Effect of the Metal of a Metallic Ionic Liquid (-butyl-methylimidazolium tetrachloroferrate) on the Oxidation of Hydrazine
Abstract
:1. Introduction
2. Results
2.1. Metallic Ionic Liquid Characterization
2.2. Morphological Characterization of Carbon Paste Electrodes
2.3. Electrochemical Impedance Spectroscopy Comparison between MWCNT/MO/IL and MWCNT/MO/ILFe
2.4. Contact Angle Measurements
2.5. Electrochemical Characterization
2.6. Electroanalytical Measurement to Evaluate MWCNT/MO/ILFe as a Possible Hydrazine Sensor
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis
4.3. Carbon Paste Electrode Preparation
4.4. Characterization Measurements
4.5. Equipment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Setzler, B.P.; Wang, J.H.; Nash, J.; Wang, T.; Xu, B.J.; Yan, Y.S. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule 2019, 3, 2472–2484. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc.-Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Goutham, R.; Rohit, P.; Vigneshwar, S.S.; Swetha, A.; Arun, J.; Gopinath, K.P.; Pugazhendhi, A. Ionic liquids in wastewater treatment: A review on pollutant removal and degradation, recovery of ionic liquids, economics and future perspectives. J. Mol. Liq. 2022, 349, 118150. [Google Scholar] [CrossRef]
- Wei, G.T.; Yang, Z.S.; Chen, C.J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Nazari, M.; Asadollahzadeh, H.; Shahidi, M.; Rastakhiz, N.; Mohammadi, S. An electrochemical nano-sensor for determination of hydrazine using modified electrode by La2O3–Co3O4 nanohybrids and ionic liquid. J. Mater. Sci. Mater. Electron. 2021, 3, 25258–25268. [Google Scholar] [CrossRef]
- Mazloum-Ardakan, M.; Khoshro, A.; Hosseinzade, L. Simultaneous determination of hydrazine and hydroxylamine based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuators B Chem. 2015, 214, 132–137. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Jahani, S. Electrocatalytic Determination of Hydrazine and Phenol Using a Carbon Paste Electrode Modified with Ionic Liquids and Magnetic Core-shell Fe3O4@SiO2/MWCNT Nanocomposite. Electroanalysis 2016, 28, 1093–1099. [Google Scholar] [CrossRef]
- Feng, G.; Kuang, Y.; Li, Y.J.; Sun, X.M. Three-dimensional porous super aerophobic nickel nanoflower electrodes for high-performance hydrazine oxidation. Nano Res. 2015, 8, 3365–3371. [Google Scholar] [CrossRef]
- Du, X.Q.; Liu, C.; Du, C.; Cai, P.; Cheng, G.Z.; Luo, W. Nitrogen-doped graphene hydrogel-supported NiPt-CeOx nanocomposites and their superior catalysis for hydrogen generation from hydrazine at room temperature. Nano Res. 2017, 10, 2856–2865. [Google Scholar] [CrossRef]
- Xia, B.Q.; Chen, K.; Luo, W.; Cheng, G.Z. NiRh nanoparticles supported on nitrogen-doped porous carbon as highly efficient catalysts for dehydrogenation of hydrazine in alkaline solution. Nano Res. 2015, 8, 3472–3479. [Google Scholar] [CrossRef]
