Abstract
With the intensification of global resource shortages and the environmental crisis, hydrogen energy has garnered significant attention as a renewable and clean energy source. Water splitting is considered the most promising method of hydrogen production due to its non-polluting nature and high hydrogen concentration. However, the slow kinetics of the two key reactions, the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER), have greatly limited the development of related technologies. Meanwhile, the scarcity and high cost of precious metal catalysts represented by Pt and Ir/RuO2 limit their large-scale commercial application. Thus, it is essential to develop catalysts based on Earth’s transition metals that have abundant reserves. Vanadium (V) is an early transition metal with a distinct electronic structure from late transition metals such as Fe, Co, and Ni, which has been emphasized and studied by researchers. Numerous vanadium-based electrocatalysts have been developed for the HER and OER. In this review, the mechanisms of the HER and OER are described. Then, the compositions, properties, and modification strategies of various vanadium-based electrocatalysts are summarized, which include vanadium-based oxides, hydroxides, dichalcogenides, phosphides, nitrides, carbides, and vanadate. Finally, potential challenges and future perspectives are presented based on the current status of V-based electrocatalysts for water splitting.
1. Introduction
With the increasing global demand for energy and the urgent need to reduce greenhouse gas emissions, it is urgent to develop clean and renewable energy solutions [1,2,3]. Hydrogen is a promising energy carrier due to its high energy density and only releasing water when burned [4,5,6]. Among many technologies for hydrogen production, electrocatalytic water splitting is an efficient and environmentally friendly approach [7,8,9,10]. However, the slow kinetics of the HER and OER limit the wide application of electrocatalytic hydrogen production. Utilizing high-efficiency electrocatalysts can decrease the energy barrier and accelerate the reaction kinetics in water electrolysis, resulting in reduced energy usage [11,12,13]. The prevailing catalysts employed for electrocatalytic hydrogen production in the industry are commonly platinum group catalysts, specifically Pt/C and Ir/RuO2. Nevertheless, their high cost and scarcity pose major challenges for large-scale applications [14,15,16,17]. Consequently, it is critical to create electrocatalysts based on transition metals. These catalysts should perform well in the actual production process, be cost-effective, and have sufficient reserves [18,19,20,21].
Vanadium, as an early transition metal element, possesses a wide range of oxidation states. This characteristic offers numerous possibilities for adjusting the electronic structure of materials, hence enhancing their catalytic performances [22,23,24]. Vanadium-based catalysts are a viable alternative to replace scarce and costly noble metals in water splitting due to their adaptable redox chemistry and plentiful availability at affordable prices on Earth [25,26,27,28]. Angnes et al. presented a comprehensive summary of the progress of NiV-LDHs as electrocatalysts for water splitting, pointing out that NiV-LDHs have significant potential in the HER and OER [29]. Yang et al. synthesized ternary Co1−xVxP nanoneedle arrays by hydrothermal low-temperature phosphorization. In 1 M KOH, the nanoneedle arrays demonstrated current densities of 10 and 400 mA cm−2, with corresponding overpotential values of 46 and 226 mV, respectively. In addition, the current densities of 10, 100, and 300 mA cm−2 were achieved at voltages of 1.58, 1.75, and 1.92 V, respectively. when Co1−xVx-HNNs were utilized as the anode and Co1−xVxP as the cathode in a device for overall water splitting [30].
In this review, we comprehensively summarize the compositions, properties, and modification strategies of vanadium-based electrocatalysts, including oxides, hydroxides, phosphides, carbides, nitrides, dichalcogenides, and vanadates (Figure 1). Additionally, we present tables to summarize the activity, stability, and preparation methods of representative vanadium-based electrocatalysts. In conclusion, we highlight the existing issues with vanadium-based electrocatalysts for water splitting and propose recommendations to promote the development of V-based electrocatalysts for the HER and OER.
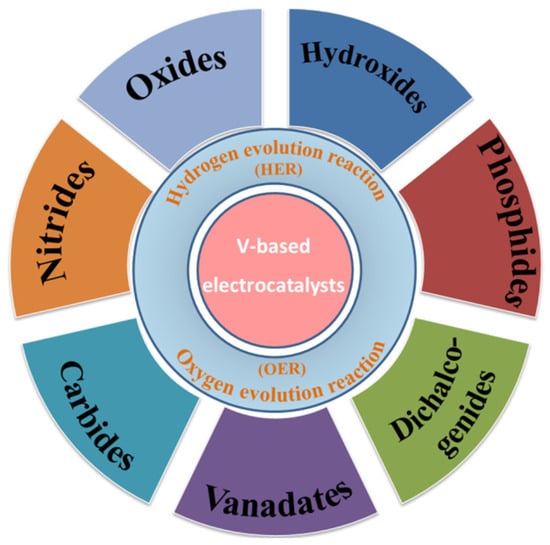
Figure 1.
Schematic organization of the vanadium-based electrocatalysts including oxides, hydroxide, phosphides, dichalcogenides, vanadates, carbides, and nitrides with the HER and OER.
2. Fundamentals of the HER and OER
As shown in Figure 2A, water splitting consists of two key half-reactions (the HER for the cathode and OER for the anode). The thermodynamic theoretical voltage of water splitting is 1.23 V. However, an additional potential is dissipated for water splitting due to the slow reaction kinetics of the HER and OER, which is called overpotential (η). Therefore, the actual voltages of water splitting (E) are shown in Equation (1) as follows.
E = 1.23 V + ηHER +ηOER + iR
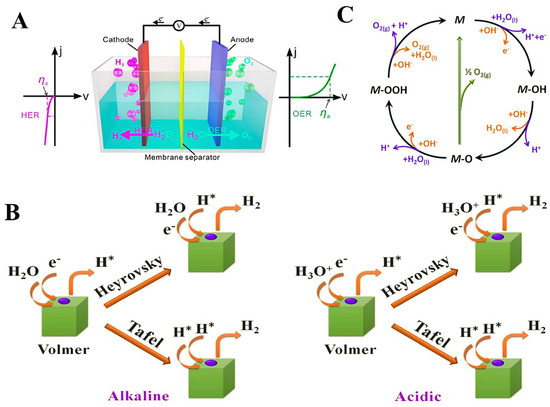
Figure 2.
(A) Schematic illustration of water splitting. Reproduced with permission [29]. Copyright 2021 Elsevier. (B) Scheme of the possible reaction steps of the OER under acidic (purple lines) and alkaline conditions (orange lines). (C) Scheme of the possible reaction steps of the OER under acidic and alkaline conditions. Reproduced with permission [31]. Copyright 2022 Elsevier.
iR is the ohmic potential drop of the system. From Equation (1), it can be seen that the focus of reducing the voltage of water splitting is to develop efficient electrocatalysts to reduce ηHER and ηOER [29].
2.1. Mechanism of the HER
The HER is a two-electron transfer reduction reaction involving the adsorption and desorption of H intermediates (H*). The difference in electrolyte pH affects the source of protons (mainly from H2O under alkaline conditions and from H+ under acidic conditions). Therefore, the hydrogen production mechanism is different in acidic and basic conditions [31].
2.1.1. Reaction Mechanism of the HER under Acidic Conditions
Volmer reaction (electrochemical adsorption):
H+ + M + e− ⇋ M-H*
Heyrovsky reaction (electrochemical desorption):
M-H* + H+ + e− ⇋ M + H2
Tafel reaction (chemical desorption):
2M-H* ⇋ 2M + H2
A large amount of H+ is present in acidic conditions. Initially, the H+ ions adhere to the surface of the catalyst and create M-H* (where H* represents the attachment of hydrogen atoms to the active site M on the catalyst surface), which is known as the Volmer reaction (Equation (2)). The subsequent desorption step may be electrochemical desorption (Equation (3)) or chemical desorption (Equation (4)) depending on the nature of the electrocatalyst (Figure 2B).
2.1.2. Reaction Mechanism of the HER under Alkaline Conditions
Volmer reaction (electrochemical adsorption):
H2O + M + e− ⇋ M-H* + OH−
Heyrovsky reaction (electrochemical desorption):
M-H* +H2O + e− ⇋ M +H2 + OH−
Tafel reaction (chemical desorption):
2M-H* ⇋ 2M +H2
The process of the HER in alkaline solutions is analogous to that in acidic solutions. It involves the Volmer reaction (Equation (5)), Heyrovsky reaction (Equation (6)), and Tafel reaction (Equation (7)). However, due to the minimal concentration of H+ in alkaline solutions, the process of water molecule dissociation is necessary to acquire H*. Consequently, the HER kinetics of electrocatalysts in an alkaline environment are significantly slower than that of an acidic environment.
2.2. Mechanism of the OER
The OER is a four-electron transfer process that occurs at the anode in water splitting. Similar to the HER, the mechanism of the OER is different in acidic and alkaline solutions [31].
2.2.1. Reaction Mechanism of the OER under Acidic Conditions
H2O + M ⇋ M-OH* + H+ + e−
M-OH* ⇋ M-O* + H+ + e−
M-O* + H2O ⇋ M-OOH* + H+ + e−
M-OOH* ⇋ O2 + H+ + e− + M
Water molecules are required to dissociate OH− under acidic conditions because trace amounts of OH− are present in acidic solutions (Figure 2C). Initially, OH− attaches to the catalyst surface to generate M-O* (Equation (8)), which is subsequently deprotonated to form M-O* (Equation (9)), and M-O* is attacked by the nucleophilic attack of water molecules to generate M-OOH* (Equation (10)) and finally further oxidized to release oxygen (Equation (11)).
2.2.2. Reaction Mechanism of the OER under Alkaline Conditions
M + OH− ⇋ M-OH* + e−
M-OH* + OH− ⇋ M-O* + H2O + e−
M-O* + OH− ⇋ M-OOH* + e−
M-OOH* + OH− ⇋ O2 + H2O + e− + M
The process of the OER in alkaline solutions is analogous to that in acidic solutions. It involves the generation of intermediates such as OH*, O*, and OOH*. Considering that the OER is an oxidation reaction, metals prone to high valence valorization are beneficial for enhancing the performance of the OER [32,33].
3. Vanadium-Based Electrocatalysts for the HER and OER
3.1. Vanadium-Based Oxides
Benefiting from the fact that V has different valence states, vanadium-based oxides have adjustable valence states, which provides more options and flexibility for the design and optimization of electrocatalysts for water splitting [34,35]. Nevertheless, improving the electrocatalytic efficiency of V-based oxides is still a significant obstacle due to the low intrinsic conductivity of the oxides [36]. Presently, the most widely used vanadium-based oxide systems include V2O3, VO2, and V2O5. Their performance in terms of the HER and OER, as well as the modification strategies, are summarized here.
3.1.1. HER Performance
V-based oxides typically exhibit semiconductor behavior, and their inadequate electrical conductivity hampers their HER performance. Presently, the main modification approach is to compound transition metal monomers or alloys with V-based oxides. This has two benefits: firstly, it enhances the electrical conductivity of V-based oxides, and secondly, it takes advantage of the strong affinity of VOx for oxygen. This affinity allows VOx to act as a center for dissociating water molecules, thereby improving the kinetics of the HER [37,38]. Li et al. successfully synthesized NiCo/V2O3/C by hydrothermal and thermal reduction. On the one hand, a Mott–Schottky junction was formed between the NiCo alloy and V2O3 (Figure 3A,B), which enhanced the charge transfer rate, and the HER activity was significantly increased. In addition, the graphite phase carbon layer acts as a support for the heterostructure, which greatly improves the stability of the material during the HER. Meanwhile, DFT calculations showed that the heterogeneous interface optimized both the hydrogen adsorption free energy and H2O adsorption energy, which promoted the HER kinetics (Figure 3C) [39].
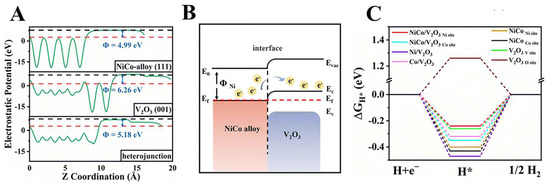
Figure 3.
(A) The electrostatic potential for NiCo (111), V2O3 (001), and the Mott–Schottky heterojunction formed from NiCo (111) and V2O3 (001). (B) Energy band diagram of Ni and V2O3 with the Mott–Schottky interface after Schottky contact. (C) The hydrogen adsorption Gibbs free energy diagrams of NiCo/V2O3, Ni/V2O3, Co/V2O3, and V2O3. Reproduced with permission [39]. Copyright 2023 Royal Society of Chemistry.
