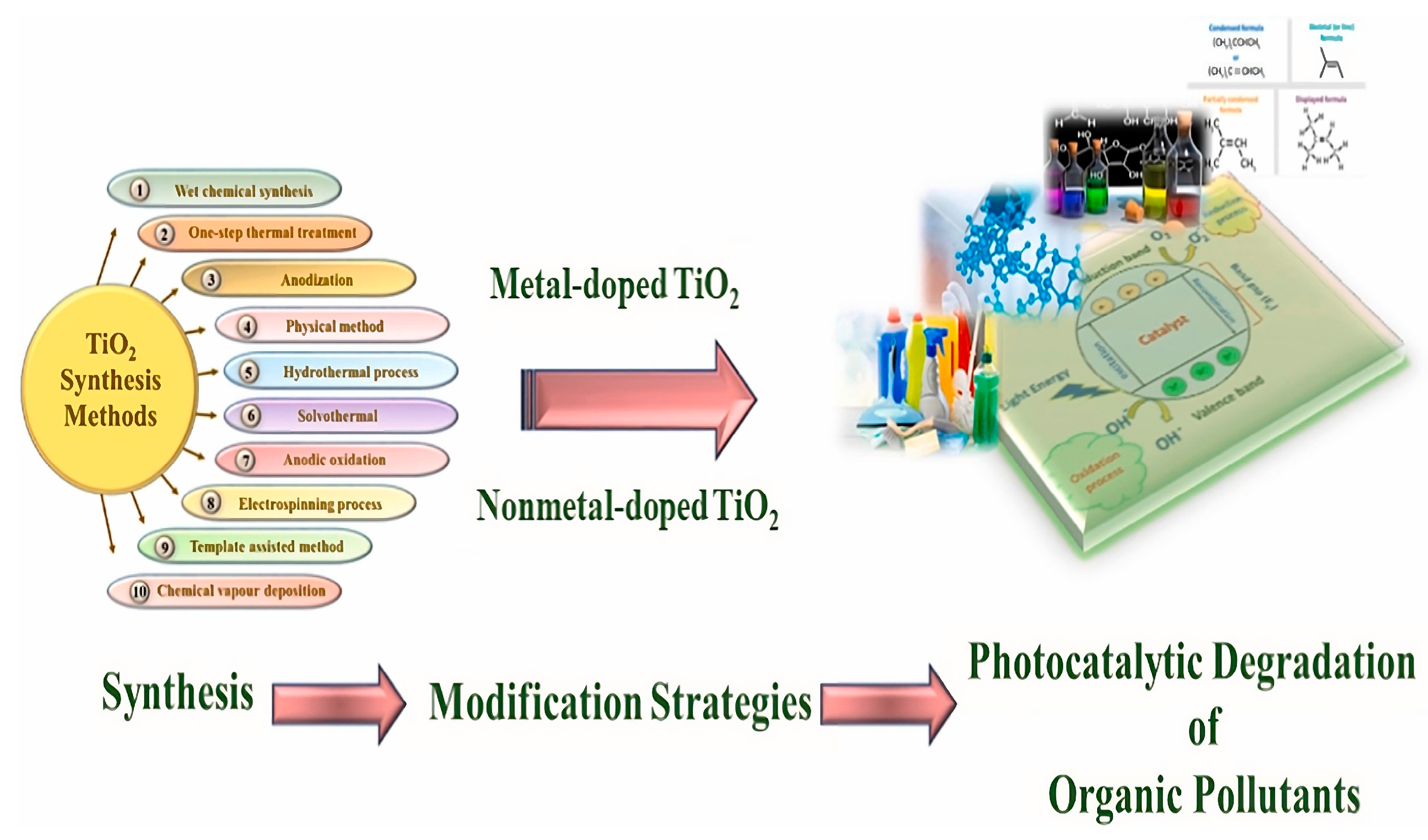
Abstract
In the relentless pursuit of sustainable energy solutions, the petroleum industry faces the imperative challenge of mitigating sulfur emissions. This comprehensive review scrutinizes Titanium Dioxide (TiO2) as an extraordinary catalyst, pushing the boundaries of desulfurization performance in petroleum refining. The abstract begins by underscoring the urgent need for advanced desulfurization technologies, driven by stringent environmental mandates and escalating global energy demands. The spotlight then shifts to the unparalleled physicochemical attributes of TiO2, showcasing its inherent advantages such as exceptional surface area, stability, and photocatalytic process. A profound exploration of TiO2’s catalytic mechanisms follows, unraveling its capacity to disintegrate stubborn sulfur–carbon bonds, thereby elevating desulfurization efficiency to unprecedented levels. This review meticulously dissects diverse forms of TiO2, ranging from nanoparticles to mesoporous structures, and provides a critical analysis of their respective strengths and limitations in catalyzing sulfur removal. Delving into operational nuances, this review examines the impact of temperature, pressure, and catalyst loading on TiO2 performance, offering crucial insights for optimizing desulfurization processes. The narrative then unfolds to explore cutting-edge developments in TiO2-based catalysts, encompassing ingenious modifications, composites, and hybrid materials designed to augment catalytic activity and selectivity. Anticipating the road ahead, this review contemplates the challenges and prospects of deploying TiO2 on an industrial scale, pointing toward avenues for future research and development. This abstract encapsulates a wealth of knowledge, serving as an indispensable resource for researchers, engineers, and policymakers navigating the dynamic landscape of sustainable petroleum refining. TiO2 emerges as a transformative force, propelling the industry toward cleaner, greener, and more efficient energy production.
1. Introduction
This versatile and well-researched mineral, titanium dioxide (TiO2), has emerged as a significant option for tackling the essential problem of sulfur removal in petroleum refining operations. Titanium dioxide is a material that has been investigated extensively [1]. As environmental regulations throughout the world grow more rigorous, there has been an increase in the need for cleaner fuel technologies [2]. This has led to an increased focus on the development of desulfurization catalysts that are efficient [3]. By diving into its one-of-a-kind qualities, synthesis processes, and the novel modification strategies that boost its efficacy in removing sulfur from petroleums, this study offers a complete investigation of the function that titanium dioxide (TiO2) plays as a catalyst for desulfurization [4]. The relevance of titanium dioxide in this field is derived from the fact that it has remarkable photocatalytic characteristics, strong stability, and is safe for the environment [5]. In spite of this, the use of titanium dioxide in the desulfurization of petroleum goes beyond the photocatalytic capabilities that it has on its own [6]. This is because of its large surface area and its capacity to be modified and adjusted via a variety of different synthesis and modification processes [7]. These factors are essential for improving the interaction between sulfur-containing compounds and titanium dioxide, which will ultimately facilitate the removal of these compounds in an effective manner [8]. The purpose of this study is to shed light on the many processes that may be used to enhance the catalytic performance of titanium dioxide (TiO2) [9]. These processes include doping with metals or non-metals, the creation of composite materials, and the manipulation of its crystal structure [10]. The purpose of each of these tactics is not only to improve the catalytic activity of titanium dioxide (TiO2) but also to overcome the intrinsic constraints of this material, such as its poor conductivity and broad band gap, which limit its activity when exposed to visible light [11,12]. Through the synthesis of the most recent developments and discoveries from research, the purpose of this study is to give a comprehensive perspective on the potential of titanium dioxide (TiO2) as a catalyst for desulfurization [13]. It conducts an in-depth analysis of the issues that are now being encountered in the industry, investigates the environmental and economic repercussions that will result from the implementation of technologies based on titanium dioxide, and seeks to foresee future avenues for study and application [14]. The study highlights the crucial role that titanium dioxide plays in upgrading petroleum refining processes, with the goal of future fuel choices that are cleaner and more sustainable being widely accessible [15].
The current state-of-the-art innovation at the forefront, therefore, has to be titanium dioxide (TiO2) for the pressing challenge of sulfur removal during upstream petroleum-refining operations. Despite many studies, a very important challenge is how to tailor and optimize the catalytic behavior of TiO2 for efficient sulfur removal in petroleum refining, meeting the increasingly stringent needs of environmental regulations [16,17].
Global environmental cleaning norms are tightening day by day; the need for cleaner fuel technologies has increased by leaps and bounds. Highlighting the demand, the market shows a high need for extremely effective desulfurization catalysts; research and development efforts were thus focused on TiO2 [18].
This review will, hence, describe the unique characteristics of TiO2, preparation processes, and advanced modification techniques available for efficient performance improvement during sulfur removal from petroleum feedstocks. Although TiO2 is recognized as one of the most powerful environmentally benign photocatalysts [19], the potential of this semiconductor in the field of petroleum desulfurization is by no means limited to the area of photoactivity.
Additionally, the relatively large surface area of TiO2 and its tunable properties allow its surface modification by many synthesis methods. Such processes are also important in providing more active sites for sulfur species, which will interact with the TiO2 to facilitate the effective removal of such species from petroleum streams [20].
In this review article, we are going to present various strategies for enhancing TiO2 catalytic activity, including metal and non-metal doping, composite material synthesis, and crystal structure manipulations. Each of these approaches separately aims to increase TiO2 catalytic activity, overcoming the intrinsic weaknesses of low conductivity and a wide band gap that limits performance under visible light illumination.
Our work is intended to provide a full perspective on the potential role of these TiO2-based materials as catalysts in petroleum desulfurization by reflecting the state-of-the-art in developing TiO2 catalysis. Critically looking at challenges in the industry, environmental implications, and economic considerations, we are going to lead, hoping for broad-based acceptance of TiO2 technologies in processes related to petroleum refining. Special attention will be paid to the central place for TiO2 in developing petroleum-refining processes for cleaner, more sustainable fuel choices to be realized in the not-too-distant future. This work conducts an in-depth analysis of the issues now being encountered in the industry, investigates the environmental and economic repercussions that will result from implementing technologies based on TiO2, and seeks to foresee future avenues for study and application. The study highlights the crucial role of TiO2 in upgrading petroleum-refining processes, with the goal of cleaner and more sustainable future fuel choices being widely accessible.
2. Advancements in Desulfurization Techniques Using TiO2
A lot of research has been done on the function of TiO2 nanoparticles in the ever-changing landscape of petroleum desulfurization technology [21]. These nanoparticles have been intensively examined because of their potential to improve the removal of sulfur compounds dramatically [22]. The efficacy of TiO2 nanoparticles has been highlighted by a recent study [23]. This effectiveness may be due to the high surface area-to-volume ratio of these nanoparticles, which also increases their catalytic activity [24]. This property, in conjunction with their capacity to distribute evenly in the reaction medium, makes it possible for the catalyst and sulfur compounds to interact more effectively, which ultimately results in superior desulfurization outcomes [25]. Furthermore, developments in modifying TiO2 nanoparticles have opened up new paths for improving their performance. Techniques such as doping with metal ions (for example, Ag and Cu) and the deposition of metal nanoparticles onto TiO2 surfaces have been found as potential solutions to boost both the activity and the selectivity of TiO2 in sulfur removal procedures [26]. These techniques have been recognized as successful in achieving the desired results [27]. Not only do these alterations affect the electrical structure, but they also affect the surface properties of TiO2, ultimately increasing the catalytic efficiency [28]. Combining TiO2 with other materials, such as activated carbon, to create hybrid catalysts synergizes desulfurization efforts [29]. These hybrids combine adsorptive and catalytic capabilities, greatly increasing the efficiency of sulfur removal [30]. Furthermore, TiO2 in photocatalysis offers an environmentally friendly method of desulfurization [31]. This method uses ultraviolet (UV) light to oxidize sulfur compounds selectively, thereby highlighting the versatility and potential of TiO2 in developing more sustainable desulfurization technologies [32]. The aforementioned developments shed light on the growing potential of TiO2 nanoparticles in revolutionizing desulfurization procedures within the petroleum sector [33]. Through formulations, researchers are paving the road for more efficient and ecologically sustainable desulfurization solutions [34]. The desulfurization by using TiO2 is shown by Figure 1.
Figure 1.
Desulfurization Techniques Using TiO2 [35]. Reprinted with permission from Elsevier @2024.
3. Comparative Analysis of Catalysts in Petroleum Desulfurization
Within the realm of potential catalysts for the desulfurization of petroleum, TiO2 stands out as a particularly promising option [36]. This prominence is because it has amazing photocatalytic characteristics and a huge surface area, both of which assist in the efficient oxidation of sulfur compounds [37]. That is why it is so useful. In contrast to conventional catalysts, TiO2 uses UV light to activate photocatalytic reactions [38]. According to Kondamareddy (2018), this method of removing sulfur harms the environment less [39]. The photocatalytic regeneration capacity of TiO2 offers an operational benefit, as shown by the findings of a 2020 study by Shang and colleagues. This advantage might reduce the expenses of catalyst turnover [40]. Zeolites, on the other hand, are characterized by their porous structure, which enables them to absorb sulfur compounds effectively [41,42,43]. This is in contrast to the situation described above. Zeolites are a good example of this [44].