- Burshtein, T.Y.; Farber, E.M.; Ojha, K.; Eisenberg, D. Revealing structure–activity links in hydrazine oxidation: Doping and nanostructure in carbide–carbon electrocatalysts. J. Mater. Chem. A 2019, 7, 23854–23861. [Google Scholar] [CrossRef]
- Zhang, T.; Asefa, T. Heteroatom-doped carbon materials for hydrazine oxidation. Adv. Mater. 2019, 31, 1804394. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Zhang, T.; Silva, T.L.; Almeida, V.C.; Asefa, T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal. B Environ. 2018, 225, 30–39. [Google Scholar] [CrossRef]
- Jeong, J.; Choun, M.; Lee, J. Tree-bark-shaped n-doped porous carbon anode for hydrazine fuel cells. Angew. Chem. Int. Ed. 2017, 56, 13513–13516. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Y.; Zou, X.X.; Huang, X.X.; Goswami, A.; Liu, Z.W.; Asefa, T. Polypyrrole-derived nitrogen and oxygen Co-doped mesoporous carbons as efficient metal-free electrocatalyst for hydrazine oxidation. Adv. Mater. 2014, 26, 6510–6516. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.; Farber, E.M.; Burshtein, T.Y.; Eisenberg, D. A multi-doped electrocatalyst for efficient hydrazine oxidation. Angew. Chem. Int. Ed. 2018, 57, 17168–17172. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.T.; Li, Z.J.; Wang, H.N.; Cui, L.R.; Zhang, J.; Lu, S.F.; Xiang, Y. Atomically dispersed Cu–N–C as a promising support for low-Pt loading cathode catalysts of fuel cells. ACS Appl. Energy Mater. 2020, 3, 3807–3814. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Watanabe, M.; Miyatake, K. Carbon segregation-induced highly metallic Ni nanoparticles for electrocatalytic oxidation of hydrazine in alkaline media. ACS Appl. Mater. Interfaces 2014, 6, 18445–18449. [Google Scholar] [CrossRef]
- Fragal, V.H.; Fragal, E.H.; Zhang, T.; Huang, X.X.; Cellet, T.S.P.; Pereira, G.M.; Jitianu, A.; Rubira, A.F.; Silva, R.; Asefa, T. Deriving efficient porous heteroatom-doped carbon electrocatalysts for hydrazine oxidation from transition metal ions-coordinated casein. Adv. Funct. Mater. 2019, 29, 1808486. [Google Scholar] [CrossRef]
- Liu, C.B.; Zhang, H.; Tang, Y.H.; Luo, S.L. Controllable growth of graphene/Cu composite and its nanoarchitecture-dependent electrocatalytic activity to hydrazine oxidation. J. Mater. Chem. A 2014, 2, 4580–4587. [Google Scholar] [CrossRef]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured catalysts for organic transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Perez-Juste, J.; Xu, Q.H.; Liz-Marzan, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Rhee, J.H.; Chung, C.C.; Diau, E.W.G. A perspective of mesoscopic solar cells based on metal chalcogenide quantum dots and organometal-halide perovskites. NPG Asia Mater. 2013, 5, 68–85. [Google Scholar] [CrossRef]
