Copper- and Manganese-Based Bimetallic Layered Double Hydroxides for Catalytic Reduction of Methylene Blue
Abstract
:1. Introduction
2. Results and Discussion
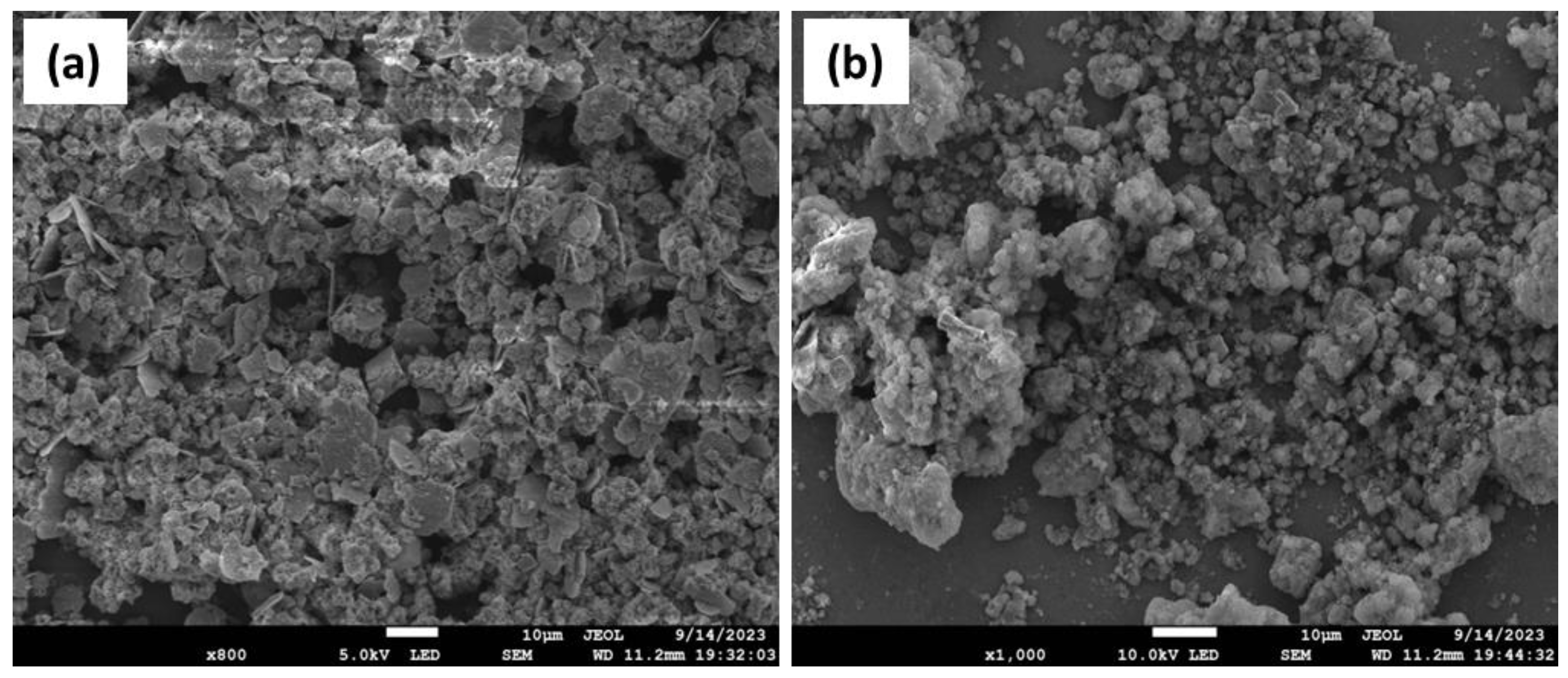
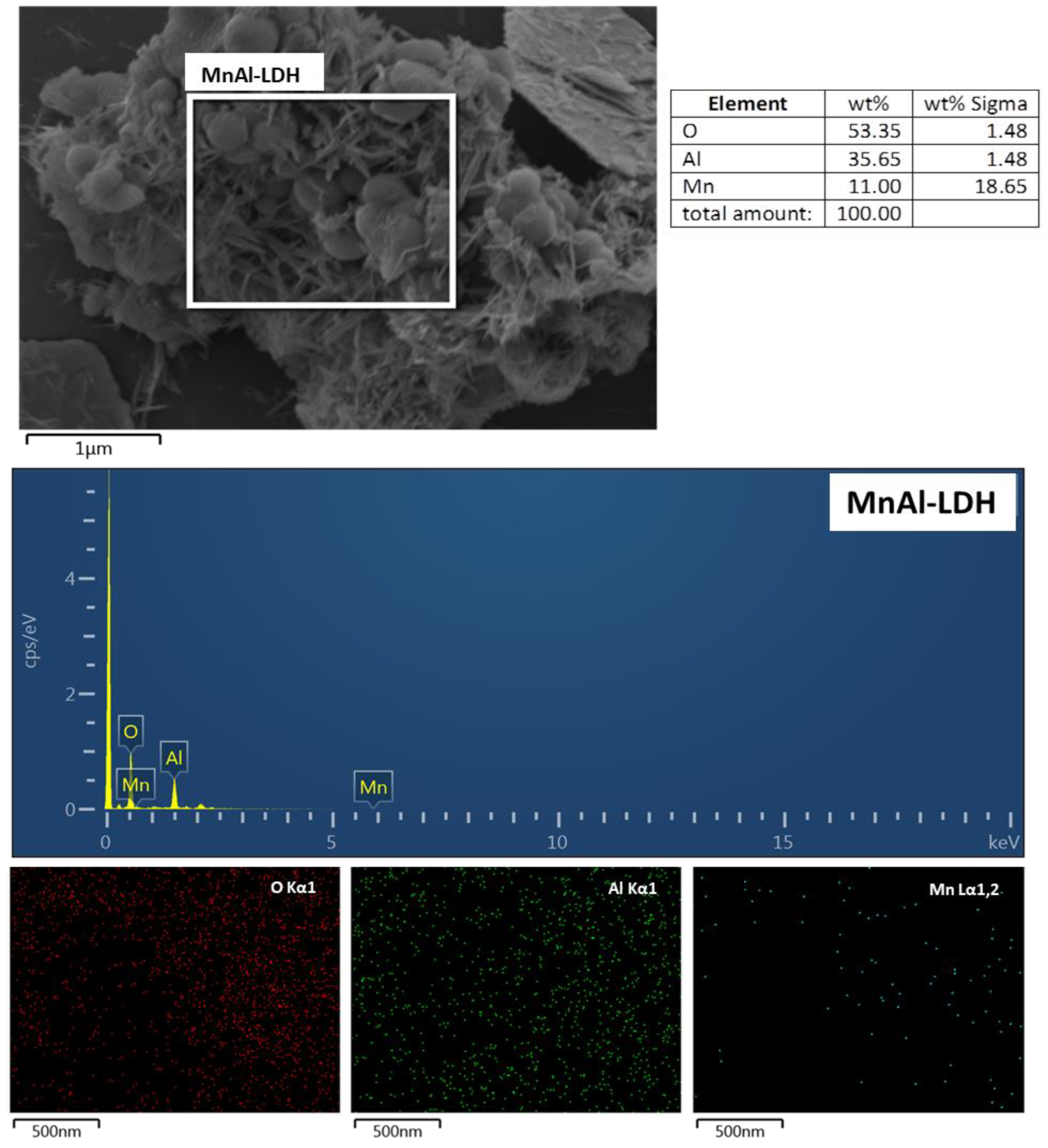
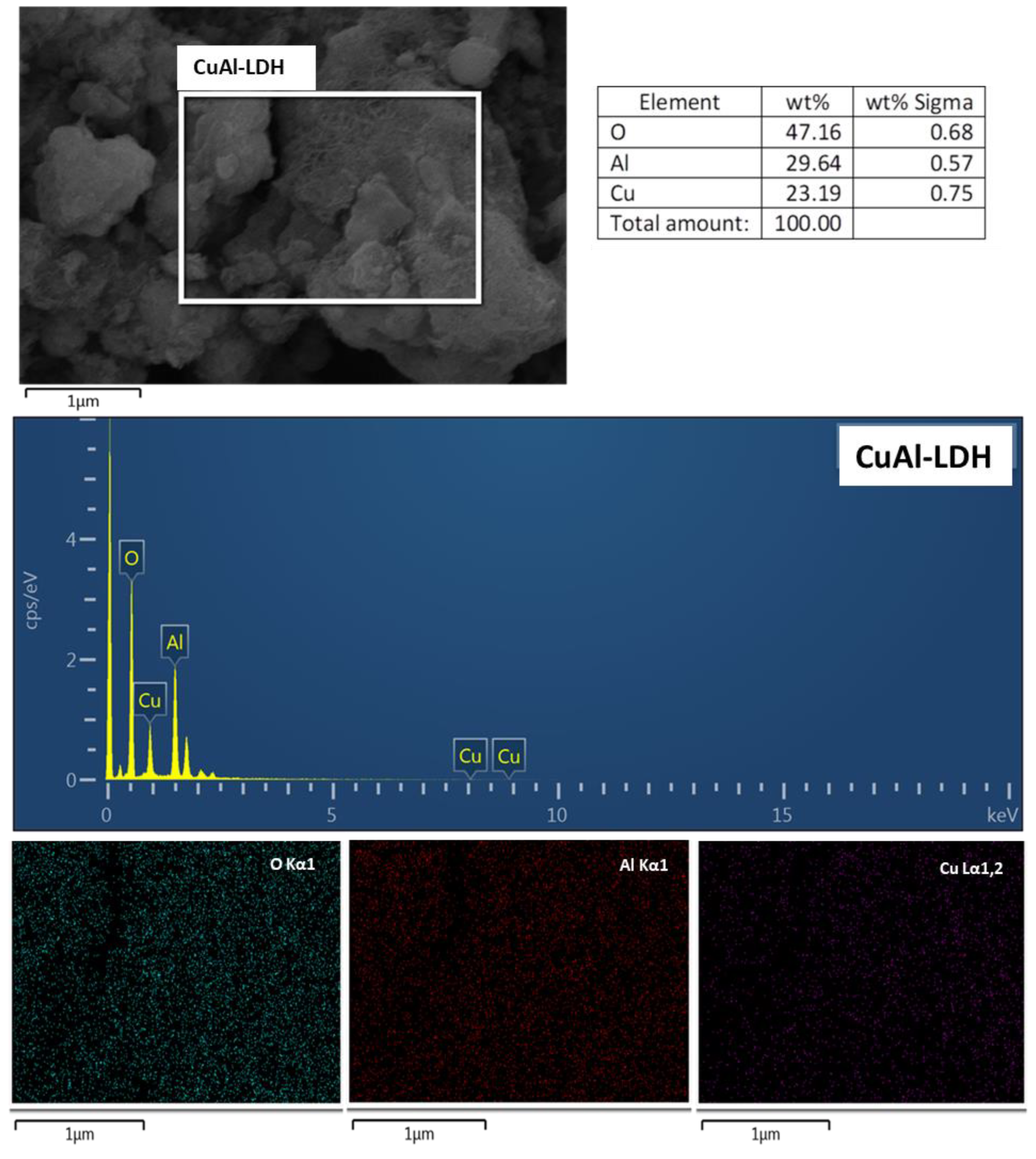
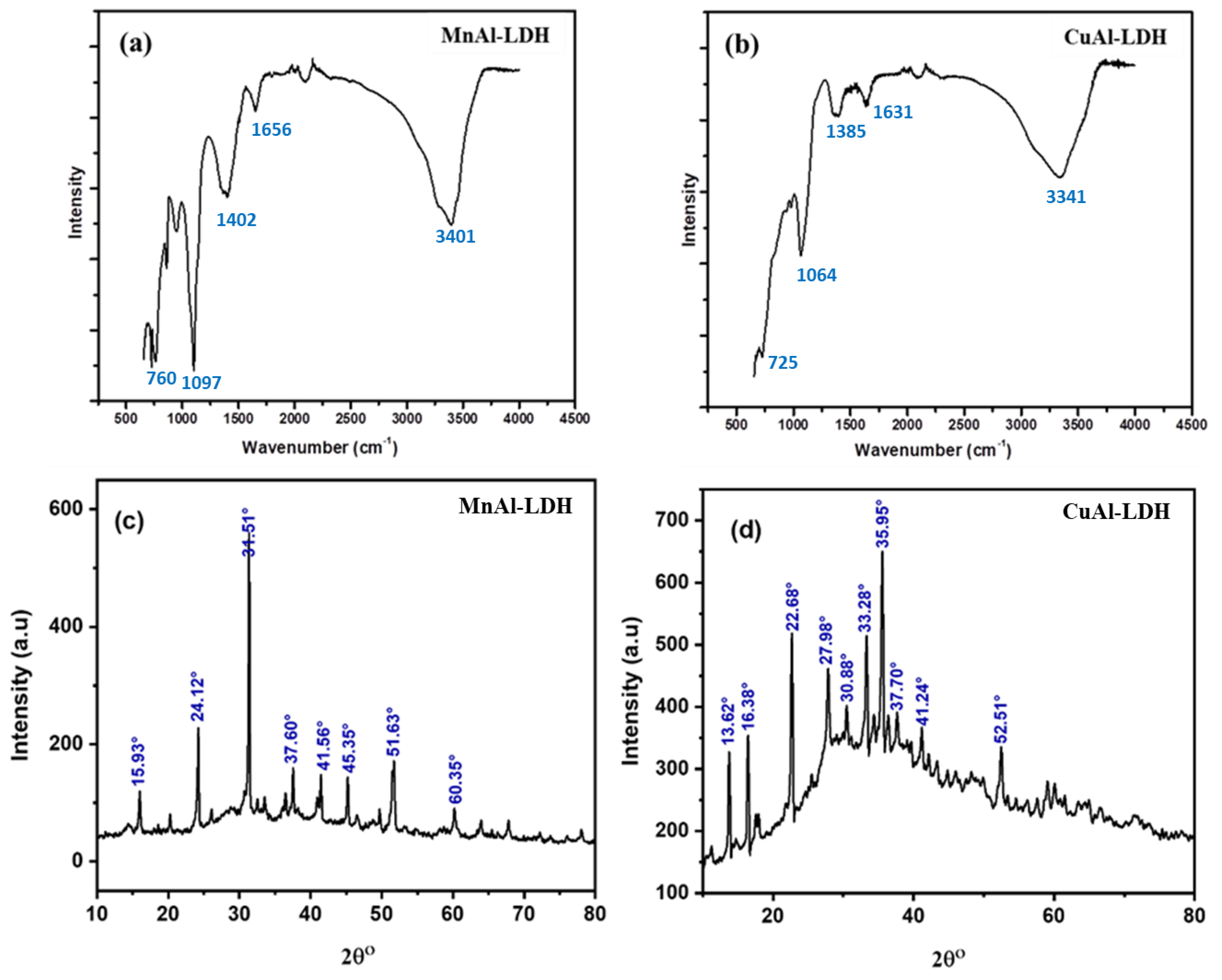
2.1. Characterization
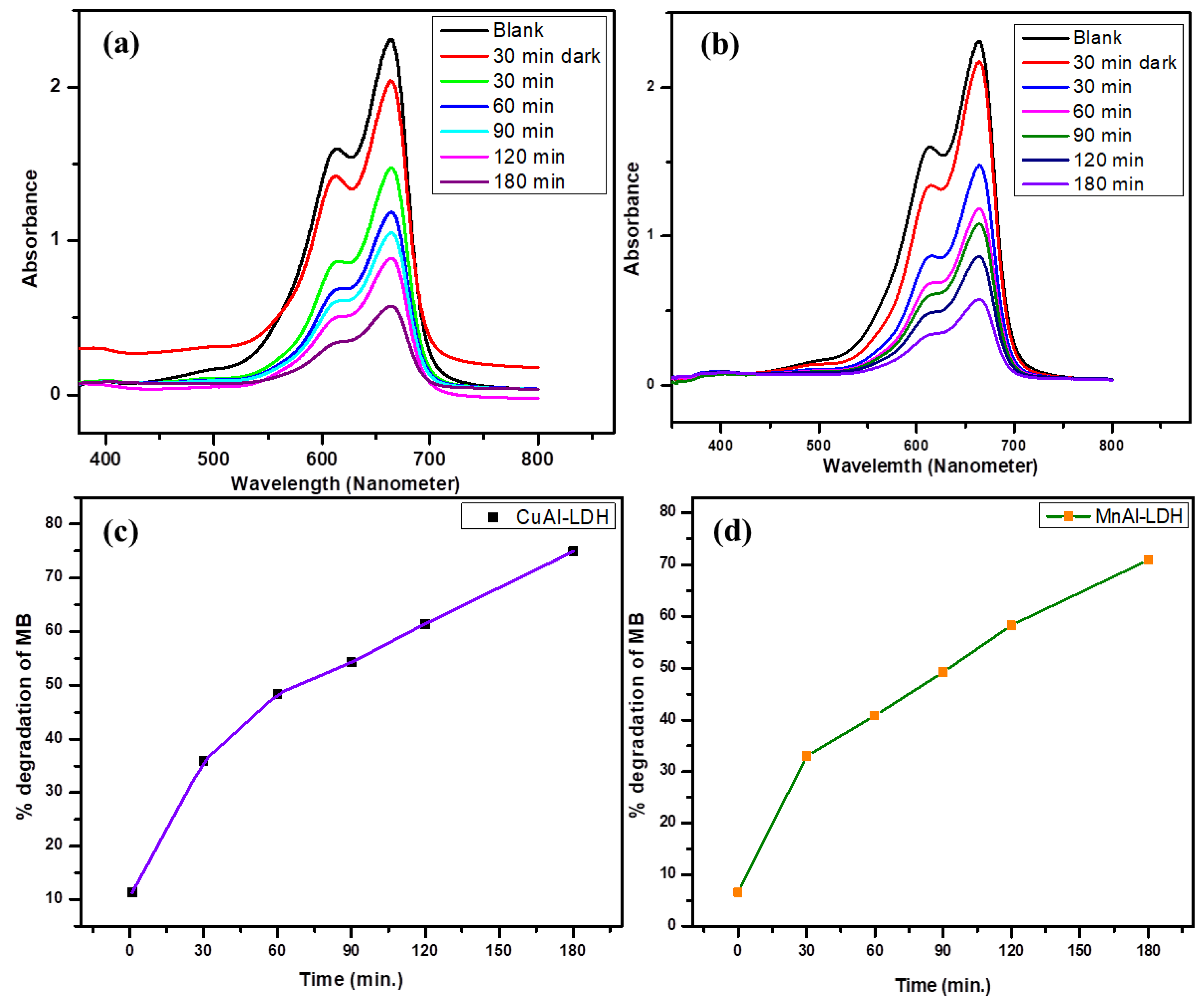
2.2. Photocatalytic Study
2.3. Effect of pH of MB Solution
2.4. Effect of Initial Concentration of MB
2.5. Kinetics Studies
| Catalyst | Reaction Parameters | % Degradation | Ref. |
|---|---|---|---|
| CuAl-LDH | 180 min, visible light | 74.95 | Current study |
| MnAl-LDH | 180 min, visible light | 70.93 | Current study |
| Ca0.5Pb0.5−xYbxZnyFe12−yO19 hexaferrite | 90 min, visible light | 96.1 | [41] |
| g-C3N4/ZnO-W/Co0.010 composite | 90 min, visible light | 90 | [42] |
| Zn-PMOS | 60 min, Tungsten bulb (200 W) | 48 | [43] |
| ZnO | 90 min, visible light | 88 | [44] |
| ZIF-67@wood | -- | 90 | [45] |
| CMO/CFO/PMS | 30 min, | 99 | [46] |
| Biosynthesized ZnO | 240 min, UV-light | 80 | [47] |
| MoS2/TiO2 | 120 min, visible light | 98.5 | [48] |
| ZnS/Zn(CO3)2(OH)6 | 80 min, sunlight | 56 | [49] |
| TiO2-decorated CNTs | 180 min, visible light | 85 | [50] |
2.6. Proposed Degradation Mechanism
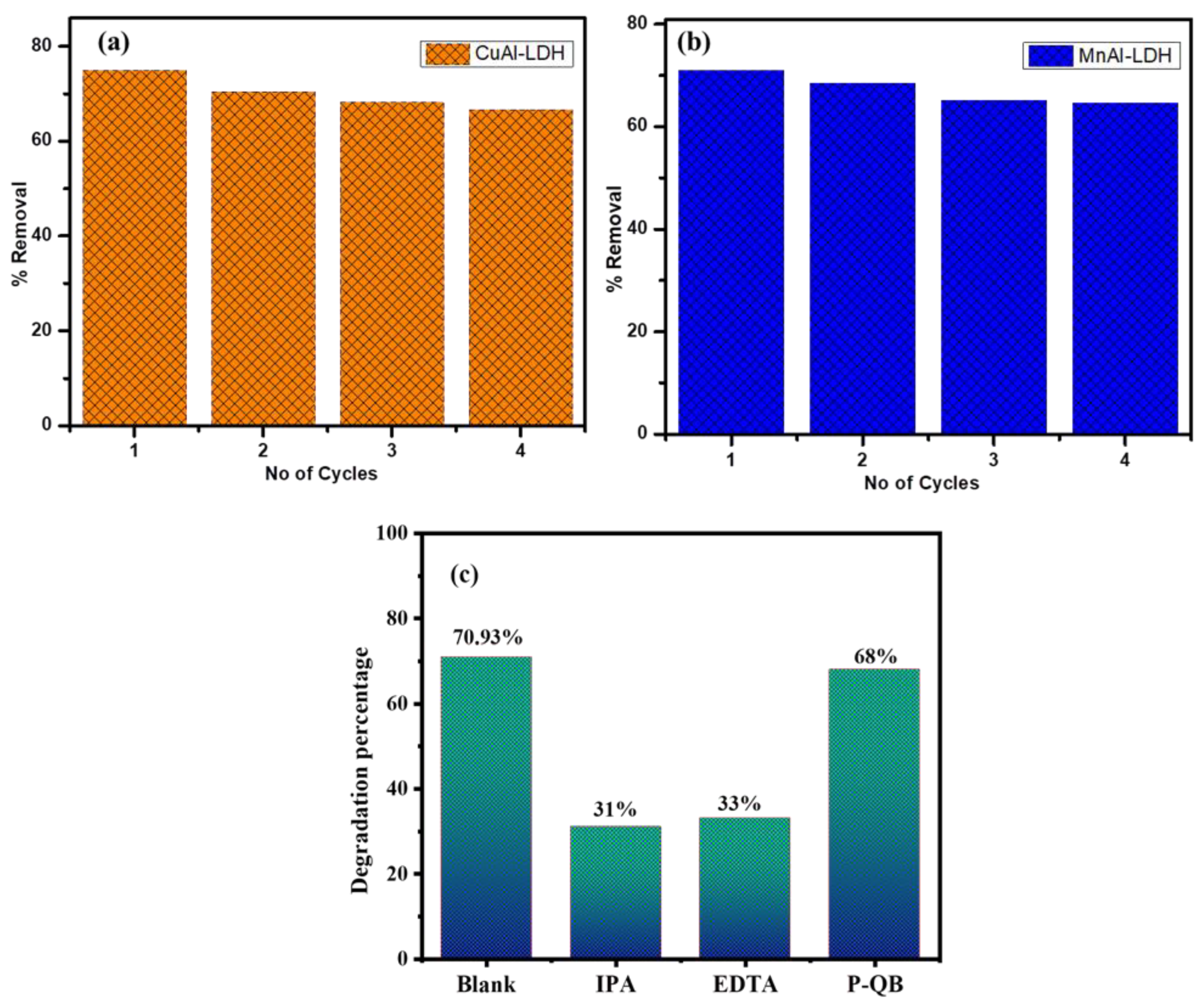
2.7. Quenching Active Species Trapping Experiment
2.8. Reusability Test
3. Experimental Section
3.1. Materials
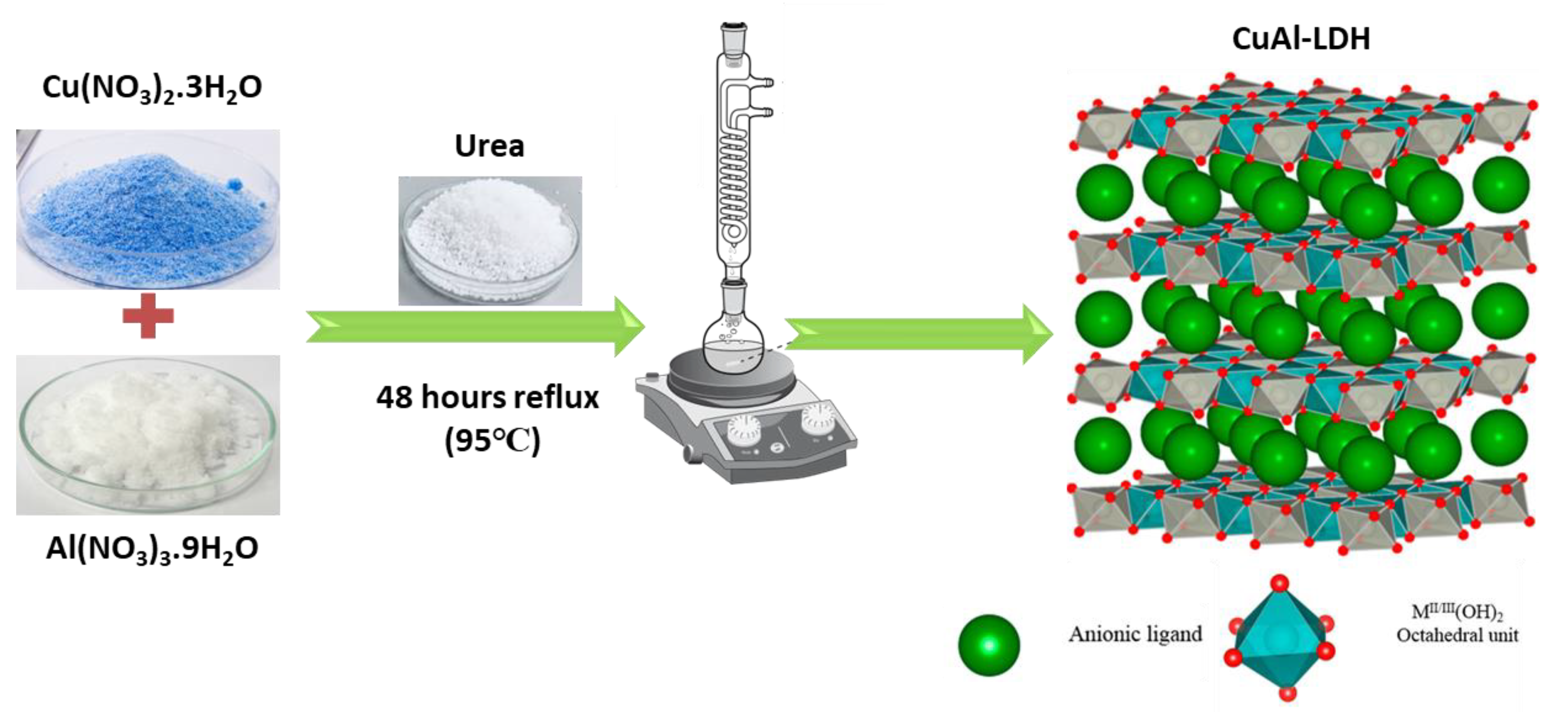
3.2. Synthesis of CuAl-LDH and MnAl-LDH
3.3. Photocatalytic Degradation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, M.A.; Yasar, A.; Bashir, M.A.; Siyal, S.H.; Najam, T.; Javed, M.S.; Ahmad, K.; Hussain, S.; Anjum, S.; Hussain, E.; et al. Quality assessment of the noncarbonated-bottled drinking water: Comparison of their treatment techniques. Int. J. Environ. Anal. Chem. 2022, 102, 8195–8206. [Google Scholar] [CrossRef]
