Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Ag-NPs Utilizing Bacterial Metabolites
2.2. Characterization
2.2.1. Optical Analysis
2.2.2. Morphological Properties
2.2.3. Elementary Mapping of Bacterial-Based Ag-NPs
2.2.4. Crystallographic Assessment
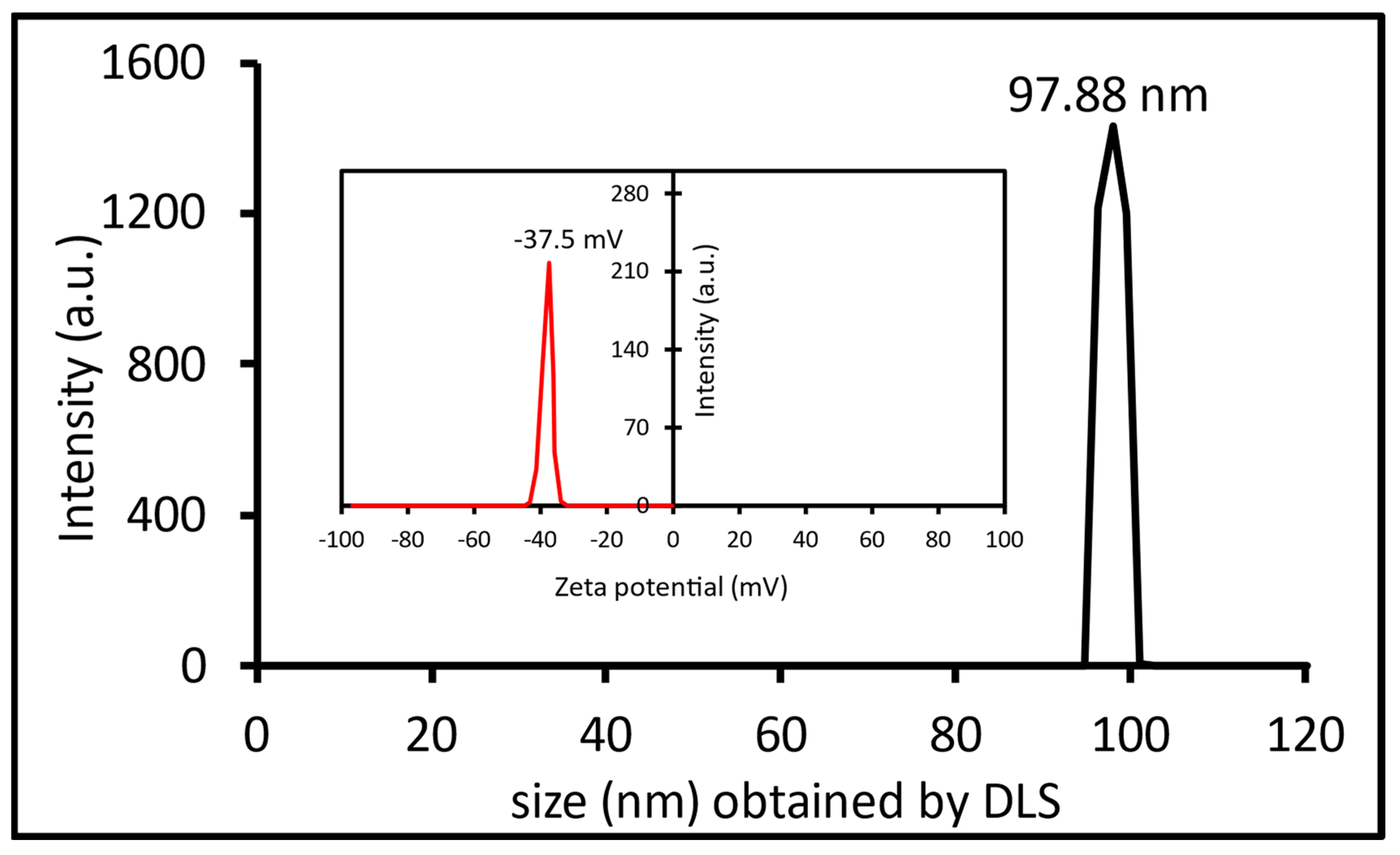
2.2.5. Ag-NPs Stability Test
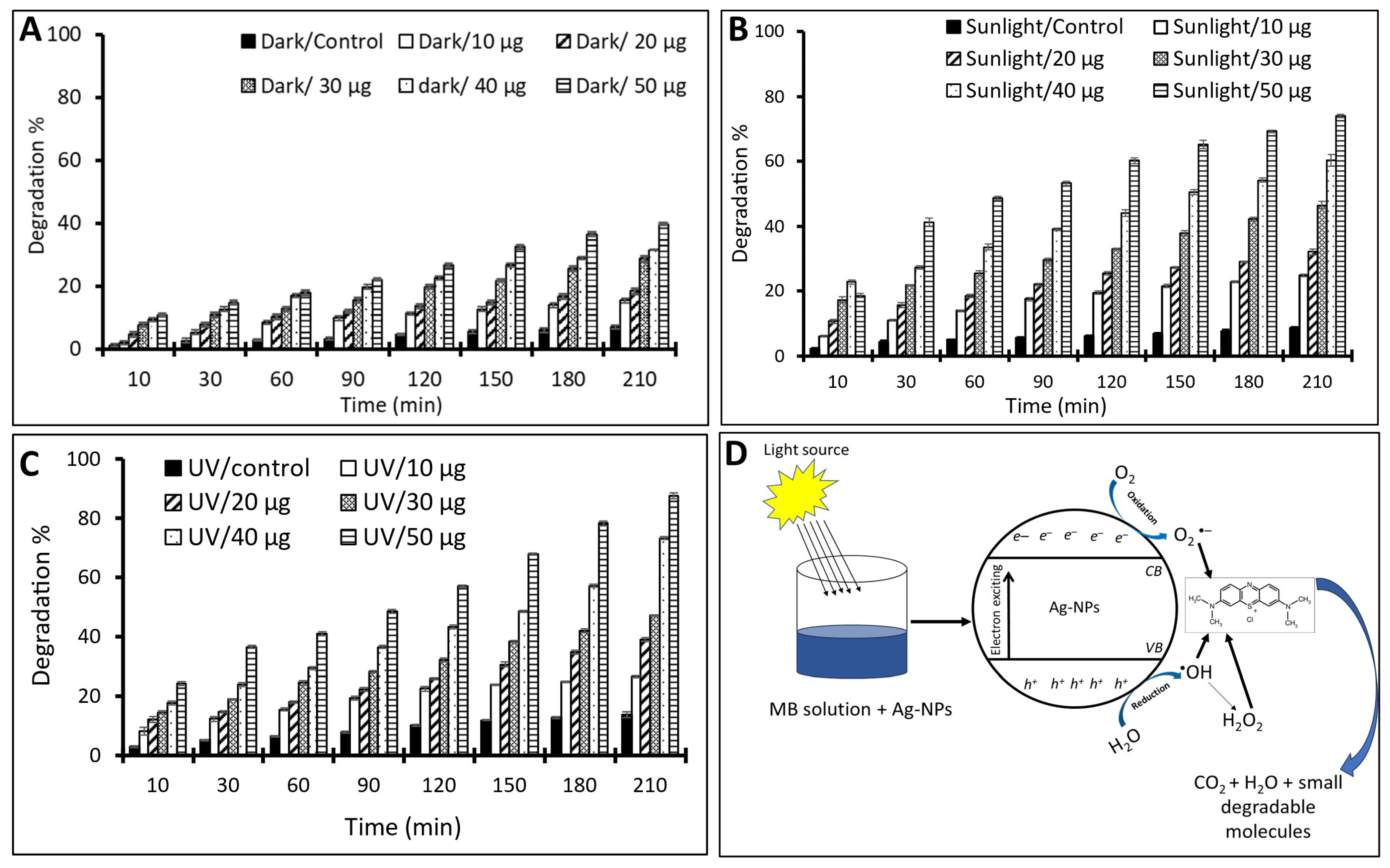
2.3. Environmental Investigation
Photocatalytic Activity
2.4. Biomedical Investigations
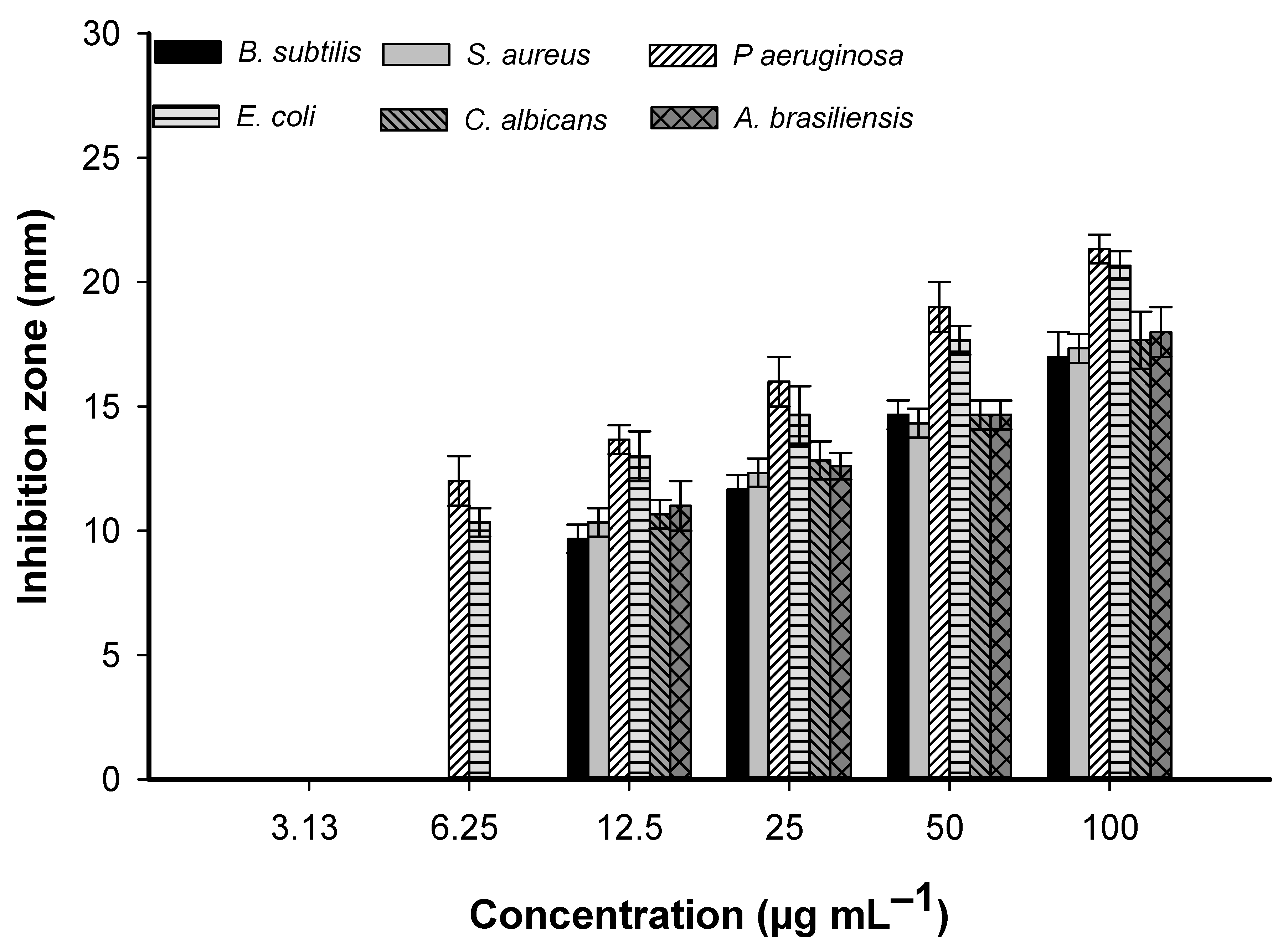
2.4.1. Antimicrobial Activity
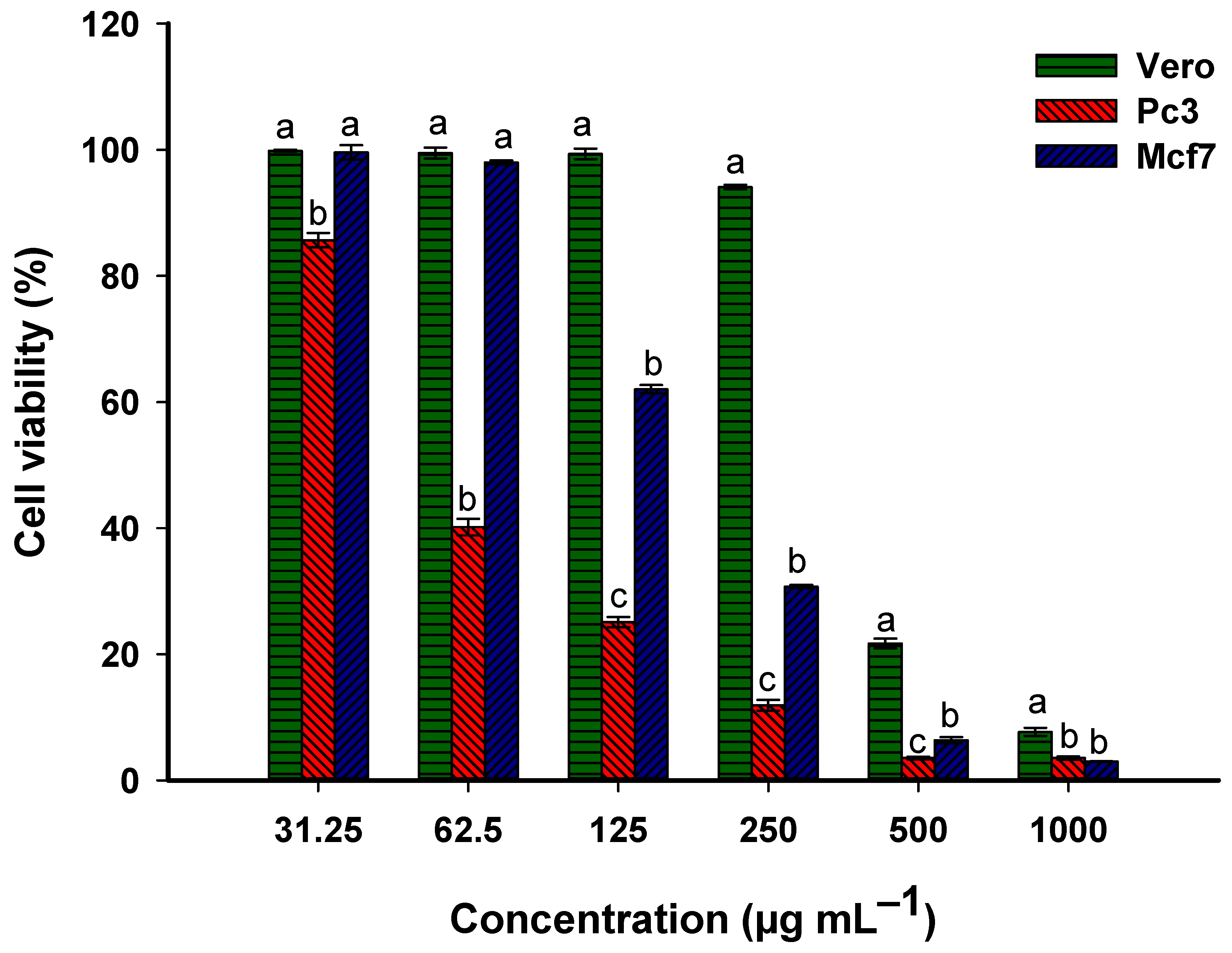
2.4.2. Anticancer Activity
3. Materials and Methods
3.1. Bacterial Strain
3.2. Ag-NPs Synthesis
3.3. Characterization
3.3.1. Optical Properties
3.3.2. Morphological Properties
3.3.3. Chemical Composition Analysis
3.3.4. Crystallographic Assessment
3.3.5. Stability and Surface Charge
3.4. Photocatalytic Activity
3.5. Antimicrobial Activity
3.6. Anticancer Activity
3.7. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, C.; Ning, J.; Dai, R.; Liu, Y.; Wu, Q.; Zhang, F.; Zhang, W.; Dou, S.; Liu, X. Unusual aliovalent Cd doped γ-Bi2MoO6 nanomaterial for efficient photocatalytic degradation of sulfamethoxazole and rhodamine B under visible light irradiation. Carbon Neutralization 2023, 2, 646–660. [Google Scholar] [CrossRef]
- Alphandéry, E. Natural metallic nanoparticles for application in nano-oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm Agrotis ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.F.; Hozzein, W.N.; Chen, W.; Li, W.J. Antibacterial Activity of Silver Nanoparticles against Staphylococcus warneri Synthesized Using Endophytic Bacteria by Photo-irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; F. Hetta, H.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- dos Santos, O.A.L.; de Araujo, I.; Dias da Silva, F.; Sales, M.N.; Christoffolete, M.A.; Backx, B.P. Surface modification of textiles by green nanotechnology against pathogenic microorganisms. Curr. Res. Green Sustain. Chem. 2021, 4, 100206. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Solís-Sandí, I.; Cordero-Fuentes, S.; Pereira-Reyes, R.; Vega-Baudrit, J.R.; Batista-Menezes, D.; Montes de Oca-Vásquez, G. Optimization of the biosynthesis of silver nanoparticles using bacterial extracts and their antimicrobial potential. Biotechnol. Rep. 2023, 40, e00816. [Google Scholar] [CrossRef]
