Cu-MOF-74-Derived CuO-400 Material for CO2 Electroreduction
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Performance of Cu-MOF-74 and CuO-400
2.2. Morphological and Structural Characterization of Cu-MOF-74 and CuO-400
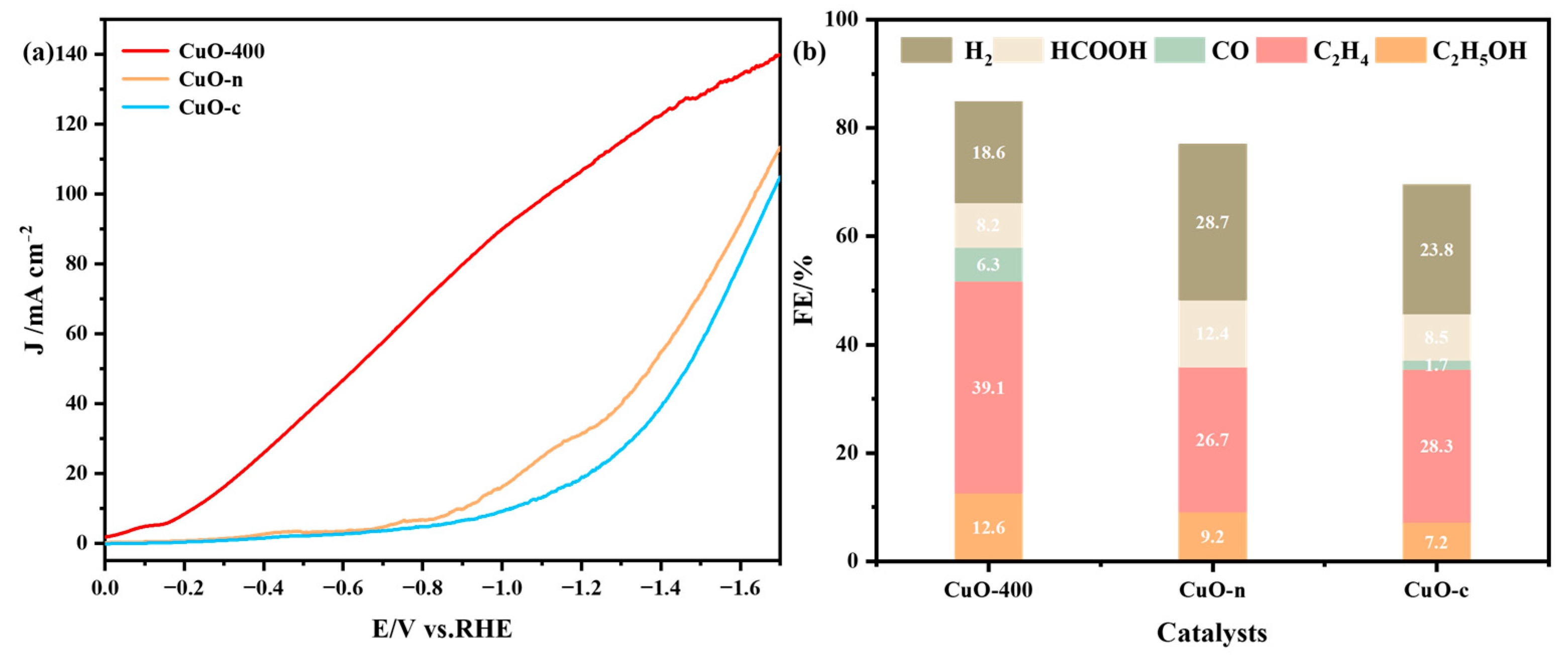
2.3. Electrochemical Performance of CuO-400, CuO-n, and CuO-c
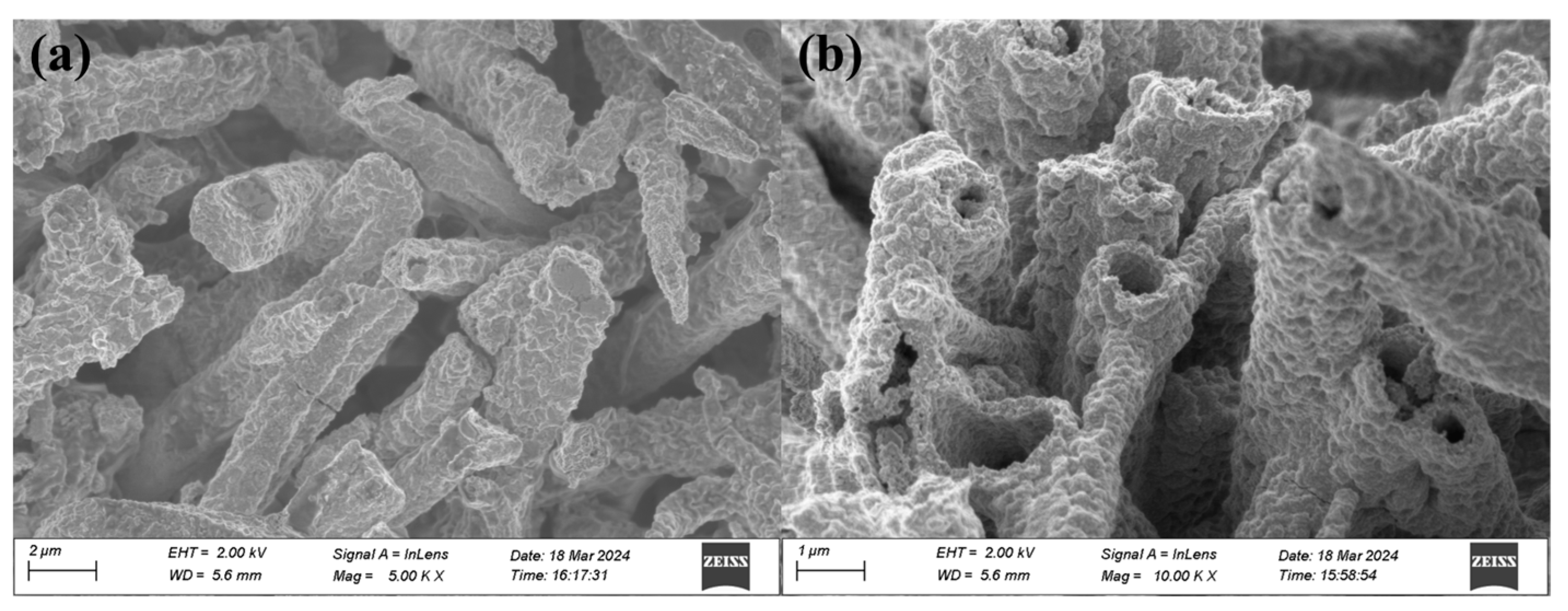
2.4. Morphological and Structural Characterization of CuO-400, CuO-n, and CuO-c
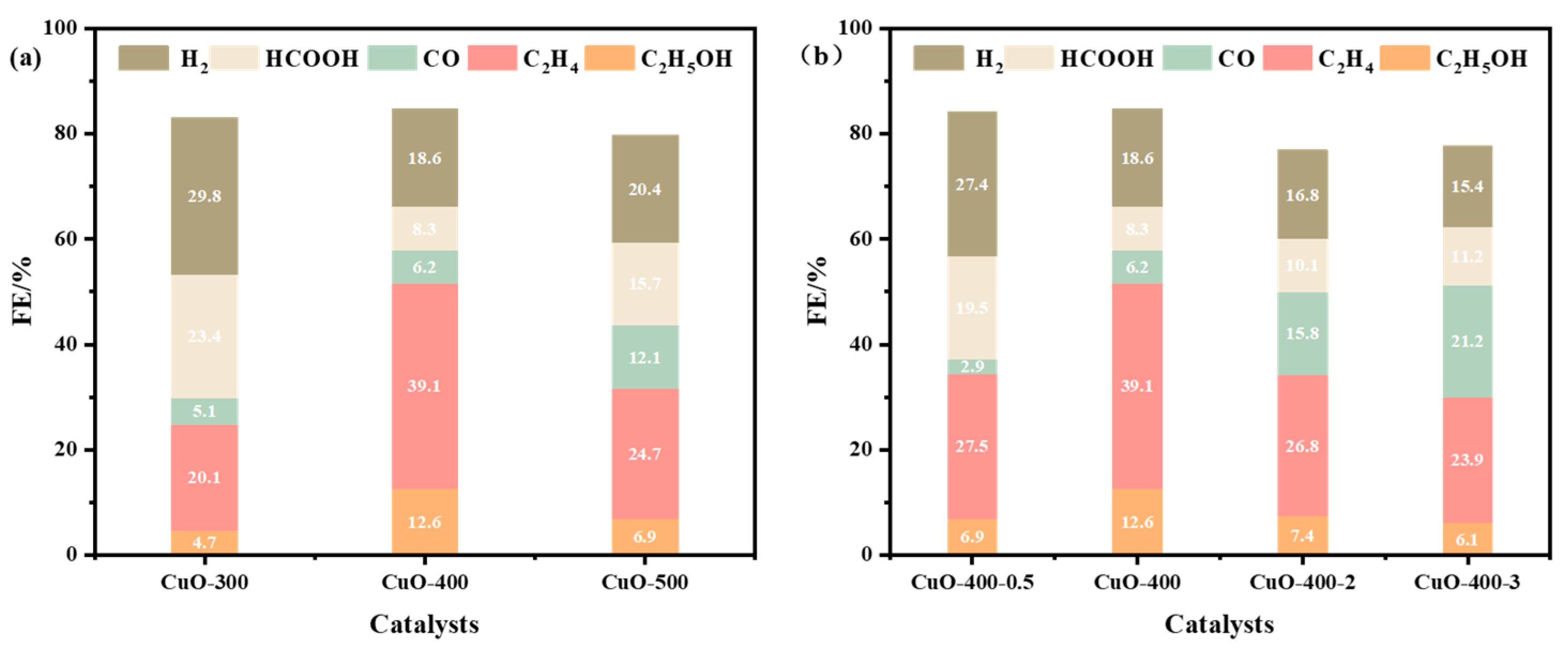
2.5. The Influence of Calcination Conditions on Material Properties
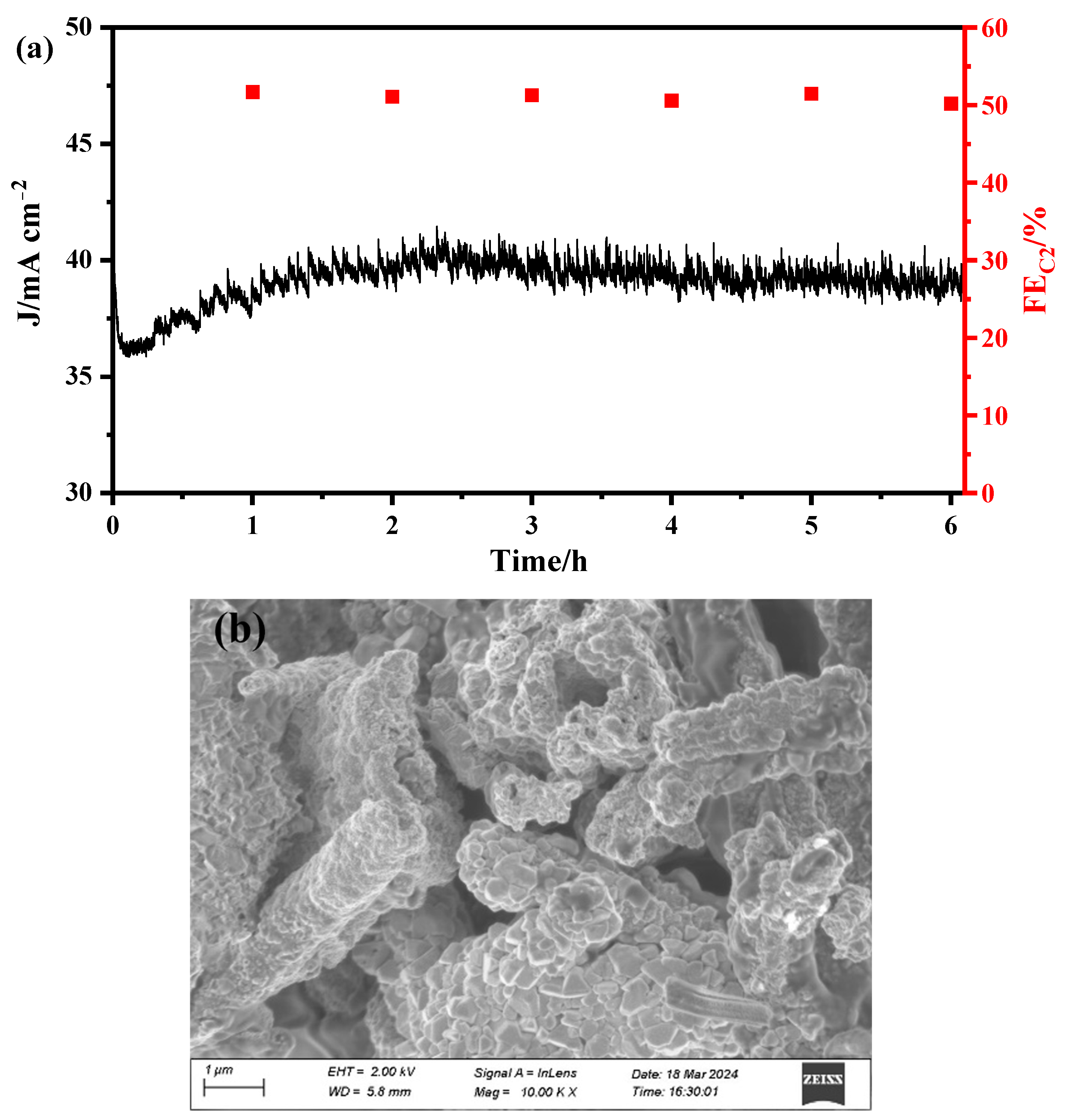
2.6. Electrode Stability in CO2 Electroreduction
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis and Electrode Preparation
3.2.1. Synthesis of Cu-MOF-74
3.2.2. Synthesis of CuO-T with Different Calcination Temperatures
3.2.3. Synthesis of CuO-400-X with Different Calcination Times
3.2.4. Synthesis of CuO-n
3.2.5. Electrode Preparation
3.3. Material Characterization
3.4. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Yao, X.; Zhang, K.; Qu, F.; Qin, L.; Huang, Y.; Li, J. Recent Progress and Development in Inorganic Halide Perovskite Quantum Dots for Photoelectrochemical Applications. Small 2019, 16, 1903398. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Chou, T.-C.; Chang, C.-C.; Yu, H.-L.; Yu, W.-Y.; Dong, C.-L.; Velasco-Vélez, J.-J.; Chuang, C.-H.; Chen, L.-C.; Lee, J.-F.; Chen, J.