- Bobde, P.; Sharma, A.K.; Panchal, D.; Patel, R.K.; Dhodapkar, R.S.; Pal, S. Layered double hydroxides (LDHs)-based photocatalysts for dye degradation: A review. Int. J. Environ. Sci. Technol. 2023, 20, 5733–5752. [Google Scholar] [CrossRef]
- Sun, H.; Lee, S.-Y.; Park, S.-J. MgAl-layered double hydroxides decorated with Pd-doped SnS nanoparticles: A novel photocatalyst for efficient dye degradation and Cr(VI) reduction in water. J. Ind. Eng. Chem. 2023, 126, 510–519. [Google Scholar] [CrossRef]
- Kumar, O.P.; Nazir, M.A.; Shah, S.S.A.; Hashem, A.; Kumar, A.; Abd_Allah, E.F.; ur Rehman, A. Ternary metal conjugated ZIF-67 coordination with Ag and Ce for the efficient Fenton-like remediation of dyes under visible light. Opt. Mater. 2024, 150, 115228. [Google Scholar] [CrossRef]
- Ishfaq, M.; Khan, S.A.; Nazir, M.A.; Ali, S.; Younas, M.; Mansha, M.; Shah, S.S.A.; Arshad, M.; ur Rehman, A. The in situ synthesis of sunlight-driven Chitosan/MnO2@MOF-801 nanocomposites for photocatalytic reduction of Rhodamine-B. J. Mol. Struct. 2024, 1301, 137384. [Google Scholar] [CrossRef]
- Sun, L.; Mo, Y.; Zhang, L. A mini review on bio-electrochemical systems for the treatment of azo dye wastewater: State-of-the-art and future prospects. Chemosphere 2022, 294, 133801. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Han, T.H.; Khan, M.M.; Kalathil, S.; Lee, J.; Cho, M.H. Simultaneous Enhancement of Methylene Blue Degradation and Power Generation in a Microbial Fuel Cell by Gold Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 8174–8181. [Google Scholar] [CrossRef]
