La-Modified SBA-15 Prepared by Direct Synthesis: Importance of Determining Actual Composition
Abstract
1. Introduction
2. Results and Discussion
2.1. Materials Textural Properties
2.2. X-ray Diffraction
2.3. FTIR Characterization
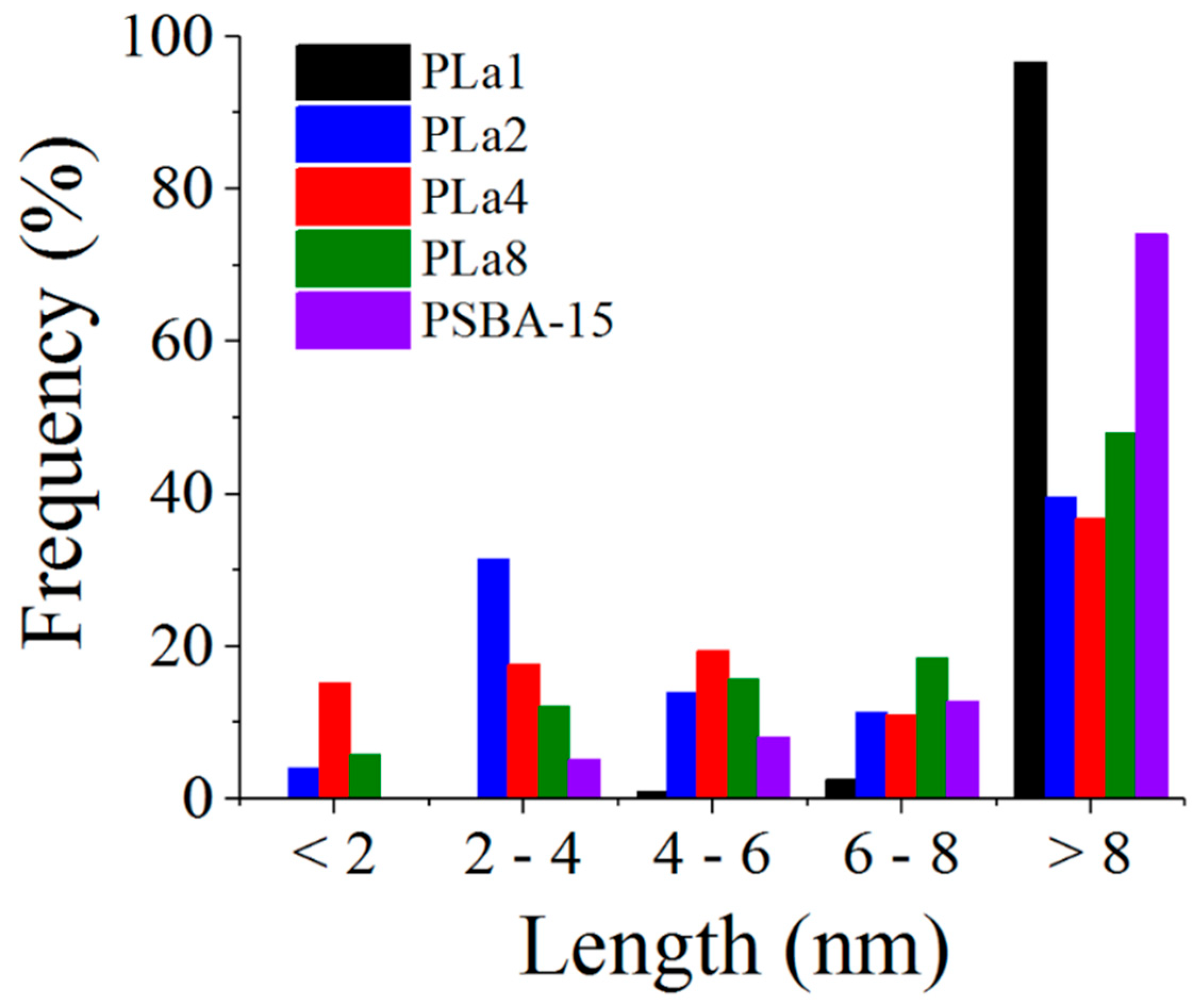
2.4. STEM (HAADF) Studies
2.5. HDO Reaction Test
2.6. NH3 and CO2 Temperature-Programmed Desorption (TPD)
2.7. Thermal Analysis (TG-DTG)
2.8. SEM-EDS Analysis
3. Experimental
3.1. Material Synthesis
3.1.1. La-Modified SBA-15
3.1.2. Pt-Containing La-Modified SBA-15
3.2. Materials Characterization
3.3. Phenol HDO Reaction Test
- x = P conversion at time t;
- t = reaction time (s).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar, J.; Colín-Luna, J.A.; Barrera, M.C. Thioresistant PdPt/Al/SBA-15 for naphthalene hydrogenation. Ind. Eng. Chem. Res. 2024, 63, 1248–1260. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of Ti-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Morales Hernández, G.; Pacheco Sosa, J.G.; Escobar Aguilar, J.; Torres Torres, J.G.; Pérez Vidal, H.; Lunagómez Rocha, M.A.; De la Cruz Romero, D.; del Ángel Vicente, P. Improving platinum dispersion on SBA-15 by titania addition. Rev. Mex. Ing. Chem. 2020, 19, 997–1010. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Santes, V.F.; Fouconnier, B. Guaiacol HDO on La-modified Pt/Al2O3: Influence of rare-earth loading. Can. J. Chem. Eng. 2023, 101, 5772–5784. [Google Scholar] [CrossRef]