- Yu, L.; Shi, M.; Yue, X.; Qu, L. Detection of allura red based on the composite of poly(diallyldimethylammonium chloride) functionalized graphene and nickel nanoparticles modified electrode. Sens. Actuators B Chem. 2016, 225, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.A.; Rahman, M.M.; Asiri, A.M.; Marwani, H.M.; Awual, M.R. 4-Hexylresorcinol sensor development based on wet-chemically prepared Co3O4@Er2O3 nanorods: A practical approach. J. Ind. Eng. Chem. 2018, 66, 446–455. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Baghayeri, M.; Rouhi, J.; Senthil Kumar, P.; Show, P.-L.; et al. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass derived activated carbon frameworks: A promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostruct. Chem. 2022, 12, 429–439. [Google Scholar] [CrossRef]
- Awual, M.R.; Alharthi, N.H.; Hasan, M.M.; Karim, M.R.; Islam, A.; Znad, H.; Hossain, M.A.; Halim, M.E.; Rahman, M.M.; Khaleque, M.A. Inorganic-organic based novel nano-conjugate material for effective cobalt(II) ions capturing from wastewater. Chem. Eng. J. 2017, 324, 130–139. [Google Scholar] [CrossRef]
- Amar, I.; Sharif, A.; Ali, M.; Alshareef, S.; Altohami, F.; Abdulqadir, M.; Ahwidi, M. Removal of methylene blue from aqueous solutions using nano-magnetic adsorbent based on zinc-doped cobalt ferrite. Chem. Methodol. 2020, 4, 1–18. [Google Scholar] [CrossRef]
- Awual, M.R. Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem. Eng. J. 2016, 300, 264–272. [Google Scholar] [CrossRef]
- Afzali, D.; Karimi-Maleh, H.; Khalilzadeh, M.A. Sensitive and selective determination of phenylhydrazine in the presence of hydrazine at a ferrocene-modified carbon nanotube paste electrode. Environ. Chem. Lett. 2011, 9, 375–381. [Google Scholar] [CrossRef]
- Rastakhiz, N.; Kariminik, A.; Soltani-Nejad, V.; Roodsaz, S. Simultaneous Determination of Phenylhydrazine, Hydrazine and Sulfite Using a Modified Carbon Nanotube Paste Electrode. Int. J. Electrochem. Sci. 2010, 5, 1203–1212. [Google Scholar] [CrossRef]
- Akhgar, M.R.; Salari, M.; Zamani, H.; Changizi, A.; Mahdiabad, H.-H. Electrocatalytic and Simultaneous Determination of Phenylhydrazine and Hydrazine Using Carbon Paste Electrode Modified with Carbon Nanotubes and Ferrocene-dicarboxylic Acid. Int. J. Electrochem. Sci. 2010, 5, 782–796. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Lua, F.; Zhang, D.; Chen, K.; Zheng, K. CoS2 -decorated ionic liquid-functionalized graphene as a novel hydrazine electrochemical sensor. Talanta 2018, 182, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahmed, J.; Asiri, A.; Siddiquey, I.; Hasnat, M. Development of highly sensitive hydrazine sensor based on facile CoS2–CNT nanocomposites. RSC Adv. 2016, 6, 90470–90479. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Li, S.; Huang, Y.; Sun, Y.; Chen, Z.; Wang, W.; Liu, X. One-step electroreduction preparation of multilayered reduced graphene oxide/gold-palladium nanohybrid as a proficient electrocatalyst for development of sensitive hydrazine sensor. J. Colloid. Interface Sci. 2020, 566, 473–484. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Bani Asadi, E. Electrochemical determination of hydrazine using a ZrO2 nanoparticles-modified carbon paste electrode. Environ. Monit. Assess. 2015, 187, 122–132. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, C.; Zheng, J. Controlled synthesis of Material of Institute Lavoisier-53(Fe) for amperometric determination of hydrazine. J. Electro-Anal. Chem. 2020, 873, 114407–114414. [Google Scholar] [CrossRef]
- Adara, O.; Oyinbo, S.; Jen, T. Density functional theory simulation and modeling of the electrical and mechanical properties of Al2O3-CAO-CNT(3,3) nanomaterial. Comput. Mater. Sci. 2023, 218, 111939–111950. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Material for Molecular Electrochemistry. Am. Chem. Soc. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Xiong, H.I. Sensitive and Selective Determination of Riboflavin in Milk and Soymilk Powder by Multi-walled Carbon Nanotubes and Ionic Liquid [BMPi]PF6 Modified Electrode. Food Anal. Methodes 2017, 10, 399–406. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Khoshroo, A. An electrochemical study of benzofuran derivative in modified electrode-based CNT/ionic liquids for deter-mining nanomolar concentration of hydrazine. Electrochim. Acta 2013, 103, 77–84. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; AlSaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic Liquid-Carbon Nanomaterial Hybrids for Electrochemical Sensor Applications: A Review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Gidi, L.; Honores, J.; Ibarra, J.; Aguirre, M.J.; Arce, R.; Ramírez, G. Electrodetermination of gallic acid using multi-walled carbon nanotube paste electrodes and N-octylpyridinium hexafluorophosphate. Electroanalysis 2022, 34, 1163–1173. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M.; Mei, T.X. Investigating the electrochemical windows of ionic liquids. J. Ind. Eng. Chem. 2013, 19, 106–112. [Google Scholar] [CrossRef]
- Lin, J.B.; Vasam, C.H. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005, 690, 3498–3512. [Google Scholar] [CrossRef]
- Rezaee, E.; Honarasa, F. Determination of Tryptophan by using of activated multiwalled carbon nanotube ionic liquid electrode. Russ. J. Electrochem. 2019, 54, 1073–1080. [Google Scholar] [CrossRef]
- Benvidi, A.; Kakoolaki, P.; Zare, H.R.; Vafazadeh, R. Electrocatalytic oxidation of hydrazine at a Co(II) complex multi-wall carbon nanotube modified carbon paste electrode. Electrochim. Acta 2010, 56, 2045–2050. [Google Scholar] [CrossRef]
- Tajik, S.; Hosseinzadeh, R.; Beitollahi, H.; Varma, R.S. Electrochemical Detection of hydrazine by Carbon Paste Modified with Ferrocene Derivates, Ionic Liquid, and CoS2-Carbon Nanotubes. ACS Omega 2021, 6, 4641–4648. [Google Scholar] [CrossRef]
