Effect of the Second-Shell Coordination Environment on the Performance of P-Block Metal Single-Atom Catalysts for the Electrosynthesis of Hydrogen Peroxide
Abstract
1. Introduction
2. Results and Discussion
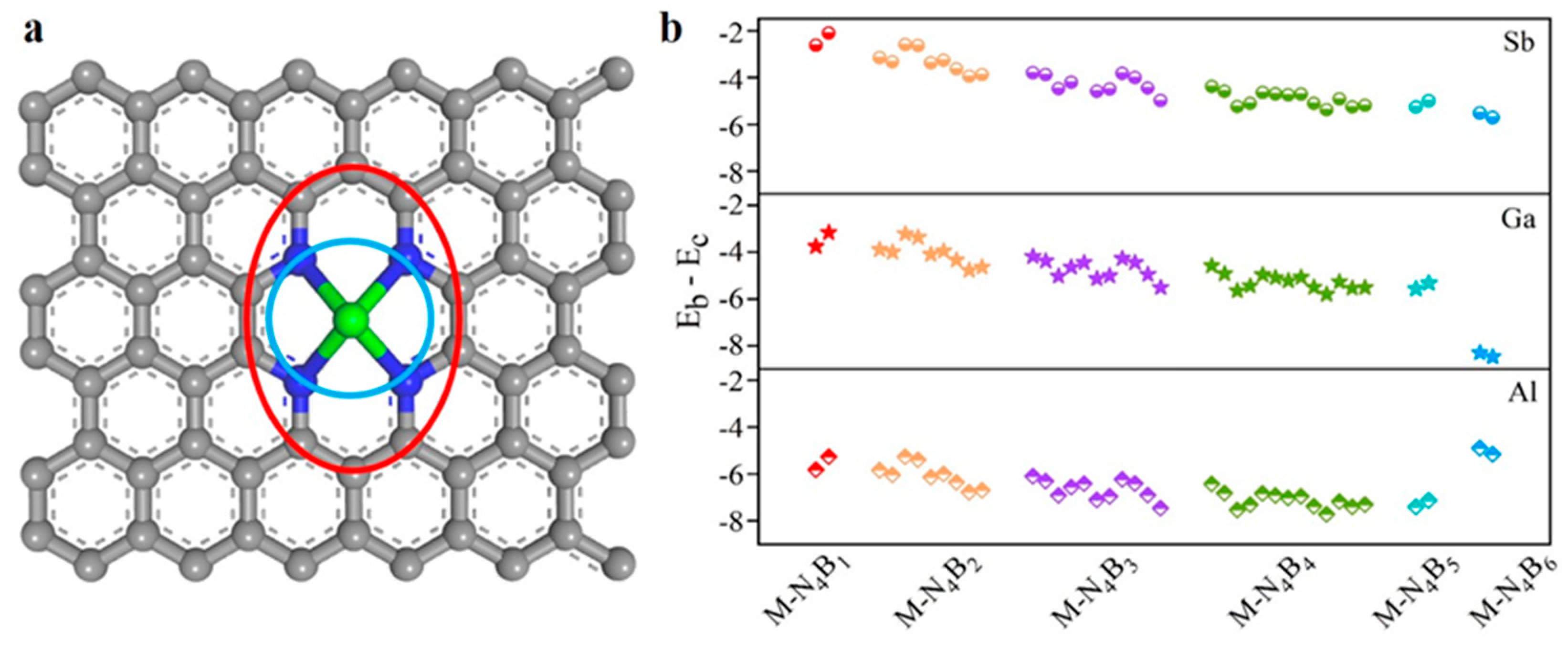
2.1. Structures and Stability
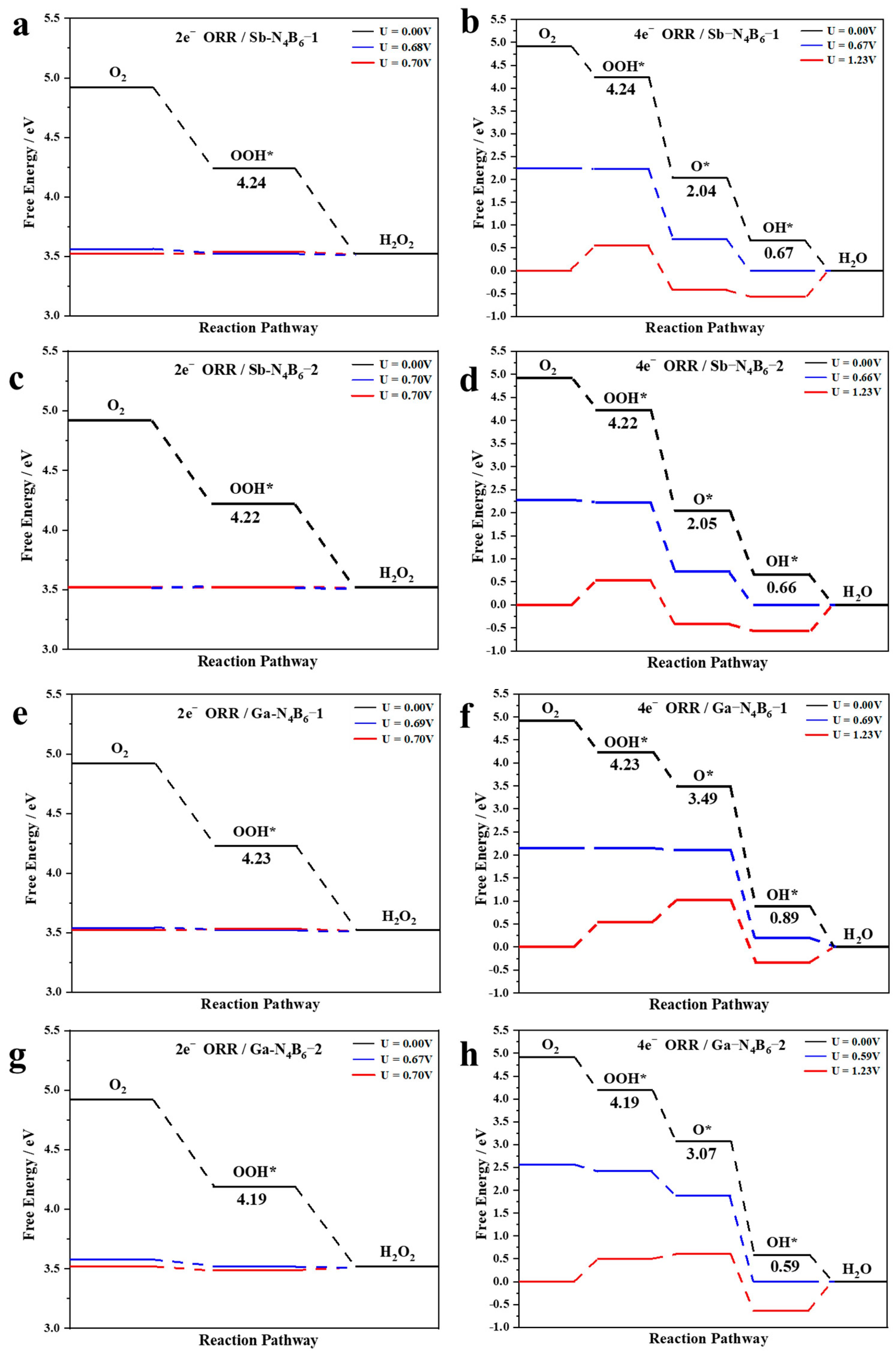
2.2. Activity and Selectivity of the Catalysts
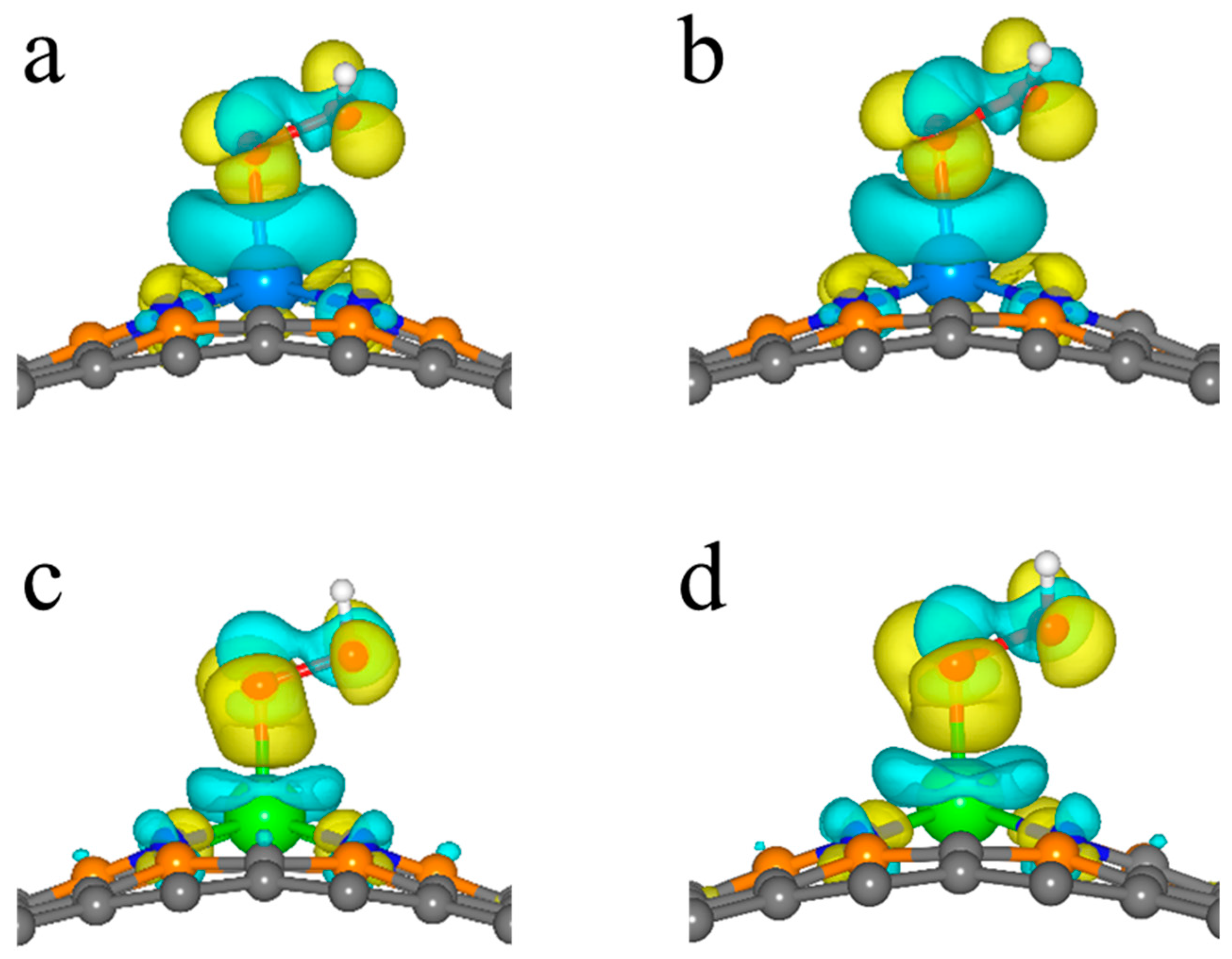
2.3. ORR Activity Origin
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, C.H.; Kim, M.; Kwon, H.C.; Cho, S.J.; Yun, S.; Kim, H.-T.; Mayrhofer, K.J.J.; Kim, H.; Choi, M. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 2016, 7, 10922. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Unifying the 2e– and 4e– Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. Lett. 2012, 3, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fang, Q.; Xia, Q.; Hu, A.; Sun, F.; Zhang, W.; Yu, Y.; Zhuang, G.; Jiang, J.; Wang, J. Dual effect of the coordination field and sulphuric acid on the properties of a single-atom catalyst in the electrosynthesis of H2O2. Phys. Chem. Chem. Phys. 2021, 23, 21338–21349. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Qu, X.; Li, P.; Wang, F.; Li, Q.; Song, L.; Zhao, Y.; Kang, X. Photocatalytic oxygen reduction to hydrogen peroxide over copper doped graphitic carbon nitride hollow microsphere: The effect of Cu(I)-N active sites. Chem. Eng. J. 2018, 334, 410–418. [Google Scholar] [CrossRef]
- Zhu, Z.; Pan, H.; Murugananthan, M.; Gong, J.; Zhang, Y. Visible light-driven photocatalytically active g-C3N4 material for enhanced generation of H2O2. Appl. Catal. B Environ. 2018, 232, 19–25. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, L.; Gao, J.; Nazari, R.; Zhao, H.; Meng, X.; Sun, F.; Zhao, G.; Ma, J. Selective H2O2 electrosynthesis by O-doped and transition-metal-O-doped carbon cathodes via O2 electroreduction: A critical review. Chem. Eng. J. 2021, 410, 128368. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Coleman, E.J.; Co, A.C. The Complex Inhibiting Role of Surface Oxide in the Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7299–7311. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wei, Z. Transition-metal-oxide-based catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 8194–8209. [Google Scholar] [CrossRef]
- An, G.-H.; Lee, Y.-G.; Ahn, H.-J. Multi-active sites of iron carbide nanoparticles on nitrogen@cobalt-doped carbon for a highly efficient oxygen reduction reaction. J. Alloys Compd. 2018, 746, 177–184. [Google Scholar] [CrossRef]
- Reda, M.; Hansen, H.A.; Vegge, T. DFT Study of the Oxygen Reduction Reaction on Carbon-Coated Iron and Iron Carbide. ACS Catal. 2018, 8, 10521–10529. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, Y.; Wu, Q.; Du, L.; Zhang, Z.; Yang, L.; Wang, X.; Hu, Z. Is iron nitride or carbide highly active for oxygen reduction reaction in acidic medium? Catal. Sci. Technol. 2017, 7, 51–55. [Google Scholar] [CrossRef]
- Fritz, K.E.; Yan, Y.; Suntivich, J. Influence of 3d transition-metal substitution on the oxygen reduction reaction electrocatalysis of ternary nitrides in acid. Nano Res. 2019, 12, 2307–2312. [Google Scholar] [CrossRef]
- Jing, S.; Luo, L.; Yin, S.; Huang, F.; Jia, Y.; Wei, Y.; Sun, Z.; Zhao, Y. Tungsten nitride decorated carbon nanotubes hybrid as efficient catalyst supports for oxygen reduction reaction. Appl. Catal. B Environ. 2014, 147, 897–903. [Google Scholar] [CrossRef]