-M.; et al. Controlling the Oxidation State of the Cu Electrode and Reaction Intermediates for Electrochemical CO2 Reduction to Ethylene. J. Am. Chem. Soc. 2020, 142, 2857–2867. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Ma, S.; Gold, J.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper Films by Additive-Controlled Electrodeposition: CO2 Reduction Catalysis. ACS Catal. 2017, 7, 3313–3321. [Google Scholar] [CrossRef]
- Vasileff, A.; Zhi, X.; Xu, C.; Ge, L.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Selectivity Control for Electrochemical CO2 Reduction by Charge Redistribution on the Surface of Copper Alloys. ACS Catal. 2019, 9, 9411–9417. [Google Scholar] [CrossRef]
- Morimoto, M.; Takatsuji, Y.; Iikubo, S.; Kawano, S.; Sakakura, T.; Haruyama, T. Experimental and Theoretical Elucidation of Electrochemical CO2 Reduction on an Electrodeposited Cu3Sn Alloy. J. Phys. Chem. C 2019, 123, 3004–3010. [Google Scholar] [CrossRef]
- Jia, F.; Yu, X.; Zhang, L. Enhanced selectivity for the electrochemical reduction of CO2 to alcohols in aqueous solution with nanostructured Cu–Au alloy as catalyst. J. Power Sources 2014, 252, 85–89. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Cho, A.; Kim, S.; Han, J.W.; Lee, J. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2019, 246, 82–88. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X. Laser-Prepared CuZn Alloy Catalyst for Selective Electrochemical Reduction of CO2 to Ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, D.