- Bendahou, K.; Cherif, L.; Siffert, S.; Tidahy, H.L.; Benaïssa, H.; Aboukaïs, A. The effect of the use of lanthanum-doped mesoporous SBA-15 on the performance of Pt/SBA-15 and Pd/SBA-15 catalysts for total oxidation of toluene. Appl. Catal. A Gen. 2008, 351, 82–87. [Google Scholar] [CrossRef]
- Roy, S.; Newalkar, B.L.; Datta, S. Vanadium Substituted SBA-15 Supported Bimetallic Pt, Pd Catalysts for Hydrogenation of Toluene to Methylcyclohexane. Can. J. Chem. Eng. 2014, 92, 1034–1040. [Google Scholar] [CrossRef]
- Flodström, K.; Alfredsson, V. Influence of the block length of triblock copolymers on the formation of mesoporous silica. Microporous Mesoporous Mater. 2003, 59, 167–176. [Google Scholar] [CrossRef]
- Pinzón-Ramos, I.; Castillo-Araiza, C.O.; Tavizón-Pozos, J.A.; de los Reyes, J.A. On a Response Surface Analysis: Hydrodeoxygenation of Phenol over a CoMoS-Based Active Phase. Catalysts 2022, 12, 1139. [Google Scholar] [CrossRef]
- Leofanti, S.G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–210. [Google Scholar] [CrossRef]
