Rational Design of Z-Scheme Heterostructures Composed of Bi/Fe-Based MOFs for the Efficient Photocatalytic Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Results and Discussion
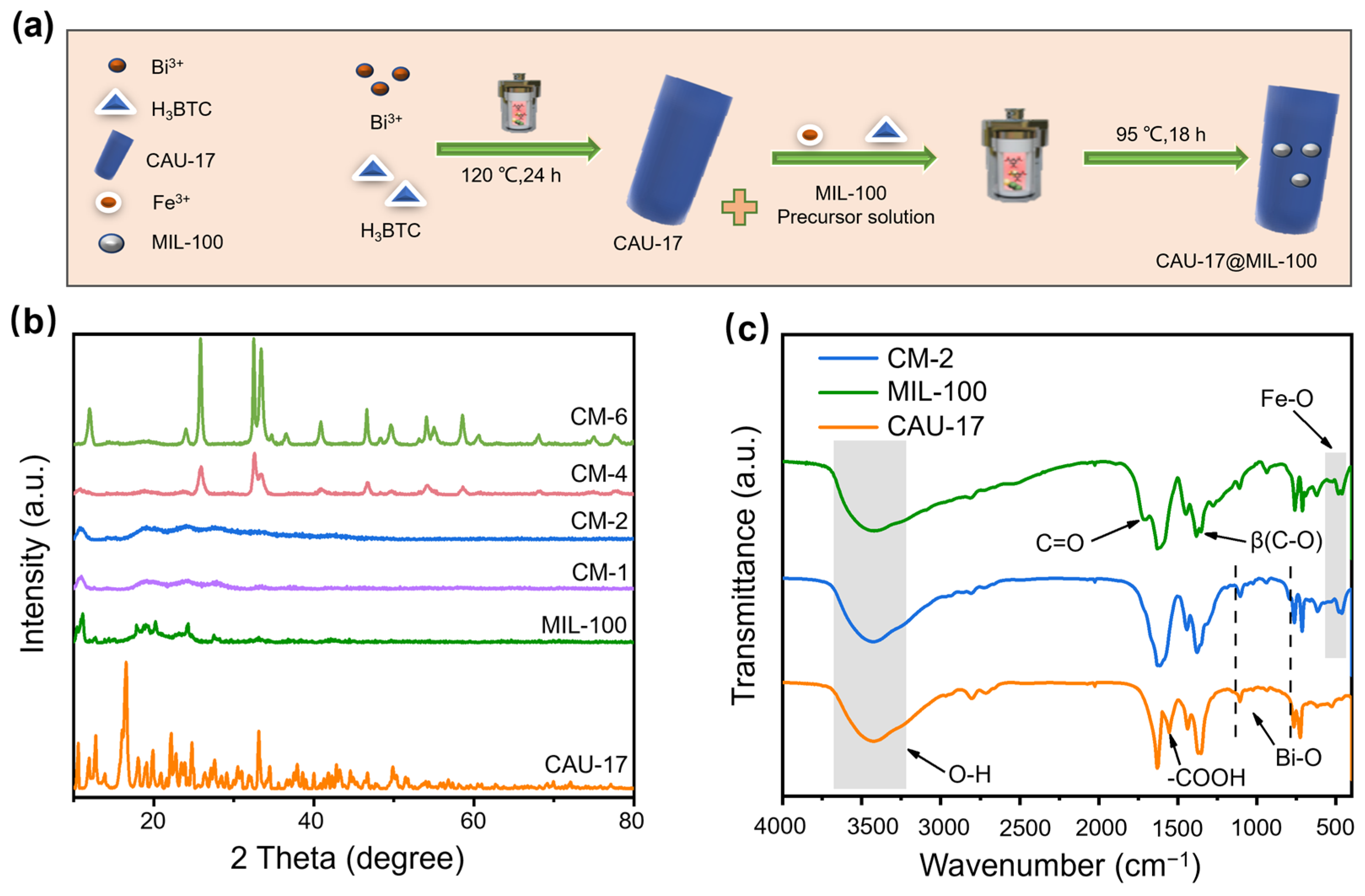
2.1. Characterization of Structure and Morphology
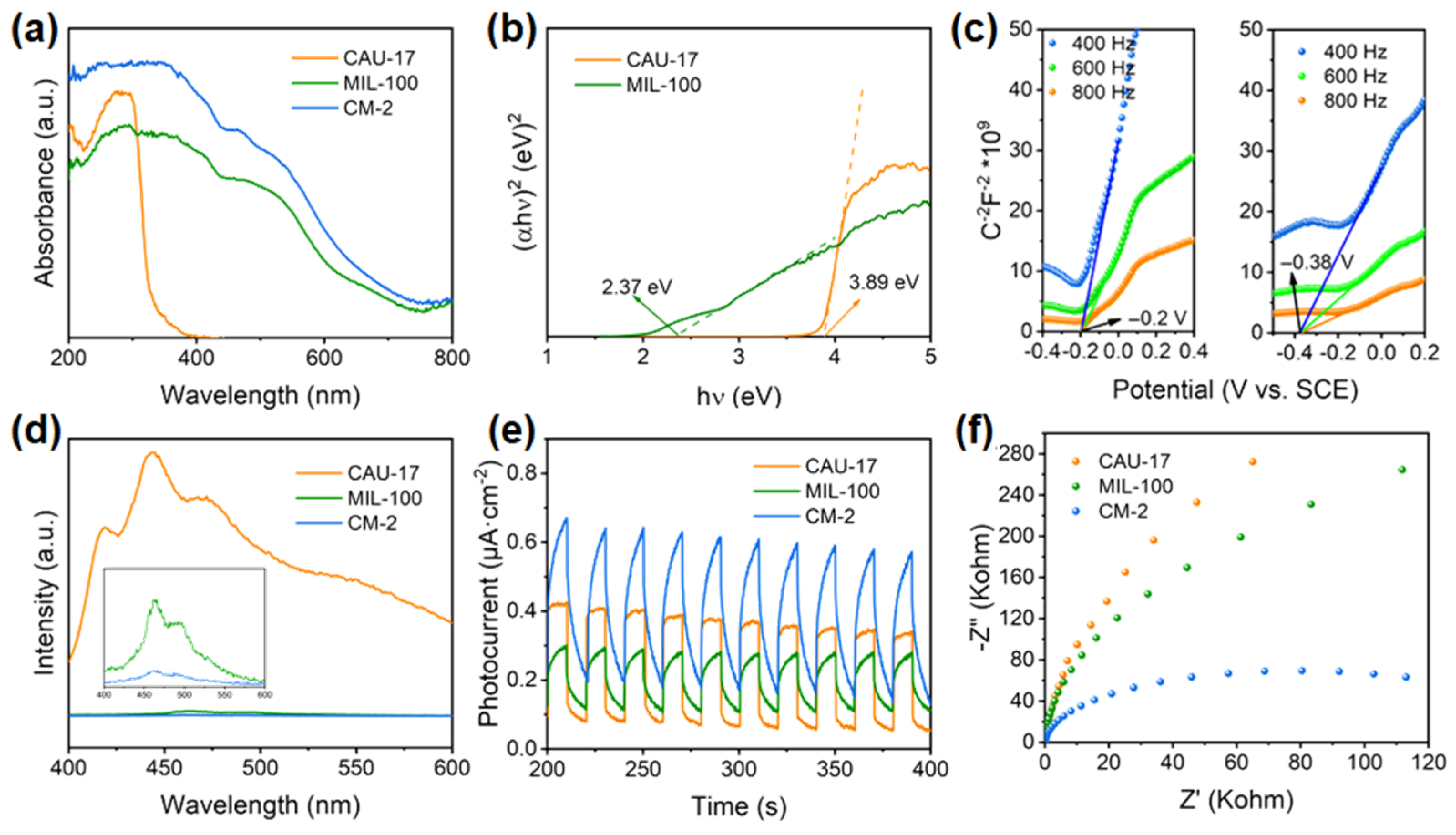
2.2. Optical and Electrochemical Properties of Photocatalysts
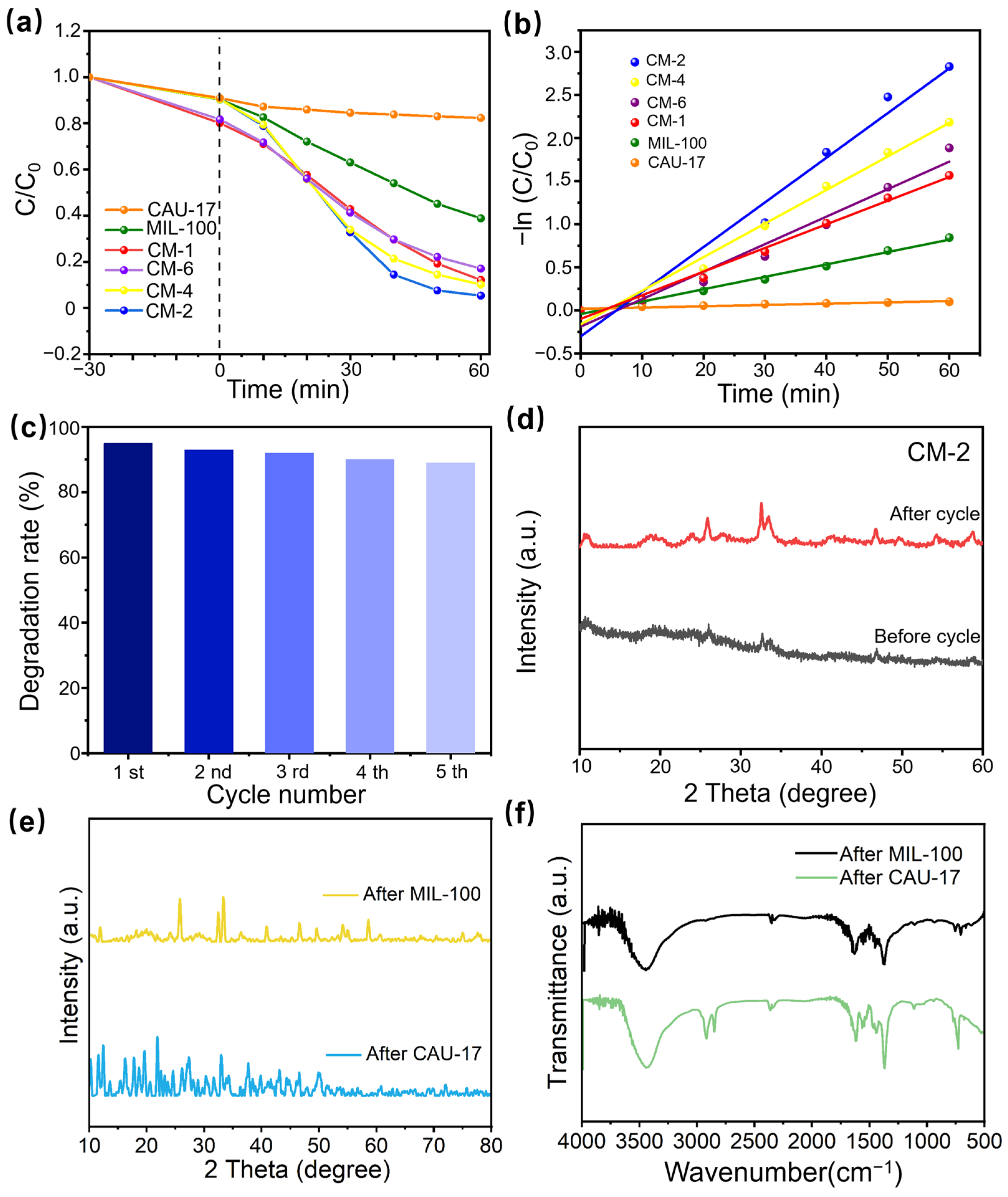
2.3. Degradation Performance
2.4. Stability and Reusability of Photocatalysts
2.5. Degradation Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of CAU-17
3.3. Synthesis of CAU-17/MIL-100(Fe)
3.4. The Method for Preparing a Working Electrode
3.5. Characterization
3.6. Photocatalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M. Titanium dioxide nanostructures as efficient photocatalyst: Progress, challenges and perspective. Int. J. Energy Res. 2021, 45, 3569–3589. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.Y.; Han, C.; Tang, Z.R.; Xu, Y.J. Photocorrosion Inhibition of Semiconductor-Based Photocatalysts: Basic Principle, Current Development, and Future Perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Xu, J.; Huang, Z.; Lao, H.; Xu, X.; Xu, J.; Tang, H.; Yuan, R.; Wang, Z.; et al. Dual non-metal atom doping enabled 2D 1T-MoS2 cocatalyst with abundant edge-S active sites for efficient photocatalytic H2 evolution. Int. J. Hydrogen Energy 2023, 48, 16987–16999. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Tang, C.; Du, S.; Huang, H.; Tan, S.; Zhao, J.; Zhang, H.; Ni, W.; Yue, X.; Ding, Z.; Zhang, Z.; et al. Au–Pd Tandem Photocatalysis for Nonoxidative Coupling of Methane toward Ethylene. ACS Catal. 2023, 13, 6683–6689. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar]
- Garcia-Sanchez, A.; Gomez-Mendoza, M.; Barawi, M.; Villar-Garcia, I.J.; Liras, M.; Gandara, F.; de la Peña O’Shea, V.A. Fundamental Insights into photoelectrocatalytic hydrogen production with a hole-transport bismuth metal–organic framework. J. Am. Chem. Soc. 2019, 142, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ye, P.; Wang, M.; Wang, L.; Wu, C.; Xu, J.; Chen, Y. 2D/2D Bi-MOF-derived BiOCl/MoS2 nanosheets S-scheme heterojunction for effective photocatalytic degradation. J. Environ. Chem. Eng. 2022, 10, 108436. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zeng, Z.; Zeng, G.; Xu, P. Metal-organic frameworks derived Bi2O2CO3/porous carbon nitride: A nanosized Z-scheme systems with enhanced photocatalytic activity. Appl. Catal. B Environ. 2020, 267, 118700. [Google Scholar] [CrossRef]
- Ouyang, H.; Chen, N.; Chang, G.; Zhao, X.; Sun, Y.; Chen, S.; Yang, D. Selective capture of toxic selenite anions by bismuth-based metal–organic frameworks. Angew. Chem. Int. Ed. 2018, 57, 13197–13201. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Verhaeghe, D.; Weng, B.; Ghosh, B.; Zhang, H.; Hofkens, J.; Steele, J.A.; Roeffaers, M.B.J. Metal Halide Perovskite Based Heterojunction Photocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202203261. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, C.; Zou, Y.; Bao, T.; Wang, J.; Tang, C.; Liu, C. AS-Scheme MOF-on-MOF Heterostructure. Adv. Funct. Mater. 2023, 33, 2214627. [Google Scholar] [CrossRef]
- Silva, C.G.; Corma, A.; García, H. Metal–organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Yue, X.; Guo, W.; Li, X.; Zhou, H.; Wang, R. Core-shell Fe3O4@ MIL-101 (Fe) composites as heterogeneous catalysts of persulfate activation for the removal of Acid Orange 7. Environ. Sci. Pollut. 2016, 23, 15218–15226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, Y.; Guo, Y.; Wu, B.; Ying, Y.; Sofer, Z.; Wang, S. Surfactant-Mediated Crystalline Structure Evolution Enabling the Ultrafast Green Synthesis of Bismuth-MOF in Aqueous Condition. Small 2023, 20, 2307484. [Google Scholar] [CrossRef]
- Köppen, M.; Dhakshinamoorthy, A.; Inge, A.K.; Cheung, O.; Ångström, J.; Mayer, P.; Stock, N. Synthesis, Transformation, Catalysis, and Gas Sorption Investigations on the Bismuth Metal–Organic Framework CAU-17. Eur. J. Inorg. Chem. 2018, 30, 3496–3503. [Google Scholar] [CrossRef]
- Wang, R.; Xu, H.; Liu, X.; Fang, D.; Wei, S.; Yu, A.N. In-situ growth of iron oxides with MIL-100(Fe) enhances its adsorption for selenite. Surf. Interfaces 2022, 34, 102325. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Guo, H.; Weng, B.; Zhang, H.; Saha, R.A.; Zhang, M.; Lai, F.; Zhou, Y.; Juan, R.Z.; et al. Noble-Metal-Free High-Entropy Alloy Nanoparticles for Efficient Solar-Driven Photocatalytic CO2 Reduction. Adv. Mater. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, P.; Wang, H.; Almatrafi, E.; Zhou, C.; He, Y.; Zeng, G. Environmentally persistent free radicals in bismuth-based metal–organic layers derivatives: Photodegradation of pollutants and mechanism unravelling. Chem. Eng. J. 2022, 430, 133026. [Google Scholar] [CrossRef]
- Bale, S.; Rahman, S. Glass structure and transport properties of Li2O containing zinc bismuthate glasses. Opt. Mater. 2008, 31, 333–337. [Google Scholar] [CrossRef]
- Yang, L.; Xin, Y.; Yao, C.; Miao, Y. In situ preparation of Bi2WO6/CAU-17 photocatalyst with excellent photocatalytic activity for dye degradation. J. Mater. Sci.-Mater. Electron. 2021, 32, 13382–13395. [Google Scholar] [CrossRef]
- He, H.; Wen, H.M.; Li, H.K.; Zhang, H.W. Recent advances in metal–organic frameworks and their derivatives for electrocatalytic nitrogen reduction to ammonia. Coord. Chem. Rev. 2022, 471, 214761. [Google Scholar] [CrossRef]
- Wang, L.; Tang, G.; Liu, S.; Dong, H.; Liu, Q.; Sun, J.; Tang, H. Interfacial active-site-rich 0D Co3O4/1D TiO2 pn heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 428, 131338. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Meng, S.; Wang, Z.; Wang, P.; Wang, Z.; Qiu, C.; Chen, S.; Weng, B.; Zheng, Y.M. Metal Sulfide S-Scheme Homojunction for Photocatalytic Selective Phenylcarbinol Oxidation. Adv. Sci. 2024, 11, 2400099. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ye, L.; Chen, T.; Wang, L.; Shi, X.; Zhang, X.; Chen, D. Facet-dependent photocatalytic N2 fixation of bismuth-rich Bi5O7I nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 27661–27668. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 1983, 16, 723. [Google Scholar] [CrossRef]
- Anyama, C.A.; Ita, B.I.; Ayi, A.A.; Louis, H.; Okon, E.E.; Ogar, J.O.; Oseghale, C.O. Experimental and density functional theory studies on a zinc (II) coordination polymer constructed with 1, 3, 5-benzenetricarboxylic acid and the derived nanocomposites from activated carbon. ACS Omega 2021, 6, 28967–28982. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Van Der Veer, M.; Yang, X.; Weng, B.; Shen, L.; Huang, H.; Dong, X.; Wang, G.; Roeffaers, M.B.; Yang, M.Q. Twin boundary defect engineering in Au cocatalyst to promote alcohol splitting for coproduction of H2 and fine chemicals. J. Colloid Interface Sci. 2024, 657, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, Y.; Liu, B.; Weng, B.; Hofkens, J.; Roeffaers, M.B.J. Crystal structure engineering of metal halide perovskites for photocatalytic organic synthesis. Chem. Commun. 2023, 59, 3122–3125. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Yan, W.; Xie, T.; Chen, Y.; Wei, Y. Optimizing Electronic Density at Active W Sites for Boosting Photocatalytic H2 Evolution. Inorg. Chem. 2024, 63, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Gao, X.; Zhang, L.; Wang, D.; Zou, X.; Xie, T. Enhanced photocatalytic hydrogen evolution of NiCoP/g-C3N4 with improved separation efficiency and charge transfer efficiency. ChemSusChem 2018, 11, 276–284. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Chen, L.; Arshad, M.; Chuang, Y.; Hong, Y.L.; Nguyen, T.B.; Wu, C.H.; Dong, C.D. Facile fabrication of efficient tungsten disulfide nanoparticles for enhanced photocatalytic removal of tetracycline (TC) and Pb (II) photoreduction. Colloid Surfaces A 2023, 662, 131004. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.; Zhang, Q.; Jin, X.; Li, D.; Liu, H.; Chen, P.; Lv, W.; Liu, G. Bi2O2CO3/Bi2O3 Z-scheme photocatalyst with oxygen vacancies and Bi for enhanced visible-light photocatalytic degradation of tetracycline. Environ. Sci.-Nano 2022, 9, 2104–2120. [Google Scholar] [CrossRef]
- Li, J.; Lv, X.; Weng, B.; Roeffaers, M.B.; Jia, H. Engineering light propagation for synergetic photo-and thermocatalysis toward volatile organic compounds elimination. Chem. Eng. J. 2023, 461, 142022. [Google Scholar] [CrossRef]