Nitrogen compounds can poison molybdenum-based catalysts, such as Co-Mo/Al2O3which are often used in hydrodesulfurization processes [45]. These catalysts are more sensitive to being poisoned than others [46]. The reason is that, compared to other catalysts, nitrogen molecules are more reactive [47]. According to Bhatkhande (2002), this widespread problem reduces the efficiency of these catalysts in processing heavy petroleum fractions [48]. This problem has existed for quite some time. TiO2 is immune to this kind of poisoning, which is one aspect contributing to its endurance and flexibility across a larger variety of desulfurization applications [49]. TiO2 also resists corrosion that may occur when exposed to high temperatures. Various doping strategies have been developed to enhance the photocatalytic activity of TiO2 when subjected to visible light. Consequently, TiO2 use has been enlarged beyond the limitations imposed by the band gap inherent to the material [50]. This is a noteworthy achievement.
TiO2 is a viable option that balances performance, cost, and environmental impact in the petroleum-refining industry [51]. This selection is made possible because of the research demonstrating the progression towards the development of sustainable and efficient desulfurization technologies [52]. This research also illustrates the movement toward developing technology for desulfurization [53]. The purpose of this endeavor is a more optimal equilibrium between the operational efficiency of refining technologies and their environmental impact [54]. To achieve this goal, a significant amount of attention will be placed on research and development in this particular field [55].
4. The Efficiency of TiO2 in Removing Sulfur Compounds
The effectiveness of TiO2 as a photocatalyst in the removal of sulfur compounds has been extensively investigated, and the results have been verified across a wide range of environmental applications [56]. TiO2 has a significant amount of potential in the fight against pollution and the enhancement of both air and water quality, as this illustrates [57]. The ability of TiO2 to harness UV light and use it to initiate a chain of chemical reactions that ultimately decompose sulfur compounds significantly contributes to the photocatalytic capabilities of this material [58,59]. The procedure in question has been the focus of a significant amount of studies carried out to lower environmental pollution [60].
Under the influence of UV light, TiO2 can form electron-hole pairs. Electron-hole pairs are a key component in creating reactive oxygen species (ROS), which include hydroxyl radicals and superoxide anions [61,62]. ROS are produced when electrons and holes are paired together. With the help of these ROS, sulfur compounds may be oxidized with great efficiency, transforming these compounds into less harmful molecules. For instance, sulfur dioxide (SO2), which significantly contributes to air pollution, can be oxidized to sulfate (SO42−) ions, so diminishing its impact on the environment when released into the atmosphere. This shift is vital to improving air quality, particularly in urban and industrial areas, where large concentrations of sulfur dioxide emissions are prevalent [63]. TiO2 has shown remarkable effectiveness in water treatment operations, especially when it comes to the elimination of sulfides and other substances that include sulfur from wastewater. This capability has been particularly impressive [64]. The photocatalytic activity of TiO2, which may break down these compounds, can prevent the release of toxic substances and unpleasant odors often associated with industrial effluents. This can be accomplished by preventing the release of these compounds. It is simpler to reuse and recycle wastewater that has been treated, thanks to this capacity, which not only improves the quality of the water but also contributes to the long-term viability of water resources [65]. This capacity also makes it possible to reuse and recycle treated wastewater. Additionally, the production of nanostructured TiO2 has increased the photocatalytic efficiency of the material because the nanostructured TiO2 has a greater surface area and a higher reactivity. The rate at which sulfur compounds decompose is sped up because of the improved capability of nanostructured TiO2 to absorb UV light and form ROS. The findings of Fujishima, Zhang, and Tryk (2008) [66] indicate that this discovery has significant repercussions for developing more efficient photocatalytic systems to clean up the environment. Additionally, the utilization of TiO2 is not devoid of challenges, even if it is effective. These challenges include optimizing its activity under visible light and restricting the recombination of electron-hole pairs generated by photons. Both challenges are tough to overcome [67]. Doping TiO2 with non-metals or combining it with other semiconductors is being investigated for expanding the photocatalytic activity of TiO2 to include the visible light spectrum [68]. The practical use of the material and its efficiency in environmental applications would both rise consequently [69].
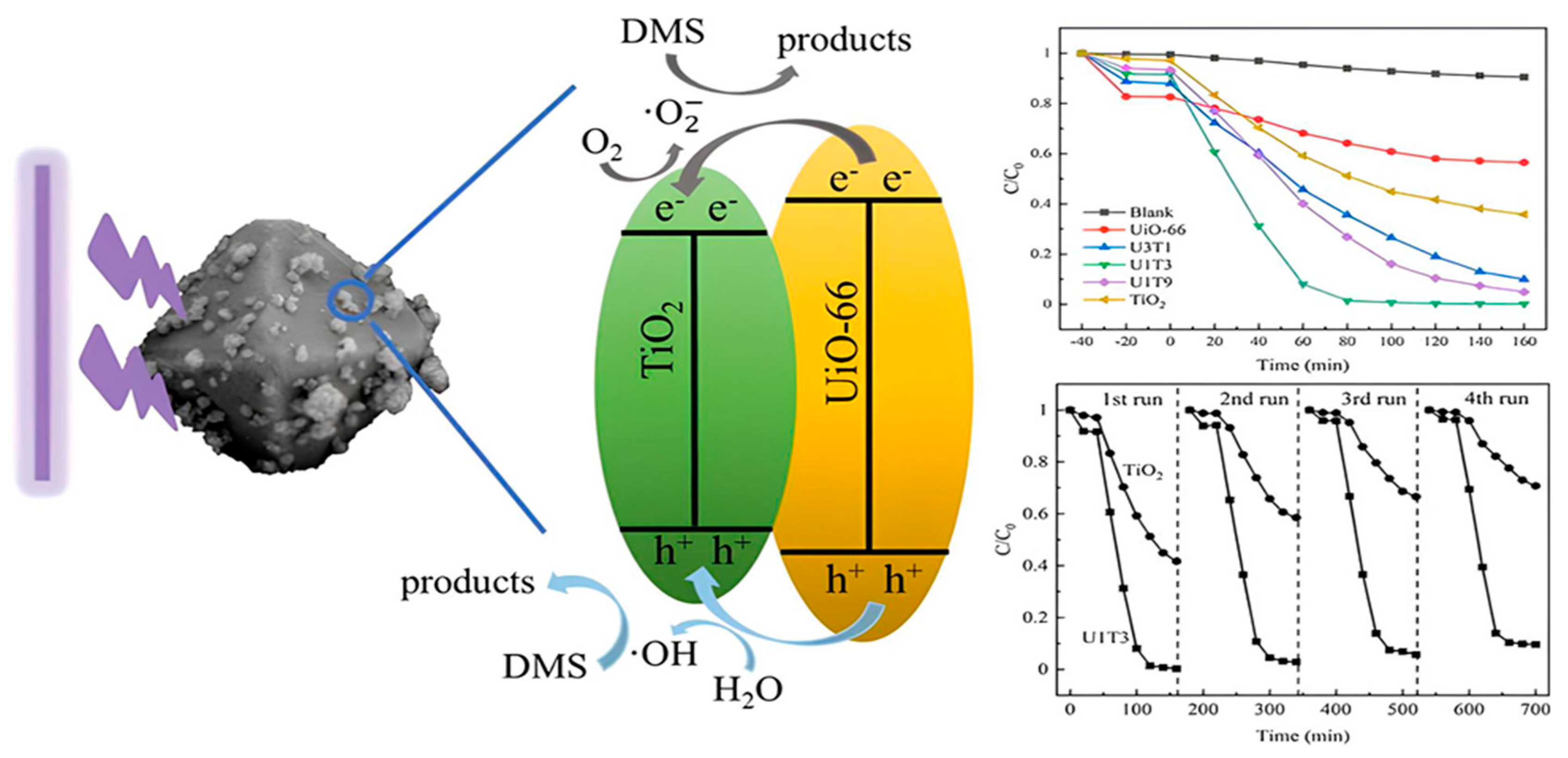
In the process of eliminating sulfur compounds from the environment, TiO2 stands out as a not only highly efficient but also very flexible photocatalyst [70]. Combined with the ongoing advancements in material science, the fact that it can destroy impurities when exposed to UV light exemplifies its potential to contribute to developing environmentally friendly management strategies [71]. Hence, future research and development efforts are anticipated to continue to focus on enhancing the photocatalytic efficiency of TiO2 and increasing its use across a wide range of environmental remediation situations [72]. The photocatalytic activity of Titanium Dioxide described in Figure 2.
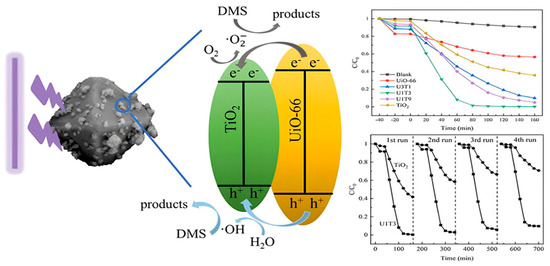
Figure 2.
The Photocatalytic Activity of Titanium Dioxide (TiO2). The purification of odor pollutants containing reduced sulfur compounds could be largely improved by coupling porous materials. Herein, UiO_66@TiO2 nanocomposites with different blending ratios were synthesized via a readily solvothermal method and characterized experimentally by X_ray diffraction and scanning electron microscope [73]. Reprinted with permission from Elsevier @2024.
5. Titanium Dioxide: A Sustainable Option for the Petroleum Industry
Incorporating TiO2 into the petroleum sector highlights a multi-dimensional approach to improving sustainability [74]. This method leverages the unique features of TiO2 to contribute majorly across a variety of dimensions. In hydrocracking operations, TiO2 as a catalyst support material not only improves the conversion of heavy crude fractions into more valuable lighter products but also emphasizes a commitment to energy efficiency and decreased carbon emissions [75]. Moreover, its involvement in environmental remediation, especially in photocatalytic applications, demonstrates its promise in reducing the environmental footprint of petroleum operations by speeding up the degradation of organic pollutants [76]. This potential was shown by its ability to mitigate the environmental impact of petroleum operations. TiO2 in protective coatings and paints serves two purposes. It improves the durability and resistance to corrosion of infrastructure and equipment, thereby reducing the amount of maintenance and potential environmental hazards [77]. Additionally, it contributes to the aesthetic and functional aspects of UV protection and thermal regulation. Hinting at the broader implications of TiO2 in facilitating a transition toward renewable energy sources, the energy sector’s pivot toward sustainability finds an ally in TiO2, particularly in reflective coatings and dye-sensitized solar cells [78]. TiO2 is particularly useful in reflective coatings.
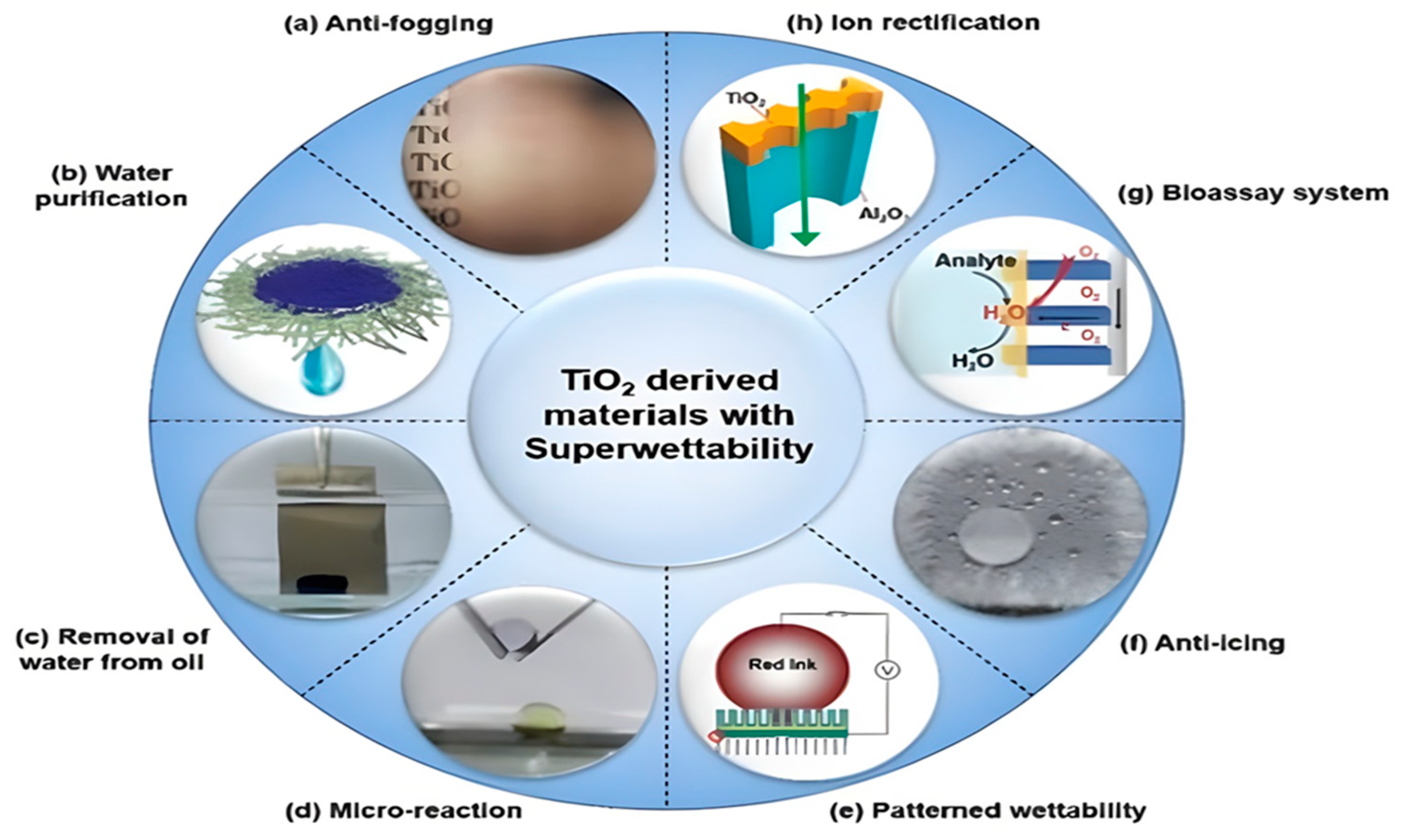
These many uses demonstrate the role that TiO2 plays not only in improving operations within the petroleum sector but also in guiding the industry toward not only a more environmentally responsible but also more sustainable future [79]. Nevertheless, the story of TiO2’s sustainability is complex, and it is necessary to analyze in-depth the environmental costs connected with its manufacturing compared to its advantages [80]. Finding a happy medium between these two elements is necessary to make the most of the promise of TiO2 as a sustainable alternative. The miscellaneous functions of TiO2 are presented by Figure 3.