- Yusuf, L.A.; Ertekin, Z.; Fletcher, S.; Symes, M.D. Enhanced ultrasonic degradation of methylene blue using a catalyst-free dual-frequency treatment. Ultrason. Sonochem. 2024, 103, 106792. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; ur Rehman, A. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Anum, A.; Nazir, M.A.; Ibrahim, S.M.; Shah, S.S.A.; Tahir, A.A.; Malik, M.; Wattoo, M.A.; ur Rehman, A. Synthesis of Bi-Metallic-Sulphides/MOF-5@ graphene Oxide Nanocomposites for the Removal of Hazardous Moxifloxacin. Catalysts 2023, 13, 984. [Google Scholar] [CrossRef]
- Cerqueira, A.; Russo, C.; Marques, M. Electroflocculation for textile wastewater treatment. Braz. J. Chem. Eng. 2009, 26, 659–668. [Google Scholar] [CrossRef]
- Ullah, S.; ur Rehman, A.; Najam, T.; Hossain, I.; Anjum, S.; Ali, R.; Shahid, M.U.; Shah, S.S.A.; Nazir, M.A. Advances in metal-organic framework@activated carbon (MOF@AC) composite materials: Synthesis, characteristics and applications. J. Ind. Eng. Chem. 2024; in press. [Google Scholar] [CrossRef]
- Anum, A.; Ibrahim, S.M.; Tahir, A.A.; Nazir, M.A.; Malik, M.; Shah, S.S.A.; Ehsan, A.; Wattoo, M.A.; ur Rehman, A. Construction of hybrid sulfur-doped MOF-235@g-C3N4 photocatalyst for the efficient removal of nicotine. Inorg. Chem. Commun. 2023, 157, 111268. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Tahir, A.A.; Nazir, M.A.; Shah, S.S.A.; Wattoo, M.A.; Kousar, R.; ur Rehman, A. Novel approach towards ternary magnetic g-C3N4/ZnO-W/Snx nanocomposite: Photodegradation of nicotine under visible light irradiation. Int. J. Environ. Anal. Chem. 2023, 1–19. [Google Scholar] [CrossRef]
- Nazir, M.A.; Javed, M.S.; Islam, M.; Assiri, M.A.; Hassan, A.M.; Jamshaid, M.; Najam, T.; Shah, S.S.A.; ur Rehman, A. MOF@graphene nanocomposites for energy and environment applications. Compos. Commun. 2024, 45, 101783. [Google Scholar] [CrossRef]
- Shahzad, K.; Khan, M.I.; Shanableh, A.; Elboughdiri, N.; Jabeen, S.; Nazir, M.A.; Farooq, N.; Ali, H.; Abdelfattah, A.; Rehman, A.U. Silver supported-Ag@PMOS onto thumb structured porous organosilica materials with efficient hetero-junction active sites for photo-degradation of methyl orange dye. Inorg. Nano-Met. Chem. 2021, 52, 407–416. [Google Scholar] [CrossRef]