Utilizing crystalline phase engineering is an effective method for enhancing the efficiency of electrocatalysts. VO2 exhibits several crystalline forms, such as VO2 (A) and VO2 (R) with tetragonal structures, and VO2 (B) and VO2 (M1) with monoclinic structures [40]. Although their chemical composition is identical, there are notable distinctions in their optoelectronic characteristics. The reason for this is that the insulator VO2 (M1), which has a monoclinic structure, converts into the metallic phase VO2 (R) with a rutile structure by insulator-to-metal transition (IMT). This transition causes a substantial decrease in the resistivity of the material [41]. Kim et al. successfully applied the IMT of VO2 to the electrocatalytic field. Firstly, VO2 (M1) was successfully synthesized by hydrothermal and solid-phase sintering methods. As shown in Figure 4A,B, DSC and in situ XRD verified that VO2 (M1) was induced to be converted to VO2 (R) by a homemade electrode-heating system. The overpotential of the HER (η10) was reduced from 411 mV to 136 mV in 0.5 M H2SO4. The significant improvement in the HER performance was attributed to the increase in the ECSA induced by the transformation of the insulator’s VO2 into metal-phase VO2, as well as the decrease in the charge transfer resistance [42]. Given that the IMT effect in VO2 occurs at 68 °C, it aligns with the temperatures achievable under industrial water electrolysis conditions (60–80 °C). Consequently, there is promising potential to further investigate VO2 as a water-splitting electrocatalyst.
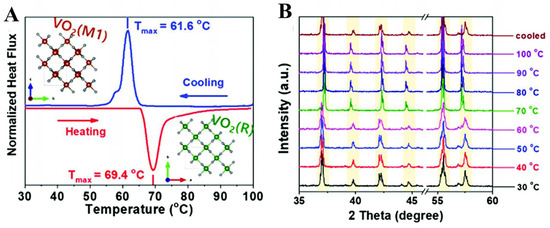
Figure 4.
(A) DSC analysis and (B) in situ heating XRD patterns of as-synthesized VO2 (M1) nanobelts in the temperature range of 30–100 °C. Reproduced with permission [42]. Copyright 2022 Royal Society of Chemistry.
Due to its inherent difficulty in adsorbing hydrogen and its limited electrical conductivity, the HER performance of pure V2O5 is not ideal. Currently, V2O5 is mainly complexed with metal sulfides to promote the dissociation of water molecules through the strong electrostatic attraction generated by its own V5+, which in turn improves electrocatalytic activity [43]. Zhong and colleagues utilized a two-step hydrothermal method to cultivate V2O5 on the surface of Ni2S3. The V5+ species at the V2O5@Ni2S3 interface exhibited a strong electrostatic affinity, allowing them to selectively absorb OH− ions from the electrolyte. The presence of Ni3S2 at the interface enhances the adsorption of H and speeds up the Volmer process, hence enhancing the electrocatalytic kinetics of the HER [44]. Species with a valence of V 5+ in V2O5 can hinder the oxidation of metal sulfides by selectively binding with oxygen (O2). Wu et al. successfully applied V2O5 colloidal nanoclusters onto CoS2, resulting in a significant decrease in the surface oxidation of CoS2 and an enhanced dissociation of water molecules [45]. The HER performance of representative V-based oxides are listed in Table 1.

Table 1.
Representative V-based oxide and hydroxide electrocatalysts for HER and OER.
3.1.2. OER Performance
Najafi et al. obtained the metallic rutile phase VO2 by topochemically transforming liquid-phase exfoliated VSe2 in an Ar-H2 atmosphere. The obtained 2D VO2 (R) nanosheets had a porous morphology, increasing the specific surface area of the material while introducing defect sites. When tested in 1 M KOH solution, the 2D VO2 (R) only acquired the overpotential of 209 mV to obtain the current density of 10 mA cm−2 [56]. Crystalline–amorphous composite engineering is a highly efficient approach to improving the OER performance of electrocatalysts. Zhou et al. designed amorphous–crystalline VO2/NiS2 heterostructures with good OER performance. The authors found that amorphous VO2 was easily dissolved in the KOH electrolyte during the OER, which induced abundant oxygen vacancies in the NiOOH active phase generated in situ by NiS2, thus greatly improving the OER activity. A current density of 10 mA cm−2 can be acquired with an overpotential of only 272 mV [1]. Tang et al. prepared V2O5/Co(OH)2 composites at room temperature. The V5+ of V2O5 can oxidize the Co2+ of Co(OH)2 to Co3+, inducing the easier generation of CoOOH species favorable for the OER [46]. The OER performance of representative V-based oxides are listed in Table 1.
In summary, the high valence species of vanadium-based oxides will have a synergistic effect with the transition metal-based hydroxides and sulfides that are composited with them, which is favorable for allowing the electrocatalysts to more easily generate the true high-valence OER active species.
3.2. Vanadium-Based Hydroxides
Pure V monometallic hydroxides in water splitting have rarely been studied, and the V-based hydroxide electrocatalysts reported for the HER and OER are mainly V ions and other transition metal ions (Fe, Co, Ni, etc.) forming bimetallic layered hydroxides (LDHs) as well as derived trimetallic layered hydroxides (LTHs) [57,58,59,60].
LDHs are simple to synthesize and have a multilayered, relatively open structure with the following chemical formula: [MⅡ1−xMⅢx(OH)2]x+An−x/n·yH2O (M is the metal cation and An− is the anion,) allowing for the rapid diffusion of reactants and products, accelerating the mass transfer process of the reaction [61,62] Therefore, more and more studies have been conducted on V-based hydroxide electrocatalysts in the field of water electrolysis, especially in the OER [29]. The role played by the element V in LDHs and LTHs has also been explored more by researchers at the same time [63]. In this paper, the performance, modification strategies, and the role of V elements in the above-mentioned V-based hydroxides for the HER and OER are summarized.
3.2.1. HER Performance
The main V-based hydroxides that have been reported for the HER are layered bimetallic hydroxides, such as NiV and CoV, and derived ternary layered hydroxides [49,64]. Generally, the element V should theoretically be in the trivalent state; however, due to oxidation during synthesis, trivalent vanadium is easily oxidized to higher valence states of tetravalent and pentavalent vanadium in NiV LDHs. Therefore, the influence of the valence state of V in the NiV system on its HER performance is worth exploring. Based on this, Feng’s team synthesized in-NiV-LDHs (the Ni source was nickel foam) and ex-NiV-LDHs (the Ni source was NiCl2.6H2O) by adjusting the type of nickel source in a one-step hydrothermal synthesis process. The main difference is that the valence state of V in in- NiV-LDHs is significantly lower than that in ex-NiV-LDHs. The authors analyzed Ni-V-O as a model (Figure 5A) and concluded that the low valence states of vanadium (V3+ and V4+) accelerated the electron aggregation, which was favorable for the dissociation of water molecules. On the other hand, the low valence states of vanadium (V3+ and V4+) regulated the adsorption/desorption capacity of Hads, thus enhancing the HER performance [48].
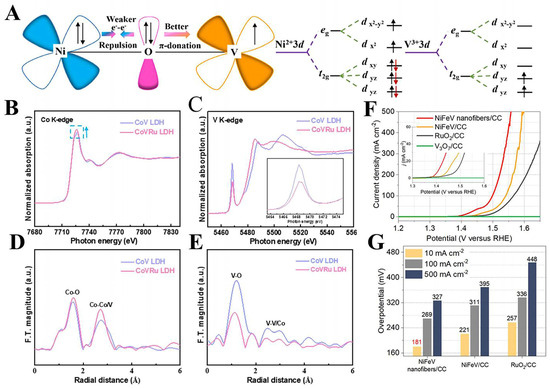
Figure 5.
(A) Schematic representations of the electronic coupling between Ni and V. Reproduced with permission [48]. Copyright 2022 Elsevier. (B,C) Normalized XANES spectra of Co K–edge and V K–edge. (D,E) Fourier–transformed EXAFS spectra for CoV LDH and CoVRu LDH. Reproduced with permission [65]. Copyright 2023 Elsevier. (F) LSV curves and (G) the overpotentials at 10, 100, and 500 mA cm−2 for NiFeV nanofibers.
In addition, it has been shown that a change in V coordination structure has a large impact on its HER performance in V-based LDHs; Zeng et al. synthesized CoV-LDHs and CoVRu LDHs with hexagonally shaped nanosheets by a hydrothermal method, and the test results showed that the HER performance was greatly enhanced after Ru was anchored in the CoV LDHs. As shown in Figure 5B,E, XANES and EXAFS from the CoV LDHs and CoVRu LDHs indicate that there is no significant change in the coordination environment of Co after the addition of Ru. However, there is a considerable change in the coordination environment of V. This suggests that the introduction of atomically dispersed Ru with high electronegativity leads to the structural distortion and space charge redistribution of V octahedra, which optimizes the HER kinetics [65]. Similarly, Li et al. also observed from XPS that the doping of Ru has a significant effect on the electronic structure of CoV-LDHs. The Tafel slope indicates that for the HER, when Ru is doped effectively it reduces the energy barrier of the Volmer step, which in turn improves the electrocatalytic hydrogen precipitation performance [49]. The HER performance of representative V-based hydroxides are listed in Table 1. In conclusion, the valence state and coordination of V have a great influence on the HER performance of V-based hydroxides.
3.2.2. OER Performance
Angnes et al. systematically summarized the progress of NiV LDHs as electrocatalysts for the HER and OER, and multiple studies have demonstrated that NiV-LDHs have optimal OER performance when Ni:V = 3:1 [29]. Although many works show that V-doped Ni-based and NiFe-based materials have good OER effects, there is a lack of in-depth discussion and characterization on whether the V ions are actually doped into the lattice in the subject materials to replace the position of Ni ions, and how to improve the OER catalytic activity of the subject materials.
Jiang et al. developed a series of Fe/V-doped a-Ni(OH)2 ultrathin nanosheet arrays (Ni3Fe1−xVx/CFP), it can be concluded that both Fe and V ions occupy the position of Ni in Ni(OH)2 using Wavelet variation curves (WT) from EXAFS. In addition, in situ XAFS tests indicate that V in Ni3Fe0.5V0.5 has a highly distorted coordination structure with a short V-O bond length, and further contraction during the OER. Meanwhile, DFT + U theoretical calculations show that the V site at the aggregation of Fe and V atoms has the optimal OER oxygen intermediate state binding energy and the lowest theoretical overpotential. Additionally, the synergistic electronic interactions between Ni, Fe, and V are well explained by metal ion valence electron structure analysis. As some electrons are transferred from Ni and Fe to V via the oxygen bridge, the electron density of the V center increases, and thus, the high-valence V intermediate is stabilized under OER conditions. More importantly, according to Sabatier’s principle, the strong bond between the high-valence-state V and adsorbed oxygen in the reaction intermediates can be weakened to a certain extent. This facilitates the release of oxygen formed by the oxidation of water from the V site [57].
Zhang et al. developed a novel interfacial atom substitution strategy to prepare V atom-doped NiFeV nanofibers for efficient alkaline OER electrocatalysis. It was found that the interfacial atom substitution growth of V can maximize the effect of V atoms on the valence states of Fe and Ni atoms and increase the proportion of high-valence Fe atoms compared to the traditional one-pot method of adding V atoms. This greatly enhances the OER kinetics of the NiFeV and ultimately improves the OER performance. As shown in Figure 5F,G, the overpotential of the NiFeV material is only 181 mV (10 mA cm−2), and it can be stably operated for more than 20 h at 100 mA cm−2 [66]. As demonstrated by the examples above, the vanadium in vanadium-based hydroxides plays a dual role: it acts as an active site and enhances the OER performance by modulating the electronic structures of the neighboring metal ions. The OER performance of representative V-based hydroxides are listed in Table 1.
However, it is worth noting that V ions are not necessarily active sites in hydroxides such as LDHS, LTHS, etc. Nejati et al. synthesized ZnFe-VO4-LDH by co-precipitation, although ZnFe-VO4-LDH had better OER properties than ZnFe-NO3-LDH and ZnFe-MoO4-LDH. The authors concluded that the vanadate ions only act as spacers, favoring the mass-transfer process, while the real electrocatalytic sites are the Zn2+ and Fe3+ sites [67].