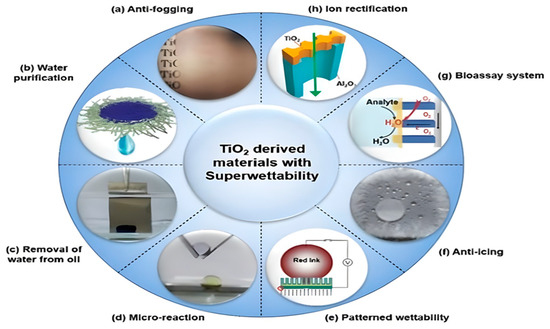
Figure 3.
The Diverse Functional Applications of Titanium Dioxide Related to its Superwettability Characteristic. Typical aplications of TiO2 based on photo-induced super wettability. (a) Glass substrate coated with nano-TiO2 shows anti-fogging properties. (b) Water can be purified through a TiO2 nanomembrane by adsorption of organic pollutants. (c) Water separation from oil due to UV-illumination on superhydrophilic TiO2 nanotube array (TiO2-NTA) surface. (d) Under-oil microreaction on fabricated superhydrophilic TiO2-NTA surface. (e) Patterned wettability, used for printing on the TiO2 surface via the photoelectric cooperative effect. (f) The anti-icing property is shown on a superhydrophobic TiO2-NTA surface modified by 1H,1H,2H,2H-perfluorooctyltriethoxysilane. (g) The solid–liquid–air triphase bio-photoelectrode was fabricated by single-crystalline TiO2 nanowire arrays (NWs) for the bioassay system. (h) Artificial ion channels can be created using the TiO2 nanochannel [81].
6. Challenges and Opportunities in TiO2-Based Desulfurization
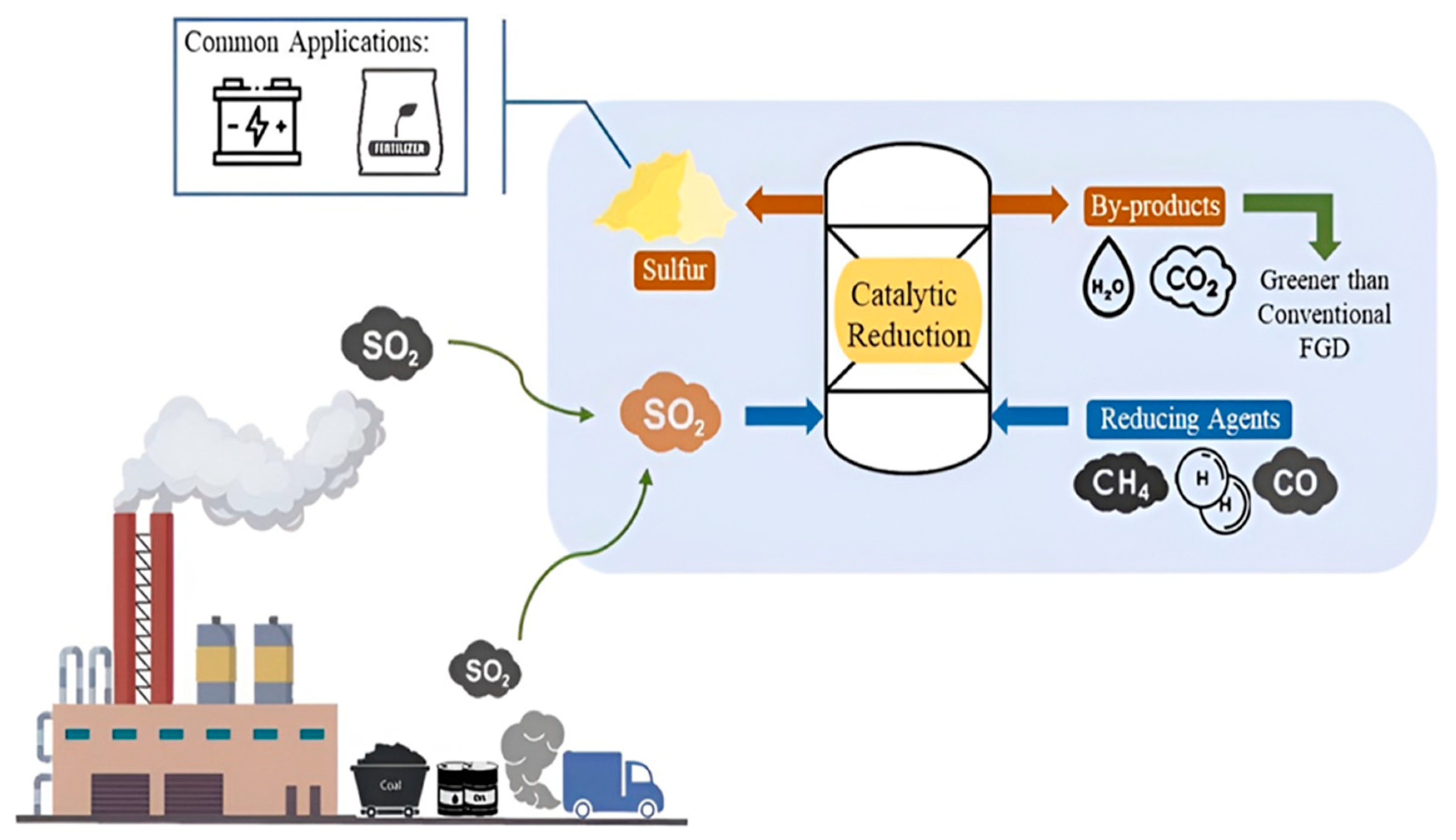
Desulfurization based on TiO2 is a persuasive approach for removing sulfur compounds from several industrial streams, such as flue gases and fuels [82]. This effectiveness is because TiO2 has photocatalytic properties, which allow it to remove sulfur compounds from these streams [83]. The decrease in emissions of sulfur dioxide (SO2), which significantly contributes to acid rain and air pollution, is one of the significant opportunities of this technology for preserving the environment [84]. Because of its high efficiency, stability under light irradiation, and powerful oxidizing capability, TiO2 has been the subject of a significant amount of research as a photocatalyst, as it can convert sulfur compounds into sulfates that are safe when exposed to ambient conditions [85].
On the other hand, desulfurization accomplished by TiO2 is not devoid of its fair share of challenges. TiO2 has a substantial band gap, which limits its photocatalytic activity to the UV region of the sun’s spectrum [86]. This portion of the sun’s spectrum accounts for only around five percent of the whole solar spectrum. This is one of the most important challenges. TiO2 requires alteration to increase its absorption in the visible light range to accomplish this goal. Doping the material with metals or non-metals or sensitizing the material with organic dyes is a potential method for accomplishing this goal [87]. There are also more methods. Another challenge to be conquered is the recombination of electron-hole pairs throughout the process. This phenomenon reduces the efficiency of the photocatalytic process. According to Kim et al. (2021) [88] one of the potential approaches that might solve this problem is the development of composite materials capable of facilitating charge separation and transfer. This is one of the recommended tactics.
TiO2-based desulfurization in practical applications is associated with many challenges, including those pertaining to scalability and cost-effectiveness. However, to apply these discoveries to industrial settings, it is necessary to construct both robust and long-lasting photocatalytic systems capable of functioning well over extended periods in conditions representative of the real world. This is the case despite experiments carried out in the laboratory showing encouraging results. Furthermore, reactor designs need optimization to enhance the interaction between TiO2 and sulfur-containing compounds, which will improve the efficiency of desulfurization [89].
Solar energy has the potential to be a viable source for photocatalytic desulfurization, which is a prospect in TiO2-based desulfurization [90]. Other potential uses include the creation of novel materials and techniques that can operate in the presence of visible light. Recent discoveries in nanotechnology have opened the door to producing such quantum dots [91]. Adsorption of sulfur compounds and photocatalytic conversion would both be improved due to this, which would eventually improve their efficiency. Additionally, the use of photocatalysis based on TiO2 in conjunction with other treatment procedures results in more comprehensive and effective desulfurization systems. These systems can manage a greater range of sulfur compounds, which further reduces the adverse impacts on the environment.
Although TiO2-based desulfurization encounters hurdles concerning photocatalytic efficiency, light absorption, and scalability, continual research and technological developments are opening up new chances for solving these problems. More efficient, cost-effective, and environmentally benign desulfurization solutions can be produced [92]. This potential can be realized through more efficient changes to TiO2, as well as through creating unique reactor designs and incorporating other processes [93].
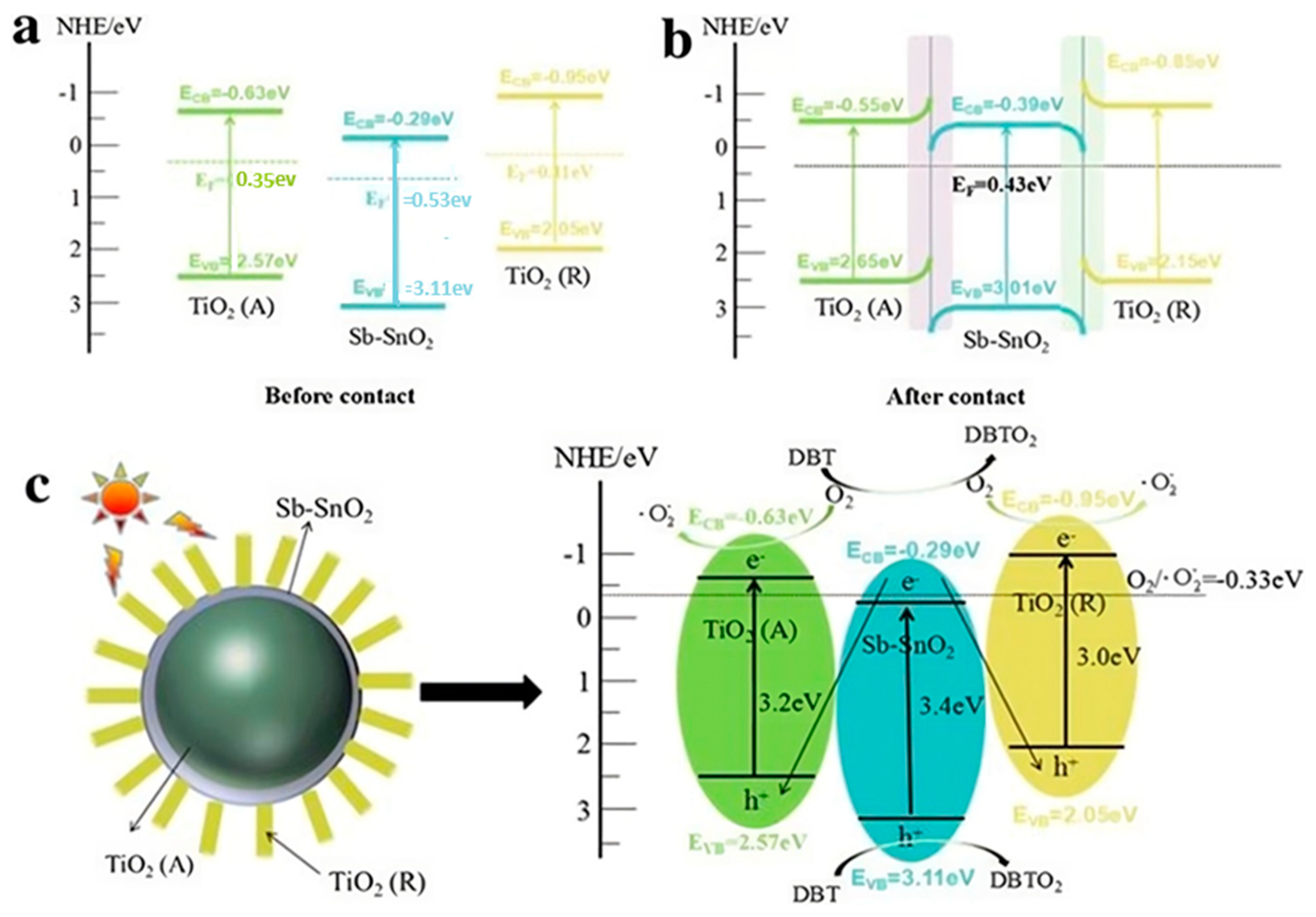
Figure 4 represents sunlight as the source of energy because it is the energy source needed for the photocatalytic reaction.
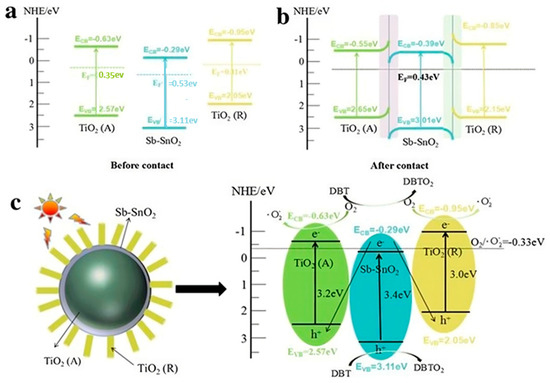
Figure 4.
Photocatalytic Desulfurization Mechanism Diagram of TiO2 (R)/C-TiO2 (A) under sunlight. (a)when TiO2 (A), TiO2 (R), SbSnO2 are before contact with light Fermi level, (b) The semiconductor energy band with high Fermi level after contact, (c) This photocatalytic mechanism of the TiO2(R)/C-TiO2 Reprinted from Ref. [94] with permission from Springer Nature@2024.
1. TiO2(R): This stands for TiO2 particles, sometimes as a surface, or particles. You can make use of small circles for TiO2 or particles. Call this TiO2(R) to represent the reactant state.
2. Adsorption of Sulfur Species: Illustrate adsorption of sulfur species onto TiO2 nanoparticle surfaces. This can be indicated via arrows or labeling for the interaction.
3. Photocatalytic Reaction: Show a scheme of the photocatalytic reaction, where sunlight is used to activate TiO2 nanoparticles in order to oxidize sulfur species to less harmful compounds. This can be shown by either arrows indicating the flow of electrons or chemical transformations.
4. C-TiO2 (A): Display the TiO2 nanoparticles under illumination, usually denoted by C-TiO2 (A), to represent the catalyst in the active state.
5. Desorption of Products: Explain how the desorption of reaction products is obtained from the surface of a TiO2 nanoparticle [93].
7. Future Perspectives of TiO2 Catalysis in Fuel Purification
Considering the growing demand for cleaner fuels and the increasing stringency of environmental restraints, the prospects for future TiO2 catalysis in fuel purification are relatively optimistic [95]. To a certain extent, the present situation may be attributed to both elements. When removing sulfur and nitrogen molecules from fuels with hazardous emissions, TiO2 is an appealing choice. TiO2 in fuel purification processes is going to be expanded as a result of this study. A few instances of improvements have the potential to increase the photocatalytic activity of TiO2, which will result in it being more successful in the removal of pollutants at lower energy inputs [96]. These innovations can improve the efficiency of the removal of pollutants. Two examples of these developments are the formation of heterojunctions with other semiconductors and the doping of semiconductors with elements that are not metallic.
According to Joo et al. (2012), the synthesis of nanostructured titanium dioxide also results in a rise in the surface area and the number of active sites [97]. This, in turn, leads to an increase in the catalytic activity and efficiency of the processes that are engaged in the purification of fuel. There is still another area of study that has the potential to result in purifying systems that are more efficient and cost-effective [98]. This field of research involves combining TiO2 with modern separation technologies, such as membrane filtration. A large number of challenges are still involved in bringing these discoveries from the laboratory to the commercial level. This process is more complicated than it seems. To guarantee that TiO2-based catalysis in fuel purification is both economically possible and sustainable, major breakthroughs in reactor design and process optimization are important [99] because it is necessary to ensure that the process is optimized.
Additionally, the shift to a low-carbon economy and the drive toward renewable energy sources needs the development of fuel purification techniques that are not only kind to the environment but also sustainable in the long run. In line with the objectives of global sustainability, TiO2 catalysis provides an option that is not harmful to the environment by using solar energy for photocatalytic processes [100]. This needs consideration. Continued research and development efforts are necessary to overcome the limitations now in place and unleash the full potential of TiO2 in fuel purification. This situation is because of the existing limitations. Hence, the energy industry can progress toward a future that is not only more environmentally friendly but also more sustainable [101].
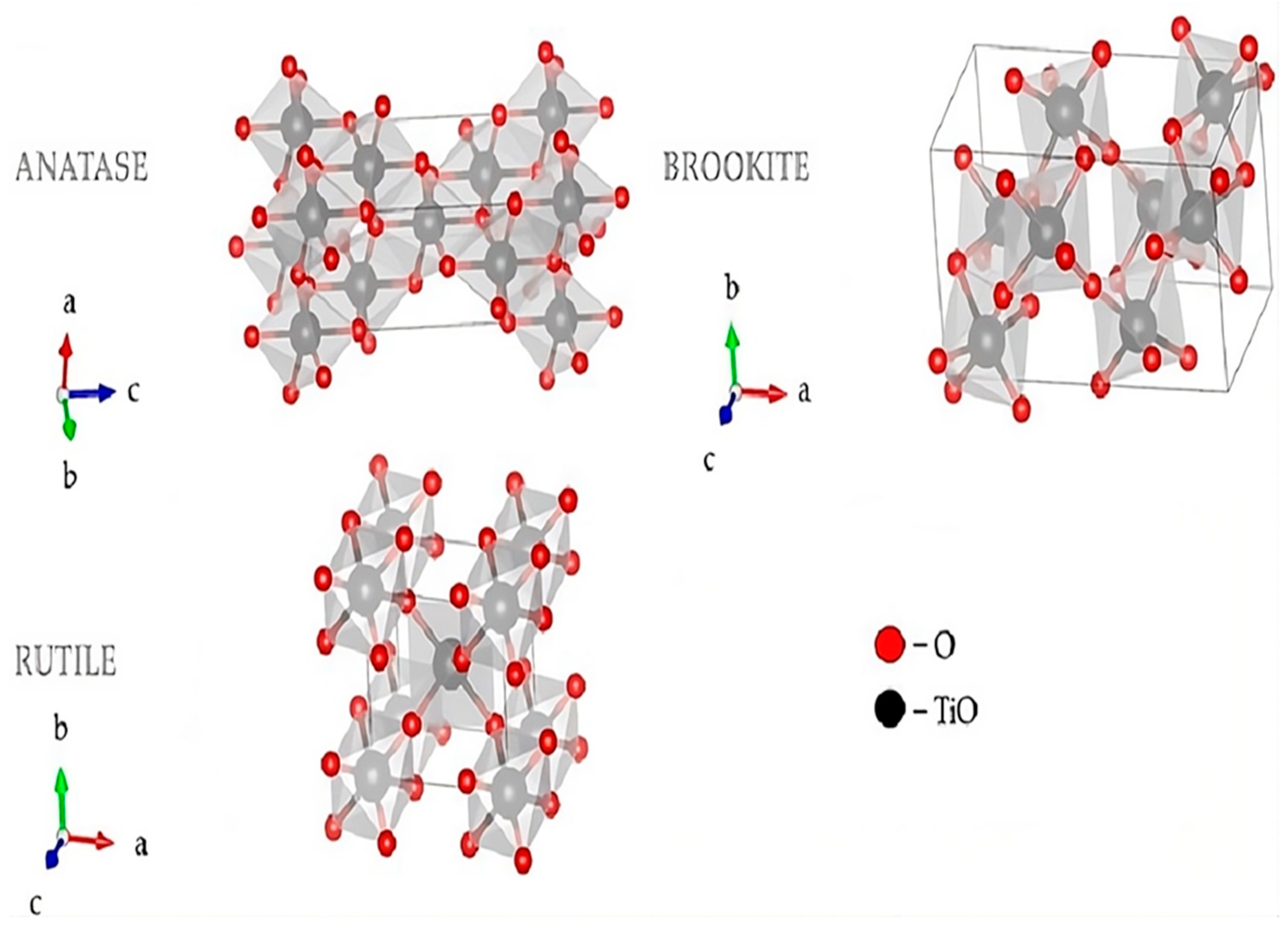
Figure 5 shows the visualization crystal structure of TiO2, used to view the structural and electronic properties of TiO2; therefore, their importance is enormous for their application to photocatalysis and semiconductor devices.
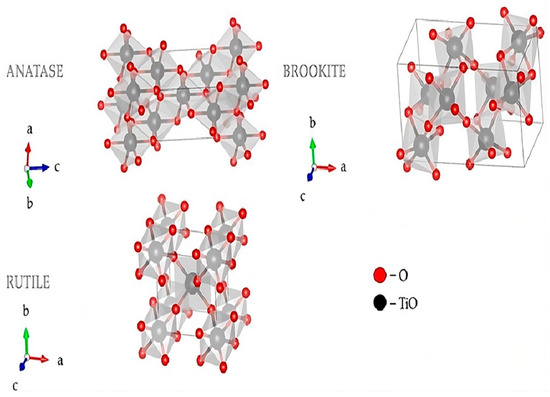
Figure 5.
3D Visualization of Crystal Structures of TiO2 Using Visualization for Electronic and Structural Analysis [102].
8. Environmental Impact of TiO2 Catalytic Desulfurization
Not only does the catalytic desulfurization of TiO2 significantly and positively impact the environment, but it also offers a sustainable approach to lowering pollutants in the air and protecting ecosystems. This effect is because it reduces the SO2 released into the atmosphere [103]. To lessen the SO2 released into the environment from industrial operations and the combustion of fossil fuels, desulfurization procedures are used. The fact that these pollutants substantially contribute to acid rain, respiratory difficulties, and the damaged ecosystem is something that has to be considered. When decreasing these emissions, desulfurization technologies are very important techniques to implement. TiO2 catalysis plays a crucial role in the transformation of sulfur compounds into molecules that are less dangerous to the environment, such as sulfates [104]. This outcome is due to the photocatalytic capabilities of TiO2, which converts sulfur compounds effectively. The process being explained here is one shown to protect the environment. This contribution has been of great use to the community of researchers, and they have profited much from it.
The desulfurization process based on TiO2 can be performed in ambient circumstances without requiring additional harmful chemicals. This decreases the carbon footprint associated with activities that include sulfur removal [105]. One of the most significant benefits of this specific kind of desulfurization is its beneficial effect on the environment. TiO2 can absorb solar energy for photocatalysis, which is in line with the global trend toward environmentally friendly energy sources. The fact that this is an extra topic of interest is something that should be mentioned. Several studies, such as Moma et al. (2018), have shown that this modification contributes to reducing emissions of greenhouse gases and enhances the sustainability of energy [106].
That being said, extensive research on the durability of TiO2 is of the utmost importance while also considering the impact on the environment. In the study by Gatou et al. (2024), [107]. The production of TiO2 nanoparticles was hypothesized to include processes that need substantial energy as well as the consumption of possibly dangerous compounds. If these processes are not handled appropriately, there is a possibility that they may undermine some of the positive consequences that they have on the environment. This scenario is because they can harm the environment. It has been shown in recent studies [108], that the primary focus of their investigation is on reducing the negative impacts of these outcomes. There are three primary core areas of emphasis for this research project. These include the development of environmentally friendly synthesis methodologies, the recycling of TiO2 catalysts, and the recycling of nanoparticles. Figure 6 represents the effect of different catalytic desulphurization on environment.
Figure 6.
Environmental Impact of Catalytic Desulfurization [109]. Reprinted with permission from Elsevier @2024.
Additionally, implementing TiO2 catalytic desulfurization on a large scale can significantly enhance the quality of both air and water. Specifically, this is because of the possibility of treating SO2. To be more precise, this is because the produced sulfates are often less harmful to the environment and simpler to control in contrast to the sulfur compounds they are generated from. The many beneficial consequences of this approach on the environment are shown by this particular example. The harm caused by acid rain might probably be very destructive to the ecosystems of both aquatic life and woods, and this is something that deserves consideration.
