 113.22 | 388.65 | ||
| r2 | 0.49 | 0.52 | 0.53 | 0.88 | ||
| First-order | KF (min−1) | 0.0227 | 0.0154 | 0.0047 | 0.0013 | |
| t0.5 (min) | 30.53 | 48.13 | 147.45 | 533.08 | ||
| r2 | 0.55 | 0.62 | 0.58 | 0.96 | ||
| Second-order | KS (L mg−1 min−1) | 0.0069 | 0.0037 | 0.0011 | 0.0002 | |
| t0.5 (min) | 13.91 | 25.95 | 87.28 | 480.03 | ||
| r2 | 0.8 | 0.8 | 0.82 | 0.99 |
| Thermodynamics Variables | T, °C | |||
|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | |
| Ea (kJmol−1) | 98.66 | |||
| ∆G° (kJmol−1) | 86.78 | 91.35 | 97.61 | 105.44 |
| ∆H° (kJmol−1) | 96.14 | 96.05 | 95.97 | 95.89 |
| ∆S° (Jmol−1K−1) | 30.88 | 15.03 | −5.07 | −28.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tony, M.A.; El-Gendy, N.S.; Hussien, M.; Ahmed, A.A.S.; Xin, J.; Lu, X.; El-Sayed, I.E.T. Nano-Magnetic Sugarcane Bagasse Cellulosic Composite as a Sustainable Photocatalyst for Textile Industrial Effluent Remediation. Catalysts 2024, 14, 354. https://doi.org/10.3390/catal14060354
Tony MA, El-Gendy NS, Hussien M, Ahmed AAS, Xin J, Lu X, El-Sayed IET. Nano-Magnetic Sugarcane Bagasse Cellulosic Composite as a Sustainable Photocatalyst for Textile Industrial Effluent Remediation. Catalysts. 2024; 14(6):354. https://doi.org/10.3390/catal14060354
Chicago/Turabian StyleTony, Maha A., Nour Sh. El-Gendy, Mohamed Hussien, Abdullah A. S. Ahmed, Jiayu Xin, Xingmei Lu, and Ibrahim El Tantawy El-Sayed. 2024. "Nano-Magnetic Sugarcane Bagasse Cellulosic Composite as a Sustainable Photocatalyst for Textile Industrial Effluent Remediation" Catalysts 14, no. 6: 354. https://doi.org/10.3390/catal14060354