- Jamshaid, M.; Khan, H.; Nazir, M.A.; Wattoo, M.A.; Shahzad, K.; Malik, M.; Rehman, A.-U. A novel bentonite–cobalt doped bismuth ferrite nanoparticles with boosted visible light induced photodegradation of methyl orange: Synthesis, characterization and analysis of physiochemical changes. Int. J. Environ. Anal. Chem. 2022, 104, 1186–1201. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.S.A.; Altaf, M.; Hossain, I.; El Sayed, M.E.; Kallel, M.; El-Bahy, Z.M.; ur Rehman, A.; Najam, T.; Nazir, M.A. Activated carbon derived from biomass for wastewater treatment: Synthesis, application and future challenges. J. Anal. Appl. Pyrolysis 2024, 179, 106480. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Tonda, S.; Jo, W.-K. Plasmonic Ag nanoparticles decorated NiAl-layered double hydroxide/graphitic carbon nitride nanocomposites for efficient visible-light-driven photocatalytic removal of aqueous organic pollutants. Catal. Today 2018, 315, 213–222. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Khazani, Y.; Khaloo, S.S.; Ghalkhani, M. Highly effectual photocatalytic degradation of tartrazine by using Ag nanoparticles decorated on Zn-Cu-Cr layered double hydroxide@ 2D graphitic carbon nitride (C3N5). Environ. Sci. Pollut. Res. 2023, 30, 12903–12915. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Jabeen, S.; Wattoo, M.A.; Bashir, M.S.; Shah, S.S.A.; ur Rehman, A. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 2022, 145, 110008. [Google Scholar] [CrossRef]