The TiO2 catalytic desulfurization technology is one of the most promising options for preserving the environment as it offers a technique that is not only more effective but also more ecologically friendly and renewable. This tech makes it one of the most promising alternatives. Hence, it is widely regarded as one of the most fascinating methods for preserving the natural environment. The research by Shafiq et al. (2022), [110] indicates its potential to cut down pollution of the environment significantly and to contribute to the efforts being undertaken to promote global sustainability. Both these conclusions are based on the findings of researchers described before. It is not out of the question that this promise will be fulfilled via implementing technologies advantageous to the environment, as well as through ongoing research to enhance the production and use of TiO2 [111]. Precious metal deposition modification high selectivity for photocatalytic degradation of organics revealed in Figure 7.
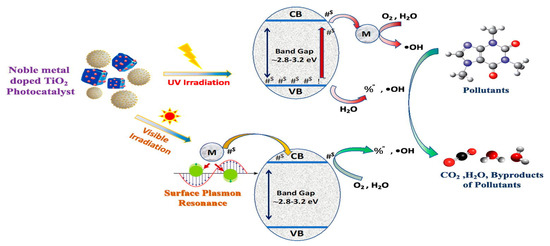
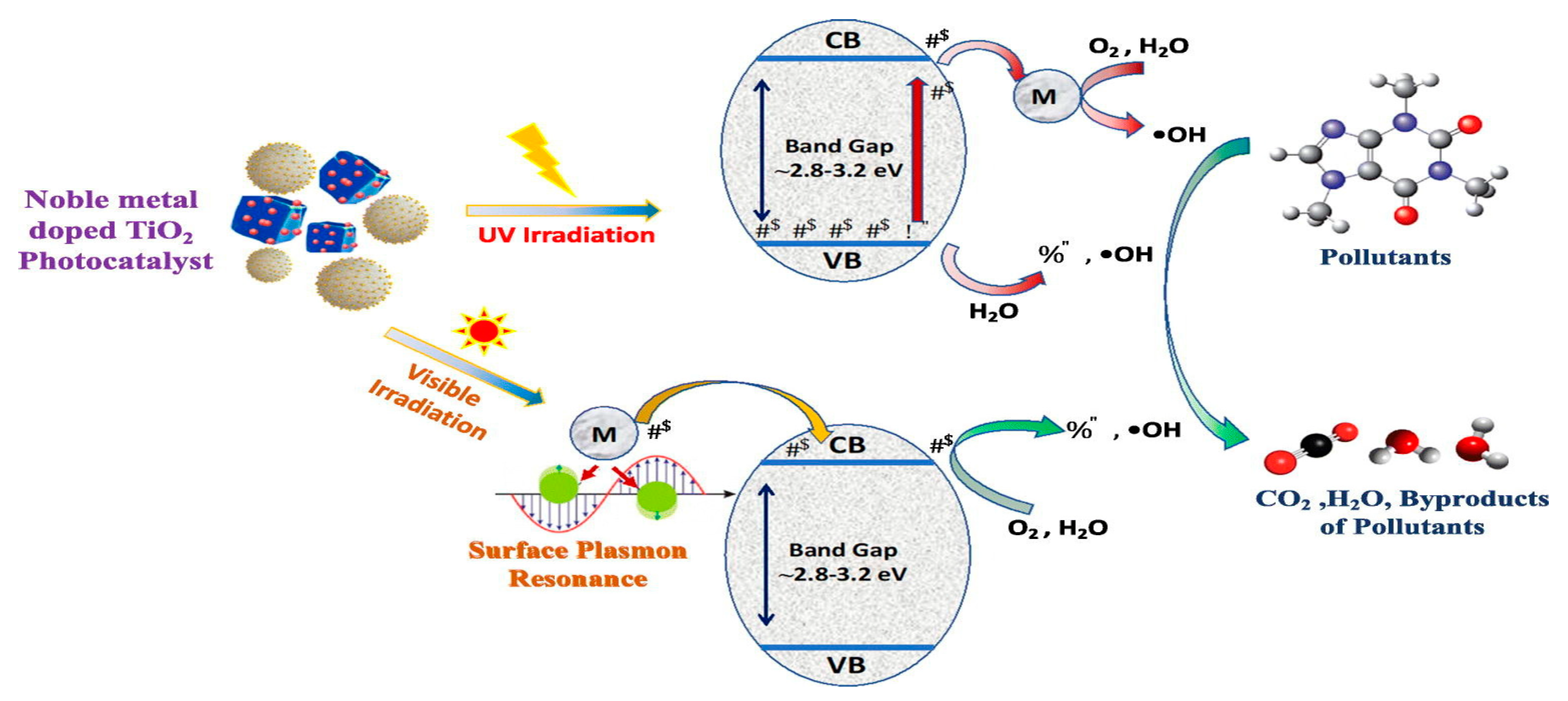
Figure 7.
The Mechanism of Precious Metal Materials Deposition in doping TiO2 and Photocatalytic Reaction [112]. Reprinted with permission from Elsevier @2024.
These precious metals can be deposited through a few methods, which include electroplating, physical vapor deposition (PVD), chemical vapor deposition (CVD), and electrodeposition. In the electroplating procedure, metal ions from the electrolyte solution are electrochemically reduced at the electrode surface while an electrical current passes through the bath. In PVD methods, the metal source is in the solid form, and the atoms are mostly transported to the substrate through sputtering or evaporation. Precursor gases deposit metal films by a chemical reaction at the substrate surface in CVD. In electrodeposition, metal ions in solution are deposited on the substrate by an applied external electrical potential. The techniques generally make it possible to deposit thin and uniform coatings of precious metals on several surfaces.
9. Cost-Benefit Analysis of TiO2 as a Desulfurization Agent
TiO2 as a desulfurization agent is an example of a cost-benefit study that illustrates the complex relationship between the progress of technology, the preservation of the environment, and the growth of the economy. The findings of the study indicate a positive correlation between these three components, which proves the case [113]. How these three qualities interact reveals a quite obviously complicated connection between them. The initial expenditures in manufacturing catalysts from technologies based on TiO2 could be more than those for classic desulfurization agents. This possibility is supported by current evidence. At this point, it is possible to do this. The fact is that there is a chance that this situation adds credibility to the concept being addressed in this particular context. The manufacturing processes required for TiO2 nanoparticles are more complicated than the procedures required for conventional desulfurization agents, as stated by Gaetano et al. (2014) This conclusion can be drawn from the investigations conducted [114]. This is the reason things transpire in the manner they do. Furthermore, the incorporation of light sources into industrial applications is of utmost importance because the photocatalytic characteristics of TiO2 need UV light to be activated. If this incorporation happens, there is a risk that the monetary expenses may increase.
On the other hand, with desulfurization activities, the utilization of TiO2 is linked to a wide range of advantageous outcomes that are considered significant and intricate. In the desulfurization process, the high efficiency and stability of TiO2 provide economic advantages that are maintained over an extended period. Researchers Haghighi et al. found that one advantage is the reduced frequency of replacement of catalysts, and another one is the eliminated need for additional chemicals [115]. The entire cost of the therapy is decreased as a direct consequence. Because of this, the quantity of extra chemicals required to fulfill the criteria is reduced. Wang et al. (2020) pointed out that the capability of the system to function in mild circumstances decreases energy consumption, which further reduces operating expenses [116]. The fact that this is an extra topic of interest is something that should be mentioned.
Therefore, it is possible to conclude that the use of TiO2 conforms to the principles of green chemistry, which indicates that it is either ecologically responsible or helpful to the environment. The use of this resource results in various advantages. Along with reduced production of secondary pollutants, the emission of greenhouse gases may also decrease, which is one of the potential advantages. Various studies, such as Verma et al. (2022), have shown that these advantages can reduce the costs associated with environmental compliance and improve the sustainability profiles of enterprises. Furthermore, these advantages can decrease the pricing of the product [117]. Because TiO2 can use solar energy for photocatalytic processes, cost reductions can take place over a longer period. According to Tsuyoshi (2012), renewable energy sources can be used to further lessen the effect of desulfurization operations on the environment [118]. The battle against the deteriorating environment has taken a huge stride ahead with this measure. This results from the fact that the action in question can now be carried out once it has been made feasible.
Two examples of the improved TiO2 modification methods now being carried out to boost photocatalytic activity under visible light are doping with non-metals and producing heterojunctions. Both examples are the improvements being made at this very moment around the globe. According to Asahi et al. (2001), these changes can reduce the reliance on UV light sources, as well as the costs of the use of energy. Additionally, this can positively impact the environment [119]. Furthermore, this can reduce the burden of energy use, which is a significant benefit. Additionally, to make this technology more cost-effective, it is necessary to do research and development on TiO2 catalysts that are not only more effective but also recyclable [120].
Because of the environmental and economic benefits of these technologies, desulfurization methods based on TiO2 constitute a compelling case in their favor. Some of these advantages include the reduced amount of energy used, a decrease in emissions, and conformity with environmental regulations. Although the initial and continuing costs connected with these technologies may be higher than those associated with traditional methods, the benefits of these technologies offer a solid argument for their usage [114]. This is the case even though these technologies may be more expensive than conventional procedures. According to Zheng et al. (2002), ongoing research and technological advancements are anticipated to further improve the cost-benefit ratio of TiO2 as a desulfurization agent, thereby making it an increasingly feasible alternative for environmentally responsible industrial operations [121]. TiO2 is particularly successful in lowering the quantity of SO2 emitted into the environment. The fact that titanium dioxide (TiO2) is a desulfurization agent will shed light on the reason why this is the case. Titanium dioxide (TiO2) is a chemical that is particularly successful in lowering the quantity of sulfur dioxide that is emitted into the environment [122]. A comprehensive analysis for the production of TiO2 presented in Figure 8.
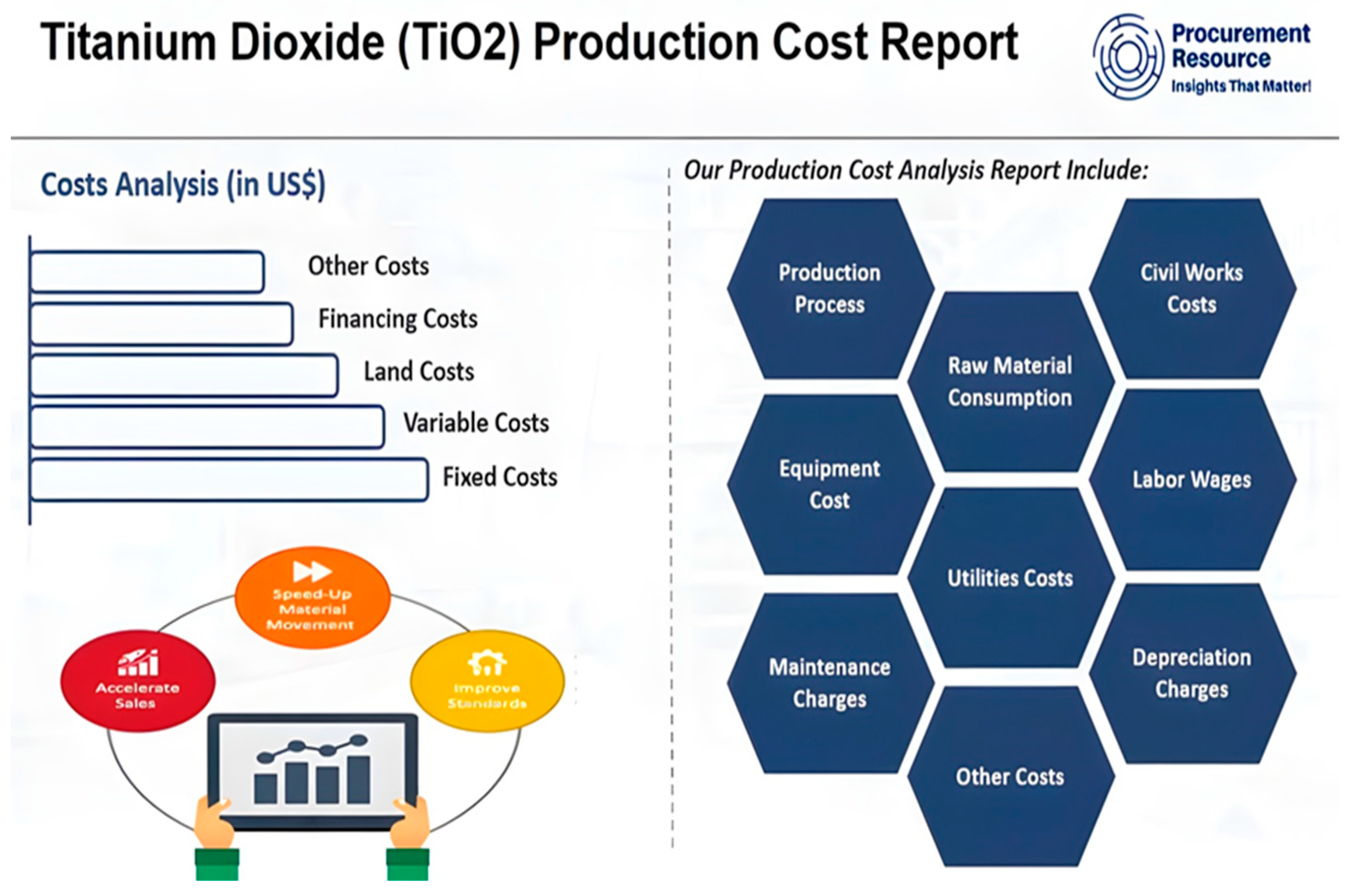
Figure 8.
