Structural Effect of Cu-Mn/Al2O3 Catalysts on Enhancing Toluene Combustion Performance: Molecular Structure of Polyols and Hydrothermal Treatment
Abstract
1. Introduction
2. Results and Discussions
2.1. Catalytic Activity of Toluene Combustion
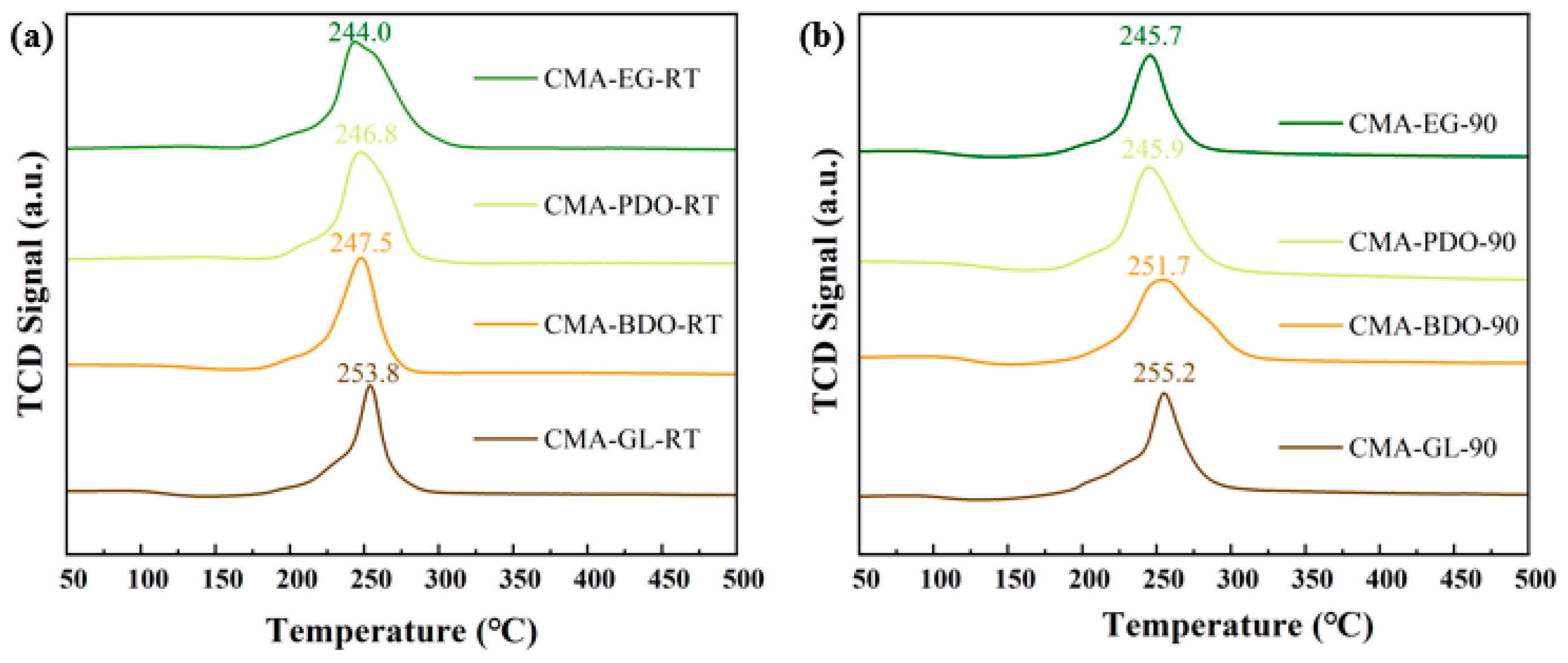
2.2. Textural and Structural Characterization
2.3. XPS and H2-TPR Analysis
2.4. Catalytic Mechanism
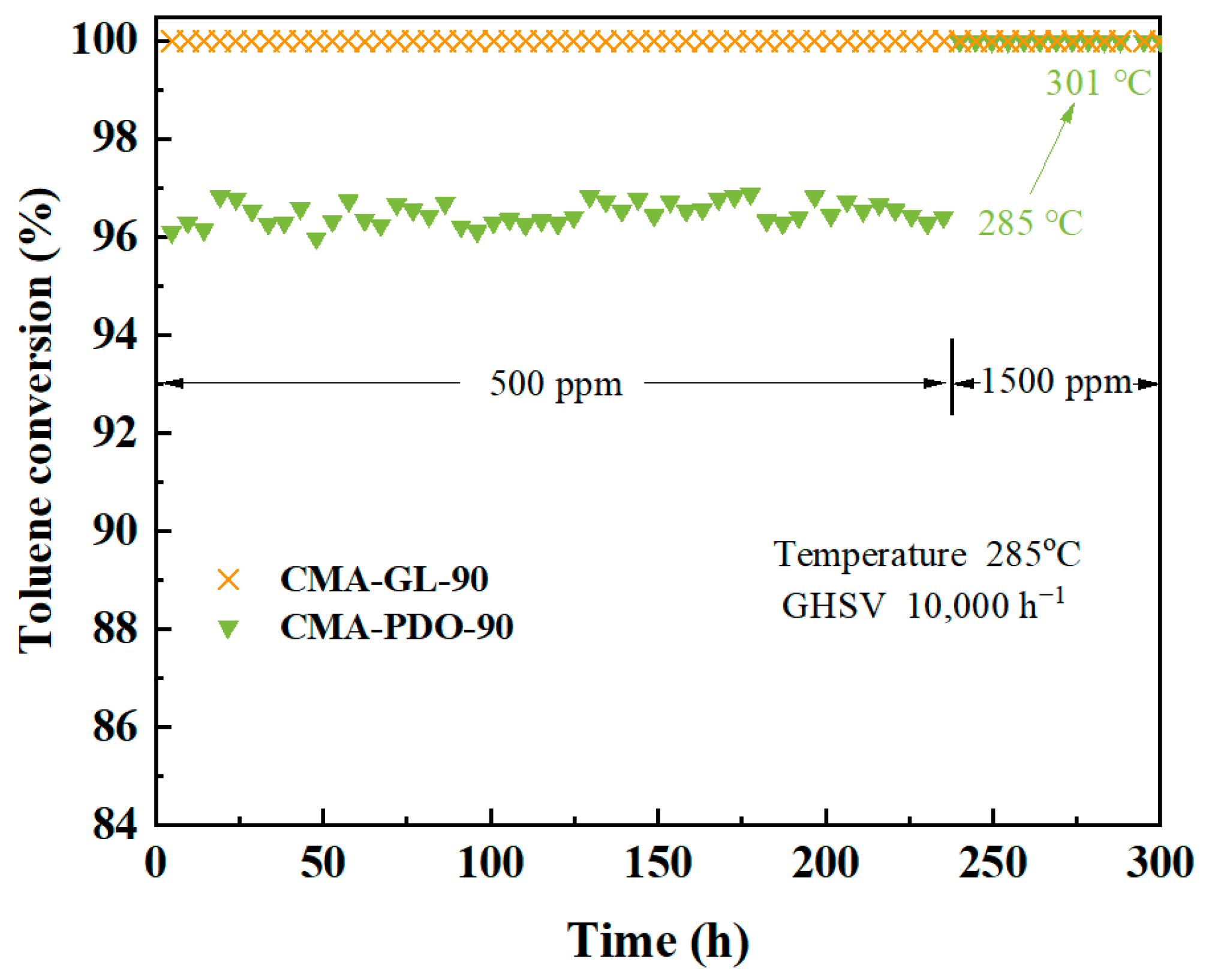
2.5. Catalytic Stability
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Evaluation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malik, N.; Elumalai, S.P.; Kumar, K. Health risk assessment from exposure to ambient VOCs and particulate matter in different functional zones in Dhanbad, India. Sci. Total Environ. 2023, 891, 164573. [Google Scholar] [CrossRef]
- Carriero, G.; Neri, L.; Famulari, D.; Di Lonardo, S.; Piscitelli, D.; Manco, A.; Esposito, A.; Chirico, A.; Facini, O.; