Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. The Activity of Catalysts for Hydrogenolysis of THFA to 1,5-PeD
2.3. Discussion
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization of Catalysts
3.3. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carolin, C.F.; Kamalesh, T.; Kumar, P.; Hemavathy, R.V.; Rangasamy, G. A critical review on sustainable cellulose materials and their multifaceted applications. Ind. Crops Prod. 2023, 203, 203–222. [Google Scholar] [CrossRef]
- Fajobi, M.O.; Lasode, O.A.; Adeleke, A.A.; Ikubanni, P.P.; Balogun, A.O. Investigation of physicochemical characteristics of selected lignocellulose biomass. Sci. Rep. 2022, 12, 2918–2932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Bal, R.; Srivastava, R. Ni-nanoparticles decorated CePO4 for the selective hydrogenation of furfural to tetrahydrofurfuryl alcohol. Mol. Catal. 2022, 531, 112712–112724. [Google Scholar] [CrossRef]
- Insyani, R.; Barus, A.F.; Gunawan, R.; Park, J.; Jaya, G.T.; Cahyadi, H.S.; Sibi, M.G.; Kwak, S.K.; Verma, D.; Kim, J. RuO2–Ru/Hβ zeolite catalyst for high-yield direct conversion of xylose to tetrahydrofurfuryl alcohol. Appl. Catal. B Environ. 2021, 291, 120120–120140. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, D.; Zhang, Y.; Wu, Y.; Tian, X.; Ding, M. Selective hydrogenolysis of tetrahydrofurfuryl alcohol over Ni/Y2O3 catalyst to produce 1,5-pentanediol. Ind. Eng. Chem. Res. 2024, 63, 8044–8053. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Luo, W.; Zhao, Y.; Pan, X.; Zheng, M. Catalytic Conversion of tetrahydrofurfuryl alcohol over stable Pt/MoS2 catalysts. Catal. Lett. 2021, 151, 2734–2747. [Google Scholar] [CrossRef]