The Titanium Dioxide (TiO2) Production Cost [123].
The manufacturing cost of TiO2 is highly likely to vary very much, given the production process, price of energy, and, to a lower extent, the availability of raw materials and labor costs. TiO2’s estimated production cost is currently between USD 1500 and USD 2000 per metric ton in September 2021. Per-ton cost divided by 1000 works out to be roughly USD 1.50 to USD 2.00 per kilogram. Remember, these are very rough numbers, and all the other factors mentioned above can easily move the results to the upside [123].
10. Challenges in Future and Practical Applications
In the realm of petroleum refining, TiO2 integration as a catalyst for desulfurization has emerged as a promising avenue, heralding a new era of sustainability and efficiency. While the manuscript delves into the multifaceted applications and advantages of TiO2 in petroleum desulfurization, it is imperative to acknowledge the potential challenges that may arise in its practical implementation and propose appropriate solutions.
- Photocatalytic Efficiency Under Visible Light: A significant hurdle lies in optimizing TiO2’s photocatalytic activity beyond the UV spectrum, as its current efficiency is limited to a small portion of solar irradiation. Solution: Investigating novel techniques such as doping TiO2 with non-metals or coupling it with other semiconductors could expand its light absorption range into the visible spectrum, thereby enhancing its overall efficiency [124].
- Recombination of Electron-Hole Pairs: The recombination of electron-hole pairs during the photocatalytic process diminishes TiO2’s efficiency in degrading sulfur compounds. Solution: Developing composite materials or heterojunctions that facilitate efficient charge separation and transfer can mitigate electron-hole pair recombination, thereby improving TiO2’s efficacy
- Scalability and Cost-Effectiveness: Transitioning TiO2-based desulfurization from laboratory-scale experiments to industrial applications poses challenges in terms of scalability and cost-effectiveness [125]. Solution: Investing in the development of robust, long-lasting photocatalytic systems and optimizing reactor designs to enhance TiO2-sulfur compound interactions can improve the efficiency and economic viability of TiO2-based desulfurization processes.
Future Directions and Solutions
Solar Energy Utilisation: Leveraging solar energy as a viable power source for TiO2 photocatalysis presents a promising avenue for sustainable desulfurization. Solution: Continued research into solar-driven photocatalytic systems and the development of efficient reactor designs tailored for solar energy utilization can enhance the feasibility and sustainability of TiO2-based desulfurization.
Nanotechnology Advancements: Recent advancements in nanotechnology offer opportunities to enhance TiO2’s catalytic efficiency by synthesizing nanostructured TiO2 with increased surface area and reactivity. Solution: Further research into nanostructured TiO2 synthesis techniques and their integration into desulfurization processes can lead to more efficient and cost-effective purification systems [126].
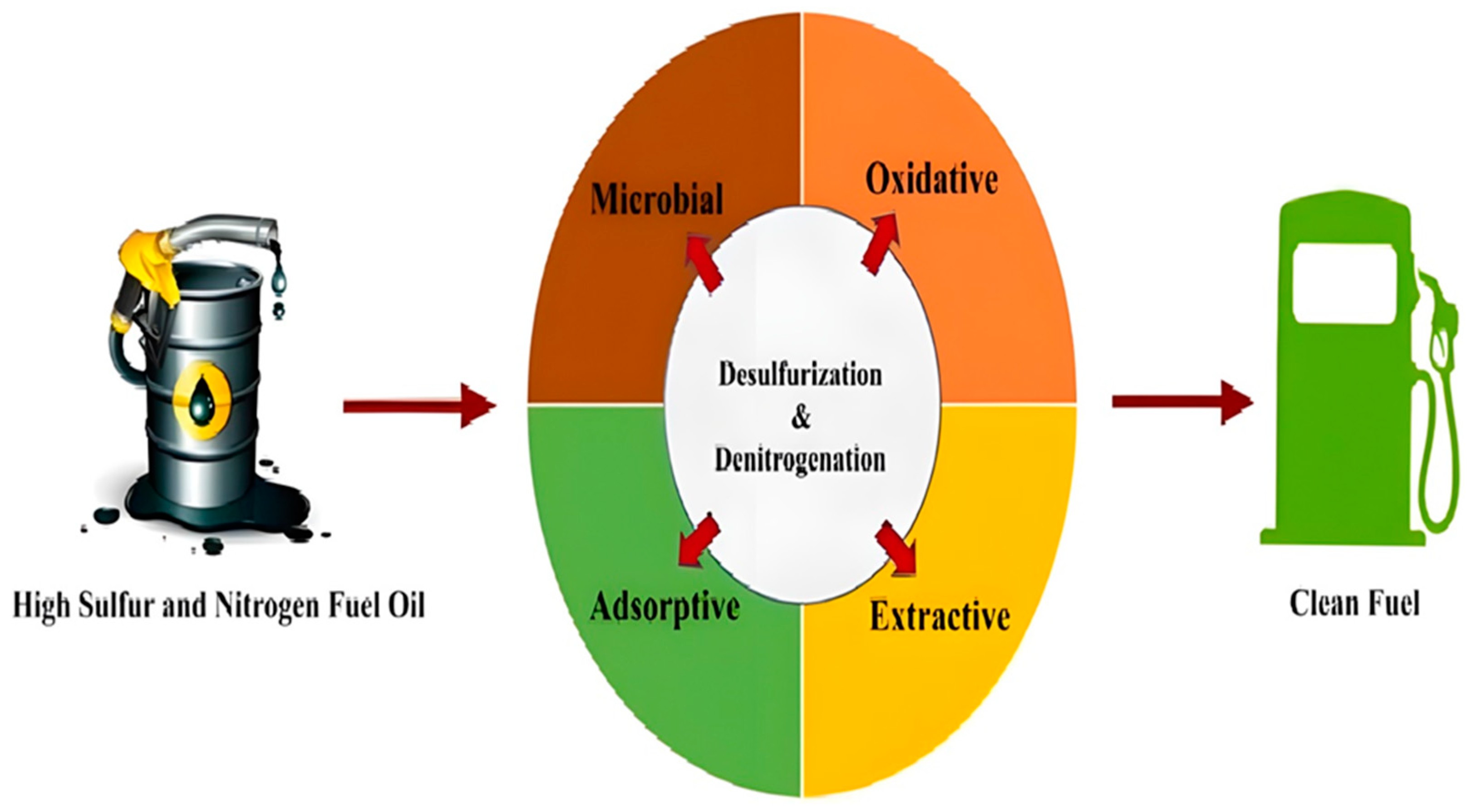
While TiO2 holds immense promise as a sustainable catalyst for petroleum desulfurization, addressing the challenges of photocatalytic efficiency, scalability, and cost-effectiveness is crucial for its successful implementation in practical applications. By embracing innovative solutions and continuing to push the boundaries of research and development, the petroleum industry can unlock the full potential of TiO2 for achieving cleaner, more sustainable energy production. The removal of sulfur- and nitrogen-containing compounds present in petroleum fractions described in Figure 9.
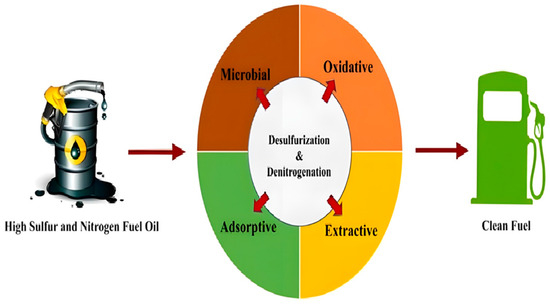
Figure 9.
The Process of Changing Sulfur and Nitrogen Fuel Oil to Clean Fuel [127]. Reprinted with permission from Springer Nature @2024.
TiO2 catalyzes the conversion of sulfur and nitrogen-containing compounds in fuel oil into less harmful forms. It, therefore, reduces air pollution as the pollutants change into forms easier to capture or destroy in industrial processes.
11. Conclusions
TiO2 has shown a tremendous amount of promise as a catalyst in the process of desulfurizing petroleum, as shown by the findings of this extensive inquiry. Additionally, it has shed light on the vital role that TiO2 plays in creating ecologically friendly energy solutions within the petroleum sector. This role is something that has been an important contribution. The rigorous environmental rules and the rising demand for energy all over the world have increased the need for improved desulfurization procedures. This demand has been on the rise because of the combination of these two factors. Because of this demand, there has been a need for research into innovative materials, such as TiO2, which provide greater performance in sulfur removal methods.
Compared to other materials, TiO2 stands out for its amazing physicochemical qualities. These properties include its enormous surface area, outstanding photocatalytic efficacy, and long-lasting stability in a wide range of environments. During the process of breaking down the very strong sulfur–carbon bonds in crude oil, TiO2 has unparalleled effectiveness due to its unique properties (for more information, see TiO2). Therefore, the quality of petroleum products is greatly improved by lowering the amount of sulfur they contain to conform to the regulations that the government has established for the environment.
The examination of the catalytic processes of TiO2 within this research comprehensively looks at the many available forms of TiO2 in the context of desulfurization. The nanoparticles, mesoporous structures, and composites that fall within this category are all examples. Each and every one of these forms comes with its unique collection of advantages and difficulties associated with the application procedure. For enhancing the methods of desulfurization, fully grasping the interactions of these TiO2 forms with sulfur molecules is of the utmost importance. This information is brought to light in a way that is both obvious and convincing by examining various TiO2 forms. A variety of operational factors, including temperature, pressure, and catalyst loading, have been investigated to shed light on the effect of these variables on the desulfurization performance of TiO2. It is especially important to have this knowledge to enhance the operational parameters of desulfurization operations as it offers the highest potential level of efficiency and cost-effectiveness. Consequently, it is very significant. The study also studies the most current developments in manufacturing TiO2 catalysts, which include novel modifications and hybrid-material synthesis. These developments are among the items being investigated. Furthermore, the research investigates the consequences of these discoveries in the technological field. These developments are being made to improve the catalytic activity, selectivity, and durability of the catalyst over time.
The study offers insight into the obstacles inherent in scaling desulfurization processes based on TiO2 for industrial applications. These issues are known as scaling challenges. This activity is done with an eye on future prospects. The management of catalyst deactivation, the incorporation of TiO2 catalysts into the infrastructures of refineries that are already in place, and the requirement for cost-efficient production procedures are some things that fall under this category. Despite the challenges, TiO2 seems to have a promising future in petroleum desulfurization. This optimism is shown by the various study avenues that may be pursued, as well as the anticipated potential technology advances. Using TiO2 as a catalyst for desulfurization in the petroleum sector indicates the continuous change toward developing cleaner, more environmentally friendly, and more efficient energy. This transition is enabled by the use of TiO2. As researchers, engineers, and government officials continue to investigate and improve technologies based on TiO2, the prospect of significantly reducing sulfur emissions and developing environmentally responsible methods for refining petroleum is becoming more apparent. The wealth of information presented in this research demonstrates the transformational capacity of TiO2 in tackling the environmental and energy concerns faced presently. A vast store of information is provided as a foundation for future study. Additionally, this review offers a wealth of information to the reader. TiO2 catalysis is expected to play a huge role in creating a sustainable future for refining petroleum, which will represent a substantial step forward in terms of environmental stewardship and energy security. This outcome is something that is predicted to have a fair probability of being accomplished via ongoing innovation and collaboration actions throughout the scientific and industrial sectors.