- Najam, T.; Aslam, M.K.; Rafiq, K.; Altaf Nazir, M.; Rehman, A.u.; Imran, M.; Javed, M.S.; Cai, X.; Shah, S.S.A. Nanostructure engineering by surficial induced approach: Porous metal oxide-carbon nanotube composite for lithium-ion battery. Mater. Sci. Eng. B 2021, 273, 115417. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Qiu, L.; Lan, X.; Zhu, C.; Duan, J.; Liu, Y.; Li, H.; Yu, Y.; Yang, W. In-situ synthesis of dual Z-scheme heterojunctions of cuprous oxide/layered double hydroxides/nitrogen-rich graphitic carbon nitride for photocatalytic sterilization. J. Colloid Interface Sci. 2022, 620, 313–321. [Google Scholar] [CrossRef]
- Jin, Z.-L.; Wang, Y.-P. Strategy of Graphdiyne (g−CnH2n−2) Preparation Coupling with the Flower-Like NiAl-LDH Heterojunctions for Efficient Photocatalytic Hydrogen Evolution. Chem. Eur. J. 2021, 27, 12649–12658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Efficient Photocatalytic Nitrogen Fixation over Cuδ+-Modified Defective ZnAl-Layered Double Hydroxide Nanosheets. Adv. Energy Mater. 2020, 10, 1901973. [Google Scholar] [CrossRef]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Coogan, Á.; Doménech, N.G.; Mc Ginley, D.; Simonian, T.; Rafferty, A.; Fedix, Q.; Donlon, A.; Nicolosi, V.; Gun’ko, Y.K. Layered double hydroxide/boron nitride nanocomposite membranes for efficient separation and photodegradation of water-soluble dyes. J. Mater. Chem. A 2023, 11, 12266–12281. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; ur Rehman, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; ur Rehman, A. Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Hanifah, Y.; Mohadi, R.; Lesbani, A. Polyoxometalate Intercalated MgAl-Layered Double Hydroxide for Degradation of Malachite Green. Ecol. Eng. Environ. Technol. 2023, 24, 109–119. [Google Scholar] [CrossRef]