3.3. Vanadate
Transition metal vanadates (MVXOY) can be regarded as a combination of transition metal ions and vanadium oxides. Currently, CoVXOY, NiVXOY, and other material systems have received wide attention for their good electrocatalytic catalytic performance [68,69]. Their excellent performance can be attributed to the following three aspects: Firstly, the doping of transition metal ions such as Ni and Co makes the vanadates have better electrical conductivity than vanadium oxides and vanadium hydroxides. Secondly, in the elemental composition of vanadate, V is a pre-transition metal element, while the doped transition metal elements are mostly Fe, Co, Ni, and other late-transition metal elements. Since there is a prominent difference between the adsorption of pre-transition metal elements and late-transition metal elements on the reaction intermediates [65,70], the rational design of vanadate materials can optimize the adsorption of the reaction intermediates, and then promote the reaction kinetics. Finally, the electronic structure of MVXOY can be optimized by taking advantage of the multiple oxidation valence states of V and the adjustable stoichiometric ratio of MVXOY to improve the performance of electrocatalytic hydrogen production. The following is a summary of the HER and OER performances of MVXOY and their modification strategies in recent years.
3.3.1. HER Performance
Constructing a heterostructure of vanadate and transition metal can optimize the electronic structure of the material and thus optimize the HER kinetics. Pan et al. synthesized Ni-Co2VO4 on NF by hydrothermal and solid-phase sintering and only required 50, 267, and 329 mV to reach current densities of 10, 500, and 1000 mA cm−2, respectively. The authors concluded that the excellent HER performance of Ni-Co2VO4 can be ascribed to two aspects: Firstly, the mesoporous nanosheets with a large specific surface area possess a large electrochemically active area, which also contributes to the desorption of surface bubbles and promotes the mass transfer kinetics. On the other hand, the heterostructure formed by Ni and Co2VO4 can facilitate the adsorption of water molecules and the intrinsic activity of HER by modulating the interfacial electronic structure to form electron-poor/rich species [71]. Zhou et al. anchored hollow CoV2O6 nanocubes to Mxene (V2CTx). The V2CTx lattice tensile strain could promote the ion transfer of transition metals due to the increased layer spacing. Meanwhile, the heterostructure between CoV2O6 and V2CTx Mxene could optimize the adsorption energies of H- and O-containing intermediates to obtain the optimal ΔGH* for the HER [72]. The HER performance of representative vanadate are listed in Table 2.

Table 2.
Representative vanadate electrocatalysts for HER and OER.
3.3.2. OER Performance
Non-metallic element doping is an important approach to enhance the OER performance of cobalt vanadate systems. Luo et al. synthesized nitrogen-doped Co2V2O7 on NF by hydrothermal and low-temperature ammoniation. As shown in Figure 6A, DFT calculations show that N doping can fine-tune the d-band center and band gap to promote intermediate adsorption and electron movement (Figure 6B,C). As depicted in Figure 6D,E, the N-Co2V2O7/NF only required an overpotential of 310 mV to reach a current density of 100 mA cm−2 with a Tafel slope of only 56.3 mV dec−1 [74]. Gyanprakash D et al. synthesized S-Co3V2O8 by hydrothermal synthesis, which had higher electrical conductivity than Co3V2O8, reaching a current density of 100 mA cm−2 with an overpotential of only 227 mV for S-Co3V2O8 [75].
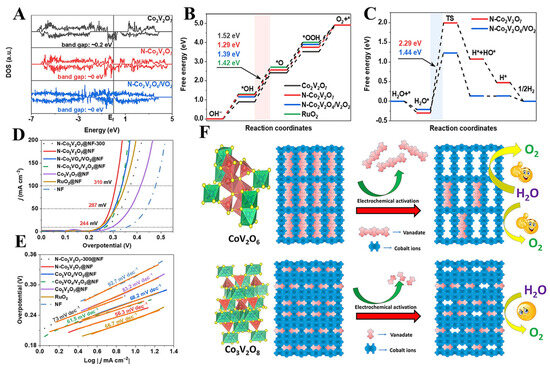
Figure 6.
(A) The DOS of Co2V2O7, N-Co2V2O7, and N-Co2V2O4/VO2. (B) Gibbs free energy diagrams of Co2V2O7, N-Co2V2O7, and N-Co2V2O4/VO2 for the OER. (C) Gibbs free energy diagrams of N-Co2V2O7 and N-Co2V2O4/VO2 for the HER. (D) The OER polarization curves. (E) Tafel plot. Reproduced with permission [74]. Copyright 2023 Elsevier. (F) Schematic diagram of the structure and electrochemical activation of Co3V2O8 and CoV2O6. Reproduced with permission [79]. Copyright 2021 American Chemical Society.
It is well known that V ions are soluble in aqueous electrolytes, which can easily lead to the surface reconstruction of catalysts. Therefore, the form of V ions in cobalt vanadate in alkaline environments and their effect on the activity and stability of the electrocatalyst need to be explored. Li et al. prepared Fe-modified Co2VO4 by hydrothermal and hydrogen reduction to induce controlled surface reconstruction by activating lattice oxygen and cation leaching. Through a combination of theoretical and experimental results, the authors found that Fe substitution plays a crucial role in accelerating Co peroxidation and introduces a great deal of structural flexibility due to elevated O 2p levels and optimized Co-O covalency. Meanwhile, V ions are present in the base-resistant form of V2O3 during CV cycling and are incorporated into the surface-constructed CoOOH layer to efficiently prevent the excessive loss of reactive species and lattice oxygen. Thanks to the modification of Fe and the stabilizing effect of V ions, Co2Fe0.25V0.75O4 requires only an overpotential of 205 mV to acquire a current density of 10 mA cm−2 for the OER [76].
Mondal et al. found that the state of polymerization of vanadate has a significant effect on its OER performance. The authors first synthesized Co3V2O8 and CoV2O6 by the sol–gel method. The difference between Co3V2O8 and CoV2O6 is that the element V in Co3V2O8 is in a discrete [VO4]3− form, while in CoV2O6 it exists as polymerized VO3 units ([VO3]n)n−). The authors suggest that the activity of cobalt vanadate arises from the etching of vanadate in the precatalyst by electrochemical activation (Figure 6F). The resulting higher surface reconstruction exposes more catalytically active cobalt sites. As shown in the figure, compared to the discrete [VO4]3− in Co3V2O8, the polymer structure of the vanadate bias ([VO3]n)n− in CoV2O6 promotes the etching phenomenon, thereby exposing a larger electrochemically active area [79]. The construction of multicomponent oxide heterostructures is an effective strategy to improve the performance of the OER. Li et al. obtained NiCoVOX (Ni(VO3)2/Co2V2O7) by a hydrothermal method. The NiCoVOX requires only an overpotential of 217 mV to reach a current density of 50 mA cm−2, thanks to the synthesized flower-like arrays and synergistic effect of Ni(VO3)2 and Co2V2O7 [80]. In conclusion, vanadates, as a highly promising OER electrocatalyst, require further investigation into their mechanisms during the OER process. The OER performance of representative vanadate are listed in Table 2.
3.4. Vanadium-Based Dichalcogenides
3.4.1. Vanadium-Based Sulfides
Transition metal disulfides have a graphene-like two-dimensional layered structure with the general structural formula MS2, where M stands for the transition metal (M = Mo, W, V, Nb, etc.) MS2 is constructed in the S-M-S pattern, where the metal atoms provide four electrons to bond with two sulfur atoms to achieve a bonded state within the molecular layer of the transition metal sulfide. In addition, the sulfur atoms have a lone pair of electrons on their surface which do not have any unpaired bonds. This prevents them from reacting to other environments [81,82,83]. Compared to MoS2, the typical TMDs, 1T-VS2 and 2H-VS2, have metallic properties and good conductivity. Moreover, their graphene-like structures provide a larger specific surface area [84,85]. In recent years, the above characteristics of VS2 have led to a rapid increase in its study for water splitting, especially in the HER [86,87]. The following summarizes the recent HER and OER performance of VS2, as well as the modification strategies used.
HER Performance
A series of computational and experimental results illustrate that VS2 has metallic properties and great potential for the HER. For example, Zhang et al. used DFT to calculate the adsorption capacity of VS2 for hydrogen atoms and found that the ΔGH* of the pristine VS2 bases in the 2H and 1T phases were 0.26 eV and −0.16 eV, respectively. These results are close to the bases of Pt. (ΔGH = −0.09 eV), suggesting that VS2 has similar HER activity to Pt [88]. Currently, VS2, along with MoS2 and other TMDs, mostly exhibits its active sites at the edge, whereas the unreactive basal plane restricts the further improvement of VS2 HER activity. Consequently, significant efforts have been dedicated to enhancing the VS2 basal plane activity in order to improve the HER performance of VS2 [88,89]. Zhang et al. synthesized VS2 with a larger layer spacing (~1 nm) by a simple hydrothermal method, which was expanded by ~74% compared with the layer spacing of pristine VS2 (0.575 nm). Due to the expanded layer spacing, VS2 introduces more in-plane defects. Meanwhile, theoretical calculations and experimental results show that modulation of the intrinsic defects excites the catalytic activity of the VS2 basal plane and improves the stability. For the HER, an overpotential of only 43 mV is required to obtain the current density of 10 mA cm−2. After a 60 h stability test, no significant current decay was found, indicating the excellent long-term stability of VS2 with expanded layer spacing [90]. Transition metal ion doping is also an important strategy to enhance the basal plane activity of VS2 [91,92]. He et al. prepared Mo-doped VS2 using a one-step hydrothermal method. The results showed that the Mo-VS2 HER performance was greatly improved with respect to VS2. The theoretical calculations mean that the enhanced performance of the catalyst is due to the fact that the Mo dopant reduces the free energy of hydrogen adsorption on the S-site (Figure 7A–C), which results in the enhancement of the catalytic activity for the basal plane [93].
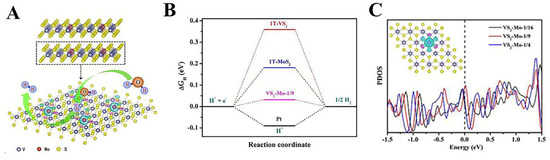
Figure 7.
(A) Schematic illustration of the catalytic HER sites for the Mo-doped VS2. (B) ΔGH* of S sites on the basal plane for 1T-VS2, 1T-MoS2, and VS2-Mo-1/9. (C) Projected p-orbital density of states of S for the Mo-doped VS2. Reproduced with permission [93]. Copyright 2020 Elsevier.
In addition to activating the basal activity of VS2 through doping and modulating intrinsic defects, fully exposing the VS2 edge active sites through composite strategies as well as morphology modulation strategies is also an effective way to enhance the HER performance of VS2-based electrocatalysts. For example, Pandurangan’s group successfully synthesized VS2/rGO by one-step hydrothermal synthesis of VS4/rGO, and then the target material VS2/rGO was synthesized by calcination. The presence of rGO resulted in more dispersed VS2 nanosheets, thus exposing more edge structures [94]. Patil et al. used a solvent–thermal method to prepare micrometer floral VS2. The HER performance of VS2 was enhanced by varying the ratio of ethylene glycol and water in the solvent to modulate the catalyst morphology, which in turn exposed more edge active sites [95]. The HER performance of representative Vanadium-based sulfides are listed in Table 3. In conclusion, VS2 emerges as a promising electrocatalyst for HER. Nevertheless, how to effectively activate its basal plane is still worth researching.

Table 3.
Representative V-based dichalcogenides electrocatalysts for HER and OER.
OER Performance
There are fewer studies about the OER performance of VS2. Some calculations have shown that monolayer VS2 is a good catalyst candidate for OER [102]. Qin et al. systematically explored the OER activity of a series of single transition metal atoms (Fe, Co, Ni, Ti, Mn, Hf, Zr, etc.) through first-principles calculations. The results showed that Ni@VS2 has the lowest OER overpotential (0.31 V) and its favorable OER performance can be attributed to the superior ΔG(O*) [103].
Hou et al. synthesized Ru-VS2 on carbon cloth by the one-step hydrothermal method. The in situ Raman spectroscopy showed that the Ru atomic clusters would be electro-oxidized to RuO2 during the OER process, offering a large number of active sites. The VS2 substrate mainly plays the role of protecting the Ru nucleus. Theoretical calculations show that electrons crossing the Ru/VS2 interface converge toward the electro-oxidized Ru clusters, while the electronic coupling of the Ru 3p and O 2p orbitals promotes the positive shift of the Ru Fermi energy level and optimizes the adsorption capacity of Ru for intermediates. As a result, the Ru-VS2 @CC required only an overpotential of 245 mV to obtain the current density of 50 mA cm−2 [97].
Dhakal et al. synthesized 3D CoMnS2@1T-Fe-VS2 by a two-step hydrothermal method, which acquired only an overpotential of 260 mV to reach the current density of 20 mA cm−2. Its excellent OER performance can be ascribed to the synergistic catalysis of the activated basal and edge sites of the CoMnS2 nucleus and the 1T-Fe-VS2 shell. The OER performance of representative Vanadium-based sulfides are listed in Table 3. Overall, the current OER research about VS2 mainly focuses on using it as a substrate, loading and protecting active sites such as single atoms and clusters, or compounding it with other materials to improve the OER performance. The performance of VS2 as an OER electrocatalyst by itself is not too satisfactory [104].