Author Contributions
The authors confirm contribution to the paper as follows: study conception: Z.A.H., data collection: Z.A.H. and J.J.D.; analysis and interpretation of results: Z.A.H., J.J.D. and M.A.J.; draft manuscript preparation: Z.A.H., J.J.D. and M.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krakowiak, R.; Musial, J.; Bakun, P.; Spychała, M.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Koczorowski, T.; Sobotta, L.; Stanisz, B.; Goslinski, T. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants Including Pharmaceutical Pollutants. Appl. Sci. 2021, 11, 8674. [Google Scholar] [CrossRef]
- Elgohary, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; El Jery, A.; Assadi, A.A.; Amrane, A. A Review of the Use of Semiconductors as Catalysts in the Photocatalytic Inactivation of Microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Guerrero, M.; Altube, A.; García-Lecina, E.; Rossinyol, E.; Baró, M.D.; Pellicer, E.; Sort, J. Facile in Situ Synthesis of BiOCl Nanoplates Stacked to Highly Porous TiO2: A Synergistic Combination for Environmental Remediation. ACS Appl. Mater. Interfaces 2014, 6, 13994–14000. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Bahadoran, A.; De Lile, J.R.; Masudy-Panah, S.; Sadeghi, B.; Li, J.; Sabzalian, M.H.; Ramakrishna, S.; Liu, Q.; Cavaliere, P.; Gopinathan, A. Photocatalytic Materials Obtained from E-Waste Recycling: Review, Techniques, Critique, and Update. J. Manuf. Mater. Process. 2022, 6, 69. [Google Scholar] [CrossRef]
- Robert, D.; Malato, S. Solar Photocatalysis: A Clean Process for Water Detoxification. Sci. Total Environ. 2002, 291, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Gowland Dan, C.A.; Neil, R.; Efthalia, C. Photocatalytic Oxidation of Natural Organic Matter in Water. Water 2020, 13, 288. [Google Scholar] [CrossRef]
- Uzelac, M.M.; Srđenović Čonić, B.; Kladar, N.; Armaković, S.; Armaković, S.J. Removal of Hydrochlorothiazide from Drinking and Environmental Water: Hydrolysis, Direct and Indirect Photolysis. Energy Environ. 2022; in press. [Google Scholar] [CrossRef]
- Burrows, H.D.; Santaballa, J.A.; Steenken, S. Reaction Pathways and Mechanisms of Photodegradation of Pesticides. J. Photochem. Photobiol. B Biol. 2002, 67, 71–108. [Google Scholar] [CrossRef]
- Menacherry, S.P.M.; Aravind, U.K.; Aravindakumar, C.T. Oxidative Degradation of Pharmaceutical Waste, Theophylline, from Natural Environment. Atmosphere 2022, 13, 835. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium Dioxide Photocatalysis: Present Situation and Future Approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Seba, S.; Zainab, Y.; Mohammad, F. Use of TiO2 in Photocatalysis for Air Purification and Wastewater Treatment: A Review. Eng. Technol. J. 2022, 40, 1131–1143. [Google Scholar]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef]
- Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent Organic Pollutants in Water Resources: Fate, Occurrence, Characterization and Risk Analysis. Sci. Total Environ. 2022, 831, 154808. [Google Scholar]
- Marathe, D.; Balbudhe, S.; Kumari, K. Persistent Organic Pollutants: A Global Issue, a Global Response. In Persistent Organic Pollutants; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–32. ISBN 1003046800. [Google Scholar]
- Song, Z.; Bi, M.; Li, J.; Guo, Y.; Xu, Q.; He, Y.; Zhao, N.; Chen, L.; Ren, D. Synthesis of TiO2-Modified Y Zeolite and its Adsorption-Catalytic Oxidative Desulfurization Performance. Silicon 2024, 1–14. [Google Scholar] [CrossRef]
- Wang, S.H.; Qin, Y.C.; Zhang, X.T.; Song, L.J. Fabrication of effective desulfurization species active sites in CeY zeolites and the adsorption desulfurization mechanisms. J. Fuel Chem. Technol. 2020, 48, 52–62. [Google Scholar] [CrossRef]
- Mordor Intelligence Research & Advisory. Titanium Dioxide Market Size & Share Analysis—Growth Trends & Forecasts (2024–2029). Mordor Intelligence. 2023. Available online: https://www.mordorintelligence.com/industry-reports/titanium-dioxide-market (accessed on 9 May 2024).
- Tomić, J.; Malinović, N. Titanium dioxide photocatalyst: Present situation and future approaches. AIDASCO Rev. 2023, 1, 26–30. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef]
- Singh, G.; Singh, A.; Singh, P.; Mishra, V.K. Organic Pollutants in Groundwater Resource. Groundw. Geochem. Pollut. Remediat. Methods 2021, 1, 139–163. [Google Scholar]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol Oxidation from Aqueous Solutions by Means of Ozonation and H2O2/UV System. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Radovnikovic, A. Ozonation and H2O2/UV Treatment of Clofibric Acid in Water: A Kinetic Investigation. J. Hazard. Mater. 2003, 103, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajova, I.; Venglovsky, J.; Szaboova, T.; Kovacova, S. Pollution of Surface and Ground Water by Sources Related to Agricultural Activities. Front. Sustain. Food Syst. 2018, 2, 42. [Google Scholar] [CrossRef]
- Ma, Y.; Halsall, C.J.; Crosse, J.D.; Graf, C.; Cai, M.; He, J.; Gao, G.; Jones, K. Persistent Organic Pollutants in Ocean Sediments from the N Orth P Acific to the A Rctic O Cean. J. Geophys. Res. Ocean. 2015, 120, 2723–2735. [Google Scholar] [CrossRef]
- Dachs, J.; Méjanelle, L. Organic Pollutants in Coastal Waters, Sediments, and Biota: A Relevant Driver for Ecosystems during the Anthropocene? Estuaries Coasts 2010, 33, 1–14. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N. Kinetic Modeling on Photooxidative Degradation of C.I. Acid Orange 7 in a Tubular Continuous-Flow Photoreactor. Chemosphere 2006, 62, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Liang, Y.; Wang, Z.; Fan, J.; Luo, Y.S.; Jia, Z.J. Synthesis and Photocatalytic Properties of TiO2 Nanostructures. Mater. Res. Bull. 2008, 43, 2187–2195. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Rodríguez-Pérez, M.; Ruiz-Gómez, M.Á.; Rodríguez-Gattorno, G.; Villanueva-Cab, J. Synergistic Correlation in the Colloidal Properties of TiO2 Nanoparticles and Its Impact on the Photocatalytic Activity. Inorganics 2022, 10, 125. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; KR, S.D. A Review of Hierarchical Nanostructures of TiO2: Advances and Applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 Nanostructures: A Review of Efficient Synthesis, Growth Mechanism, Probing Capabilities, and Applications in Bio-Safety and Health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.; Schmidt, W.G. The Electronic Structure and Optical Response of Rutile, Anatase and Brookite TiO2. J. Phys. Condens. Matter 2012, 24, 195503. [Google Scholar] [CrossRef]
- Siddiqui, H. Modification of Physical and Chemical Properties of Titanium Dioxide (TiO2) by Ion Implantation for Dye Sensitized Solar Cells. In Ion Beam Techniques and Applications; IntechOpen: London, UK, 2019; ISBN 1789845718. [Google Scholar]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.; Oskam, G. Phase-Pure TiO2 Nanoparticles: Anatase, Brookite and Rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Gilani, M.R.H.S.; Maaz, M.; Akhter, P.; Hussain, M.; Jeong, K.E.; Kwon, E.E.; Bae, S.; Park, Y.K. Advancements in TiO2-based photocatalysis for environmental remediation: Strategies for enhancing visible-light-driven activity. Chemosphere 2023, 349, 140703. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetics of Nanoparticle Oxides: Interplay between Surface Energy and Polymorphism. Geochem. Trans. 2003, 4, 34. [Google Scholar] [CrossRef]
- Li, J.-G.; Ishigaki, T.; Sun, X. Anatase, Brookite, and Rutile Nanocrystals via Redox Reactions under Mild Hydrothermal Conditions: Phase-Selective Synthesis and Physicochemical Properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Pottier, A.; Chanéac, C.; Tronc, E.; Mazerolles, L.; Jolivet, J.-P. Synthesis of Brookite TiO2 Nanoparticlesby Thermolysis of TiCl4 in Strongly Acidic Aqueous Media. J. Mater. Chem. 2001, 11, 1116–1121. [Google Scholar] [CrossRef]
- Kondamareddy, K.K.; Neena, D.; Lu, D.; Peng, T.; Lopez, M.A.M.; Wang, C.; Yu, Z.; Cheng, N.; Fu, D.J.; Zhao, X.-Z. Ultra-Trace (Parts per Million-Ppm) W6+ Dopant Ions Induced Anatase to Rutile Transition (ART) of Phase Pure Anatase TiO2 Nanoparticles for Highly Efficient Visible Light-Active Photocatalytic Degradation of Organic Pollutants. Appl. Surf. Sci. 2018, 456, 676–693. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Yang, Y.; Wang, N.; Zhuang, X.; Zhou, Z. Optimized photocatalytic regeneration of adsorption-photocatalysis bifunctional composite saturated with Methyl Orange. J. Environ. Sci. 2020, 94, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ammar, T.K.; Talib, M.A. Desulfurization of Real Diesel Fuel onto Mesoporous Silica MCM-41 Implementing Batch Adsorption Process: Equilibrium, Kinetics, and Thermodynamic Studies. Eng. Technol. J. 2022, 9, 1144–1157. [Google Scholar]
- Neran, K.I.; Samar, K.A. Desulfurization and Kinetic Study of Diesel Fuel by Batch Adsorption on Activated Carbon. Eng. Technol. J. 2015, 33, 8. [Google Scholar]
- Kwak, C.; Lee, J.J.; Bae, J.S.; Moon, S.H. Poisoning effect of nitrogen compounds on the performance of CoMoS/Al2O3 catalyst in the hydrodesulfurization of dibenzothiophene, 4-methyldibenzothiophene, and 4,6-dimethyldibenzothiophene. Appl. Catal. B Environ. 2001, 35, 59–68. [Google Scholar] [CrossRef]
- Liu, K.; Ng, F.T.T. Effect of the nitrogen heterocyclic compounds on hydrodesulfurization using in situ hydrogen and a dispersed Mo catalyst. Catal. Today 2010, 149, 28–34. [Google Scholar] [CrossRef]
- Patra, S.; Davoisne, C.; Bouyanfif, H.; Foix, D.; Sauvage, F. Phase Stability Frustration on Ultra-Nanosized Anatase TiO2. Sci. Rep. 2015, 5, 10928. [Google Scholar] [CrossRef]
- Holm, A.; Hamandi, M.; Simonet, F.; Jouguet, B.; Dappozze, F.; Guillard, C. Impact of Rutile and Anatase Phase on the Photocatalytic Decomposition of Lactic Acid. Appl. Catal. B Environ. 2019, 253, 96–104. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic Degradation for Environmental Applications–a Review. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini Review on Photocatalysis of Titanium Dioxide Nanoparticles and Their Solar Applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Lydakis-Simantiris, N.; Riga, D.; Katsivela, E.; Mantzavinos, D.; Xekoukoulotakis, N.P. Disinfection of Spring Water and Secondary Treated Municipal Wastewater by TiO2 Photocatalysis. Desalination 2010, 250, 351–355. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Berberidou, C.; Paspaltsis, I.; Kyriakou, G.; Sklaviadis, T.; Poulios, I. Photocatalytic Degradation and Drug Activity Reduction of Chloramphenicol. Water Res. 2008, 42, 386–394. [Google Scholar] [CrossRef]
- Vijayabalan, A.; Selvam, K.; Krishnakumar, B.; Swaminathan, M. Photocatalytic Degradation of Reactive Orange 4 by Surface Fluorinated TiO2 Wackherr under UV-A Light. Sep. Purif. Technol. 2013, 108, 51–56. [Google Scholar] [CrossRef]
- Selvam, K.; Swaminathan, M. Photocatalytic Synthesis of 2-Methylquinolines with TiO2 Wackherr and Home Prepared TiO2—A Comparative Study. Arab. J. Chem. 2017, 10, S28–S34. [Google Scholar]
- Vione, D.; Minero, C.; Maurino, V.; Carlotti, M.E.; Picatonotto, T.; Pelizzetti, E. Degradation of Phenol and Benzoic Acid in the Presence of a TiO2-Based Heterogeneous Photocatalyst. Appl. Catal. B Environ. 2005, 58, 79–88. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Könenkamp, R. Carrier Transport in Nanoporous TiO2 Films. Phys. Rev. B 2000, 61, 11057. [Google Scholar]
- Zhang, K.; Lin, Y.; Muhammad, Z.; Wu, C.; Yang, S.; He, Q.; Zheng, X.; Chen, S.; Ge, B.; Song, L. Active {010} Facet-Exposed Cu2MoS4 Nanotube as High-Efficiency Photocatalyst. Nano Res. 2017, 10, 3817–3825. [Google Scholar] [CrossRef]
- Che, M.; Védrine, J.C. Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527645330. [Google Scholar]
- Sattar, J.H.; Khaleel, I.H.; Odai, N.S. Structural Properties of Fe Doped TiO2 Nanorods Prepared by Low Cost Hydrothermal Method. Eng. Technol. J. 2020, 38, 177–183. [Google Scholar]
- Kowalska, E.; Wei, Z.; Janczarek, M. Band-Gap Engineering of Photocatalysts: Surface Modification versus Doping. In Visible-Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanisms, and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 449–484. [Google Scholar]
