A Green Synthesis of 3-Selanyl-Isoflavones via Lipase Mediated Selenylation/Cyclization of Enaminones
Abstract
:1. Introduction
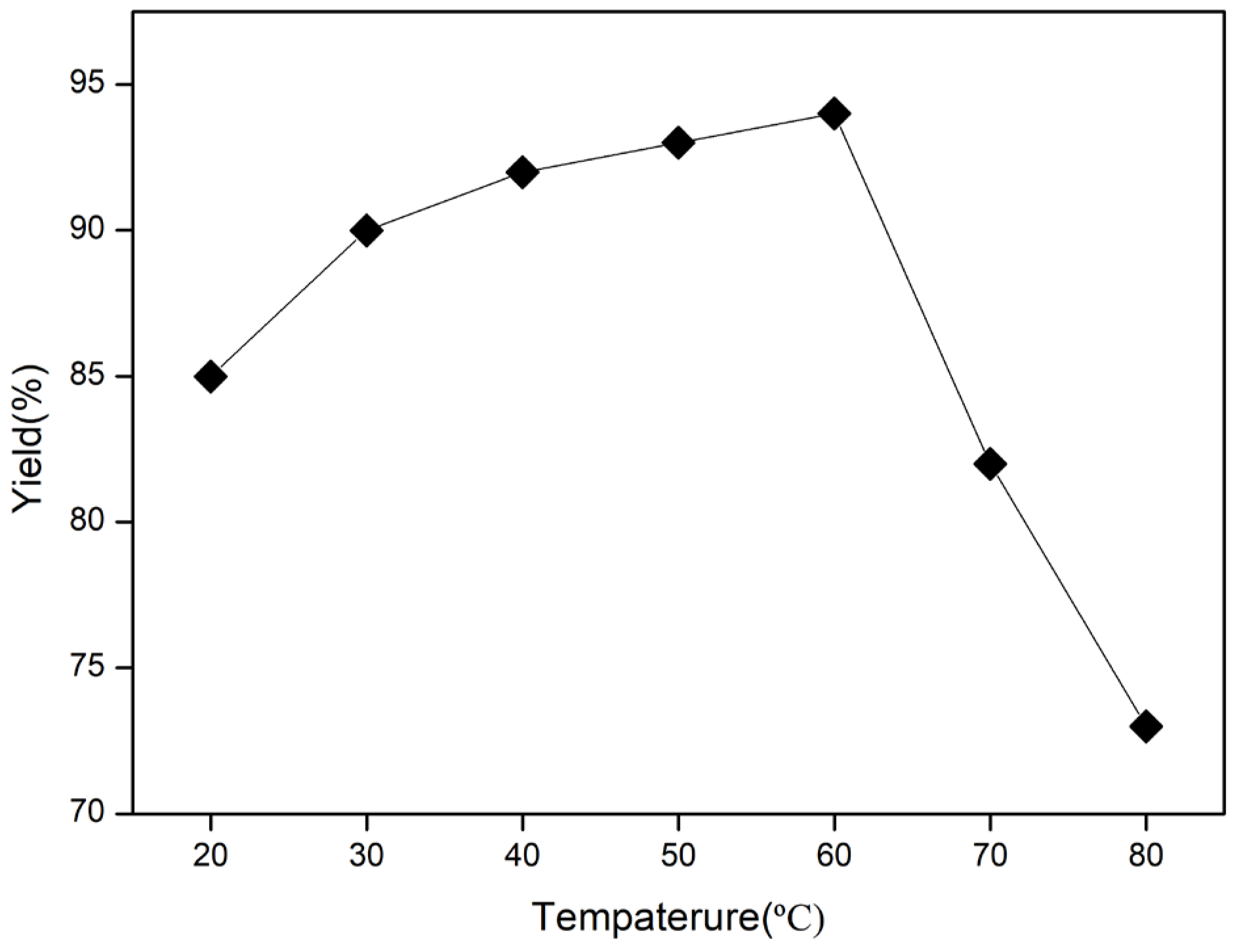
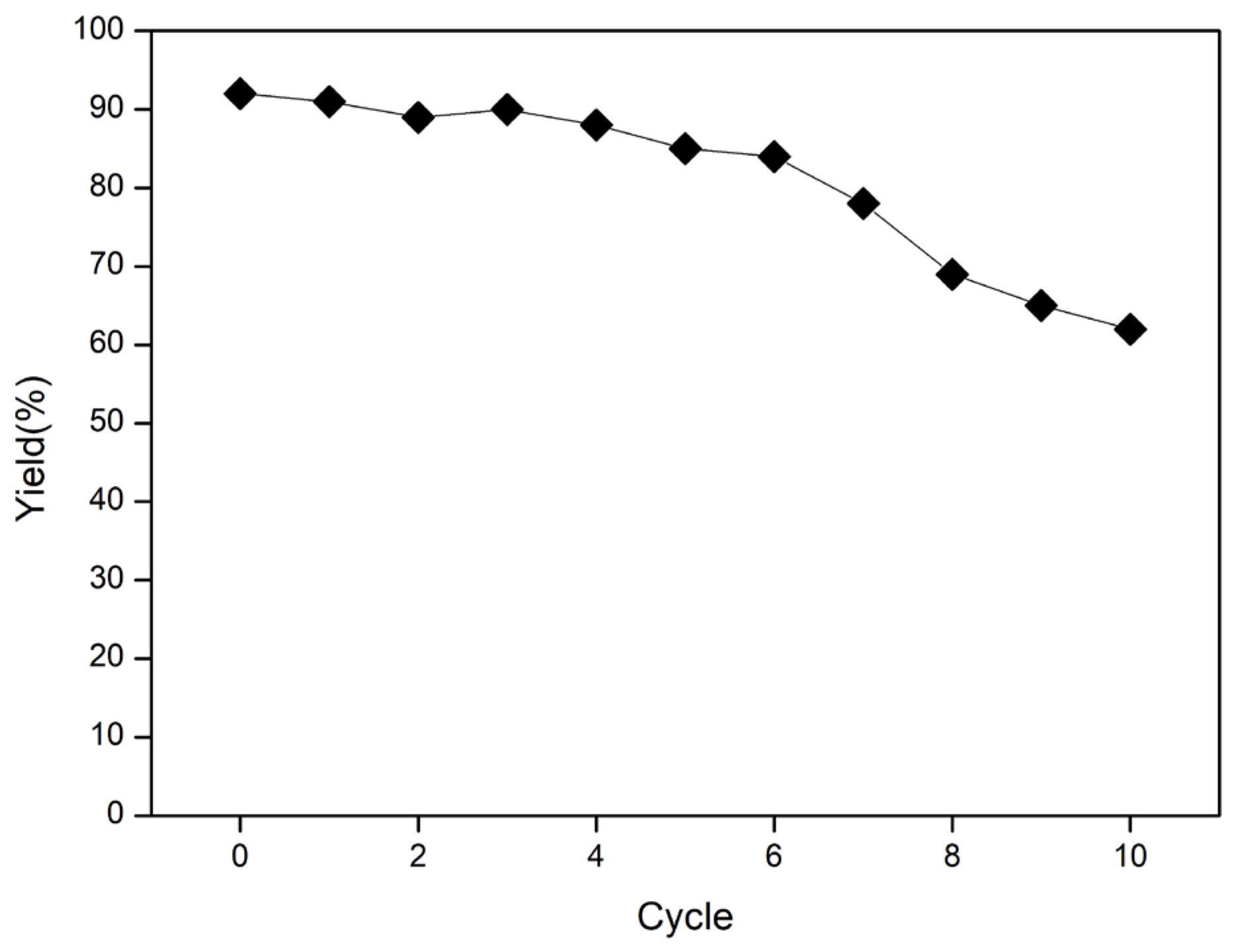
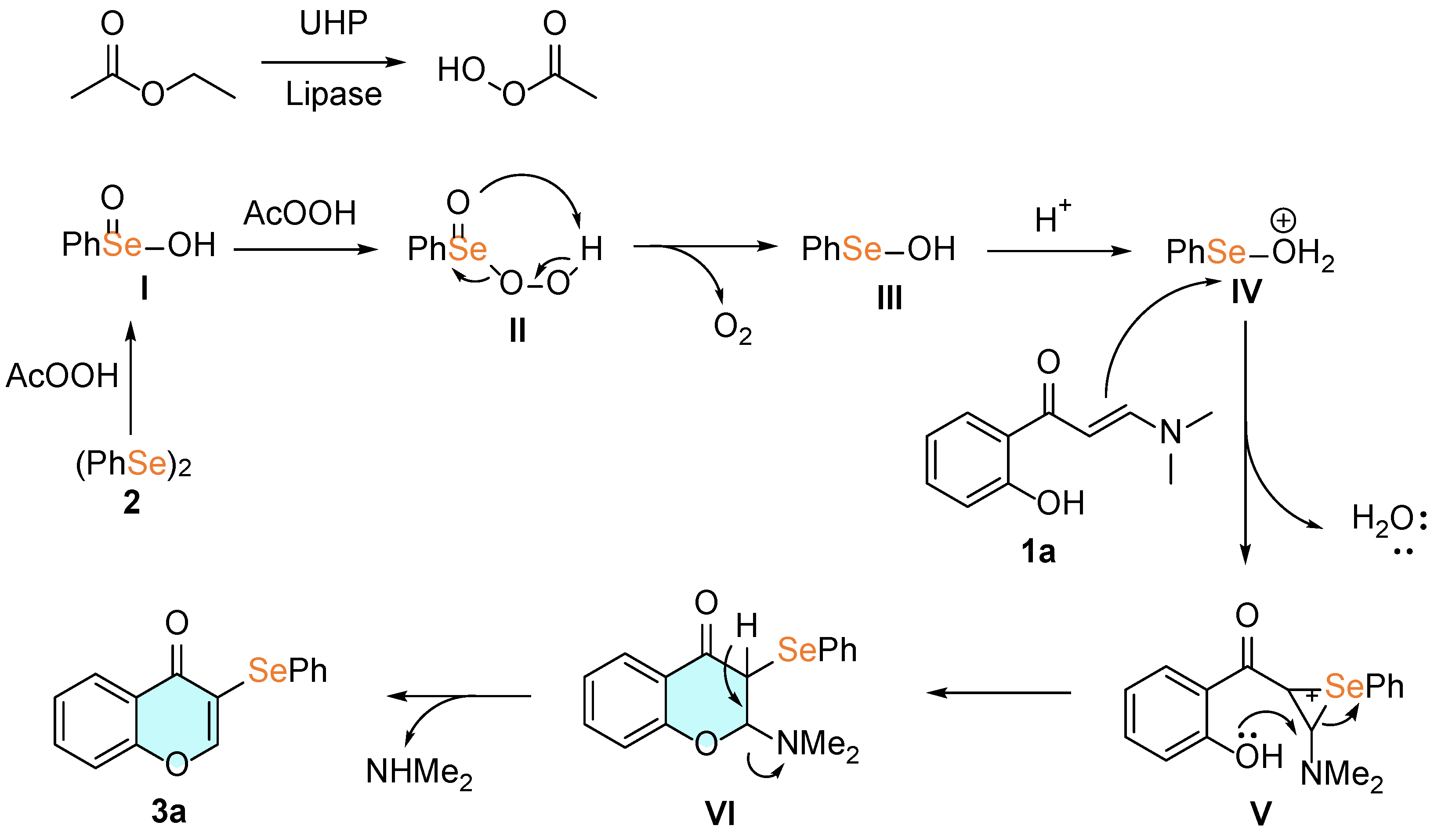
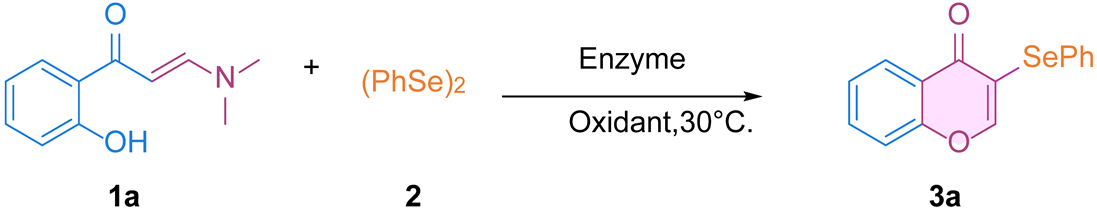
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Procedure for Lipase-Catalyzed Synthesis of 3-Selanyl-Isoflavones (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Cheng, F.-B.; Wu, X.-R.; Zhu, W.; Liao, J.-W.; Jiang, Y.; Zhang, C.; Niu, W.-Y.; Yu, Y.; Duan, H.-Q.; et al. Flavonoid derivatives synthesis and anti-diabetic activities. Bioorg. Chem. 2020, 95, 103501. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, B.; Chen, H.; Huang, H.; Hu, Y. Promiscuous enzyme-catalyzed cascade reaction: Synthesis of xanthone derivatives. Bioorg. Chem. 2018, 80, 555–559. [Google Scholar] [CrossRef]
- Li, H.; Lyv, Y.; Zhou, S.; Yu, S.; Zhou, J. Microbial cell factories for the production of flavonoids–barriers and opportunities. Bioresour. Technol. 2022, 360, 127538. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Altunay, N.; Tuzen, M. A simple and green ultrasound liquid–liquid microextraction method based on low viscous hydrophobic deep eutectic solvent for the preconcentration and separation of selenium in water and food samples prior to HG-AAS detection. Food Chem. 2021, 364, 130371. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; He, Z.; Zhang, J.; Cao, Y.; Mao, D.; Feng, C.; Tian, B.; Chen, G. Molecular docking-assisted design and synthesis of an anti-tumor quercetin–Se(iv) complex. New J. Chem. 2020, 44, 8434–8441. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, B.; Yu, J.; Ren, Y.; Wang, J.; Xie, P.; Pittman, C.U.; Zhou, A. Copper-catalyzed generation of flavone selenide and thioether derivatives using KSeCN and KSCN via C–H functionalization. Org. Biomol. Chem. 2018, 16, 5999–6005. [Google Scholar] [CrossRef]
- Ding, C.; Yu, Y.; Yu, Q.; Xie, Z.; Zhou, Y.; Zhou, J.; Liang, G.; Song, Z. NIS/TBHP Induced Regioselective Selenation of (Hetero)Arenes via Direct C−H Functionalization. ChemCatChem 2018, 10, 5397–5401. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhang, J.-R.; Huang, G.-B.; Zhou, Y.-H.; Chen, Y.-Y.; Xu, Y.-L. Visible Light-Promoted Selenylation/Cyclization of Enaminones toward the Formation of 3-Selanyl-4H-Chromen-4-Ones. Adv. Synth. Catal. 2021, 363, 1656–1661. [Google Scholar] [CrossRef]
- Xu, P.; Zhong, Z.; Huang, H.; Zhou, A. Selenation of 2-Hydroxyphenyl Enaminones with Se Powder to Generate ArSe-subsituted Chromone Derivatives. Chem. Select 2022, 7, e202202854. [Google Scholar] [CrossRef]
- Xia, J.-H.; Chen, Q.; Yuan, J.-W.; Shi, W.-S.; Yang, L.-R.; Xiao, Y.-M. Selectfluor-mediated tandem cyclization of enaminones with diselenides toward the synthesis of 3-selenylated chromones. RSC Adv. 2023, 13, 26948–26959. [Google Scholar] [CrossRef]
- Doerner, C.V.; Neto, J.S.S.; Cabreira, C.R.; Saba, S.; Sandjo, L.P.; Rafique, J.; Braga, A.L.; de Assis, F.F. Synthesis of 3-selanyl-isoflavones from 2-hydroxyphenyl enaminones using trichloroisocyanuric acid (TCCA): A sustainable approach. New J. Chem. 2023, 47, 5598–5602. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Varma, R.S.; Naicker, K.P. The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles. Org. Lett. 1999, 1, 189–192. [Google Scholar] [CrossRef]