- Palcheva, R.; Kaluža, L.; Dimitrov, L.; Tyuliev, G.; Avdeev, G.; Jirátová, K.; Spojakina, A. NiMo catalysts supported on the Nb modified mesoporous SBA-15 and HMS: Effect of thioglycolic acid addition on HDS. Appl. Catal. A Gen. 2016, 520, 24–34. [Google Scholar] [CrossRef]
- Miller, J.T.; Schreier, M.; Kropf, A.J.; Regalbuto, J.R. A fundamental study of platinum tetraammine impregnation of silica 2. The effect of method of preparation, loading, and calcination temperature on (reduced) particle size. J. Catal. 2004, 225, 203–212. [Google Scholar] [CrossRef]
- Vít, Z.; Gulková, D.; Kaluža, L.; Boaro, M. Effect of catalyst precursor and its pretreatment on the amount of β-Pd hydride phase and HDS activity of Pd-Pt/silica-alumina. Appl. Catal. B Environ. 2014, 146, 213–220. [Google Scholar] [CrossRef]
- Hernández Morales, R.; Pacheco Sosa, J.G.; Torres Torres, J.G.; Pérez Vidal, H.; Lunagómez Rocha, M.A.; De la Cruz Romero, D. Pt/Ga-SBA-15 composites synthesis and characterization. Superf. Vacío 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wen, B.; Zhu, M.; Dai, B. Lanthanum incorporated in MCM-41 and its application as a support for a stable Ni-based methanation catalyst. J. Rare Earths 2018, 36, 367–373. [Google Scholar] [CrossRef]
- Giraldo, L.; Bastidas-Barranco, M.; Moreno-Piraján, J.C. Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules 2014, 19, 20594–20612. [Google Scholar] [CrossRef]