- Lu, B.; Wang, S.; Zhao, L.; Zhou, D.; Dong, S.; Wang, G. Selective and superior capture of phosphate by using bimetallic bismuth-based metal-organic frameworks. Chem. Eng. J. 2021, 425, 131514. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Singhal, N.K.; Hassan, A.A.; Kim, K.H.; Kumar, S. Metal organic frameworks MIL-100 (Fe) as an efficient adsorptive material for phosphate management. Environ. Res. 2019, 169, 229–236. [Google Scholar] [CrossRef]


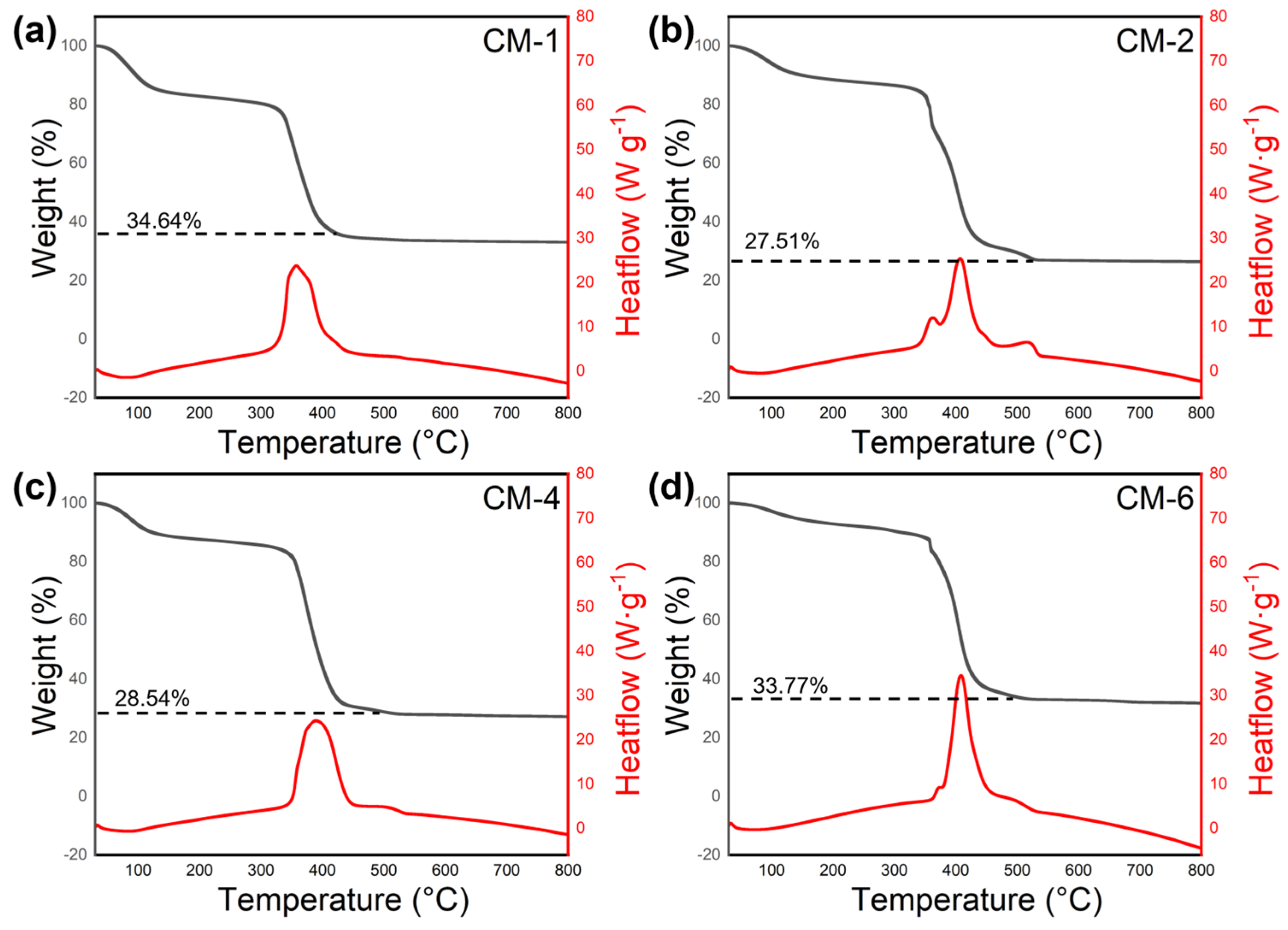


| Wt/% | CM-1 | CM-2 | CM-3 | CM-4 |
|---|---|---|---|---|
| Bi | 6.92 | 7.14 | 8.96 | 15.96 |
| Fe | 19.25 | 14.66 | 13.66 | 11.09 |
| Bi:Fe | 0.36 | 0.49 | 0.66 | 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhu, S.; Zhou, H.; Hou, M.; Wan, K.; Zhang, X.; Yan, W.; Wei, Y.; Chen, Y. Rational Design of Z-Scheme Heterostructures Composed of Bi/Fe-Based MOFs for the Efficient Photocatalytic Degradation of Organic Pollutants. Catalysts 2024, 14, 356. https://doi.org/10.3390/catal14060356
Xu J, Zhu S, Zhou H, Hou M, Wan K, Zhang X, Yan W, Wei Y, Chen Y. Rational Design of Z-Scheme Heterostructures Composed of Bi/Fe-Based MOFs for the Efficient Photocatalytic Degradation of Organic Pollutants. Catalysts. 2024; 14(6):356. https://doi.org/10.3390/catal14060356
Chicago/Turabian StyleXu, Jing, Songlin Zhu, Huizhi Zhou, Minghao Hou, Kangle Wan, Xueqi Zhang, Wei Yan, Yingcong Wei, and Yuanping Chen. 2024. "Rational Design of Z-Scheme Heterostructures Composed of Bi/Fe-Based MOFs for the Efficient Photocatalytic Degradation of Organic Pollutants" Catalysts 14, no. 6: 356. https://doi.org/10.3390/catal14060356