- Fayadoglu, M.; Fayadoglu, E.; Er, S.; Koparal, A.T.; Koparal, A.S. Determination of biological activities of nanoparticles containing silver and copper in water disinfection with/without ultrasound technique. J. Environ. Health Sci. Eng. 2023, 21, 73–83. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, F.; Li, M.; Wang, P.; Fu, Y.; Wang, G.; Liu, X. Construction of hollow binary oxide heterostructures by Ostwald ripening for superior photoelectrochemical removal of reactive brilliant blue KNR dye. Adv. Powder Mater. 2023, 2, 100117. [Google Scholar] [CrossRef]
- Zhou, R.; Srinivasan, M.P. Photocatalysis in a packed bed: Degradation of organic dyes by immobilized silver nanoparticles. J. Environ. Chem. Eng. 2015, 3, 609–616. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.S.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, B.; Siqin, L.; Liu, G.; Wu, Q.; Xue, S.; Shao, T.; Zhang, F.; Zhang, W.; Liu, X. Designing advanced S-scheme CdS QDs/La-Bi2WO6 photocatalysts for efficient degradation of RhB. Exploration 2023, 3, 20230050. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.-D. Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Kumari, P.; Deepa, N.; Trivedi, P.K.; Singh, B.K.; Srivastava, V.; Singh, A. Plants and endophytes interaction: A “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites. Microb. Cell Factories 2023, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-mediated green synthesis of silver nanoparticles and evaluation of their antibacterial, anti-candida, acaricides, and photocatalytic activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E. Harnessing Bacterial Endophytes for Promotion of Plant Growth and Biotechnological Applications: An Overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic Nanotechnology: An Approach to Study Scope and Potential Applications. Front. Chem. 2021, 9, 613343. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.S.; Hussein, G.; Brza, M.A.; Mohammed, S.J.; Abdulwahid, R.T.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green approach to overcome the resistance pattern of Candida spp. using biosynthesized silver nanoparticles fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar] [CrossRef]
- Taha, Z.K.; Hawar, S.N.; Sulaiman, G.M. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol. Lett. 2019, 41, 899–914. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; León-González, M.E.; Madrid, Y. Simultaneous determination of the size and concentration of AgNPs in water samples by UV–vis spectrophotometry and chemometrics tools. Talanta 2018, 188, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Jianchu, M. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Bagur, H.; Poojari, C.C.; Melappa, G.; Rangappa, R.; Chandrasekhar, N.; Somu, P. Biogenically synthesized silver nanoparticles using endophyte fungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J. Clust. Sci. 2020, 31, 1241–1255. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; Hassan, S.E.-D.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current research on silver nanoparticles: Synthesis, characterization, and applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- R El Shanshoury, A.E.; Z Sabae, S.; A El Shouny, W.; M Abu Shady, A.; M Badr, H. Extracellular biosynthesis of silver nanoparticles using aquatic bacterial isolate and its antibacterial and antioxidant potentials. Egypt. J. Aquat. Biol. Fish. 2020, 24, 183–201. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef]
- Musarrat, J.; Dwivedi, S.; Singh, B.R.; Al-Khedhairy, A.A.; Azam, A.; Naqvi, A. Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU-09. Bioresour. Technol. 2010, 101, 8772–8776. [Google Scholar] [CrossRef]