- Gregg, S.J.; Ramsay, J.D.F. A Study of the Adsorption of Carbon Dioxide by Alumina Using Infrared and Isotherm Measurements. J. Phys. Chem. 1969, 73, 1243–1247. [Google Scholar] [CrossRef]
- Parkyns, N.D. The Influence of Thermal Pretreatment on the Spectrum of Carbon Dioxide Adsorbed on Alumina. J. Phys. Chem. 1971, 76, 526–531. [Google Scholar] [CrossRef]
- Escobar, J.; Ramírez, J.; Cuevas, R.; Ángeles, C.; Barrera, M.C.; Gutiérrez, A. Thiophene HDS on La-Modified CoMo/Al2O3 Sulfided Catalysts. Effect of Rare-Earth Content. Top. Catal. 2020, 63, 529–545. [Google Scholar] [CrossRef]
- Hungría, A.B.; Calvino, J.J.; Hernández-Garrido, J.C. HAADF STEM Electron Tomography in Catalysis Research. Top. Catal. 2019, 62, 808–821. [Google Scholar] [CrossRef]
- Ballesteros-Plata, D.; Infantes-Molina, A.; Rodríguez-Castellón, E. Study of bifunctionality of Pt/SBA-15 catalysts for HDO of Dibenzofuran reaction: Addition of Mo or use of an acidic support. Appl. Catal. A Gen. 2019, 580, 93–101. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ra, J.; Shen, K.; Vohs, J.M.; Gorte, R.J. Hydrodeoxygenation of m-Cresol over WOx-Pt/SBA-15 Using Alkanes as Hydrogen Carriers. ACS Catal. 2023, 13, 10908–10915. [Google Scholar] [CrossRef]