3.4.2. Vanadium-Based Selenides
VSe2 is also a member of TMDs, and Se belongs to the same main group (VIA) as S. The chemical properties are similar between S and Se, but at the same time, there are many differences. Se is more metallic than S, which exhibits better electrical conductivity. At the same time, the ionization energy of Se is smaller than that of S. Therefore, VSe2 and VS2 have a similar ionization energy. Therefore, the physicochemical properties and electrocatalytic performances of VSe2 and VS2 are different [105,106]. The following is a summary of the HER and OER performances of VSe2, as well as the modification strategies in recent years.
HER Performance
Contrary to the metallic nature of the 1T-VS2 and 2H-VS2, the 2H-VSe2 phase predominantly displays semiconducting characteristics [107]. Hence, the conversion of 2H-VSe2 to the metallic 1T phase of VSe2 through crystalline phase engineering can enhance the conductivity of the material and accelerate the electron transfer for HER. Zhao et al. prepared metallic VSe2 nanosheets with a thickness of about 0.4 nm by a one-pot colloidal route and tested the powder on a rotating disk electrode, which required an overpotential of only 206 mV to reach the density of 10 mA cm−2 [108]. Kwak et al. prepared the ReSe2-VSe2 (Re1−xVxSe2) alloy nanosheets by colloidal reaction. The authors found that increasing x makes the nanosheets more metallic and transforms VSe2 to 1T phase at x = 0.5–0.6. It requires only an overpotential of 77 mV under acidic conditions to reach the current density of 10 mA cm−2 [99].
VSe2 has a layered structure with active sites mainly found at the edges like VS2. Therefore, the activation of the inert basal plane inside VSe2 through defect engineering and the increase in the density of active sites at the edges of VSe2 through morphology modification strategies are important ways to enhance the HER performance of VSe2 [109,110,111]. Zhang et al. reported a VSe2 nanosheet with single-atom (SA) V defects and Se vacancy defects. The experimental and computational results showed that the presence of double defects effectively activated the inert substrate of VSe2, and the HER performance was enhanced. Its overpotentials to reach the current densities of 10 mA cm−2 under acidic, alkaline, and neutral conditions were 67.2, 72.3, and 122.3 mV, respectively [112]. Wang et al. obtained VSe2 quantum dots (3–6 nm) with a high density of edge sites by the tip sonication of VSe2 nanosheets. Compared with VSe2 nanosheets, VSe2 quantum dots have a high density of edge-active sites, which significantly improves their HER performance [113]. The HER performance of representative Vanadium-based selenides are listed in Table 3.
OER Performance
Recent work has shown that VSe2 has room-temperature ferromagnetism [114]. For ferromagnetic catalysts, an external magnetic field can optimize the kinetics of OER by modulating the electronic state of the material [115,116]. Inspired by this, Chen et al. confined monodisperse 1T-VSe2 nanoparticles on an amorphous carbon substrate by simple pulsed laser deposition (PLD) with rapid thermal annealing (RTA) (Figure 8A). Stimulated by an external magnetic field of 800 mT, the confined 1T-VSe2 nanoparticles exhibited highly efficient OER performance with an overpotential of 228 mV for the densities of 10 mA cm−2, which is superior to that of 1T-VSe2 without an applied magnetic field. Theoretical calculations showed that the magnetic field could promote the surface charge transfer kinetics of 1T-VSe2 and change the free energy of adsorption of *OH (Figure 8B,C), thereby ultimately increasing the intrinsic activity of the catalyst [117]. The OER performance of representative Vanadium−based selenides are listed in Table 3. Consequently, the intrinsic OER activity of electrocatalysts such as VS2 and VSe2, which is not ideal, can be enhanced by optimizing their adsorption towards oxygen-containing intermediates through the application of external fields during testing.
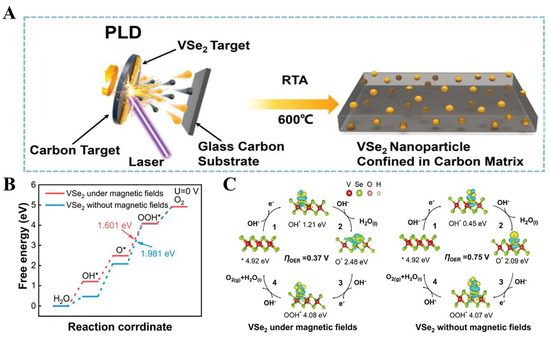
Figure 8.
(A) The schematic illustration for the synthesis process of confined 1T-VSe2 nanoparticles. (B) Gibbs free-energy diagram and (C) calculated charge density difference plot of OER steps of 1T-VSe2 under magnetic fields and without magnetic fields. Reproduced with permission [117]. Copyright 2023, Wiley-VCH.
3.5. Vanadium-Based Nitrides
Transition metal nitrides (TMNs), with their Pt-like electronic structure and catalytic behavior, are strong contenders for replacing noble metal catalysts for water splitting [18,118,119]. VN has a face-centered cubic crystal structure. The bonding between vanadium (V) and nitrogen (N) atoms results in a reduction in the d-band of the metallic vanadium, leading to an increase in the density of states at the Fermi energy level. As a result, VN exhibits electrical conductivity and resistance to corrosion. The aforementioned characteristics of VN make it a strong contender to replace precious metal catalysts [120,121,122]. However, the HER performance of VN is limited by its excessively strong hydrogen adsorption-free energy [123,124]. Meanwhile, the development of VN-based bifunctional electrocatalysts for water splitting remains challenging. The performance of VN in the HER, as well as in the OER and its modification strategies, are presented below.
3.5.1. HER Performance
The excessively strong hydrogen adsorption-free energy of VN limits the further enhancement of its HER performance. Thus, regulating the hydrogen adsorption-free energy of VN by modulating the electronic structure of VN is an effective strategy to improve the VN-based HER performance. Feng et al. employed Co, V-MIL-88B as a precursor, and subjected it to high-temperature ammoniation, resulting in the formation of shuttle-like ends composed of Co/VN (Figure 9A,B). Experimental results and density-functional theory demonstrate that the anchoring of Co single atoms on the VN surface can lower the position of V -3d at the center of the d-band of Co-VN (Figure 9C), which facilitates the desorption of OH species as well as the dissociation of water molecules (Figure 9D,E). Thanks to the improved HER kinetics, the Co-VN can reach a current density of 10 mA.cm−2 for HER with an overpotential of only 59 mV [125]. Researchers have also found that the metal monomer Ni can optimize the hydrogen adsorption-free energy of VN [126]. The construction of porous heterostructures can expose additional active sites and modulate the electronic structure of the material. r-MoC/VN electrocatalysts with heterostructures were prepared by in situ carbonization and nitridation by Pi et al. r-MoC/VN showed a three-dimensional porous structure as revealed by SEM and TEM, which is favorable for the exposure of the active sites as well as for the contact with the electrolyte. DFT calculations and XPS reveal that there is a strong electronic interaction between r-MoC and VN, which optimizes the M-H binding energy and facilitates the desorption of H*, thus enhancing the HER performance [127]. The HER performance of representative Vanadium-based nitrides are listed in Table 4.
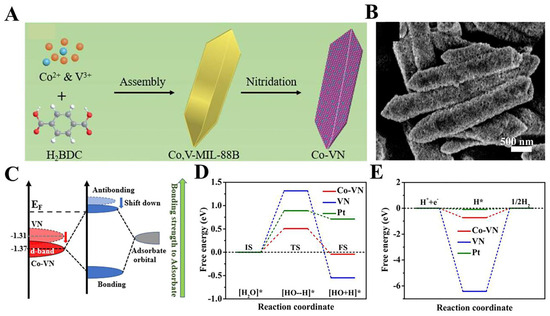
Figure 9.
(A) Schematic illustration of the synthetic procedure of Co-VN. (B) SEM image. (C) Schematic illustration of the interaction between catalyst and adsorbate. (D) Water dissociation, and (E) H adsorption of Co-VN, VN, and Pt (1 1 1). Reproduced with permission [125]. Copyright 2023 Elsevier.

Table 4.
Representative V-based nitrides, carbides, and phosphides for HER and OER.
3.5.2. OER Performance
VN plays two main roles in the field of the OER: one is to be compounded with other electrocatalysts as a precatalyst to generate amorphous hydroxides or oxides as OER active species. Yang et al. synthesized VN-Co-P by hydrothermal and high-temperature nitriding. The TEM of the samples after the OER test showed amorphous mixed hydroxides and oxides, suggesting that VN itself is not really acting as an OER active species, but only as a precatalyst [138].
On the other hand, the high conductivity of VN is utilized as a carrier for other OER-active species. For example, Meng et al. grew CoFe-PBA in situ on the surface of highly conductive vanadium nitride (VN) particles by a facile co-precipitation method. CoFe−PBAs/VN hybridization improves the poor conductivity of pure CoFe-PBAs and due to the electronic interactions increases the active site of Co2+ (Figure 10A). Compared to bare CoFe-PBAs (Figure 10B,C), CoFe-PBAs/VNs exhibited significantly enhanced electrocatalytic activity towards OER (a lower overpotential of 290 mV at 10 mA cm−2 current density with a smaller Tafel slope of 39.72 mV dec−1) [129]. The OER performance of representative Vanadium-based nitrides are listed in Table 4.
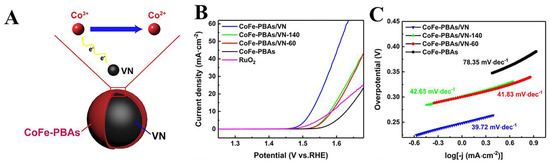
Figure 10.
(A) Schematic diagram of electronic interaction between CoFe-PBAs and VN in CoFe-PBAs/VN. (B) LSV curves and (C) Tafel plots of RuO2, CoFe-PBAs, CoFe-PBAs/VN-60, CoFe-PBAs/VN-140, and CoFe-PBAs/VN. Reproduced with permission [129]. Copyright 2020 Elsevier.
3.6. Vanadium-Based Carbides
Vanadium-based carbides, as transition metal carbides, have unique metal-like properties, making the limited electron transfer from the catalyst body to the surface possible. In addition, they have the advantages of wide pH range suitability and corrosion resistance. The main vanadium-based carbides reported so far are VC, V4C3, and V8C7 [139,140,141,142]. VC, V4C3, and V8C7 exhibit excellent HER performance but significantly poor OER performance according to the calculation results [142]. Therefore, there is a great challenge to develop a bifunctional vanadium-based carbide electrocatalyst for overall water splitting. The performance in the HER and OER, as well as the modification strategies, of vanadium-based carbons are presented below.
3.6.1. HER Performance
Some calculations have shown that V8C7 with natural carbon vacancies has the best hydrogen precipitation ability due to its larger surface energy and appropriate hydrogen adsorption energy compared with VC and V4C3 [142]. However, VCs are currently receiving significantly more research and attention as HER electrocatalysts. It has been shown that the interaction of VC with reaction intermediates can be effectively improved by doping engineering. Zheng et al. prepared Al-VC@C/NF for the first time by chemical vapor carbonization for efficient electrocatalytic hydrogen production (Figure 11A), which required only the overpotential of 97 mV in 1 M KOH to reach the current density of 10 mA cm−2. As shown in Figure 11B–D, theoretical calculations indicate that the doped Al atoms can induce electron redistribution on the vanadium carbide surface to form electron-rich carbon sites, which significantly reduces the energy barrier for the HER [131].
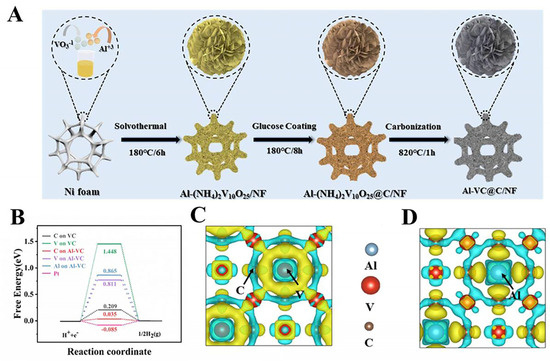
Figure 11.
(A) Illustration of the synthesis process of Al-VC@C/NF. (B) Free energy diagram for HER of VC and Al-VC. (C,D) Charge density differences of VC and Al-VC. Reproduced with permission [131]. Copyright 2024 Elsevier.