- Mujaddidi, N.; Nisa, S.; Al Ayoubi, S.; Bibi, Y.; Khan, S.; Sabir, M.; Zia, M.; Ahmad, S.; Qayyum, A. Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 2021, 28, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Eid, A.M.; Elsheikh, T.M.Y.; Al-Faifi, Z.E.; Saad, N.; Sultan, M.H.; Selim, S.; Al-Khalaf, A.A.; Fouda, A. Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain. J. Fungi 2022, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Kumar Shahi, S.; Kanwar, L.; Kumar Yadaw, R.; Kumar Soni, D. Biochemical profiling of microbes inhibiting Silver nanoparticles using symbiotic organisms. Int. J. Nano Dimens. 2018, 9, 273–285. [Google Scholar]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Lotina, A.; Portela, R.; Baeza, P.; Alcolea-Rodriguez, V.; Villarroel, M.; Ávila, P. Zeta potential as a tool for functional materials development. Catal. Today 2023, 423, 113862. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Shokoohinia, Y.; Khoshroo, A.; Fattahi, A. One-step Synthesized Silver Nanoparticles Using Isoimperatorin: Evaluation of Photocatalytic, and Electrochemical Activities. Sci. Rep. 2020, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Jian, L.; Wang, G.; Liu, X.; Ma, H. Unveiling an S-scheme F–Co3O4@Bi2WO6 heterojunction for robust water purification. eScience 2024, 4, 100206. [Google Scholar] [CrossRef]
- Nga, N.K.; Hong, P.T.T.; Lam, T.D.; Huy, T.Q. A facile synthesis of nanostructured magnesium oxide particles for enhanced adsorption performance in reactive blue 19 removal. J. Colloid Interface Sci. 2013, 398, 210–216. [Google Scholar] [CrossRef]
- Fouda, A.; Saied, E.; Eid, A.M.; Kouadri, F.; Alemam, A.M.; Hamza, M.F.; Alharbi, M.; Elkelish, A.; Hassan, S.E. Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. J. Funct. Biomater. 2023, 14, 205. [Google Scholar] [CrossRef]
- Jose, P.P.A.; Kala, M.S.; Kalarikkal, N.; Thomas, S. Silver-attached reduced graphene oxide nanocomposite as an eco-friendly photocatalyst for organic dye degradation. Res. Chem. Intermed. 2018, 44, 5597–5621. [Google Scholar] [CrossRef]