-K.; Han, M.-K.; Janani, G.; Surendran, S.; Kim, J.H.; Kim, J.K.; Cho, H.; Sim, U. Review—Non-Noble Metal-Based Single-Atom Catalysts for Efficient Electrochemical CO2 Reduction Reaction. J. Electrochem. Soc. 2020, 167, 164503. [Google Scholar] [CrossRef]
- Karapinar, D.; Huan, N.T.; Ranjbar Sahraie, N.; Li, J.; Wakerley, D.; Touati, N.; Zanna, S.; Taverna, D.; Galvão Tizei, L.H.; Zitolo, A.; et al. Electroreduction of CO2 on Single-Site Copper-Nitrogen-Doped Carbon Material: Selective Formation of Ethanol and Reversible Restructuration of the Metal Sites. Angew. Chem. Int. Ed. 2019, 58, 15098–15103. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Lim, J.; Kim, N.-Y.; Kim, Y.-H.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017, 8, 1090–1096. [Google Scholar] [CrossRef]
- Qin, T.; Qian, Y.; Zhang, F.; Lin, B.-L. Cloride-derived copper electrode for efficient electrochemical reduction of CO2 to ethylene. Chin. Chem. Lett. 2019, 30, 314–318. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; García de Arquer, F.P.; Dinh, C.-T.; Ozden, A.; Li, Y.C.; Nam, D.-H.; Li, J.; Liu, Y.-S.; Wicks, J.; et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 2020, 5, 478–486. [Google Scholar] [CrossRef]