- Kreider, M.E.; Gallo, A.; Back, S.; Liu, Y.; Siahrostami, S.; Nordlund, D.; Sinclair, R.; Nørskov, J.K.; King, L.A.; Jaramillo, T.F. Precious Metal-Free Nickel Nitride Catalyst for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2019, 11, 26863–26871. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Tran, D.T.; Singh, T.I.; Kim, N.H.; Lee, J.H. Mesoporous iron sulfide nanoparticles anchored graphene sheet as an efficient and durable catalyst for oxygen reduction reaction. J. Power Sources 2019, 427, 91–100. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, D.-J.; Tong, Y.J. Mechanistic Insight into Sulfide-Enhanced Oxygen Reduction Reaction Activity and Stability of Commercial Pt Black: An in Situ Raman Spectroscopic Study. ACS Catal. 2016, 6, 5000–5004. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Simultaneously Achieving High Activity and Selectivity toward Two-Electron O2 Electroreduction: The Power of Single-Atom Catalysts. ACS Catal. 2019, 9, 11042–11054. [Google Scholar] [CrossRef]
- Baughman, R.H.; Eckhardt, H.; Kertesz, M. Structure-property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms. J. Chem. Phys. 1987, 87, 6687–6699. [Google Scholar] [CrossRef]
- Lian, K.; Wan, Q.; Jiang, R.; Lin, S. Electrocatalytic Oxygen Reduction to Hydrogen Peroxide on Graphdiyne-Based Single-Atom Catalysts: First-Principles Studies. Catalysts 2023, 13, 307. [Google Scholar] [CrossRef]
- Lin, L.; Ma, R.; Jiang, R.; Lin, S. Design of high performance nitrogen reduction electrocatalysts by doping defective polyoxometalate with a single atom promoter. Phys. Chem. Chem. Phys. 2024, 26, 8494–8503. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, F.; Wei, F.; Zhao, J.; Lin, S. Revealing the Origin of Nitrogen Electroreduction Activity of Molybdenum Disulfide Supported Iron Atoms. J. Phys. Chem. C 2022, 126, 5180–5188. [Google Scholar] [CrossRef]
- Ge, B.; Wei, F.; Wan, Q.; Zhang, H.; Yuan, P.; Lin, S. Peripheral Coordination-Dependent Descriptor for Selective Interactions between Near-Frontier Molecular Orbitals and Single-Atom Catalysts. Precis. Chem. 2023, 1, 429–436. [Google Scholar] [CrossRef]