- Canales, C.; Gidi, L.; Arce, R.; Ramirez, G. Hydrazine electrooxidation mediated by transition metal octaethylporphyrins-modified electrode. New J. Chem. 2016, 40, 2813–2821. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Li, Z.; Lin, Q.; Ma, X.; Zhang, Z.; Xiang, S. MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium. Sensors 2019, 20, 140. [Google Scholar] [CrossRef]
- Haghighi, B.; Hamidi, H.; Bozorgzadeh, S. Sensitive and selective determination of hydrazine using glassy carbon electrode modified with Pd nanoparticles decorated multiwalled carbon nanotubes. Anal. Bioanal. Chem. 2010, 398, 1411–1416. [Google Scholar] [CrossRef]
- Teymoori, N.; Raoof, J.B.; Khalilzadeh, M.A.; Ojani, R. An electrochemical sensor based on CuO nanoparticle for simultaneous determination of hydrazine and bisphenol A. J. Iran. Chem. Soc. 2018, 15, 271–2279. [Google Scholar] [CrossRef]
- Cardoso, A.; Maraldi, V.; Bonfim, K.; Rocha, T.; Lataro, L.; Pereira, P.; Nakamura, A.; Ribeiro do Carmo, D. Electrocatalitic Detection of Hydrazine Using Chemically Modified Electrodes with Cobalt Pentacyanonitrosylferrate Adsorbed on the 3– Aminopropylsilica Surface. Int. J. Chem. 2017, 4, 12–21. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, B.; Peng, X. Effects of additives in iron-catalyzed cross-coupling reactions involving grignard reagents. Chin. J. Org. Chem. 2018, 38, 40–50. [Google Scholar] [CrossRef]
- Yang, C.H.; Chang, J.C.; Wu, T.Y.; Sun, I.W.; Wu, J.H.; Ho, W.J. Novel Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity as Both Solvents and Catalysts for Friedel–Crafts Alkylation. Appl. Sci. 2019, 9, 4743. [Google Scholar] [CrossRef]
- Ding, S.; Guo, Y.; Hülsey, M.J.; Zhang, B.; Asakura, H.; Liu, L.; Han, Y.; Gao, M. Electrostatic Stabilization of Single-Atom Catalysts by Ionic Liquids. Chem 2019, 5, 3207–3219. [Google Scholar] [CrossRef]
- Sitze, M.; Schreiter, E.; Patterson, E.; Freeman, G. Ionic liquids based on FeCl3 and FeCl2. Raman scattering and ab initio calculations. Inorg. Chem. 2001, 40, 2298–2304. [Google Scholar] [CrossRef]
- Lvovich, V.F. Impedance Instrumentation, Testing, and Data Validation. In Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2012; pp. 163–204. [Google Scholar] [CrossRef]
- Brug, G.J.J.; van den Eeden, A.L.G.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, X.; Yang, H.; Shen, P.K. Performance improvement of air electrode for Li/air batteries by hydrophobicity adjustment. J. Mater. Chem. A 2015, 22, 11874–11879. [Google Scholar] [CrossRef]
- Figueroa, R.; Nóvoa, X.R.; Pérez, C. Hydrophobic surface treatments for improving the corrosion resistance of anodized AA2024-T3 alloys. Electrochim. Acta 2019, 303, 56–66. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Abbas, Q.; Hunt, M.; Hall, P. Pseudocapacitive Effect of Carbons Doped with Different Functional Groups as Electrode Materials for Electrochemical Capacitors. Energies 2020, 13, 5577. [Google Scholar] [CrossRef]