- Majidi, L.; Ahmadiparidari, A.; Shan, N.; Misal, S.N.; Kumar, K.; Huang, Z.; Rastegar, S.; Hemmat, Z.; Zou, X.; Zapol, P.; et al. 2D Copper Tetrahydroxyquinone Conductive Metal–Organic Framework for Selective CO2 Electrocatalysis at Low Overpotentials. Adv. Mater. 2021, 33, 2004393. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, D.; Liu, H.; Sun, X.; Chen, C.; Bi, J.; Liu, J.; Wu, H.; Han, B. Hollow Metal–Organic-Framework-Mediated In Situ Architecture of Copper Dendrites for Enhanced CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 8896–8901. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal–Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef]
- Li, C.; Ji, Y.; Wang, Y.; Liu, C.; Chen, Z.; Tang, J.; Hong, Y.; Li, X.; Zheng, T.; Jiang, Q.; et al. Applications of Metal–Organic Frameworks and Their Derivatives in Electrochemical CO2 Reduction. Nano-Micro Lett. 2023, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Hai, G.; Xue, X.; Feng, S.; Ma, Y.; Huang, X. High-Throughput Computational Screening of Metal–Organic Frameworks as High-Performance Electrocatalysts for CO2RR. ACS Catal. 2022, 12, 15271–15281. [Google Scholar] [CrossRef]
- Qu, G.; Wei, K.; Pan, K.; Zhou, J. Metal-Organic Frameworks (MOFs) and Their Derivatives for Electrocatalytic Carbon Dioxide (CO2) Reduction to Value-added Chemicals: Recent Advances and New Challenges. Chem.—Asian J. 2023, 18, e202300291. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, U.; Siahrostami, S. Copper-based metal–organic frameworks for CO2reduction: Selectivity trends, design paradigms, and perspectives. Catal. Sci. Technol. 2023, 13, 3740–3761. [Google Scholar] [CrossRef]