- Lomelí-Rodríguez, M.; Corpas-Martínez, J.; Willis, S.; Mulholland, R.; Lopez-Sanchez, J. Synthesis and characterization of renewable polyester coil coatings from biomass-derived isosorbide, FDCA, 1,5-pentanediol, succinic acid, and 1,3-propanediol. Polymers 2018, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Deshpande, S.; Denny, S.R.; Porter, W.N.; Wang, C.; Marlowe, J.; Christopher, P.; Zheng, W.; Caratzoulas, S.; Vlachos, D.G.; et al. Mechanistic understanding of ring-opening of tetrahydrofurfuryl alcohol over WOx-modified Pt model surfaces and powder catalysts. ACS Catal. 2023, 13, 8014–8024. [Google Scholar] [CrossRef]
- Feng, S.; Nagao, A.; Aihara, T.; Miura, H.; Shishido, T. Selective hydrogenolysis of tetrahydrofurfuryl alcohol on Pt/WO3/ZrO2 catalysts: Effect of WO3 loading amount on activity. Catal. Today 2018, 303, 207–212. [Google Scholar] [CrossRef]
- Soghrati, E.; Kok Poh, C.; Du, Y.; Gao, F.; Kawi, S.; Borgna, A. C−O hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Bi-functional nickel-tungsten catalysts. ChemCatChem 2018, 10, 4652–4664. [Google Scholar] [CrossRef]
- Koso, S.; Furikado, I.; Shimao, A.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol. Chem. Commun. 2009, 15, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pholjaroen, B.; Li, M.; Dong, W.; Li, N.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Ir-MoOx/SiO2 catalyst. J. Energy Chem. 2014, 23, 427–434. [Google Scholar] [CrossRef]