Although doping engineering can improve intrinsic activity by changing the electronic structure of vanadium-based carbons, it should be noted that vanadium-based carbons inevitably agglomerate during the preparation of vanadium-based carbons, which reduces their electrocatalytic activity. Therefore, modulating the morphology of vanadium-based carbons to prepare ultrafine vanadium-based carbons is an effective way to improve the HER performance. Peng et al. synthesized vanadium carbide nanodots with a size of 7.5 nm anchored on N-doped carbon nanosheets (VC/NC) by magnesiothermic reduction (MTR). The VC/NC only acquired 76 mV to obtain the current density of 10 mA cm−2 in 0.5 M H2SO4 [132]. The HER performance of representative Vanadium-based carbides are listed in Table 4.
3.6.2. OER Performance
It is well known that crystal surface exposure is an important factor affecting electrocatalytic performance. Kawashima et al. investigated the morphology and crystal surface exposure of cubic symmetric V8C7 after different electrochemical CV cycling tests using it as a precatalyst. Theoretical and experimental results indicate that the (100), (010), and (001) surfaces of V8C7 are stable during the OER process, whereas the (110) and (111) surfaces undergo autoxidation and dissolution during the OER. This leads to the transformation of V8C7 particle morphology from distorted spheres to cubes [143]. Constructing a heterogeneous structure can optimize the electron distribution of the material, which in turn improves the adsorption/desorption behavior of the material towards intermediates. Wu et al. prepared V8C7/CoP composites by hydrothermal and solid-phase phosphatization, which required only 290 mV overpotential to achieve a current density of 10 mA cm−2 in 1 M KOH due to the synergistic interaction between V8C7 and CoP [133]. The OER performance of representative Vanadium-based carbides are listed in Table 4.
3.7. Vanadium-Based Phosphides
Transition metal phosphides (TMPs) have good electrical conductivity, as well as a wide range of stoichiometric ratios and crystal shapes that can be adjusted to meet specific requirements. The positively charged metal sites and negatively charged P sites can act as hydrides and proton acceptors, respectively, to enhance HER performance [144,145,146]. In addition, the P-anion protects metal phosphides from being easily dissolved. So, TMPs have good stability over a wide pH range [147,148]. Recently, V-based phosphides used as representative transition metal phosphides have also received extensive attention. The following are the performance and modification strategies of vanadium-based phosphides for the HER and OER.
3.7.1. HER Performance
In vanadium-based phosphides, elemental vanadium has the potential to form V2P crystals with P or to be doped as a cation into the lattice of other metal phosphides. Suo et al. synthesized Fe-doped vanadium phosphide (V2P) by a two-step hydrothermal and solid-phase phosphatization method. The high valence state of V is favorable for the enhancement of the binding ability of the catalyst surface to H* during the HER process. In addition, the morphology and conductivity of vanadium phosphides can be modified by introducing Fe doping. Thanks to these advantages, FexVy-PC/NF can reach 10 mA cm−2 current density in 1 M KOH with only 66 mV of overpotential [134]. Han et al. prepared vanadium-doped cobalt phosphide (CoVP) on carbon cloth, and the doping of V ions led to the optimization of the surface electronic structure of the CoP nanosheets, which required only an overpotential of 77 mV to obtain a current density of 10 mA cm−2 in 1 M KOH [135]. The HER performance of representative Vanadium-based phosphides are listed in Table 4.
3.7.2. OER Performance
Wang et al. synthesized CoVM (M = P, S, and Se) catalysts with nanosphere morphology consisting of nanoneedles by one-step hydrothermal and low-temperature phosphorization. Among them, the CoVP electrode showed efficient catalytic performance for the OER, which required an overpotential of only 270 mV to acquire a current density of 10 mA cm−2. The good OER performance was attributed to the fast charge transfer rate and the exposure of additional active sites by the synergistic catalytic effect of CoP and V3P. Meanwhile, DFT calculations show that the V3P material exhibits a higher density of states near the Fermi energy level, which enhances the conductivity of the material [149].
Zhu et al. prepared Co-VOx-P nanoflowers on nickel foam as oxygen-removing electrocatalysts and achieved a smaller overpotential of 230 mV for OER at 100 mA cm−2. Experiments and DFT calculations showed that on the Co-VOx-P surface, V doping changed the d-band center of the catalyst (Figure 12A), and the chemisorption of oxygen-containing intermediates on the metal atoms was greatly weakened by V doping (Figure 12B,C). Overall, the Co-VOx-P electrocatalysts showed improved intrinsic catalytic properties enhanced conductivity, easy electron transfer, and good surface adsorption strength due to the combination of P and V. The OER performance of representative Vanadium-based phosphides are listed in Table 4. In summary, V in V-based phosphides not only improves the electrical conductivity of the catalyst but also optimizes the intrinsic activity of the electrocatalyst by tuning the electronic structure [137].
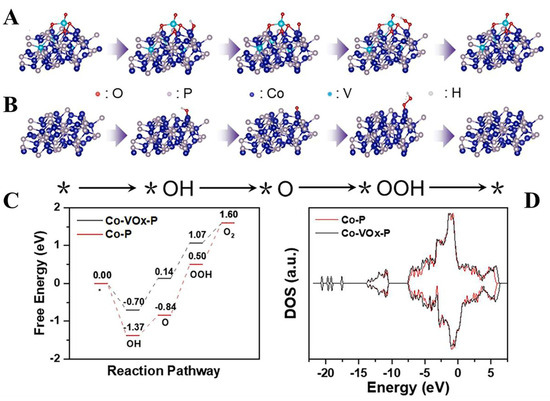
Figure 12.
(A,B) The optimized interaction between intermediates on Co-Vox-P and Co-P surfaces for the OER. (C) The free energy diagram for each elemental step of the OER for Co-Vox-P and Co-P. (D) TDOS of Co-VOx-P and Co-P. Reproduced with permission [137]. Copyright 2022 Elsevier.
4. Summary and Outlook
Vanadium-based electrocatalysts for water splitting have gained a considerable amount of attention in recent decades owing to their many benefits, such as excellent electrocatalytic performance, low cost, and adjustable electronic structure. Nevertheless, a comprehensive overview of vanadium-based electrocatalysts for water splitting is currently lacking. Thus, in this review, we firstly demonstrate the mechanisms of the HER and OER under acidic and alkaline conditions and then focus on the developments currently made in vanadium-based water-splitting electrocatalysts, including vanadium oxides, hydroxides, dichalcogenides, phosphides, nitrides, carbides, and vanadate. Despite notable developments in the research of vanadium-based electrocatalysts for the processes of the HER and OER, there are still numerous existing issues to solve. Herein, we present some valuable perspectives and suggestions on the current obstacles to V-based electrocatalysts for water splitting.
- It is recommended that new V-based water-splitting electrocatalysts, such as V-based phosphides and carbides, are further developed, along with exploring their potential applications in seawater electrolysis. Moreover, the testing conditions for V-based water-splitting electrocatalysts in the literature are often carried out at room temperature and in 1 M KOH solutions. However, higher temperatures (60–80 °C) and more concentrated alkaline environments (30 wt% KOH or 6 M KOH) are more relevant to the actual production process. Therefore, application-related conditions should be considered to comprehensively assess their suitability in the actual production process when conducting performance tests on V-based water-splitting electrocatalysts.
- Recent literature reports suggest that non-oxide/hydroxide V-based water-splitting electrocatalysts may act merely as precatalysts in the OER process, rather than being the true active species involved in the reaction. Therefore, it is necessary to employ a suite of in situ characterization techniques (such as in situ Raman, in situ FTIR, and in situ XPS) along with synchrotron radiation to further ascertain the actual active species of non-oxide/hydroxide V-based electrocatalysts during the OER process and to explore the role of elemental V therein.
- Developing bifunctional V-based nitrides and sulfides as electrocatalysts for overall water splitting presents significant challenges. We have found that vanadium-based sulfides and nitrides, particularly vanadium nitrides, exhibit excellent HER performance. However, due to the sluggish reaction kinetics of the OER, their OER performance is less satisfactory. Therefore, it is necessary to further optimize the adsorption of oxygen-containing intermediates for V-based nitrides and sulfides via doping engineering, surface modification, and other strategies, aiming to boost their OER performance for efficient overall water splitting.
- The stability of V-based electrocatalysts for the HER and OER reported so far is relatively good for small current densities. However, it is essential to recognize that the element V is soluble in aqueous electrolytes. Thus, it is essential to explore the stability of V-based electrocatalysts at high current densities. This issue can be considered from two perspectives: One is to inhibit the dissolution of V during the electrocatalysis process through carbon material encapsulation and valence engineering. Secondly, V-based water-splitting electrocatalysts with a superhydrophilic–superhydrophobic structure can be designed to prevent bubble blockage at the active sites, thereby enhancing stability.
Author Contributions
Writing—original draft preparation, H.L. writing—review and editing, J.W. and M.L. project administration, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the support of this research by the National Natural Science Foundation of China (No. 61761047 and 41876055), Yunnan University’s Practical Innovation Fund for Graduate Students (ZC-23234859), and the Program for Innovative Research Team (in Science and Technology) in the University of Yunnan Province.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, W.; Li, X.; Li, X.; Shao, J.; Yang, H.; Chai, X.; Hu, Q.; He, C. Crafting amorphous VO2–crystalline NiS2 heterostructures as bifunctional electrocatalysts for efficient water splitting: The different cocatalytic function of VO2. Chem. Eng. J. 2023, 470, 144146. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Iqbal, S. Green financing role on renewable energy dependence and energy transition in E7 economies. Renew. Energy 2022, 200, 1561–1572. [Google Scholar] [CrossRef]
- Ameer, W.; Ali, M.S.e.; Farooq, F.; Ayub, B.; Waqas, M. Renewable energy electricity, environmental taxes, and sustainable development: Empirical evidence from E7 economies. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Song, W.; Li, M.; Wang, C.; Lu, X. Electronic modulation and interface engineering of electrospun nanomaterials-based electrocatalysts toward water splitting. Carbon Energy 2021, 3, 101–128. [Google Scholar] [CrossRef]
- Pan, S.; Ma, Z.; Yang, W.; Dongyang, B.; Yang, H.; Lai, S.; Dong, F.; Yang, X.; Lin, Z. Magnesium incorporation activates perovskite cobaltites toward efficient and stable electrocatalytic oxygen evolution. Mater. Rep. Energy 2023, 3, 100212. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of hydrogen energy: Bio-hydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Daus, Y.V.; Kovalev, A.A.; Yudaev, I.V.; Litti, Y.V. Prospects for the production of green hydrogen: Review of countries with high potential. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Y.; Tian, X.; Xing, X.; Cao, M.; Wang, Y. A crystalline/amorphous FeCo alloy/FeCoNi-Pi bifunctional electrocatalyst for efficient overall water splitting. Inorg. Chem. Front. 2024. [Google Scholar] [CrossRef]