- Rashed, M.; Faisal, M.; Ahmed, J.; Alsareii, S.A.; Jalalah, M.; Harraz, F.A. Highly sensitive and selective amperometric hydrazine sensor based on Au nanoparticle-decorated conducting polythiophene prepared via oxidative polymerization and photo-reduction techniques. J. Saudi Chem. Soc. 2022, 26, 101480–104493. [Google Scholar] [CrossRef]
- Srinidhi, G.; Sudalaimani, S.; Giribabu, K.; Basha, S.J.; Suresh, C. Amperometric determination of hydrazine using a CuS-ordered mesoporous carbon electrode. Microchim. Acta 2020, 187, 359. [Google Scholar] [CrossRef]
- Saengsookwaow, C.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. Nitrogen-doped graphene-polyvinylpyrrolidone/ gold nanoparticles modified electrode as a novel hydrazine sensor. Sens. Actuators B Chem. 2016, 227, 524–532. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.A.; Abdullah, M.M.; Harraz, F.A.; Jalalah, M.; Al-Assiri, M.S. Efficient hydrazine electrochemical sensor based on PANI doped mesoporous SrTiO3 nanocomposite modified glassy carbon electrode. J. Electroanal. Chem. 2020, 879, 114805. [Google Scholar] [CrossRef]
- Avanes, A.; Hasanzadeh-Karamjavan, M.; Shokri-Jarcheloo, G. Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with β-nickel hydroxide nanoplatelets. Microchim. Acta 2019, 186, 441. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, A.; Narayanan, S.S. Fabrication of CdSe quantum dots@nickel hexacyanoferrate core-shell nanoparticles modified electrode for the electrocatalytic oxidation of hydrazine. J. Mater. Sci. Mater. Electron. 2018, 29, 20146–20155. [Google Scholar] [CrossRef]
- Daneshvar, A.; Moosavi, M.; Sabzyan, H. A molecular dynamics study on magnetic imidazolium-based ionic liquids: The effect of an external magnetic field. Phys. Chem. Chem. Phys. 2020, 22, 13070–13083. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, Z.; Yin, C.; Horike, S.; Mukaida, M. A magnetic ionic liquid redox couple for harvesting waste heat and mechanical energy. Chem. Phys. Lett. 2021, 776, 138663–138667. [Google Scholar] [CrossRef]
- Baccour, M.; Louvain, N.; Alauzun, J.C.; Stievano, L.; Mutin, P.; Boury, B.; Monconduit, L.; Brun, N. Carbonization of polysaccharides in FeCl3/BmimCl ionic liquids: Breaking the capacity barrier of carbon negative electrodes in lithium-ion batteries. J. Power Sources 2020, 474, 228575–228586. [Google Scholar] [CrossRef]
- Gidi, L.; Arce, R.; Ibarra, J.; Isaacs, M.; Aguirre, M.J.; Ramírez, G. Hydrogen evolution reaction highly electrocatalyzed by MWCNT/N-octylpyridinum hexafluorophosphate metal-free system. Electrochim. Acta 2021, 372, 137859–137866. [Google Scholar] [CrossRef]
- Bustos Villalobos, M.; Ibarra, J.; Gidi, L.; Cavieres, V.; Aguirre, M.J.; Ramírez, G.; Arce, R. Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid. Catalysts 2022, 12, 1675. [Google Scholar] [CrossRef]