- Berger, T.; Sterrer, M.; Diwald, O.; Knözinger, E.; Panayotov, D.; Thompson, T.L.; Yates, J.T. Light-Induced Charge Separation in Anatase TiO2 Particles. J. Phys. Chem. B 2005, 109, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Neran, K.I.; Shahrazad, R.R.; Zainab, A.N. Removal of SO2 over Modified Activated Carbon in Fixed Bed Reactor: II. Effect of Process Variables on the Characteristics of Mass Transfer Zone. Eng. Tech. J. 2014, 32, 1825–1842. [Google Scholar]
- Tsyshevsky, R.V.; Pagoria, P.; Kuklja, M.M. Computational Design of Novel Energetic Materials: Dinitro-Bis-Triazolo-Tetrazine. J. Phys. Chem. C 2015, 119, 8512–8521. [Google Scholar] [CrossRef]
- Perdew, J.P. Density Functional Theory and the Band Gap Problem. Int. J. Quantum Chem. 1985, 28, 497–523. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.U.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A. Understanding Band Gaps of Solids in Generalized Kohn–Sham Theory. Proc. Natl. Acad. Sci. USA 2017, 114, 2801–2806. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Perdew, J.P.; Levy, M. Physical Content of the Exact Kohn-Sham Orbital Energies: Band Gaps and Derivative Discontinuities. Phys. Rev. Lett. 1983, 51, 1884. [Google Scholar] [CrossRef]
- Mori-Sánchez, P.; Cohen, A.J.; Yang, W. Localization and Delocalization Errors in Density Functional Theory and Implications for Band-Gap Prediction. Phys. Rev. Lett. 2008, 100, 146401. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Borlido, P.; Aull, T.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Large-Scale Benchmark of Exchange–Correlation Functionals for the Determination of Electronic Band Gaps of Solids. J. Chem. Theory Comput. 2019, 15, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Borlido, P.; Schmidt, J.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Exchange-Correlation Functionals for Band Gaps of Solids: Benchmark, Reparametrization and Machine Learning. npj Comput. Mater. 2020, 6, 96. [Google Scholar] [CrossRef]
- Cococcioni, M. The LDA+ U Approach: A Simple Hubbard Correction for Correlated Ground States. Correl. Electrons Model. Mater. Model. Simul. 2012, 2, 1–33. [Google Scholar]
- Man, Z.; Meng, Y.; Lin, X.; Dai, X.; Wang, L.; Liu, D. Assembling UiO-66@TiO2 nanocomposites for efficient photocatalytic degradation of dimethyl sulfide. Chem. Eng. J. 2022, 431, 133952. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, P.; Zhang, S.; Sun, Q.; Liang, E.; Jia, Y. Electronic Properties of Anatase TiO2 Doped by Lanthanides: A DFT+ U Study. Phys. B Condens. Matter 2012, 407, 1038–1043. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zuo, C.; Fang, X. Application of Nanostructured TiO2 in UV Photodetectors: A Review. Adv. Mater. 2022, 34, 2109083. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 Single Crystals with a Large Percentage of Reactive Facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Pan, F.; Li, Y. A Review on the Effects of TiO2 Surface Point Defects on CO2 Photoreduction with H2O. J. Mater. 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Wrana, D.; Gensch, T.; Jany, B.R.; Cieślik, K.; Rodenbücher, C.; Cempura, G.; Kruk, A.; Krok, F. Photoluminescence Imaging of Defects in TiO2: The Influence of Grain Boundaries and Doping on Charge Carrier Dynamics. Appl. Surf. Sci. 2021, 569, 150909. [Google Scholar] [CrossRef]
- Wen, B.; Hao, Q.; Yin, W.-J.; Zhang, L.; Wang, Z.; Wang, T.; Zhou, C.; Selloni, A.; Yang, X.; Liu, L.-M. Electronic Structure and Photoabsorption of Ti 3+ Ions in Reduced Anatase and Rutile TiO2. Phys. Chem. Chem. Phys. 2018, 20, 17658–17665. [Google Scholar] [CrossRef]
- Dharmale, N.; Chaudhury, S.; Kar, J.K. Various Exchange-Correlation Effects on Structural, Electronic, and Optical Properties of Brookite TiO2. ECS J. Solid State Sci. Technol. 2021, 10, 83010. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, Z.; Tian, Y.; You, J.; Jiang, L. Titanium Dioxide Derived Materials with Superwettability. Catalysts 2021, 11, 425. [Google Scholar] [CrossRef]
- Armaković, S.J.; Mary, Y.S.; Mary, Y.S.; Pelemiš, S.; Armaković, S. Optoelectronic Properties of the Newly Designed 1, 3, 5-Triazine Derivatives with Isatin, Chalcone and Acridone Moieties. Comput. Theor. Chem. 2021, 1197, 113160. [Google Scholar] [CrossRef]
- Del Angel, R.; Durán-Álvarez, J.C.; Zanella, R. TiO2-Low Band Gap Semiconductor Heterostructures for Water Treatment Using Sunlight-Driven Photocatalysis. In Titanium Dioxide: Material for a Sustainable Environment; IntechOpen: London, UK, 2018; Volume 305. [Google Scholar]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Direct Observation of Surface-Mediated Electron− Hole Pair Recombination in TiO2 (110). J. Phys. Chem. C 2010, 114, 3098–3101. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119538. [Google Scholar] [CrossRef]
- Kubovics, M.; Silva, G.; Ana, M.L.; Faria, J.L. Photocatalytic Hydrogen Production Using Porous 3D Graphene-Based Aerogels Supporting Pt/TiO2 Nanoparticles. Gels 2022, 8, 719. [Google Scholar] [CrossRef]
- Mosquera-Vargas, E.; Herrera-Molina, D.; Diosa, J.E. Structural and Optical Properties of TiO2 Nanoparticles and Their Photocatalytic Behavior under Visible Light. Ing. Compet. 2021, 23, 2. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?-Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Glassford, K.M.; Chelikowsky, J.R. Optical Properties of Titanium Dioxide in the Rutile Structure. Phys. Rev. B 1992, 45, 3874. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, G.; Murugasen, P.; Sagadevan, S. Investigations on the Synthesis, Optical and Electrical Properties of TiO2 Thin Films by Chemical Bath Deposition (CBD) Method. Mater. Res. 2016, 19, 413–419. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, W.; Zuo, S.; Yuan, K.; Wu, F.; Ji, J.; Yao, C. Double Z-scheme TiO2 (R)/C-TiO2 (A) heterojunction greatly enhanced efficiency of photocatalytic desulfurization under sunlight. J. Mater. Sci. Mater. Electron. 2020, 31, 22297–22311. [Google Scholar] [CrossRef]
- Soussi, A.; Ait Hssi, A.; Boujnah, M.; Boulkadat, L.; Abouabassi, K.; Asbayou, A.; Elfanaoui, A.; Markazi, R.; Ihlal, A.; Bouabid, K. Electronic and Optical Properties of TiO2 Thin Films: Combined Experimental and Theoretical Study. J. Electron. Mater. 2021, 50, 4497–4510. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis-Materials, Mechanisms and Applications; InTech: Rang-Du-Fliers, France, 2016; pp. 31–80. [Google Scholar]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K.; Kuvarega, A.T. Fabrication of Magnetic Recoverable Fe3O4/TiO2 Heterostructure for Photocatalytic Degradation of Rhodamine B Dye. Inorg. Chem. Commun. 2022, 145, 109966. [Google Scholar] [CrossRef]
- Amin, S.; Sher, M.; Ali, A.; Rehman, M.F.; Hayat, A.; Ikram, M.; Abbas, A.; Amin, H.M.A. Sulfonamide-Functionalized Silver Nanoparticles as an Analytical Nanoprobe for Selective Ni (II) Sensing with Synergistic Antimicrobial Activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100735. [Google Scholar] [CrossRef]
- Joo, J.B.; Zhang, Q.; Dahl, M.; Zaera, F.; Yin, Y. Synthesis, crystallinity control, and photocatalysis of nanostructured titanium dioxide shells. J. Mater. Res. 2013, 28, 362–368. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent Developments and Challenges in Practical Application of Visible–Light–Driven TiO2–Based Heterojunctions for PPCP Degradation: A Critical Review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Liu, Z.; Bruce, P.G.; Grey, C.P. The Morphology of TiO2 (B) Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13612–13623. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Varshney, G.; Kanel, S.R.; Kempisty, D.M.; Varshney, V.; Agrawal, A.; Sahle-Demessie, E.; Varma, R.S.; Nadagouda, M.N. Nanoscale TiO2 Films and Their Application in Remediation of Organic Pollutants. Coord. Chem. Rev. 2016, 306, 43–64. [Google Scholar] [CrossRef]
- Žener, B.; Matoh, L.; Reli, M.; Škapin, A.S.; Korošec, R.C. Metal and Non-Metal Modified Titania: The Effect of Phase Composition and Surface Area on Photocatalytic Activity. Acta Chim. Slov. 2022, 69, 217–226. [Google Scholar] [PubMed]
- Wu, J.; Lu, S.; Ge, D.; Zhang, L.; Chen, W.; Gu, H. Photocatalytic Properties of Pd/TiO2 Nanosheets for Hydrogen Evolution from Water Splitting. RSC Adv. 2016, 6, 67502–67508. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts—Applications and Attributes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Ma, H.; Song, M.; Li, D.; Li, Y.; Li, S.; Zhang, X.; Liu, Y. Revisiting Pt/TiO2 Photocatalysts for Thermally Assisted Photocatalytic Reduction of CO2. Nanoscale 2020, 12, 7000–7010. [Google Scholar] [CrossRef]
- Lum, M.M.X.; Ng, K.H.; Lai, S.Y.; Mohamed, A.R.; Alsultan, A.G.; Taufiq-Yap, Y.H.; Koh, M.K.; Mohamed, M.A.; Vo, D.-V.N.; Subramaniam, M. Sulfur dioxide catalytic reduction for environmental sustainability and circular economy: A review. Process Saf. Environ. Prot. 2023, 176, 580–604. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Abbas, G.; Qurashi, A.; Hussain, M. Efficient catalyst development for deep aerobic photocatalytic oxidative desulfurization: Recent advances, confines, and outlooks. Catal. Rev. 2022, 64, 789–834. [Google Scholar] [CrossRef]
- Porcar-Santos, O.; Cruz-Alcalde, A.; López-Vinent, N.; Zanganas, D.; Sans, C. Photocatalytic degradation of sulfamethoxazole using TiO2 in simulated seawater: Evidence for direct formation of reactive halogen species and halogenated by-products. Sci. Total Environ. 2020, 736, 139605. [Google Scholar] [CrossRef] [PubMed]
- Chhakchhuak Vanlalhmingmawia, Seung Mok Lee, Diwakar Tiwari, Plasmonic noble metal doped titanium dioxide nanocomposites: Newer and exciting materials in the remediation of water contaminated with micropollutants. J. Water Process Eng. 2023, 51, 103360. [CrossRef]
- Cao, X.; Yang, X.; Li, H.; Huang, W.; Liu, X. Investigation of Ce-TiO2 Photocatalyst and Its Application in Asphalt-Based Specimens for NO Degradation. Constr. Build. Mater. 2017, 148, 824–832. [Google Scholar] [CrossRef]
- Dedual, G.; MacDonald, M.J.; Alshareef, A.; Wu, Z.; Tsang, D.C.W.; Yip, A. CKRequirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2014, 2, 1947–1955. [Google Scholar] [CrossRef]
- Haghighi, M.; Gooneh-Farahani, S. Insights to the oxidative desulfurization process of fossil fuels over organic and inorganic heterogeneous catalysts: Advantages and issues. Environ. Sci. Pollut. Res. 2020, 27, 39923–39945. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Umar, A.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Efficient Photocatalytic Degradation of Brilliant Green Using Sr-Doped TiO2 Nanoparticles. Ceram. Int. 2015, 41, 3533–3540. [Google Scholar] [CrossRef]
- Zheng, S.K.; Wang, T.M.; Hao, W.C.; Shen, R. Improvement of Photocatalytic Activity of TiO2 Thin Film by Sn Ion Implantation. Vacuum 2002, 65, 155–159. [Google Scholar] [CrossRef]
- Rossi, L.; Palacio, M.; Villabrille, P.I.; Rosso, J.A. V-Doped TiO2 Photocatalysts and Their Application to Pollutant Degradation. Environ. Sci. Pollut. Res. 2021, 28, 24112–24123. [Google Scholar] [CrossRef] [PubMed]
- Procurement Resource, Market Insights. Titanium Dioxide (TiO2) Production Cost Reports. Available online: https://www.procurementresource.com/ (accessed on 1 February 2024).
- Choudhury, B.; Bayan, S.; Choudhury, A.; Chakraborty, P. Narrowing of Band Gap and Effective Charge Carrier Separation in Oxygen Deficient TiO2 Nanotubes with Improved Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2016, 465, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, Y.; Kılıç, M.; Cinar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2010, 13, 281–296. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the Strategies for Enhancing the Photocatalytic Activity of TiO2: Conversion from UV-Light Active to Visible-Light Active Photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Kumari, S.; Sengupta, S. Non-hydrogen processes for simultaneous desulfurization and denitrogenation of light petroleum fuels—An elaborative review. Environ. Sci. Pollut. Res. 2021, 28, 61873–61907. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).