- Sonadia; Iqbal, Z.; Miran, W.; Ul-Hamid, A.; Ayub, K.S.; Azad, F. Enhanced Electrocatalytic Performance of Erbium-Incorporated Nickel-Based Metal–Organic Frameworks for Water Splitting. Energy Fuels 2024, 38, 5397–5406. [Google Scholar] [CrossRef]
- Yue, D.; Feng, T.; Zhu, Z.; Lu, S.; Yang, B. Ir Single Atom-Doped Ni2P Anchored by Carbonized Polymer Dots for Robust Overall Water Splitting. ACS Catal. 2024, 14, 3006–3017. [Google Scholar] [CrossRef]
- Mohili, R.; Hemanth, N.R.; Jin, H.; Lee, K.; Chaudhari, N. Emerging high entropy metal sulphides and phosphides for electrochemical water splitting. J. Mater. Chem. A 2023, 11, 10463–10472. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Li, F.; Li, L.; Wu, J.; Liu, S.; Cheng, T.; Xu, Y.; Shao, Q.; Huang, X. Single-site Pt-doped RuO2 hollow nanospheres with interstitial C for high-performance acidic overall water splitting. Sci. Adv. 2022, 8, eabl9271. [Google Scholar] [CrossRef]
- Nicole, S.L.D.; Li, Y.; Xie, W.; Wang, G.; Lee, J.-M. Heterointerface and Tensile Strain Effects Synergistically Enhances Overall Water-Splitting in Ru/RuO2 Aerogels. Small 2023, 19, 2206844. [Google Scholar] [CrossRef]
- Wang, N.; Ning, S.; Yu, X.; Chen, D.; Li, Z.; Xu, J.; Meng, H.; Zhao, D.; Li, L.; Liu, Q.; et al. Graphene composites with Ru-RuO2 heterostructures: Highly efficient Mott–Schottky-type electrocatalysts for pH-universal water splitting and flexible zinc–air batteries. Appl. Catal. B 2022, 302, 120838. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-Rich RuCu Nanosheets for pH-Universal Overall Water Splitting Electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef]
- Batool, M.; Hameed, A.; Nadeem, M.A. Recent developments on iron and nickel-based transition metal nitrides for overall water splitting: A critical review. Coord. Chem. Rev. 2023, 480, 215029. [Google Scholar] [CrossRef]
- Sabir, A.S.; Pervaiz, E.; Khosa, R.; Sohail, U. An inclusive review and perspective on Cu-based materials for electrochemical water splitting. RSC Adv. 2023, 13, 4963–4993. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhang, H.; Zou, Z.; Li, C.M. Mesoporous molybdenum carbide for greatly enhanced hydrogen evolution at high current density and its mechanism studies. Mater. Rep. Energy 2023, 3, 100215. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, e202301534. [Google Scholar] [CrossRef]
- Han, Y.H.; Lee, S.H.; Kim, K.T.; Choi, I.H.; Moon, S. Properties of Electrical Conductivity of Amorphous Tungsten-Doped Vanadium Oxide for Uncooled Microbolometers. Solid State Phenom. 2007, 124–126, 343–346. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Kim, M.; Lee, J.; Kim, K.J.; Choi, J.W. Corrosion as the origin of limited lifetime of vanadium oxide-based aqueous zinc ion batteries. Nat. Commun. 2022, 13, 2371. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Thang, A.Q.; Li, F.-L.; Chen, D.; Geng, H.; Rui, X.; Yan, Q. Vanadium-based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. SmartMat 2022, 3, 384–416. [Google Scholar] [CrossRef]
- Wu, C.; Zhong, M.; Tan, Y. Highly efficient and stable vanadium-based electrocatalysts: Stoichiometric iron vanadium sulfides for water-oxidation at large current densities. Chem. Eng. J. 2023, 477, 146981. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khan, A.; Al-Ahmed, A.; Hakeem, A.S.; Afzaal, M.; Pandey, S.; Mahar, N. Aerosol-assisted chemical vapour deposited vanadium oxide thin films on nickel foam with auspicious electrochemical water oxidation properties. Int. J. Hydrogen Energy 2024, 52, 718–727. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.-X.; Luan, R.-N.; Li, C.-R.; Liu, X.; Zhao, H.-Y.; Wang, Y.-H.; Chai, Y.-M.; Dong, B. Scalloped nickel/iron vanadium oxide-coated vanadium dioxides based on chemical etching-induced reconstruction strategy for efficient oxygen evolution. Int. J. Hydrogen Energy 2022, 47, 33352–33360. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Araki, K.; Angnes, L. Recent progress in water splitting and hybrid supercapacitors based on nickel-vanadium layered double hydroxides. J. Energy Chem. 2021, 57, 496–515. [Google Scholar] [CrossRef]
- Yang, M.; Shang, C.; Li, F.; Liu, C.; Wang, Z.; Gu, S.; Liu, D.; Cao, L.; Zhang, J.; Lu, Z.; et al. Synergistic electronic and morphological modulation on ternary Co1−xVxP nanoneedle arrays for hydrogen evolution reaction with large current density. Sci. China Mater. 2021, 64, 880–891. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yu, M.; Yu, C.; Shen, P. Amorphous metallic ultrathin nanostructures: A latent ultra-high-density atomic-level catalyst for electrochemical energy conversion. Int. J. Hydrogen Energy 2022, 47, 26956–26977. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Lu, X.F.; Zang, S.-Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, J.; He, G.; Chen, H. Current and future trends for spinel-type electrocatalysts in electrocatalytic oxygen evolution reaction. Coord. Chem. Rev. 2023, 475, 214869. [Google Scholar] [CrossRef]
- Kashif, M.; Thangarasu, S.; Murugan, N.; Magdum, S.S.; Kim, Y.A.; Kurkuri, M.; Oh, T.-H. Interatomic interaction of 2D crumpled V2O5 nanosheets layered with Ni-MOF as a bifunctional electrocatalyst for overall water splitting and supercapacitor applications. J. Energy Storage 2024, 81, 110348. [Google Scholar] [CrossRef]
- Ha, Q.-N.; Yeh, C.-H.; Gultom, N.S.; Kuo, D.-H. Industrial-scale efficient alkaline water electrolysis achieved with sputtered NiFeV-oxide thin film electrodes for green hydrogen production. J. Mater. Chem. A 2024, 12, 460–474. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, X.; Gao, Y.; Cui, Z.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Dai, Y.; Huang, B. Enhanced electrocatalytic HER performance of non-noble metal nickel by introduction of divanadium trioxide. Electrochim. Acta 2019, 320, 134535. [Google Scholar] [CrossRef]
- Liu, G.; Lv, H.; Quan, Q.; Li, X.; Lu, H.; Li, W.; Cui, X.; Jiang, L. Self-powered electrolysis systems for sustainable hydrogen generation from natural seawater via a Ni/V2O3 Schottky electrode. Chem. Eng. J. 2022, 450, 138079. [Google Scholar] [CrossRef]
- Fan, X.-Z.; Pang, Q.-Q.; Fan, F.; Yao, H.-C.; Li, Z.-J. Ultra-fine Ru nanoparticles decorated V2O3 as a pH-universal electrocatalyst for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 20577–20587. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Wang, S.; Chu, B.; Li, R.; Li, B.; Dong, L.; Fan, M.; Chen, Z. An interface engineering induced hierarchical NiCo/V2O3/C Schottky heterojunction catalyst for large-current-density hydrogen evolution reaction. J. Mater. Chem. A 2023, 11, 23397–23404. [Google Scholar] [CrossRef]
- Lee, S.; Ivanov, I.N.; Keum, J.K.; Lee, H.N. Epitaxial stabilization and phase instability of VO2 polymorphs. Sci. Rep. 2016, 6, 19621. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, X.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, K.-H.; Choi, W.; Kim, Y.-M.; Hong, S.-H.; Choi, Y.-H. Mapping the electrocatalytic water splitting activity of VO2 across its insulator-to-metal phase transition. Nanoscale 2022, 14, 8281–8290. [Google Scholar] [CrossRef]
- Haldar, K.K.; Ahmed, I.; Biswas, R.; Mete, S.; Patil, R.A.; Ma, Y.-R. Efficient MoS2/V2O5 Electrocatalyst for Enhanced Oxygen and Hydrogen Evolution Reactions. Electrocatalysis 2023, 14, 624–635. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, L.; Tang, J.; Chai, J.; Xu, J.; Cao, L.; Yang, M.; Yang, M.; Kong, W.; Wang, S.; et al. Efficient coupling of a hierarchical V2O5@Ni3S2 hybrid nanoarray for pseudocapacitors and hydrogen production. J. Mater. Chem. A 2017, 5, 17954–17962. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Xia, Y.; Zhang, Y.; Zhang, B.; Du, Y.; Wang, H.-L.; Li, S.; Xu, P. Surface oxidation protection strategy of CoS2 by V2O5 for electrocatalytic hydrogen evolution reaction. Nanoscale Horiz. 2023, 8, 338–345. [Google Scholar] [CrossRef]
- Tang, J.; Ruan, Q.; Yu, H.; Huang, C. Activating Co(OH)2 Active Sites by Coupled with V2O5 to Boost Highly Efficient Oxygen Evolution Reaction. Adv. Sustain. Syst. 2023, 7, 2200473. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, Y.; Tang, X.; Huang, Q.; Huang, L. Fast and in situ releasing of active species confined in two-dimensional layers for superior overall electrochemical water splitting. Appl. Catal. B 2024, 341, 123319. [Google Scholar] [CrossRef]
- He, D.; Cao, L.; Huang, J.; Kajiyoshi, K.; Wu, J.; Wang, C.; Liu, Q.; Yang, D.; Feng, L. In-situ optimizing the valence configuration of vanadium sites in NiV-LDH nanosheet arrays for enhanced hydrogen evolution reaction. J. Energy Chem. 2020, 47, 263–271. [Google Scholar] [CrossRef]
- Li, W.; Feng, B.; Yi, L.; Li, J.; Hu, W. Highly Efficient Alkaline Water Splitting with Ru-Doped Co−V Layered Double Hydroxide Nanosheets as a Bifunctional Electrocatalyst. ChemSusChem 2021, 14, 730–737. [Google Scholar] [CrossRef]
- De, A.; Madhu, R.; Bera, K.; Dhandapani, H.N.; Nagappan, S.; Singha Roy, S.; Kundu, S. Deciphering the amplification of dual catalytic active sites of Se-doped NiV LDH in water electrolysis: A hidden gem exposure of anion doping at the core-lattice LDH framework. J. Mater. Chem. A 2023, 11, 25055–25071. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Liu, M.; Hou, G.-Y.; Tang, Y.-P.; Wu, L.-K. The release of metal ions induced surface reconstruction of layered double hydroxide electrocatalysts. Sustain. Energy Fuels 2021, 5, 3436–3444. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, B.; Min, L.; Xu, W.; Zhang, W. Preparation of NiCo-LDH@NiCoV-LDH interconnected nanosheets as high-performance electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2022, 47, 15583–15592. [Google Scholar] [CrossRef]
- Srividhya, G.; Sangavi, T.; Viswanathan, C.; Ponpandian, N. Cobalt–Iron Co-substituted NiV Layered Double Hydroxide as a High-Performance Electrocatalyst for Oxygen Evolution Reaction in a Neutral Saline Medium. ACS Appl. Energy Mater. 2024, 7, 154–164. [Google Scholar] [CrossRef]
- Maji, M.; Dihingia, N.; Dutta, S.; Parvin, S.; Pati, S.K.; Bhattacharyya, S. Charge transfer modulated heterointerfaces for hydrogen production at all pH values. J. Mater. Chem. A 2022, 10, 24927–24937. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Zhao, Y.; Cao, L.; Li, K.; Zhang, N.; Yang, D.; Feng, L.; Feng, L. Tuning the coupling interface of ultrathin Ni3S2@NiV-LDH heterogeneous nanosheet electrocatalysts for improved overall water splitting. Nanoscale 2019, 11, 8855–8863. [Google Scholar] [CrossRef]
- Najafi, L.; Oropesa-Nuñez, R.; Bellani, S.; Martín-García, B.; Pasquale, L.; Serri, M.; Drago, F.; Luxa, J.; Sofer, Z.; Sedmidubský, D.; et al. Topochemical Transformation of Two-Dimensional VSe2 into Metallic Nonlayered VO2 for Water Splitting Reactions in Acidic and Alkaline Media. ACS Nano 2022, 16, 351–367. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, F.; Zhou, S.; Hu, W.; Zhang, H.; Dong, J.; Jiang, Z.; Zhao, J.; Li, J.; Yan, W.; et al. Atomic-level insight into super-efficient electrocatalytic oxygen evolution on iron and vanadium co-doped nickel (oxy)hydroxide. Nat. Commun. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Bera, K.; Dhandapani, H.N.; Nagappan, S.; Murugan, P.; Kundu, S. Stabilization of ruthenium nanoparticles over NiV-LDH surface for enhanced electrochemical water splitting: An oxygen vacancy approach. J. Mater. Chem. A 2022, 10, 3618–3632. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Li, R.; Wang, L.; Zhang, K.; Zhou, P.; Huang, X.; Wang, G. Constructing accelerated charge transfer channels along V-Co-Fe via introduction of V into CoFe-layered double hydroxides for overall water splitting. Appl. Catal. B 2021, 298, 120587. [Google Scholar] [CrossRef]