- Ji, X.-Y.; Sun, K.; Liu, Z.-K.; Liu, X.; Dong, W.; Zuo, X.; Shao, R.; Tao, J. Identification of Dynamic Active Sites Among Cu Species Derived from MOFs@CuPc for Electrocatalytic Nitrate Reduction Reaction to Ammonia. Nano-Micro Lett. 2023, 15, 110. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, B.-K.; Jo, Y.-L.; Shim, J.-J. Ag@graphene oxide nanocomposite as an efficient visible-light plasmonic photocatalyst for the degradation of organic pollutants: A facile green synthetic approach. Mater. Chem. Phys. 2014, 143, 1452–1461. [Google Scholar] [CrossRef]
- Rojaa, K.; Mehtaa, P.; Premalathaa, M.; Jeyadheepanb, K.; Gopalakrishnanc, C.; Meenakshisundaramd, N.; Sankaranarayanana, K. Biosynthesized silver nanoparticles as antimicrobial agents and photocatalytic degradation of methylene blue. In Proceedings of the Presented at the InDA Conference, Tiruchirappalli, India, 20–21 April 2018; pp. 1944–3986. [Google Scholar]
- Roshmi, T.; Jishma, P.; Radhakrishnan, E.K. Photocatalytic and antibacterial effects of silver nanoparticles fabricated by Bacillus subtilis SJ 15. Inorg. Nano-Met. Chem. 2017, 47, 901–908. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Koçberber KiliÇ, N.; Dönmez, G.; Tekinay, T. Green Synthesis of Silver Nanoparticles Using Cyanobacteria and Evaluation of their Photocatalytic and Antimicrobial Activity. J. Nano Res. 2016, 40, 120–127. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Elgammal, W.E.; Eid, A.M.; Alharbi, M.; Mohamed, A.E.; Alayafi, A.A.; Hassan, S.M.; Fouda, A. New Functionalized Chitosan with Thio-Thiadiazole Derivative with Enhanced Inhibition of Pathogenic Bacteria, Plant Threatening Fungi, and Improvement of Seed Germination. Chemistry 2023, 5, 1722–1744. [Google Scholar] [CrossRef]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.C.; Gupta, P.; Saini, S.; Mohiyuddin, S.; Pruthi, V.; Prasad, R. Plausible mechanistic insights in biofilm eradication potential against Candida spp. using in situ-synthesized tyrosol-functionalized chitosan gold nanoparticles as a versatile antifouling coating on implant surfaces. ACS Omega 2022, 7, 8350–8363. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Gaballah, S.; Youssef, A.M.; Eid, A.M.; Sultan, M.H.; Fouda, A. Eco-friendly approach for control of fungal deterioration of archaeological skeleton dated back to the Greco-Roman period. J. Cult. Herit. 