- Hou, M.; Shi, Y.x.; Li, J.j.; Gao, Z.; Zhang, Z. Cu-Based Organic-Inorganic Composite Materials for Electrochemical CO2Reduction. Chem.—Asian J. 2022, 17, e202200624. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Wen, X.; Guo, X.; Wei, R.; Gao, L.; Pan, X.; Zhang, J.; Xiao, G. Modulable Cu(0)/Cu(I)/Cu(II) sites of Cu/C catalysts derived from MOF for highly selective CO2 electroreduction to hydrocarbons. Vacuum 2023, 215, 112231. [Google Scholar] [CrossRef]
- Nam, D.-H.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Dinh, C.-T.; García de Arquer, F.P.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal–Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- Haldoupis, E.; Borycz, J.; Shi, H.; Vogiatzis, K.D.; Bai, P.; Queen, W.L.; Gagliardi, L.; Siepmann, J.I. Ab Initio Derived Force Fields for Predicting CO2 Adsorption and Accessibility of Metal Sites in the Metal–Organic Frameworks M-MOF-74 (M = Mn, Co, Ni, Cu). J. Phys. Chem. C 2015, 119, 16058–16071. [Google Scholar] [CrossRef]
- Calleja, G.; Sanz, R.; Orcajo, G.; Briones, D.; Leo, P.; Martínez, F. Copper-based MOF-74 material as effective acid catalyst in Friedel–Crafts acylation of anisole. Catal. Today 2014, 227, 130–137. [Google Scholar] [CrossRef]
- Andrew, R.; Millward, O.M.Y. Metal-Organic Frameworks with Exceptionally High Capacity for Storage ofCarbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar]