- Guan, J.; Li, X.; Zhu, Y.; Dai, Y.; Zhang, R.; Guo, B.; Zhang, M. Ni5P4-embedded FeV LDH porous nanosheets for enhancing oxygen evolution and urea oxidation reactions. New J. Chem. 2023, 47, 16964–16971. [Google Scholar] [CrossRef]
- Wan, X.; Song, Y.; Zhou, H.; Shao, M. Layered Double Hydroxides for Oxygen Evolution Reaction towards Efficient Hydrogen Generation. Energy Mater. Adv. 2022, 2022, 9842610. [Google Scholar] [CrossRef]
- Laipan, M.; Yu, J.; Zhu, R.; Zhu, J.; Smith, A.T.; He, H.; O’Hare, D.; Sun, L. Functionalized layered double hydroxides for innovative applications. Mater. Horiz. 2020, 7, 715–745. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Ireno da Silva, M.; Angnes, L.; Araki, K. Vanadium-containing electro and photocatalysts for the oxygen evolution reaction: A review. J. Mater. Chem. A 2020, 8, 2171–2206. [Google Scholar] [CrossRef]
- Ansari, M.S.; Kim, H. Enhanced electrocatalytic oxygen evolution reaction kinetics using dual-phase engineering of self-supported hierarchical NiCoV(OH)x nanowire arrays. Fuel 2021, 304, 121309. [Google Scholar] [CrossRef]
- Zeng, K.; Tian, M.; Chen, X.; Zhang, J.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Strong electronic coupling between single Ru atoms and cobalt-vanadium layered double hydroxide harness efficient water splitting. Chem. Eng. J. 2023, 452, 139151. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Z.; Shao, W.; Gao, Y.; Wang, W.; Ma, T.; Ma, L.; Li, S.; Cheng, C.; Zhao, C. Interfacial Atom-Substitution Engineered Transition-Metal Hydroxide Nanofibers with High-Valence Fe for Efficient Electrochemical Water Oxidation. Angew. Chem. Int. Ed. 2022, 61, e202115331. [Google Scholar] [CrossRef]
- Nejati, K.; Akbari, A.R.; Davari, S.; Asadpour-Zeynali, K.; Rezvani, Z. Zn–Fe-layered double hydroxide intercalated with vanadate and molybdate anions for electrocatalytic water oxidation. New J. Chem. 2018, 42, 2889–2895. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Xiang, M.; Yu, C.; Hui, J.; Dong, S. Coral reef structured cobalt-doped vanadate oxometalate nanoparticle for a high-performance electrocatalyst in water splitting. Int. J. Hydrogen Energy 2022, 47, 31566–31574. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, W.; Zhang, P.; Zhuang, H.; Xiang, M.; Hui, J. Ni2V2O7 dandelion microsphere for a high-performance electrocatalyst in water splitting. Int. J. Hydrogen Energy 2021, 46, 39658–39664. [Google Scholar] [CrossRef]
- Guo, W.; Yang, T.; Zhang, H.; Zhou, H.; Wei, W.; Liang, W.; Zhou, Y.; Yu, T.; Zhao, H. Charge-counterbalance modulated amorphous nickel oxide for efficient alkaline hydrogen and oxygen evolution. Chem. Eng. J. 2023, 470, 144241. [Google Scholar] [CrossRef]
- Pan, M.; Qian, G.; Yu, T.; Chen, J.; Luo, L.; Zou, Y.; Yin, S. Ni modified Co2VO4 heterojunction with poor/rich-electron structure for overall urea-rich wastewater oxidation. Chem. Eng. J. 2022, 435, 134986. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Guo, D.; Li, J.; Chu, D.; Na, S.; Yu, M.; Li, D.; Sui, G.; Chai, D.-F. Anchoring metal-organic framework-derived hollow CoV2O6 nanocubes onto lattice tensile strained V2CTx MXene for superior overall water splitting. J. Alloys Compd. 2023, 963, 171133. [Google Scholar] [CrossRef]
- Pan, M.; Chen, W.; Qian, G.; Yu, T.; Wang, Z.; Luo, L.; Yin, S. Carbon-encapsulated Co3V decorated Co2VO4 nanosheets for enhanced urea oxidation and hydrogen evolution reaction. Electrochim. Acta 2022, 407, 139882. [Google Scholar] [CrossRef]
- Luo, Z.; Peng, Q.; Huang, Z.; Wang, L.; Yang, Y.; Dong, J.; Isimjan, T.T.; Yang, X. Fine-tune d-band center of cobalt vanadium oxide nanosheets by N-doping as a robust overall water splitting electrocatalyst. J. Colloid Interface Sci. 2023, 629, 111–120. [Google Scholar] [CrossRef]
- Gyanprakash, D.M.; Sharma, G.P.; Gupta, P.K. Isovalent anion-induced electrochemical activity of doped Co3V2O8 for oxygen evolution reaction application. Dalton Trans. 2022, 51, 15312–15321. [Google Scholar] [CrossRef]
- Li, A.; Tang, X.; Cao, R.; Song, D.; Wang, F.; Yan, H.; Chen, H.; Wei, Z. Directed Surface Reconstruction of Fe Modified Co2VO4 Spinel Oxides for Water Oxidation Catalysts Experiencing Self-Terminating Surface Deterioration. Adv. Mater. 2024, 2401818. [Google Scholar] [CrossRef]
- Ma, H.; Sun, C.; Wang, Z.; Jiang, Q. Tuning the electronic structure of NiCoVOx nanosheets through S doping for enhanced oxygen evolution. Nanoscale 2021, 13, 17022–17027. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, W.; Chu, J.-F. Novel Co3(1–x)Fe3xV2O8 Nanoparticles as Highly Active and Noble-Metal-Free Electrocatalysts for Oxygen Evolution Reaction. Energy Fuels 2020, 34, 15019–15025. [Google Scholar] [CrossRef]
- Mondal, A.; Ganguli, S.; Inta, H.R.; Mahalingam, V. Influence of Vanadate Structure on Electrochemical Surface Reconstruction and OER Performance of CoV2O6 and Co3V2O8. ACS Appl. Energy Mater. 2021, 4, 5381–5387. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Shen, J.; Ji, X.; Liu, J. Bifunctional keel flower-like Ni-Co-V multicomponent oxide catalyst with enhanced electron transport for accelerating overall water splitting. J. Colloid Interface Sci. 2022, 628, 467–476. [Google Scholar] [CrossRef]
- Aftab, S.; Iqbal, M.Z.; Rim, Y.S. Recent Advances in Rolling 2D TMDs Nanosheets into 1D TMDs Nanotubes/Nanoscrolls. Small 2023, 19, 2205418. [Google Scholar] [CrossRef]
- Tang, X.; Hao, Q.; Hou, X.; Lan, L.; Li, M.; Yao, L.; Zhao, X.; Ni, Z.; Fan, X.; Qiu, T. Exploring and Engineering 2D Transition Metal Dichalcogenides toward Ultimate SERS Performance. Adv. Mater. 2024, 36, 2312348. [Google Scholar] [CrossRef]
- Sanap, P.P.; Gupta, S.P.; Kahandal, S.S.; Gunjakar, J.L.; Lokhande, C.D.; Sankapal, B.R.; Said, Z.; Bulakhe, R.N.; Man Kim, J.; Bhalerao, A.B. Exploring vanadium-chalcogenides toward solar cell application: A review. J. Ind. Eng. Chem. 2024, 129, 124–142. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, J.; Hardy, W.J.; Loya, P.; Lou, M.; Yang, Y.; Najmaei, S.; Jiang, M.; Qin, F.; Keyshar, K.; et al. Facile Synthesis of Single Crystal Vanadium Disulfide Nanosheets by Chemical Vapor Deposition for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2015, 27, 5605–5609. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic Few-Layered VS2 Ultrathin Nanosheets: High Two-Dimensional Conductivity for In-Plane Supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef]
- Karthick, K.; Bijoy, T.K.; Sivakumaran, A.; Mansoor Basha, A.B.; Murugan, P.; Kundu, S. Enhancing Hydrogen Evolution Reaction Activities of 2H-Phase VS2 Layers with Palladium Nanoparticles. Inorg. Chem. 2020, 59, 10197–10207. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Sarfraz, M.; Faizan, M.; Patil, S.A.; Batoo, K.M.; Nam, K.-W.; Kim, H.-S.; Jung, J. Design of XS2 (X = W or Mo)-Decorated VS2 Hybrid Nano-Architectures with Abundant Active Edge Sites for High-Rate Asymmetric Supercapacitors and Hydrogen Evolution Reactions. Small 2023, 19, 2205881. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Huang, Y.; Zhang, C.; Li, F.; Shu, H. The Role of Intrinsic Defects in Electrocatalytic Activity of Monolayer VS2 Basal Planes for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2017, 121, 1530–1536. [Google Scholar] [CrossRef]
- Wang, L.; Gao, W.; Chen, X.; Liu, Y.; Qureshi, A.H.; Liu, Y.; Chen, M.; Zhang, X.; Guo, Y.; Wang, J. Charge-Modulated VS2 Monolayer for Effective Hydrogen Evolution Reaction. J. Phys. Chem. C 2021, 125, 12004–12011. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Wang, Z.; Zhu, J.; Wen, Z.; Zhao, X.; Zhang, X.; Xu, J.; Lu, Z. Synergistic Interlayer and Defect Engineering in VS2 Nanosheets toward Efficient Electrocatalytic Hydrogen Evolution Reaction. Small 2018, 14, 1703098. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Yu, B.; Fang, C.; Zhang, J. Metallic 1T-VS2 nanosheets featuring V2+ self-doping and mesopores towards an efficient hydrogen evolution reaction. Inorg. Chem. Front. 2019, 6, 3510–3517. [Google Scholar] [CrossRef]
- Singh, V.K.; Nakate, U.T.; Bhuyan, P.; Chen, J.; Tran, D.T.; Park, S. Mo/Co doped 1T-VS2 nanostructures as a superior bifunctional electrocatalyst for overall water splitting in alkaline media. J. Mater. Chem. A 2022, 10, 9067–9079. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Peng, J.; Dong, H.; Wang, J.; Zhao, W. Mo-dopant-strengthened basal-plane activity in VS2 for accelerating hydrogen evolution reaction. Chem. Eng. J. 2020, 396, 125227. [Google Scholar] [CrossRef]
- Mohan, P.; Yang, J.; Jena, A.; Suk Shin, H. VS2/rGO hybrid nanosheets prepared by annealing of VS4/rGO. J. Solid State Chem. 2015, 224, 82–87. [Google Scholar] [CrossRef]
- Patil, S.A.; Rabani, I.; Hussain, S.; Seo, Y.-S.; Jung, J.; Shrestha, N.K.; Im, H.; Kim, H. A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage. Nanomaterials 2022, 12, 339. [Google Scholar] [CrossRef]
- Du, C.; Liang, D.; Shang, M.; Zhang, J.; Mao, J.; Liu, P.; Song, W. In Situ Engineering MoS2 NDs/VS2 Lamellar Heterostructure for Enhanced Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 15471–15479. [Google Scholar] [CrossRef]
- Hou, Z.; Cui, C.; Yang, Y.; Zhang, T. Electrochemical Oxidation Encapsulated Ru Clusters Enable Robust Durability for Efficient Oxygen Evolution. Small 2023, 19, 2207170. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S.; et al. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Kwak, I.H.; Kim, J.Y.; Zewdie, G.M.; Yang, J.; Lee, K.-S.; Yoo, S.J.; Kwon, I.S.; Park, J.; Kang, H.S. Electrocatalytic Activation in ReSe2-VSe2 Alloy Nanosheets to Boost Water-Splitting Hydrogen Evolution Reaction. Adv. Mater. 2024, 36, 2310769. [Google Scholar] [CrossRef]
- Pan, U.N.; Kandel, M.R.; Tomar, A.K.; Kim, N.H.; Lee, J.H. Synchronous Surface-Interface and Crystal-Phase Engineered Multifaceted Hybrid Nanostructure of Fe-(1T)-VSe2 Nanosheet and Fe-CoSe2 Nanorods Doped with P for Rapid HER and OER, Kinetics. Small 2024, 20, 2305519. [Google Scholar] [CrossRef]
- Samal, R.; Shinde, P.V.; Rout, C.S. 2D Vanadium diselenide supported on reduced graphene oxide for water electrolysis: A comprehensive study in alkaline media. Emergent Mater. 2021, 4, 1047–1053. [Google Scholar] [CrossRef]
- Tomar, S.; Sen, P.; Chakraborty, S. Single atom functionalization in vanadium dichalcogenide monolayers: Towards enhanced electrocatalytic activity. Sustain. Energy Fuels 2022, 6, 5337–5344. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Z.; Zhao, J. Computational screening of single-atom catalysts supported by VS2 monolayers for electrocatalytic oxygen reduction/evolution reactions. Nanoscale 2022, 14, 6902–6911. [Google Scholar] [CrossRef]