- Melián-Cabrera, I. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18560. [Google Scholar] [CrossRef]
- Lei, Z.; Hengliang, W.; Zhang, L.; Yang, J.; Qi, W. A study on the catalytic performance of the ZrO2@γ-Al2O3 hollow sphere catalyst for COS hydrolysis. New J. Chem. 2023, 47, 7070–7083. [Google Scholar] [CrossRef]
- Fu, R.; Fang, T.; Wang, Z.; Guo, Y.; Zhan, W.; Guo, Y.; Wang, L. Boosting vinyl chloride combustion over Pt/WOx/ZrO2: Regulating redox and acidity. Chem. Phys. Lett. 2023, 821, 140467–140475. [Google Scholar] [CrossRef]
- Kuang, B.; Zhang, Q.; Fang, Y.; Bai, Y.; Qiu, S.; Wu, P.; Qin, Y.; Wang, T. Ring opening of cyclic ether for selective synthesis of renewable 1,5-pentanediol over Pt/WO3@SiO2 catalysts. Ind. Eng. Chem. Res. 2020, 59, 9372–9381. [Google Scholar] [CrossRef]
- Wang, F.; Luo, M.; Liu, Q.; Shao, C.; Yang, Z.; Liu, X.; Guo, J. Preparation of Pt/MgAl2O4 decalin dehydrogenation catalyst for chemical hydrogen storage application. Catal. Lett. 2023, 154, 191–205. [Google Scholar] [CrossRef]
- Li, W.Z.; Kovarik, L.; Mei, D.; Liu, J.; Wang, Y.; Peden, C.H. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Dai, H.; Peng, L.; Lu, D.; Wan, X.; Zhou, C.; Zheng, J.; Dai, Y.; Wang, H.; Yang, Y. Effect of hydrotalcite interlayer water on Pt-catalyzed aqueous-phase selective hydrogenation of cinnamaldehyde. ACS Appl. Mater. Interfaces 2019, 12, 2516–2524. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Lian, L.; Zhang, G.; An, P.; Zeng, K.; He, H.; Yuan, T.; Huang, J.; Wang, L.; et al. Oxygen vacancy-reinforced water-assisted proton hopping for enhanced catalytic hydrogenation. ACS Catal. 2023, 13, 2326–2334. [Google Scholar] [CrossRef]