- Ballesteros-Plata, D.; Barroso-Martín, I.; Medina Cervantes, J.A.; Maciel, C.; Huirache-Acuña, R.; Rodríguez-Castellón, E.; Infantes-Molina, A. Bimetallic Niobium-Based Catalysts Supported on SBA-15 for Hydrodeoxygenation of Anisole. Ind. Eng. Chem. Res. 2021, 60, 18831–18840. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A.; Szczyglewska, P.; Nowak, I. The effect of metal (Nb, Ru, Pd, Pt) supported on SBA-16 on the phenol Hydrodeoxygenation. Catal. Today 2019, 325, 61–67. [Google Scholar] [CrossRef]
- Shivhare, A.; Hunns, J.A.; Durndell, L.J.; Parlett, C.M.A.; Isaacs, M.A.; Lee, A.F.; Wilson, K. Metal-Acid Synergy: Hydrodeoxygenation of Anisole over Pt/Al-SBA-15. ChemSusChem 2020, 13, 4945–4953. [Google Scholar] [CrossRef]
- Novodarszki, G.; Lónyi, F.; Csík, B.; Mihályi, M.R.; Barthos, R.; Valyon, J.; Vikár, A.; Deka, D.; Pászti, Z.; Shi, Y.; et al. Hydroconversion of lignin-derived platform compound guaiacol to fuel additives and value-added chemicals over alumina-supported Ni catalysts. Appl. Catal. A Gen. 2024, 680, 119757. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Pawelec, B.; Fierro, J.L.G.; Loricera, C.V.; Jiménez-López, A.; Rodríguez-Castellón. Effect of Ir and Pt Addition on the HDO Performance of RuS2/SBA-15 Sulfide Catalysts. Top. Catal. 2015, 58, 247–257. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Valente, J.S.; Solís-Casados, D.A.; Santes, V.; Terrazas, J.E.; Fouconnier, B.A.R. Dibenzothiophene Hydrodesulfurization over P-CoMo on Sol-Gel Alumina Modified by La Addition. Effect of Rare-Earth Content. Catalysts 2019, 9, 359. [Google Scholar] [CrossRef]
- Cui, J.-W.; Massoth, F.E.; Topsøe, N.Y. Studies of Molybdena-Alumina Catalysts XVIII. Lanthanum-Modified Supports. J. Catal. 1992, 136, 361–377. [Google Scholar] [CrossRef]
- Terrab, I.; Ouargli, R.; Boukoussa, B.; Ghomari, K.; Hamacha, R.; Roy, R.; Azzouz, A.; Bengueddach, A. Assessment of the intrinsic interactions of mesoporous silica with carbon dioxide. Res. Chem. Intermed. 2017, 43, 3775–3786. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Nunes-Beltrao, A.P.; Hamacha, R.; Azzouz, A. Assessment of the intrinsic interactions of nanocomposite polyaniline/SBA-15 with carbon dioxide: Correlation between the hydrophilic character and surface basicity. J. CO2 Util. 2018, 26, 171–178. [Google Scholar] [CrossRef]
- Bartz, W.; Górka, M.; Rybak, J.; Rutkowski, R.; Stojanowska, A. The assessment of effectiveness of SEM- EDX and ICP-MS methods in the process of determining the mineralogical and geochemical composition of particulate matter deposited on spider webs. Chemosphere 2021, 278, 130454. [Google Scholar] [CrossRef] [PubMed]