- Lu, Y.; Li, S.; Li, H.; Guo, C.; Yang, L. Facile fabrication of well-dispersed CuxO nanoneedle on porous carbonized nano sponge and its promising application in the thermal decomposition of ammonium perchlorate. Powder Technol. 2021, 391, 206–213. [Google Scholar] [CrossRef]
- Patra, A.K.; Das, S.K.; Bhaumik, A. Self-assembled mesoporous TiO2 spherical nanoparticles by a new templating pathway and its enhanced photoconductivity in the presence of an organic dye. J. Mater. Chem. 2011, 21, 3925–3930. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, Y.-L.; Chen, L.-B.; Li, M.-H.; Lu, J.-X.; Wang, H. Cu-MOF-74-Derived CuO-400 Material for CO2 Electroreduction. Catalysts 2024, 14, 361. https://doi.org/10.3390/catal14060361
Liu H, Wang Y-L, Chen L-B, Li M-H, Lu J-X, Wang H. Cu-MOF-74-Derived CuO-400 Material for CO2 Electroreduction. Catalysts. 2024; 14(6):361. https://doi.org/10.3390/catal14060361
Chicago/Turabian StyleLiu, Hua, Ya-Li Wang, Lei-Bing Chen, Meng-Han Li, Jia-Xing Lu, and Huan Wang. 2024. "Cu-MOF-74-Derived CuO-400 Material for CO2 Electroreduction" Catalysts 14, no. 6: 361. https://doi.org/10.3390/catal14060361
APA StyleLiu, H., Wang, Y.-L., Chen, L.-B., Li, M.-H., Lu, J.-X., & Wang, H. (2024). Cu-MOF-74-Derived CuO-400 Material for CO2 Electroreduction. Catalysts, 14(6), 361. https://doi.org/10.3390/catal14060361