2023, 59, 38–48. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Murtaza, G.; Rashid, F.; Iqbal, J. Eco-friendly green synthesis of silver nanoparticles and their potential applications as antioxidant and anticancer agents. Drug Dev. Ind. Pharm. 2019, 45, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Alshallash, K.S.; Eid, A.M.; Hassan, S.E.; Salih, M.; Hamza, M.F.; Fouda, A. Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L. Pharmaceuticals 2024, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tejavath, K.K. Synthesis, characterization and comparative anticancer potential of phytosynthesized mono and bimetallic nanoparticles using Moringa oleifera aqueous leaf extract. Nano 2022, 17, 2250047. [Google Scholar] [CrossRef]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Al Ali, A.A.A.L.A. Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Xu, X.; Amraii, S.A.; Toushmalani, R.; Almasi, M. Formulation of a modern anti-human breast cancer drug from silver nanoparticles green-synthesized using Allium saralicum. J. Eng. Res. 2023, 11, 288–292. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.; Agarwal, S.; Masih, M.; Chauhan, A.; Gautam, P.K. Silver nanoparticles induces apoptosis of cancer stem cells in head and neck cancer. Toxicol. Rep. 2024, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Shahidur Rahman, D.; Kumar Ghosh, S.; Sengupta, M. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, antioxidant and larvicidal activities of spherical silver nanoparticles synthesized by endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Batterjee, M.G.; Nabi, A.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Malik, M.A. Green Hydrothermal Synthesis of Zinc Oxide Nanoparticles for UV-Light-Induced Photocatalytic Degradation of Ciprofloxacin Antibiotic in an Aqueous Environment. Catalysts 2022, 12, 1347. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; EL-Belely, E.F.; Awad, M.A.; Hassan, S.E.-D.; AL-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]