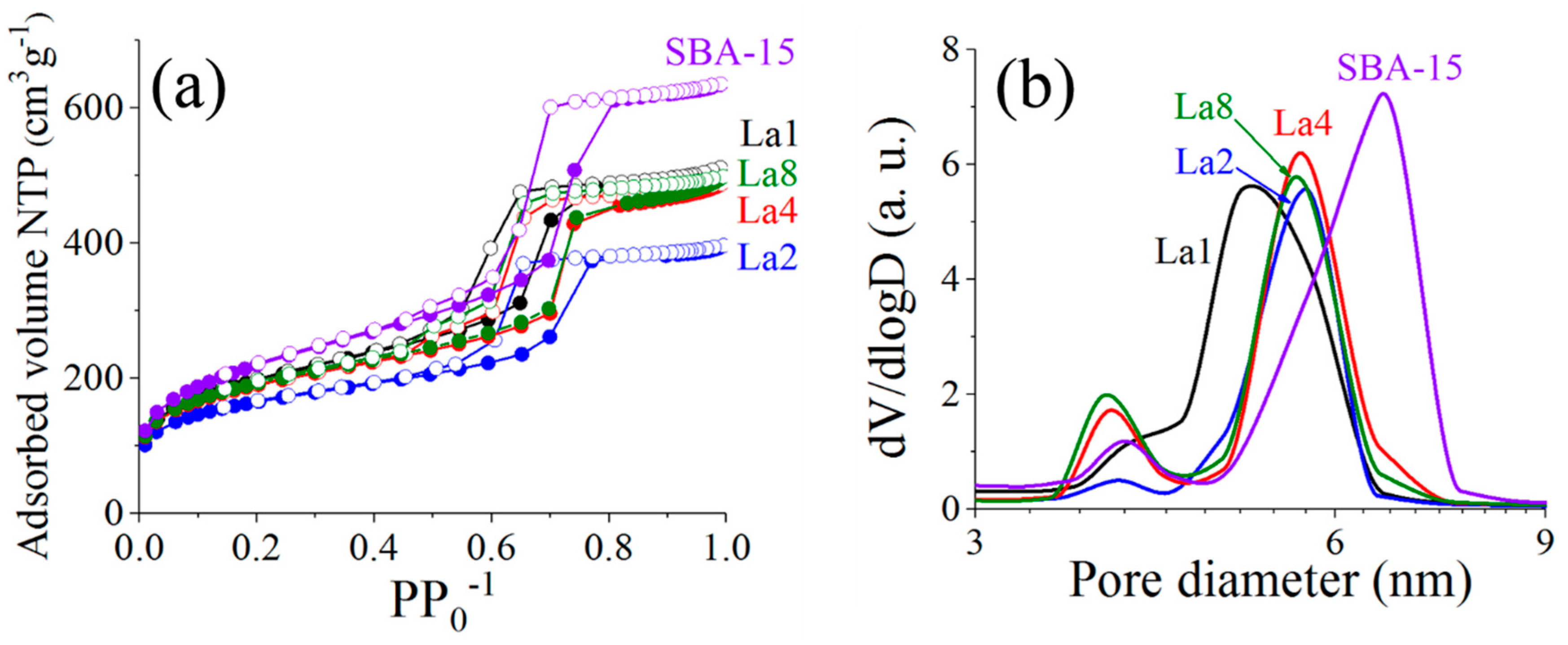
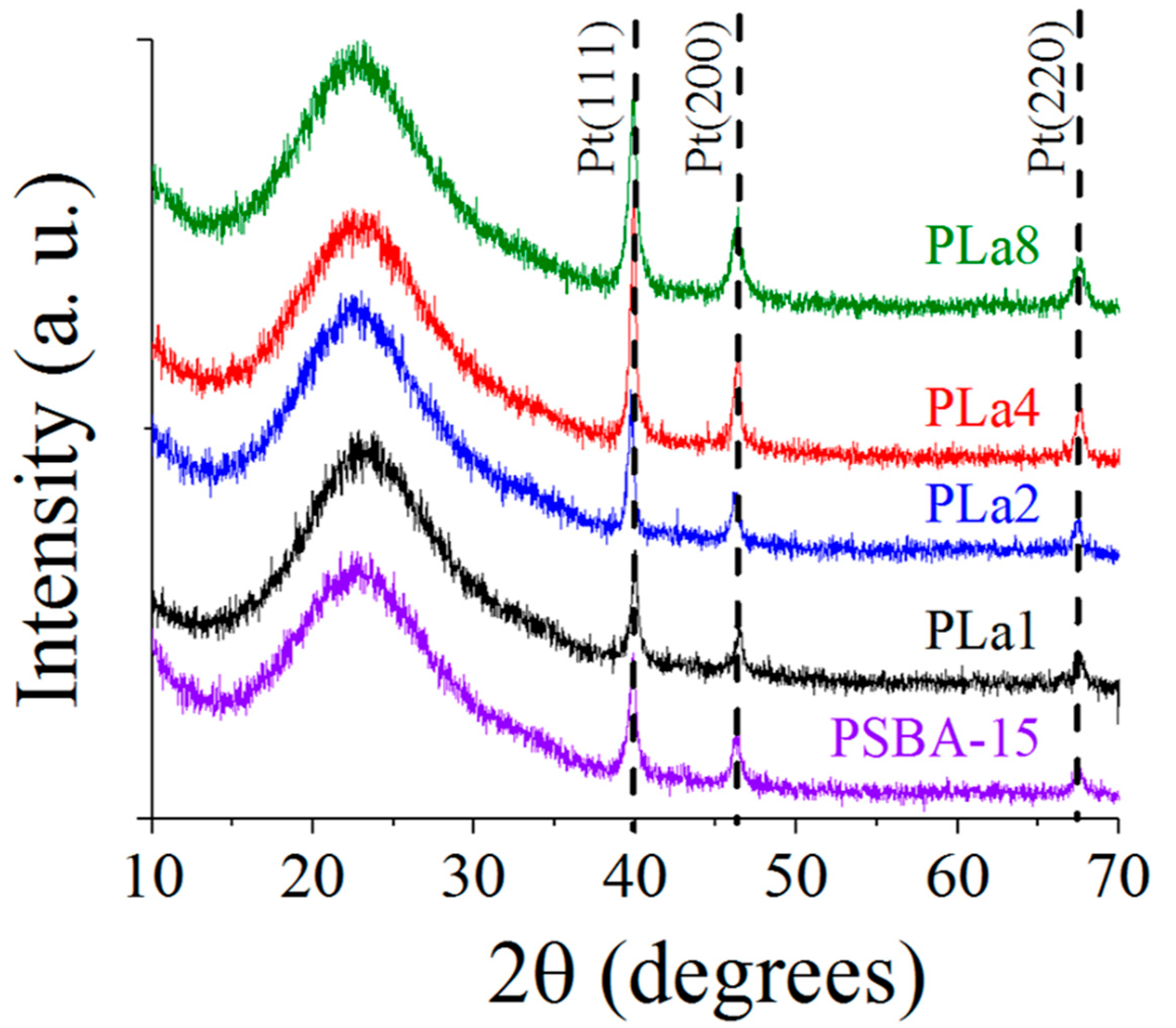
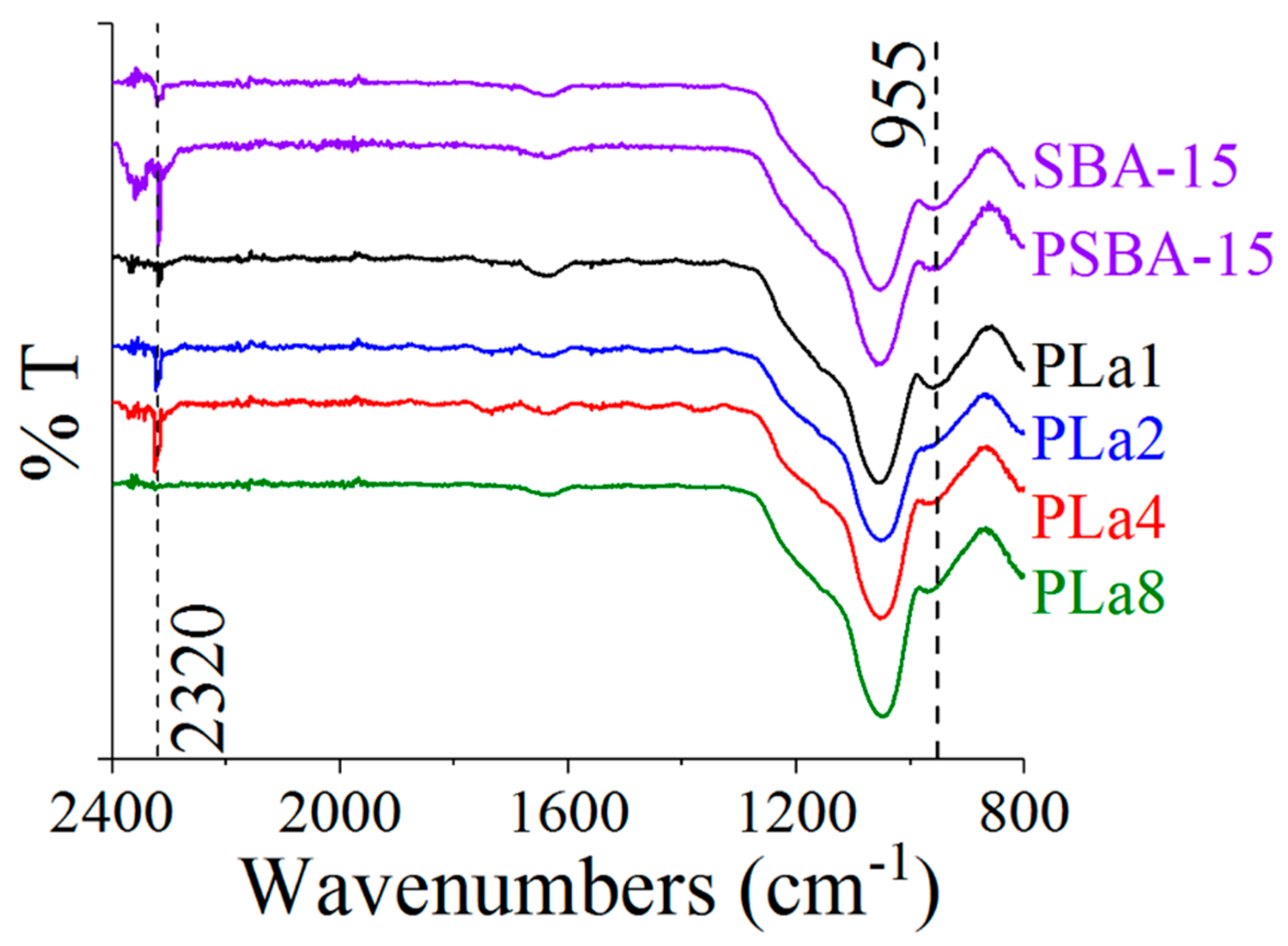
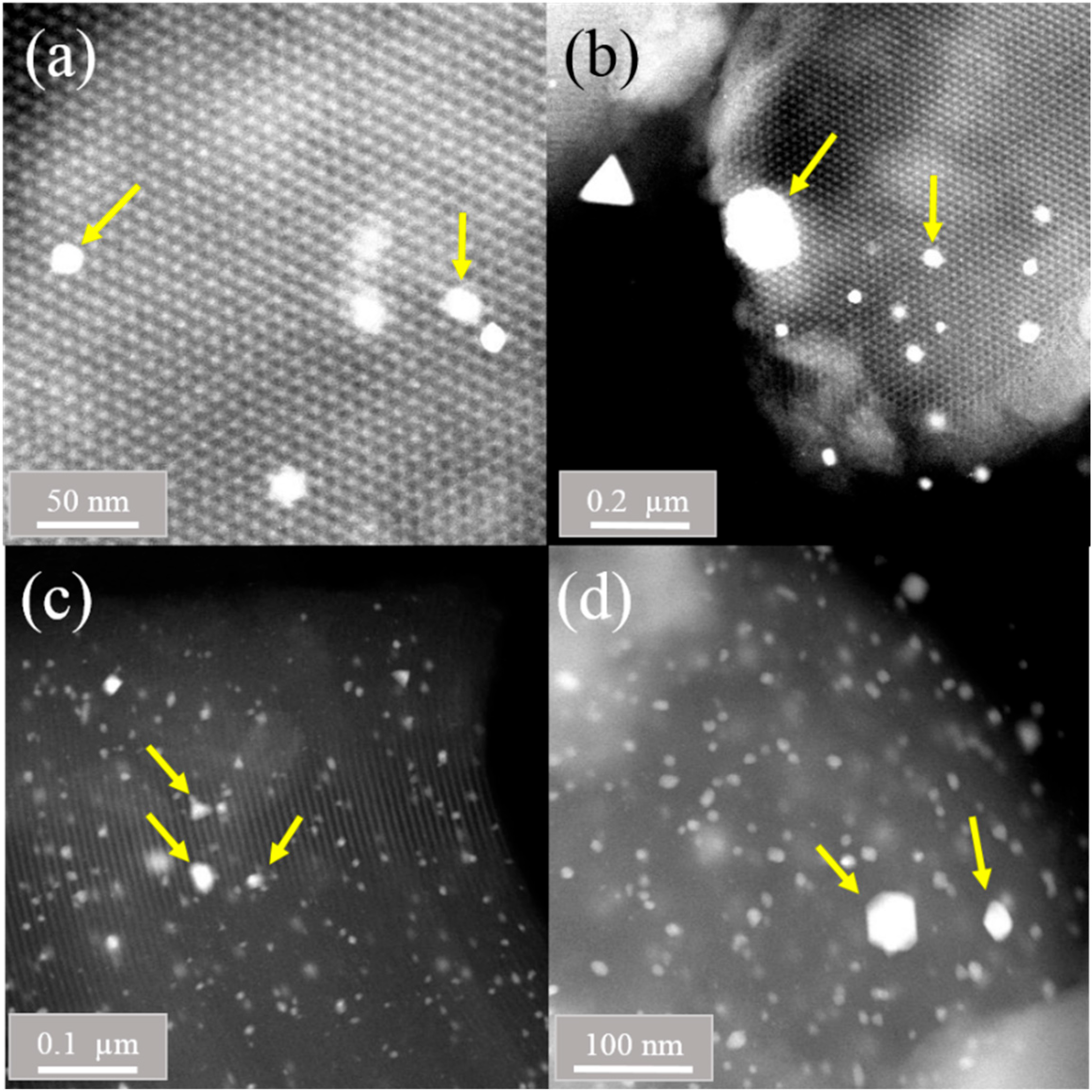
| Sample | SBET (m2g−1) | Vp (cm3g−1) | aDp Peak 1 | (nm) Peak 2 | bSBET (m2g−1) | SBET/bSBET |
|---|---|---|---|---|---|---|
| SBA-15 | 793 | 0.98 | 3.98 | 6.6 | - | |
| La1 | 706 | 0.78 | 4.09 | 5.10 | 785 | 0.90 |
| La2 | 582 | 0.61 | 3.93 | 5.66 | 777 | 0.75 |
| La4 | 674 | 0.75 | 3.89 | 5.60 | 761 | 0.89 |
| La8 | 685 | 0.76 | 3.86 | 5.58 | 730 | 0.94 |
| Catalyst | Crystal Size (nm) |
|---|---|
| PSBA-15 | 22.3 |
| PLa1 | 23.6 |
| PLa2 | 27.9 |
| PLa4 | 32.8 |
| PLa8 | 21.2 |
| Sample | Peak Position (cm−1) |
|---|---|
| SBA-15 | 955 |
| PSBA-15 | 959 |
| PLa1 | 961 |
| PLa2 | 964 |
| PLa4 | 968 |
| PLa8 | 971 |
| Sample | kHDO (×10−5) (s−1) |
|---|---|
| PSBA-15 | 0.3 |
| PLa1 | 2.0 |
| PLa2 | 2.0 |
| PLa4 | 2.0 |
| PLa8 | 0.7 |
| Sample | O (wt%) | Si (wt%) | La (wt%) |
|---|---|---|---|
| SBA-15 | 52.94 | 47.06 | 0.00 |
| La1 | 49.49 | 50.50 | 0.02 |
| La2 | 49.15 | 50.85 | 0.00 |
| La4 | 46.63 | 53.27 | 0.10 |