- Bharali, D.; Saikia, S.; Devi, R.; Choudary, B.M.; Gour, N.K.; Deka, R.C. Photocatalytic degradation of phenol and its derivatives over ZnFe layered double hydroxide. J. Photochem. Photobiol. A Chem. 2023, 438, 114509. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; Lu, Y.; Li, H.; Xu, X. Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water. Sensors 2020, 20, 4146. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liang, X.; Xu, J.; Zhao, Z.; Tian, G. Synthesis of CuZrO3 nanocomposites/graphene and their application in modified electrodes for the co-detection of trace Pb (II) and Cd (II). Sens. Actuators B Chem. 2018, 273, 1146–1155. [Google Scholar] [CrossRef]
- Berner, S.; Araya, P.; Govan, J.; Palza, H. Cu/Al and Cu/Cr based layered double hydroxide nanoparticles as adsorption materials for water treatment. J. Ind. Eng. Chem. 2018, 59, 134–140. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.-K.; Hussain, M. Efficient visible light assisted photocatalysis using ZnO/TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Nayak, S.; Kumar Das, K.; Parida, K. Indulgent of the physiochemical features of MgCr-LDH nanosheets towards photodegradation process of methylene blue. J. Colloid Interface Sci. 2023, 634, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zeng, H.-Y.; Xu, S.; Chen, C.-R.; Duan, H.-Z.; Du, J.-Z.; Hu, G.; Sun, Y.-X. Facile preparation of sepiolite@LDH composites for the visible-light degradation of organic dyes. Chin. J. Catal. 2018, 39, 1832–1841. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, L.; Wei, Y.; Zhao, Z.; Du, K.; Chen, D.; Li, X.; Zhou, C.; Liu, G. ZIF-67-derived Co@ N-PC anchored on tracheid skeleton from sawdust with micro/nano composite structures for boosted methylene blue degradation. Sep. Purif. Technol. 2021, 278, 119489. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; ur Rehman, A. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; ur Rehman, A. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; ur Rehman, A.