- Meng, X.; Guo, X.; Wang, Z.; Yang, P. Effect of Se percent on the properties of Cu2FeSn(S, Se)4 thin films prepared by sol-gel method. Mater. Lett. 2024, 355, 135484–135488. [Google Scholar] [CrossRef]
- Zarubina, V.; Melián-Cabrera, I. On the geometric trajectories of pores during the thermal sintering of relevant catalyst supports. Scr. Mater. 2021, 194, 113679–113684. [Google Scholar] [CrossRef]
- Kirichenko, O.; Nissenbaum, V.; Kapustin, G.; Redina, E.; Vikanova, K.; Davshan, N.; Kustov, L. Thermal analysis of intermediates formed during preparation of a Pt/WOx/Al2O3 catalyst for 1,3-Propanediol synthesis from glycerol. J. Therm. Anal. Calorim. 2019, 138, 2205–2218. [Google Scholar] [CrossRef]
- Jarauta-Córdoba, C.; Bengoechea, M.O.; Agirrezabal-Telleria, I.; Arias, P.-L.; Gandarias, I. Insights into the nature of the active sites of Pt-WOx/Al2O3 catalysts for glycerol hydrogenolysis into 1,3-propanediol. Catalysts 2021, 11, 1171. [Google Scholar] [CrossRef]
- Haneda, M.; Watanabe, T.; Kamiuchi, N.; Ozawa, M. Effect of platinum dispersion on the catalytic activity of Pt/Al2O3 for the oxidation of carbon monoxide and propane. Appl. Catal. B Environ. 2013, 142–143, 8–14. [Google Scholar] [CrossRef]
- Bao, W.; Chen, H.; Wang, H.; Zhang, R.; Wei, Y.; Zheng, L. Pt Nanoparticles supported on N/Ce-doped activated carbon for the catalytic oxidation of formaldehyde at room temperature. ACS Appl. Nano Mater. 2020, 3, 2614–2624. [Google Scholar] [CrossRef]
- Sun, K.; Shen, C.; Zou, R.; Liu, C.-J. Highly active Pt/In2O3-ZrO2 catalyst for CO2 hydrogenation to methanol with enhanced CO tolerance: The effects of ZrO2. Appl. Catal. B Environ. 2023, 320, 122018–122030. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Kavadiya, S.; He, X.; Wang, W.-N.; Karakocak, B.B.; Lin, Y.-C.; Berezin, M.Y.; Biswas, P. Engineering stable Pt nanoparticles and oxygen vacancies on defective TiO2 via introducing strong electronic metal-support interaction for efficient CO2 photoreduction. Chem. Eng. J. 2020, 389, 123450–123462. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, S.H.; Min, H.-K.; Seo, M.-g.; Kweon, S.; Park, M.B.; Choi, Y.H.; Lee, J.W. Catalytic pyrolysis of HDPE over WOx/Al2O3: Effect of tungsten content on the acidity and catalytic performance. Mol. Catal. 2022, 528, 112439–112447. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, G.; Ma, N.; Zhang, H.; Li, Y.; Xia, Y.; Zhang, D.; Zhan, S. Oxygen-vacancy mediated acidity and redox properties on WOx/Cu-Doped CeO2 for the removal of NOx. J. Environ. Chem. Eng. 2021, 9, 106024–106035. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Vapour Phase Hydrogenation of Phenol over Pd/C Catalysts: A Relationship between Dispersion, Metal Area and Hydrogenation Activity. Catal. Commun. 2007, 8, 471–477. [Google Scholar] [CrossRef]
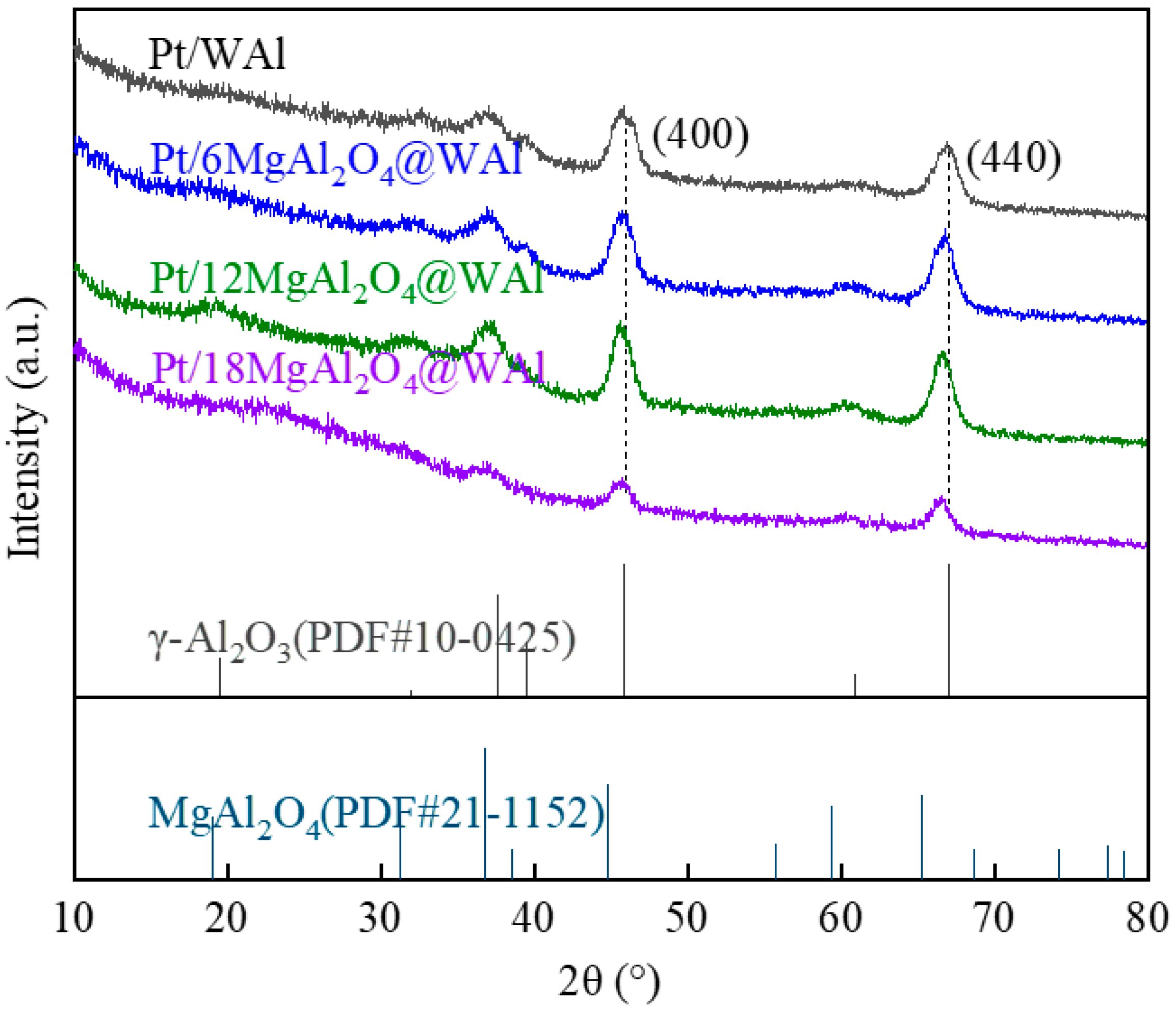