| La8 | 47.52 | 52.39 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales Hernández, G.; Escobar, J.; Pacheco Sosa, J.G.; Guzmán Cruz, M.A.; Torres Torres, J.G.; del Ángel Vicente, P.; Barrera, M.C.; Santolalla Vargas, C.E.; Pérez Vidal, H. La-Modified SBA-15 Prepared by Direct Synthesis: Importance of Determining Actual Composition. Catalysts 2024, 14, 436. https://doi.org/10.3390/catal14070436
Morales Hernández G, Escobar J, Pacheco Sosa JG, Guzmán Cruz MA, Torres Torres JG, del Ángel Vicente P, Barrera MC, Santolalla Vargas CE, Pérez Vidal H. La-Modified SBA-15 Prepared by Direct Synthesis: Importance of Determining Actual Composition. Catalysts. 2024; 14(7):436. https://doi.org/10.3390/catal14070436
Chicago/Turabian StyleMorales Hernández, Gloribel, José Escobar, José G. Pacheco Sosa, Mario A. Guzmán Cruz, José G. Torres Torres, Paz del Ángel Vicente, María C. Barrera, Carlos E. Santolalla Vargas, and Hermicenda Pérez Vidal. 2024. "La-Modified SBA-15 Prepared by Direct Synthesis: Importance of Determining Actual Composition" Catalysts 14, no. 7: 436. https://doi.org/10.3390/catal14070436
APA StyleMorales Hernández, G., Escobar, J., Pacheco Sosa, J. G., Guzmán Cruz, M. A., Torres Torres, J. G., del Ángel Vicente, P., Barrera, M. C., Santolalla Vargas, C. E., & Pérez Vidal, H. (2024). La-Modified SBA-15 Prepared by Direct Synthesis: Importance of Determining Actual Composition. Catalysts, 14(7), 436. https://doi.org/10.3390/catal14070436