- Gidi, L.; Honores, J.; Arce, R.; Arévalo, M.C.; Aguirre, M.J.; Ramírez, G. Enhanced Electrocatalysis of the Oxygen Reduction Reaction Using Cobalt and Iron Porphyrin/Ionic Liquid Systems. Energy Technol. 2019, 7, 1900698–1900706. [Google Scholar] [CrossRef]
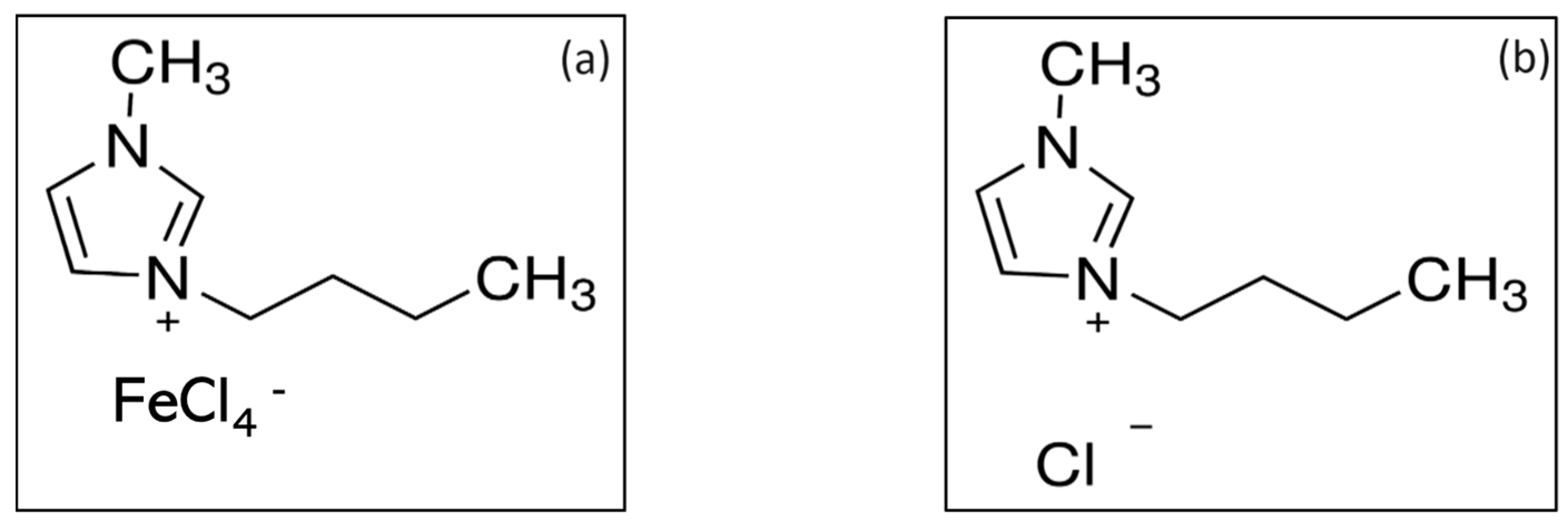
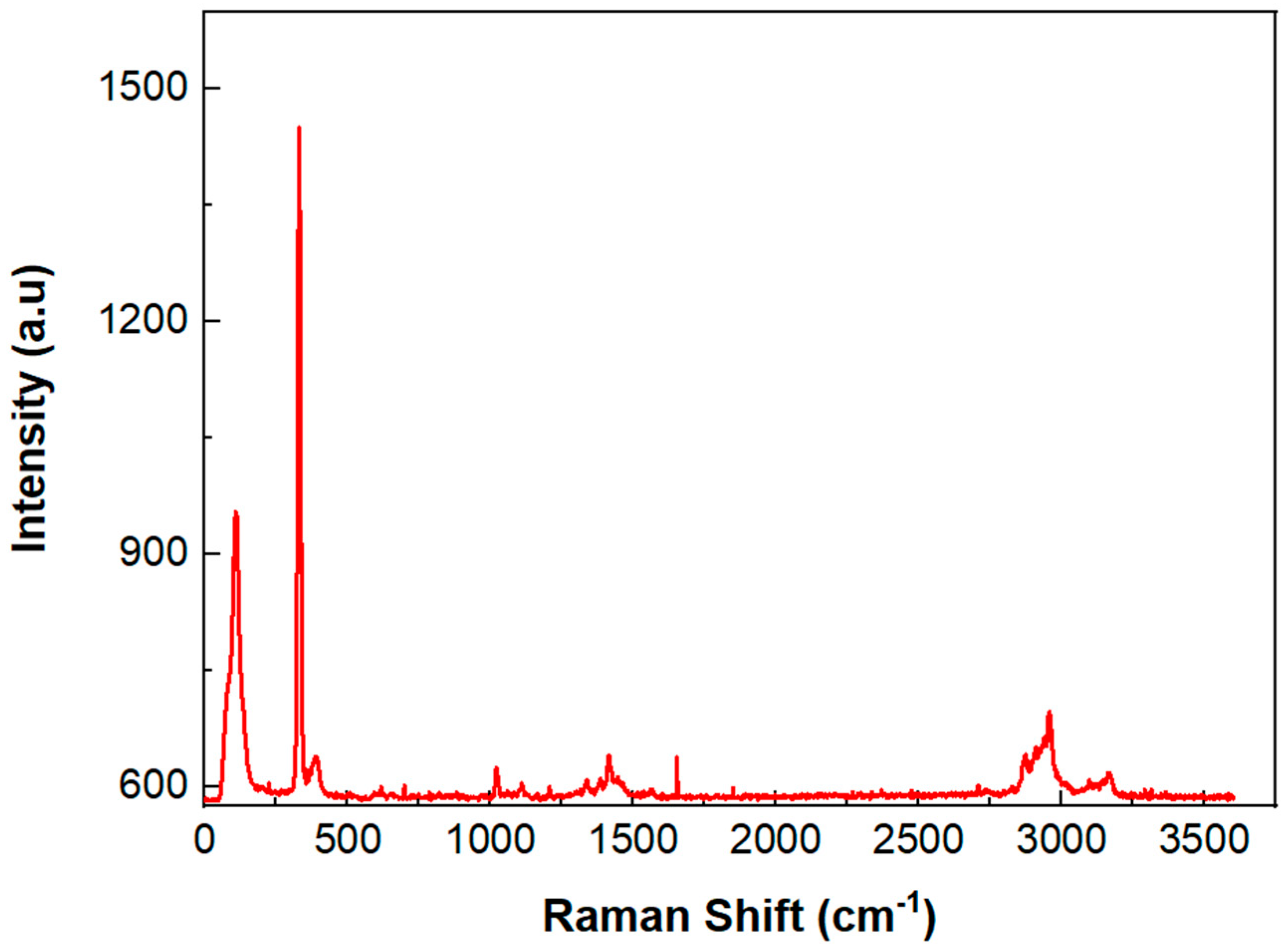






| Electrode | %C | %O | %Fe |
|---|---|---|---|
| MWCNT/MO | 96.81 ± 0.21 | 3.19 ± 0.26 | -- |
| MWCNT/MO/IL | 94.42 ± 3.82 | 4.08 ± 2.50 | -- |
| MWCNT/MO/ILFe | 86.22 ± 1.35 | 2.45 ± 1.65 | 4.19 ± 1.42 |
| Element | Before Activation | After Activation | ||
|---|---|---|---|---|
| MWCNT/MO/IL | MWCNT/MO/ILFe | MWCNT/MO/IL | MWCNT/MO/ILFe | |
| Rs (Ω) | 481 | 140 | 223 | 171 |
| CPEdl (µF) | 1.6 | 1.0 | 1.7 | 3.5 |
| Ret (Ω) | 8345 | 7373 | 6929 | 12,547 |
| CPE1 (µF) | - | - | 229 | - |
| R1 (Ω) | - | - | 10,675 | - |
| Electrode | Vonset/V vs. Ag/AgCl | Vp/V vs. Ag/AgCl | Ip/mA |
|---|---|---|---|
| MWCNT/MO | 0.16 | 0.61 | 0.56 |
| MWCNT/MO/IL | 0.14 | 0.54 | 0.56 |
| MWCNT/MO/ILFe | 0.010 | 0.49 | 0.63 |
| Electrochemical Sensor | Electrochemical Method | Linear Range, LR (μ mol L−1) | Limit of Detection, LOD (μ mol L−1) | Ref. |
|---|---|---|---|---|
| Polythiophene (PTh)/GCE | Amperometry | 0.5−48 | 0.207 | [67] |
| CuS-ordered mesoporous carbon/GCE | Amperometry | 0.25−40 | 0.10 | [68] |
| CuO NPs/IL/CPE | Differential pulse voltammetry | 0.05–150 | 0.03 | [56] |
| Nitrogen-doped graphene-polyvinylpyrrolidone-Au nanoparticles (NPs)/SPCE | Square wave voltammetry | 2−300 | 0.07 | [69] |
| Polyaniline (PANI)-doped mesoporous SrTiO3 nanocomposite/GCE | Linear sweep voltammetry/Amperometry | 200−3560 16−58 | 1.09 0.95 | [70] |
| β-nickel hydroxide nanoplatelets/carbon paste electrode (CPE) | Amperometry | 1−1300 | 0.28 | [71] |
| CdSe quantum dots (QDs)@Ni hexacyanoferrate (NiHCF) core−shell NPs modified electrode | Chronoamperometry | 1.6−1300 | 0.5 | [72] |
| MWCNT/MO/ILFe | Square wave voltammetry | 40,000–100,000 | 8000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockmann, M.; Navarro, F.; Ibarra, J.; León, C.; Armijo, F.; Aguirre, M.J.; Ramírez, G.; Arce, R. Effect of the Metal of a Metallic Ionic Liquid (-butyl-methylimidazolium tetrachloroferrate) on the Oxidation of Hydrazine. Catalysts 2024, 14, 359. https://doi.org/10.3390/catal14060359
Brockmann M, Navarro F, Ibarra J, León C, Armijo F, Aguirre MJ, Ramírez G, Arce R. Effect of the Metal of a Metallic Ionic Liquid (-butyl-methylimidazolium tetrachloroferrate) on the Oxidation of Hydrazine. Catalysts. 2024; 14(6):359. https://doi.org/10.3390/catal14060359
Chicago/Turabian StyleBrockmann, Marcela, Freddy Navarro, José Ibarra, Constanza León, Francisco Armijo, María Jesús Aguirre, Galo Ramírez, and Roxana Arce. 2024. "Effect of the Metal of a Metallic Ionic Liquid (-butyl-methylimidazolium tetrachloroferrate) on the Oxidation of Hydrazine" Catalysts 14, no. 6: 359. https://doi.org/10.3390/catal14060359