- Gu, K.; Lin, S. Sustained Hydrogen Spillover on Pt/Cu(111) Single-Atom Alloy: Dynamic Insights into Gas-Induced Chemical Processes. Angew. Chem. Int. Ed. 2023, 62, e202312796. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, J.; Lin, S. Theoretical Insights into H2 Activation and Hydrogen Spillover on Near-Surface Alloys with Embedded Single Pt Atoms. ACS Catal. 2024, 14, 2194–2201. [Google Scholar] [CrossRef]
- Yan, B.; Krishnamurthy, D.; Hendon, C.H.; Deshpande, S.; Surendranath, Y.; Viswanathan, V. Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis. Joule 2017, 1, 600–612. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, G.-F.; Noh, H.-J.; Kim, S.-J.; Lu, Y.; Jeong, H.Y.; Fu, Z.; Baek, J.-B. Boosting oxygen reduction catalysis with abundant copper single atom active sites. Energy Environ. Sci. 2018, 11, 2263–2269. [Google Scholar] [CrossRef]
- Song, P.; Luo, M.; Liu, X.; Xing, W.; Xu, W.; Jiang, Z.; Gu, L. Zn Single Atom Catalyst for Highly Efficient Oxygen Reduction Reaction. Adv. Funct. Mater. 2017, 27, 1700802. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Li, Y.; Chen, Z. Two-dimensional iron-phthalocyanine (Fe-Pc) monolayer as a promising single-atom-catalyst for oxygen reduction reaction: A computational study. Nanoscale 2015, 7, 11633–11641. [Google Scholar] [CrossRef] [PubMed]
- Bin Yang, H.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Mitchell, S.; Li, J.; Lyu, P.; Wu, X.; Pérez-Ramírez, J.; Lu, J. Design of Local Atomic Environments in Single-Atom Electrocatalysts for Renewable Energy Conversions. Adv. Mater. 2021, 33, e2003075. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, L.; Li, H.; Li, L.; Jiao, Y.; Zheng, Y.; Xu, H.; Davey, K.; Qiao, S.-Z. Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Atom-Coordinated Structure Triggers Selective H2O2 Production. Chem 2020, 6, 548–550. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wang, C.; Tao, W.; Huang, M.; Zuo, M.; Yang, Y.; Yang, K.; Zhang, L.; Chen, S.; et al. Turning main-group element magnesium into a highly active electrocatalyst for oxygen reduction reaction. Nat. Commun. 2020, 11, 938. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-Band Centers Enables Co4N Nanosheets To Be Highly Active for Hydrogen Evolution Catalysis. Angew. Chem. Int. Ed. 2018, 57, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Q.; Wang, Y.; Da, Y.; Zhou, W.; Shao, Y.; Li, D.; Zhan, S.; Yuan, J.; Wang, H. Atomically Dispersed Semimetallic Selenium on Porous Carbon Membrane as an Electrode for Hydrazine Fuel Cells. Angew. Chem. Int. Ed. 2019, 58, 13466–13471. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhang, Q.; Yang, H.; Kato, K.; Yang, W.; Lu, Y.-R.; Liu, S.; Wang, C.; Yamakata, A.; Su, C.; et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 2021, 4, 374–384. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Qin, H.; Shang, L.; Zheng, S.; Fang, F.; Jiao, L. P-Block Atomically Dispersed Antimony Catalyst for Highly Efficient Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 21237–21241. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhao, Z.; Luo, Z.; Wang, C. Unraveling the Impact of Curvature on Electrocatalytic Performance of Carbon Materials: A State-of-the-Art Review. ChemSusChem 2024, 17, e202301859. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Guo, H.; Lin, S. Corrugation-Induced Active Sites on Pristine Graphene for H2 Activation. ACS Catal. 2022, 12, 14601–14608. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. J. Phys. Rev. B Condens. Matter 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Sharada, S.M.; Karlsson, R.K.B.; Maimaiti, Y.; Voss, J.; Bligaard, T. Adsorption on transition metal surfaces: Transferability and accuracy of DFT using the ADS41 dataset. Phys. Rev. B 2019, 100, 035439. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Valdés, Á.; Qu, Z.-W.; Kroes, G.-J.; Rossmeisl, J.; Nørskov, J.K. Oxidation and Photo-Oxidation of Water on TiO2 Surface. J. Phys. Chem. C 2008, 112, 9872–9879. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]


| M-N4B6 | qM (e−) | QM (e−) | qOOH (e−) | Bond Length (Å) |
|---|---|---|---|---|
| Ga-N4B6-1 | −1.48 | −1.57 | 0.43 | 1.44 |
| Ga-N4B6-2 | −1.45 | −1.57 | 0.47 | 1.45 |
| Sb-N4B6-1 | −1.53 | −2.30 | 0.53 | 1.475 |
| Sb-N4B6-2 | −1.53 | −2.32 | 0.54 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, Y.; Lin, S. Effect of the Second-Shell Coordination Environment on the Performance of P-Block Metal Single-Atom Catalysts for the Electrosynthesis of Hydrogen Peroxide. Catalysts 2024, 14, 421. https://doi.org/10.3390/catal14070421
Wu Y, Zhang Y, Lin S. Effect of the Second-Shell Coordination Environment on the Performance of P-Block Metal Single-Atom Catalysts for the Electrosynthesis of Hydrogen Peroxide. Catalysts. 2024; 14(7):421. https://doi.org/10.3390/catal14070421
Chicago/Turabian StyleWu, Yidi, Yuxiang Zhang, and Sen Lin. 2024. "Effect of the Second-Shell Coordination Environment on the Performance of P-Block Metal Single-Atom Catalysts for the Electrosynthesis of Hydrogen Peroxide" Catalysts 14, no. 7: 421. https://doi.org/10.3390/catal14070421
APA StyleWu, Y., Zhang, Y., & Lin, S. (2024). Effect of the Second-Shell Coordination Environment on the Performance of P-Block Metal Single-Atom Catalysts for the Electrosynthesis of Hydrogen Peroxide. Catalysts, 14(7), 421. https://doi.org/10.3390/catal14070421