- Verma, P.; Chauhan, S.; Singh, V.; Singh, S.; Srivastava, V. Urea hydrogen peroxide-initiated synthesis of pyranopyrazoles through oxidative coupling under base- and metal-free conditions by physical grinding method. Mol. Divers. 2022, 26, 1769–1777. [Google Scholar] [CrossRef]
- Moraes, C.A.O.; Santos, R.B.C.; Cavalcante, M.F.O.; Guilhermi, J.S.; Ali, M.A.; Botteselle, G.V.; Frizon, T.E.A.; Shah, M.I.A.; Lião, L.M.; Beatriz, A.; et al. Urea Hydrogen Peroxide and Ethyl Lactate, an Eco-Friendly Combo System in the Direct C(sp2)–H Bond Selenylation of Imidazo [2,1-b]thiazole and Related Structures. ACS Omega 2023, 8, 39535–39545. [Google Scholar] [CrossRef]
- Page, P.C.B.; Buckley, B.R.; Elliott, C.; Chan, Y.; Dreyfus, N.; Marken, F. Chemoselective Oxidation of Sulfides to Sulfoxides with Urea-Hydrogen Peroxide Complex Catalysed by Diselenide. Synlett 2016, 27, 80–82. [Google Scholar] [CrossRef]
- Bordeaux, M.; Tyagi, V.; Fasan, R. Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1744–1748. [Google Scholar] [CrossRef]
- Xie, H.; Li, F.; Xu, Y.; Wang, C.; Xu, Y.; Wu, J.; Li, Z.; Wang, Z.; Wang, L. Vitreoscilla hemoglobin: A natural carbene transfer catalyst for diastereo- and enantioselective synthesis of nitrile-substituted cyclopropanes. Green Chem. 2023, 25, 6853–6858. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Xu, Y.; Xie, H.; Wu, J.; Wang, C.; Li, Z.; Wang, Z.; Wang, L. Engineering of Dual-Function Vitreoscilla Hemoglobin: A One-Pot Strategy for the Synthesis of Unnatural α-Amino Acids. Org. Lett. 2023, 25, 7115–7119. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Xu, Y.; Ma, J.; Xie, H.; Yang, H.; Han, W.; Wang, C.; Li, Z.; Wang, L. Novel dual-enzyme system for synthesis of 2-alkyl and 2-arylbenzoxazoles via aerobic oxidation. Org. Chem. Front 2023, 10, 3509–3514. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, N.; Li, F.; Wang, C.; Xie, H.; Wu, J.; Cheng, L.; Wang, L.; Wang, Z. Application of Vitreoscilla Hemoglobin as a Green and Efficient Biocatalyst for the Synthesis of Benzoxazoles in Water. ChemBioChem 2024, 25, e202300609. [Google Scholar] [CrossRef]
- Xu, X.; Hilberath, T.; Hollmann, F. Selective oxyfunctionalisation reactions catalysed by P450 monooxygenases and peroxygenases—A bright future for sustainable chemical synthesis. Curr. Opin. Green Sustainable Chem. 2023, 39, 100745. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Jiang, Y.; Qin, X.; Ma, N.; Yao, F.; Dong, S.; Liu, C.; Feng, Y.; Jin, L.; et al. Engineering Cytochrome P450BM3 Enzymes for Direct Nitration of Unsaturated Hydrocarbons. Angew. Chem. Int. Ed. 2023, 62, e202217678. [Google Scholar] [CrossRef]
- Fan, S.; Cong, Z. Emerging Strategies for Modifying Cytochrome P450 Monooxygenases into Peroxizymes. Acc. Chem. Res. 2024, 57, 613–624. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, B.; Wang, F.; Yu, X.; Ma, L.; Li, A.; Reetz, M.T. Chemo- and Regioselective Dihydroxylation of Benzene to Hydroquinone Enabled by Engineered Cytochrome P450 Monooxygenase. Angew. Chem. Int. Ed. 2019, 58, 764–768. [Google Scholar] [CrossRef]
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate’. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2021, 401, 123353. [Google Scholar] [CrossRef]