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Nazir, M.A.; Jamshaid, M.; ur Rehman, A.; Alkorbi, A.S.; Alsaiari, R.; Alhemiary, N.A. Versatile Ag2O and ZnO nanomaterials fabricated via annealed Ag-PMOS and ZnO-PMOS: An efficient photocatalysis tool for azo dyes. J. Mol. Liq. 2022, 356, 119036. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Guan, H.; Dai, X.; Wu, M.; Wang, X. Wood-based catalytic filter decorated with ZIF-67 for highly efficient and continuous organic pollutant removal. Chem. Eng. J. 2024, 479, 147580. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, L.; Tian, L. Non-radical-dominated catalytic degradation of methylene blue by magnetic CoMoO4/CoFe2O4 composite peroxymonosulfate activators. J. Environ. Manag. 2023, 325, 116587. [Google Scholar] [CrossRef] [PubMed]
- Şendal, K.; Üstün Özgür, M.; Gülen, J. Biosynthesis of ZnO photocatalyst and its application in photo catalytic degradation of methylene blue dyestuff. J. Dispers. Sci. Technol. 2023, 44, 2734–2747. [Google Scholar] [CrossRef]
- Chen, L.; Ou, S.-F.; Nguyen, T.-B.; Chuang, Y.; Chen, C.-W.; Dong, C.-D. In-situ hydrothermal synthesis of MoS2/TiO2 nanocomposites for enhanced and stable photocatalytic performance: Methylene blue degradation pathway and mechanism. J. Taiwan Inst. Chem. Eng. 2024, 105436. [Google Scholar] [CrossRef]
- Vicencio Garrido, M.A.; Chávez Portillo, M.; Juarez, H.; Luna, A.; Serrano-De la Rosa, L.E. Low cost chemical bath deposition synthesis of Zinc Oxide/Zinc sulfide composite and Zinc hydrozincite for methylene blue degradation. Inorg. Chem. Commun. 2024, 164, 112484. [Google Scholar] [CrossRef]
- Akhter, P.; Ali, F.; Ali, A.; Hussain, M. TiO2 decorated CNTs nanocomposite for efficient photocatalytic degradation of methylene blue. Diam. Relat. Mater. 2024, 141, 110702. [Google Scholar] [CrossRef]