| Synthesized by… | Shape and Size | MB Removal % | Recyclability with Decreasing Percentages | Ref. | ||
|---|---|---|---|---|---|---|
| UV-Light | Sunlight | Dark | ||||
| Pongamia pinnata | Spherical, 30–40 nm | - | 64.4% after 120 min | - | - | [61] |
| Green tea | Spherical, 9–12 nm | - | 73.9% after 120 min | - | - | |
| Spirulina platensis | agglomerated | - | 75% after 120 min | - | - | |
| Bacillus subtilis SJ 15 | Spherical, 10–36 nm | - | 76.5% after 900 min | - | - | [62] |
| Cyanobacteria sp. | Spherical, 5–10 nm | 18% after 240 min | - | - | - | [63] |
| Prangos ferulacea | Spherical, 79–200 nm | 96.5% after 60 min | - | - | Fourth cycle with a decrease of 8% | [51] |
| Streptomyces tuirus | Spherical, 64 nm | 11.3% after 360 min | 71.3% after 360 min | - | - | |
| Bacillus amyloliquefaciens | Spherical, 5–40 nm | 87.4% after 210 min | 73.9% after 210 min | 39.8% after 210 min | Fifth cycle with a decrease % of 11.2% | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, A.M.; Hassan, S.E.-D.; Hamza, M.F.; Selim, S.; Almuhayawi, M.S.; Alruhaili, M.H.; Tarabulsi, M.K.; Nagshabandi, M.K.; Fouda, A. Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens. Catalysts 2024, 14, 419. https://doi.org/10.3390/catal14070419
Eid AM, Hassan SE-D, Hamza MF, Selim S, Almuhayawi MS, Alruhaili MH, Tarabulsi MK, Nagshabandi MK, Fouda A. Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens. Catalysts. 2024; 14(7):419. https://doi.org/10.3390/catal14070419
Chicago/Turabian StyleEid, Ahmed M., Saad El-Din Hassan, Mohammed F. Hamza, Samy Selim, Mohammed S. Almuhayawi, Mohammed H. Alruhaili, Muyassar K. Tarabulsi, Mohammed K. Nagshabandi, and Amr Fouda. 2024. "Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens" Catalysts 14, no. 7: 419. https://doi.org/10.3390/catal14070419
APA StyleEid, A. M., Hassan, S. E.-D., Hamza, M. F., Selim, S., Almuhayawi, M. S., Alruhaili, M. H., Tarabulsi, M. K., Nagshabandi, M. K., & Fouda, A. (2024). Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens. Catalysts, 14(7), 419. https://doi.org/10.3390/catal14070419