- Könst, P.; Kara, S.; Kochius, S.; Holtmann, D.; Arends, I.W.C.E.; Ludwig, R.; Hollmann, F. Expanding the Scope of Laccase-Mediator Systems. ChemCatChem 2013, 5, 3027–3032. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.-L.; Guan, Z.; He, Y.-H. Concurrent Asymmetric Reactions Combining Photocatalysis and Enzyme Catalysis: Direct Enantioselective Synthesis of 2,2-Disubstituted Indol-3-ones from 2-Arylindoles. Angew. Chem. Int. Ed. 2019, 58, 118–124. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Zhu, H.; Shen, Y.; Le, Z.; Xie, Z. Combining enzyme and photoredox catalysis for the synthesis of quinazolines. Mol. Catal. 2023, 550, 113549. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Tang, Y.; Wu, J.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Synthesis of functionalized 1,4-dihydropyridines containing benzosultams catalyzed by lipase. New J. Chem. 2023, 47, 9708–9713. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Wang, C.; Zhang, L.; Li, F.; Zhang, W.; Chen, P.; Wang, L. A mild and efficient Dakin reaction mediated by lipase. Green Chem. Lett. Rev. 2017, 10, 269–273. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Wang, C.; Zheng, L.; Wang, Z.; Zhao, R.; Wang, L. Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage. Catalysts 2017, 7, 115. [Google Scholar] [CrossRef]
- Suo, H.; Geng, H.; Zhang, L.; Liu, G.; Yan, H.; Cao, R.; Zhu, J.; Hu, Y.; Xu, L. Covalent immobilization of lipase on an ionic liquid-functionalized magnetic Cu-based metal–organic framework with boosted catalytic performance in flavor ester synthesis. J. Mater. Chem. B 2023, 11, 1302–1311. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Z.; Fang, K.; He, X.; Xu, H.; Hu, Y. Enzymatic approach to cascade synthesis of bis(indolyl)methanes in pure water. RSC Adv. 2020, 10, 10848–10853. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Xu, Y.; Li, F.; Liu, Y.; Sun, Y.; Wang, C.; Chen, P.; Wang, L. Efficient synthesis of 3-hydroxy chromones via oxidative cyclization mediated by lipase. Green Chem. Lett. Rev. 2022, 15, 689–694. [Google Scholar] [CrossRef]
- Guo, T. Ammonium iodide-mediated regioselective chalcogenation of chromones with diaryl disulfides and diselenides. Synth. Commun. 2017, 47, 2053–2061. [Google Scholar] [CrossRef]


 | |||
|---|---|---|---|
| Entry | Enzyme 1 | Oxidant | Yield (%) |
| 1 | PPL | UHP | <5 |
| 2 | MML | UHP | <5 |
| 3 | CSL | UHP | <5 |
| 4 | Cal-B | UHP | 67 |
| 5 | Cal-B 2 | UHP | 13 |
| 6 | Cal-B 3 | UHP | 9 |
| 7 | Novozym 435 | UHP | 90 |
| 8 | Novozym 435 | H2O2 | 71 |
| 9 | Novozym 435 | None | <5 |
| 10 | None | UHP | <5 |
| 11 | None | None | <5 |
| 12 | Novozym 435 4 | UHP | <5 |
| 13 | Novozym 435 5 | UHP | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, W.; Piao, Y.; Song, W.; Chen, X.; Wang, C.; Wang, Z.; Wang, L. A Green Synthesis of 3-Selanyl-Isoflavones via Lipase Mediated Selenylation/Cyclization of Enaminones. Catalysts 2024, 14, 413. https://doi.org/10.3390/catal14070413
Kan W, Piao Y, Song W, Chen X, Wang C, Wang Z, Wang L. A Green Synthesis of 3-Selanyl-Isoflavones via Lipase Mediated Selenylation/Cyclization of Enaminones. Catalysts. 2024; 14(7):413. https://doi.org/10.3390/catal14070413
Chicago/Turabian StyleKan, Wenbo, Yuming Piao, Wenning Song, Xiaoxuan Chen, Chunyu Wang, Zhi Wang, and Lei Wang. 2024. "A Green Synthesis of 3-Selanyl-Isoflavones via Lipase Mediated Selenylation/Cyclization of Enaminones" Catalysts 14, no. 7: 413. https://doi.org/10.3390/catal14070413