- Boumeriame, H.; Cherevan, A.; Eder, D.; Apaydin, D.H.; Chafik, T.; Da Silva, E.S.; Faria, J.L. Engineering g-C3N4 with CuAl-layered double hydroxide in 2D/2D heterostructures for visible-light water splitting. J. Colloid Interface Sci. 2023, 652, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.A.; Kim, T.W.; Kim, I.Y.; Hwang, S.-J. Synthesis and lithium electrode application of ZnO−ZnFe2O4 nanocomposites and porously assembled ZnFe2O4 nanoparticles. Solid State Ion. 2011, 182, 91–97. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Pan, G.; Qian, P.; Ni, Z. Photocatalytic degradation of methylene blue with a nanocomposite system: Synthesis, photocatalysis and degradation pathways. Phys. Chem. Chem. Phys. 2015, 17, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Bashir, M.S.; Javed, M.S.; Bashir, M.A.; Imran, M.; Azhar, U.; Shah, S.S.A.; ur Rehman, A. Kinetics, isothermal and mechanistic insight into the adsorption of eosin yellow and malachite green from water via tri-metallic layered double hydroxide nanosheets. Korean J. Chem. Eng. 2022, 39, 216–226. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, M.A.; Rehman, A.u.; Najam, T.; Elsadek, M.F.; Ali, M.A.; Hossain, I.; Tufail, M.K.; Shah, S.S.A. Copper- and Manganese-Based Bimetallic Layered Double Hydroxides for Catalytic Reduction of Methylene Blue. Catalysts 2024, 14, 430. https://doi.org/10.3390/catal14070430
Nazir MA, Rehman Au, Najam T, Elsadek MF, Ali MA, Hossain I, Tufail MK, Shah SSA. Copper- and Manganese-Based Bimetallic Layered Double Hydroxides for Catalytic Reduction of Methylene Blue. Catalysts. 2024; 14(7):430. https://doi.org/10.3390/catal14070430
Chicago/Turabian StyleNazir, Muhammad Altaf, Aziz ur Rehman, Tayyaba Najam, Mohamed Farouk Elsadek, M. Ajmal Ali, Ismail Hossain, Muhammad Khurram Tufail, and Syed Shoaib Ahmad Shah. 2024. "Copper- and Manganese-Based Bimetallic Layered Double Hydroxides for Catalytic Reduction of Methylene Blue" Catalysts 14, no. 7: 430. https://doi.org/10.3390/catal14070430