- Dhakal, P.P.; Pan, U.N.; Paudel, D.R.; Kandel, M.R.; Kim, N.H.; Lee, J.H. Cobalt–manganese sulfide hybridized Fe-doped 1T-Vanadium disulfide 3D-Hierarchical core-shell nanorods for extreme low potential overall water-splitting. Mater. Today Nano 2022, 20, 100272. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Hong, M.; Jiang, S.; Zhao, G.; Shi, J.; Xie, Q.; Zhang, Y. Recent progress in the controlled synthesis of 2D metallic transition metal dichalcogenides. Nanotechnology 2019, 30, 182002. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Kan, C.-m.; He, D.; Li, Z.; Hao, Q.; Zhao, H.; Wu, C.; Jin, C.; Cui, X. Structural Phase Transition of Multilayer VSe2. ACS Appl. Mater. Interfaces 2020, 12, 25143–25149. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, B.; Guo, Z.; Su, G.; Gao, R.; Wang, W.; Cao, L. Colloidal synthesis of VSe2 single-layer nanosheets as novel electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2016, 52, 9228–9231. [Google Scholar] [CrossRef]
- Fu, J.; Ali, R.; Mu, C.; Liu, Y.; Mahmood, N.; Lau, W.-M.; Jian, X. Large-scale preparation of 2D VSe2 through a defect-engineering approach for efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 411, 128494. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Z.; Tan, X.; Zhang, J.; Yang, L.; Wang, W.; Cao, L.; Dong, B. Atmosphere plasma treatment and Co heteroatoms doping on basal plane of colloidal 2D VSe2 nanosheets for enhanced hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 32425–32434. [Google Scholar] [CrossRef]
- Kuniyil, N.; Koottumvathukkal Anil Raj, S.R.; Rout, C.S. Selective Growth of Molybdenum Sulfo-Selenides on a Defect-Rich Carbon Nanotube Skeleton for Efficient Energy Storage and Hydrogen Generation Applications. Energy Fuels 2022, 36, 13346–13355. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Huang, H.; Chen, W.; Cui, Y.; Li, Y.; Mao, W.; Zhu, X.; Li, X.a. Spatial Relation Controllable Di-Defects Synergy Boosts Electrocatalytic Hydrogen Evolution Reaction over VSe2 Nanoflakes in All pH Electrolytes. Small 2022, 18, 2204557. [Google Scholar] [CrossRef]
- Wang, C.; Jin, M.; Liu, D.; Liang, F.; Luo, C.; Li, P.; Cai, C.; Bi, H.; Wu, X.; Di, Z. VSe2 quantum dots with high-density active edges for flexible efficient hydrogen evolution reaction. J. Phys. D Appl. Phys. 2021, 54, 214006. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Yu, L.-F.; Yang, H.; Xu, X.; Wang, F.; Xu, X.-H. Room-temperature ferromagnetism enhancement in Fe-doped VSe2 nanosheets synthesized by a chemical method. Rare Met. 2021, 40, 2501–2507. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, C.; Luo, X.; Zhou, W.; Jiang, Z.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C. Field-Free Improvement of Oxygen Evolution Reaction in Magnetic Two-Dimensional Heterostructures. Nano Lett. 2021, 21, 10486–10493. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, S.; Yang, H.; Xi, S.; Gracia, J.; Xu, Z.J. Spin-Related Electron Transfer and Orbital Interactions in Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2003297. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Ye, K.; Yuan, C.; Zhu, M.; Yu, H.; Yang, H.; Huang, H.; Wu, Y.; Zhang, J.; et al. External Fields Assisted Highly Efficient Oxygen Evolution Reaction of Confined 1T-VSe2 Ferromagnetic Nanoparticles. Small 2023, 19, 2300122. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Zhang, J.; Lv, M.; Li, S.; Song, H.; Cui, Z.; Du, L.; Liao, S. Recent advances in nanostructured transition metal nitrides for fuel cells. J. Mater. Chem. A 2020, 8, 20803–20818. [Google Scholar] [CrossRef]
- Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. [Google Scholar] [CrossRef]
- Fu, C.; Feng, L.; Yin, H.; Li, Y.; Li, G.; Feng, Y.; Cao, L.; Huang, J.; Liu, Y. Synergistic Coupling of Hierarchical Heterostructured Ni3P/Ni/VN Microflower Electrocatalyst for Enhanced Hydrogen Evolution Reaction. ChemCatChem 2023, 15, e202201399. [Google Scholar] [CrossRef]
- He, X.; Tian, Y.; Deng, D.; Chen, F.; Wu, J.; Qian, J.; Li, H.; Xu, L. Engineering Antiperovskite Ni4N/VN Heterostructure with Improved Intrinsic Interfacial Charge Transfer as a Bifunctional Catalyst for Rechargeable Zinc–Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 17007–17015. [Google Scholar] [CrossRef]
- Meng, K.; Wen, S.; Liu, L.; Jia, Z.; Wang, Y.; Shao, Z.; Qi, T. Vertically Grown MoS2 Nanoplates on VN with an Enlarged Surface Area as an Efficient and Stable Electrocatalyst for HER. ACS Appl. Energy Mater. 2019, 2, 2854–2861. [Google Scholar] [CrossRef]
- Zhou, P.; Xing, D.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. Accelerated electrocatalytic hydrogen evolution on non-noble metal containing trinickel nitride by introduction of vanadium nitride. J. Mater. Chem. A 2019, 7, 5513–5521. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, F.; Xie, K. Porous single-crystalline vanadium nitride octahedra with a unique electrocatalytic performance. New J. Chem. 2022, 46, 1392–1398. [Google Scholar] [CrossRef]
- Feng, J.; Tang, R.; Liu, G.; Meng, T. Regulating d-orbital electronic character and HER free energy of VN electrocatalyst by anchoring single atom. Chem. Eng. J. 2023, 452, 139131. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, K.; Li, X.; Hui, C.; Huang, J.; Deng, Z.; Yang, D.; Cao, L.; Feng, L. Ni and VN Nanoparticles Supported on N-Doped Carbon Layer Containing Ni Single Atoms as Electrocatalyst for the Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2024, 7, 4059–4067. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, Z.; Zhang, X.; Gao, B.; Zheng, Y.; Chu, P.K.; Yang, L.; Huo, K. In situ construction of γ-MoC/VN heterostructured electrocatalysts with strong electron coupling for highly efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 416, 129130. [Google Scholar] [CrossRef]
- Li, Q.; Yang, P.; Liu, Y.; Xiao, W.; Xiao, Z.; Xu, G.; Wang, L.; Liu, F.; Wu, Z. In-situ construction of pomegranate-like nickel-vanadium nitride for hydrogen production through urea-assisted water-splitting. J. Alloys Compd. 2023, 968, 171861. [Google Scholar] [CrossRef]
- Meng, K.; Zheng, Z.; Cao, J.; Liu, L.; Jia, Z.; Wang, Y.; Qi, T. Vanadium nitride based CoFe prussian blue analogues for enhanced electrocatalytic oxygen evolution. Int. J. Hydrogen Energy 2020, 45, 31410–31417. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Feng, L.; He, D.; Liu, Z.; Li, G.; Zhang, N.; Feng, Y.; Cao, L. Molybdenum and cobalt co-doped VC nanoparticles encapsulated in nanocarbon as efficient electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2022, 9, 870–878. [Google Scholar] [CrossRef]
- Zheng, Y.; Mou, Y.; Wang, Y.; Wan, J.; Yao, G.; Feng, C.; Sun, Y.; Dai, L.; Zhang, H.; Wang, Y. Aluminum-incorporation activates vanadium carbide with electron-rich carbon sites for efficient pH-universal hydrogen evolution reaction. J. Colloid Interface Sci. 2024, 656, 367–375. [Google Scholar] [CrossRef]
- Peng, X.; Huang, C.; Zhang, B.; Liu, Y. Vanadium carbide nanodots anchored on N doped carbon nanosheets fabricated by spatially confined synthesis as a high-efficient electrocatalyst for hydrogen evolution reaction. J. Power Sources 2021, 490, 229551. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, M.; Wen, Z.; Ci, S. V8C7 decorating CoP nanosheets-assembled microspheres as trifunctional catalysts toward energy-saving electrolytic hydrogen production. Chem. Eng. J. 2020, 399, 125728. [Google Scholar] [CrossRef]
- Suo, N.; Han, X.; Chen, C.; He, X.; Dou, Z.; Lin, Z.; Cui, L.; Xiang, J. Engineering vanadium phosphide by iron doping as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2020, 333, 135531. [Google Scholar] [CrossRef]
- Han, H.; Yi, F.; Choi, S.; Kim, J.; Kwon, J.; Park, K.; Song, T. Self-supported vanadium-incorporated cobalt phosphide as a highly efficient bifunctional electrocatalyst for water splitting. J. Alloys Compd. 2020, 846, 156350. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, F.; Wang, J.; Song, Y.; Cheng, J.; Mao, M.; Ma, H.; Lu, J.; Cheng, Y. Multi-channel V-doped CoP hollow nanofibers as high-performance hydrogen evolution reaction electrocatalysts. Nanoscale 2020, 12, 9144–9151. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, K.; Guo, W.; Zhang, H.; Xiao, X.; He, M.; Yu, T.; Zhao, H.; Zhang, D.; Yang, T. Vanadium-phosphorus incorporation induced interfacial modification on cobalt catalyst and its super electrocatalysis for water splitting in alkaline media. Appl. Catal. B 2022, 304, 120985. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Huang, D.; Xiong, T.; Li, M.; Balogun, M.S.; Tong, Y. Efficient hydrogen and oxygen evolution electrocatalysis by cobalt and phosphorus dual-doped vanadium nitride nanowires. Mater. Today Chem. 2019, 11, 1–7. [Google Scholar] [CrossRef]
- Peng, L.; Shen, J.; Zhang, L.; Wang, Y.; Xiang, R.; Li, J.; Li, L.; Wei, Z. Graphitized carbon-coated vanadium carbide nanoboscages modified by nickel with enhanced electrocatalytic activity for hydrogen evolution in both acid and alkaline solutions. J. Mater. Chem. A 2017, 5, 23028–23034. [Google Scholar] [CrossRef]
- Ma, W.; Wan, J.; Fu, W.; Wu, Y.; Wang, Y.; Zhang, H.; Wang, Y. Heterostructures induced between platinum nanoparticles and vanadium carbide boosting hydrogen evolution reaction. Appl. Catal. A 2022, 633, 118512. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, N.; Feng, L.; Huang, J.; Feng, Y.; Li, W.; Yang, D.; Liu, Q. Well-dispersed ultrasmall VC nanoparticles embedded in N-doped carbon nanotubes as highly efficient electrocatalysts for hydrogen evolution reaction. Nanoscale 2018, 10, 14272–14279. [Google Scholar] [CrossRef]
- Wan, J.; Wang, C.; Tang, Q.; Gu, X.; He, M. First-principles study of vanadium carbides as electrocatalysts for hydrogen and oxygen evolution reactions. RSC Adv. 2019, 9, 37467–37473. [Google Scholar] [CrossRef]
- Kawashima, K.; Cao, C.L.; Li, H.; Márquez-Montes, R.A.; Wygant, B.R.; Son, Y.J.; Guerrera, J.V.; Henkelman, G.; Mullins, C.B. Evaluation of a V8C7 Anode for Oxygen Evolution in Alkaline Media: Unusual Morphological Behavior. ACS Sustain. Chem. Eng. 2020, 8, 14101–14108. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Ji, P.; Hu, W.; Liu, J.; Mu, S. Transition-Metal Phosphides: Activity Origin, Energy-Related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Anne Acedera, R.; Theresse Dumlao, A.; Donn Matienzo, D.J.; Divinagracia, M.; Anne Paraggua, J.; Abel Chuang, P.-Y.; Ocon, J. Templated synthesis of transition metal phosphide electrocatalysts for oxygen and hydrogen evolution reactions. J. Energy Chem. 2024, 89, 646–669. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Murthy, A.P.; Lee, S.J.; Karuppasamy, K.; Arumugam, S.R.; Yu, Y.; Hanafiah, M.M.; Kim, H.-S.; Mittal, V.; Choi, M.Y. Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion. Ceram. Int. 2021, 47, 4404–4425. [Google Scholar] [CrossRef]
- Zhang, W.; Han, N.; Luo, J.; Han, X.; Feng, S.; Guo, W.; Xie, S.; Zhou, Z.; Subramanian, P.; Wan, K.; et al. Critical Role of Phosphorus in Hollow Structures Cobalt-Based Phosphides as Bifunctional Catalysts for Water Splitting. Small 2022, 18, 2103561. [Google Scholar] [CrossRef]
- Mo, Y.; Ni, Y.; Li, X.; Pan, R.; Tang, Y.; Deng, Y.; Xiao, B.; Tan, Y.; Fang, Y. An efficient pH-universal non-noble hydrogen-evolving electrocatalyst from transition metal phosphides-based heterostructures. Int. J. Hydrogen Energy 2023, 48, 31101–31109. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Du, X.; Zhang, X.; Hu, T. Controlled synthesis of CoVP as robust electrocatalysts for water, seawater and urea oxidation. Int. J. Hydrogen Energy 2023, 48, 34370–34381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).