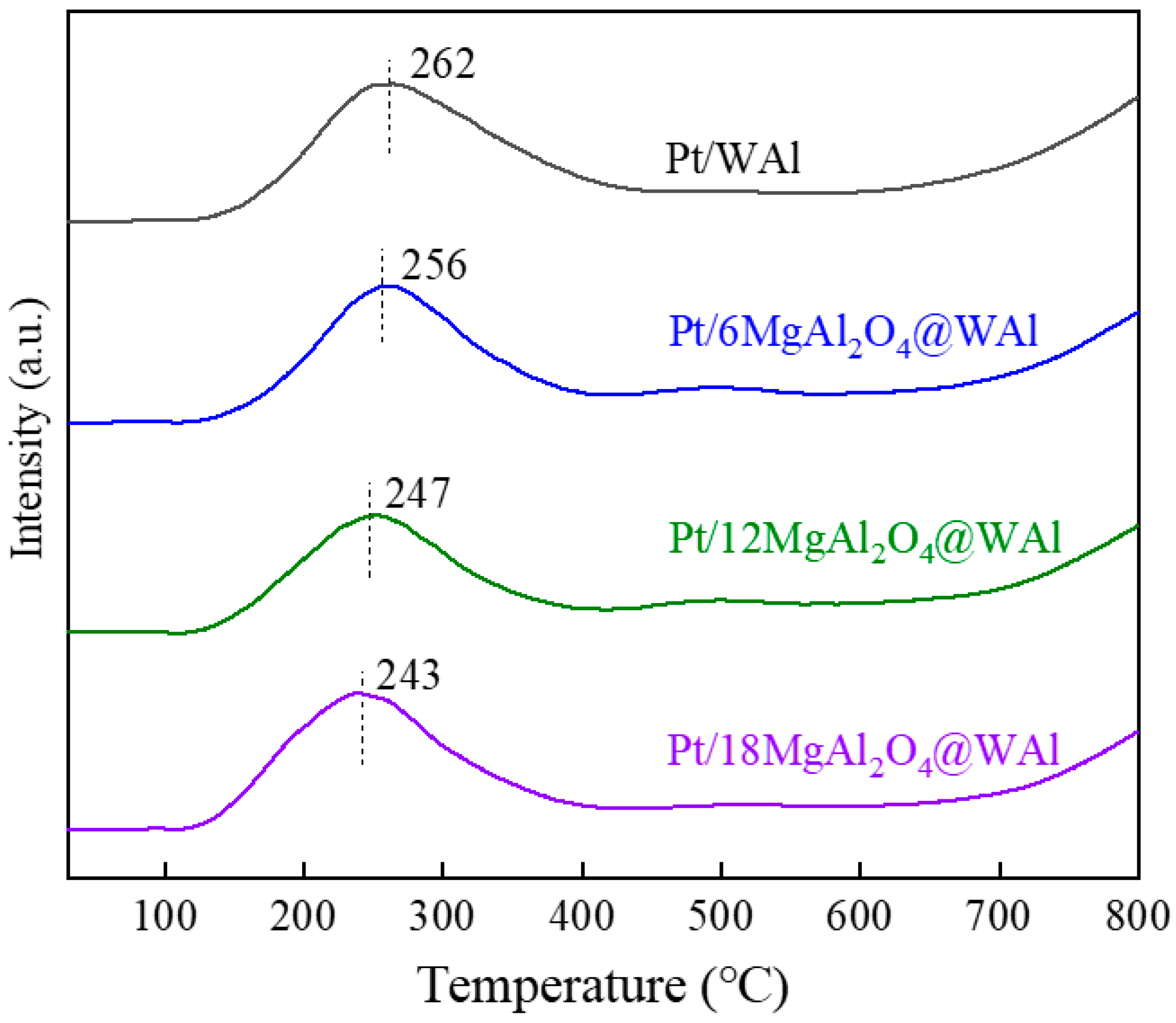
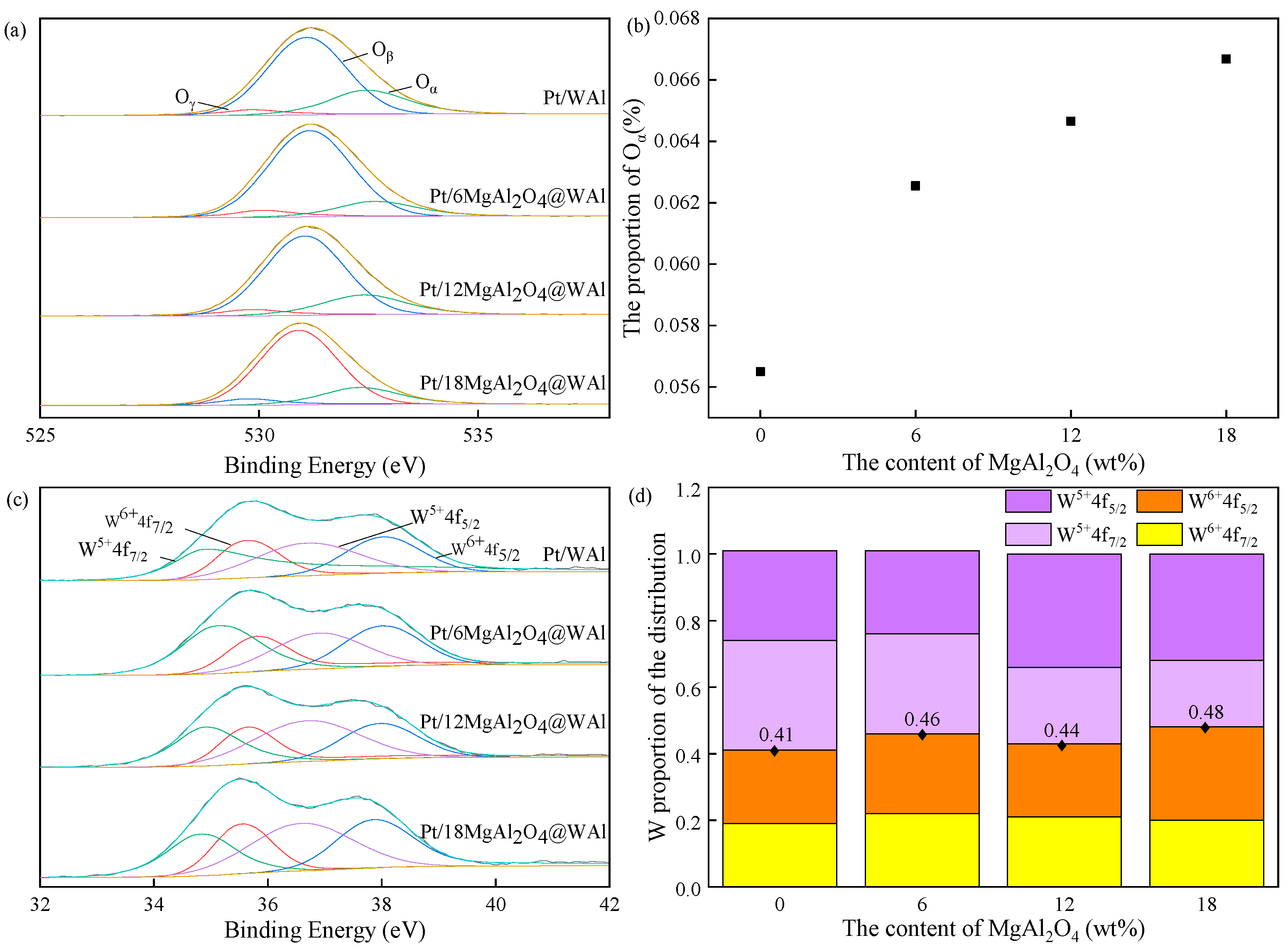
| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size Diameter (nm) | Total Acid Content (μmol/g) |
|---|---|---|---|---|
| WAl | 163 | 0.50 | 13.9 | 181 |
| 6MgAl2O4@WAl | 138 | 0.45 | 13.9 | 165 |
| 12MgAl2O4@WAl | 133 | 0.42 | 13.9 | 127 |
| 18MgAl2O4@WAl | 122 | 0.38 | 13.9 | 124 |
| Samples | CO Adsorption Capacity (μmol/g) | Pt Dispersion (%) |
|---|---|---|
| 12MgAl2O4@WAl | 0.0 | 0 |
| Pt/WAl | 15.6 | 15 |
| Pt/6MgAl2O4@WAl | 35.0 | 34 |
| Pt/12MgAl2O4@WAl | 40.5 | 40 |
| Pt/18MgAl2O4@WAl | 38.0 | 37 |
| Catalysts | THFA Conversion (%) | Selective (%) | ||
|---|---|---|---|---|
| 1,5-PeD | 1,2-PeD | n-Pentanol | ||
| Pt/WAl | 26.0 | 86.9 | 0.8 | 12.3 |
| Pt/6MgAl2O4@WAl | 43.6 | 88.9 | 0.8 | 10.3 |
| Pt/12MgAl2O4@WAl | 47.3 | 88.4 | 0.4 | 11.3 |
| Pt/18MgAl2O4@WAl | 34.7 | 90.0 | 0.6 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, C. Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst. Catalysts 2024, 14, 428. https://doi.org/10.3390/catal14070428
Wang W, Chen C. Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst. Catalysts. 2024; 14(7):428. https://doi.org/10.3390/catal14070428
Chicago/Turabian StyleWang, Weiying, and Changlin Chen. 2024. "Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst" Catalysts 14, no. 7: 428. https://doi.org/10.3390/catal14070428
APA StyleWang, W., & Chen, C. (2024). Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst. Catalysts, 14(7), 428. https://doi.org/10.3390/catal14070428





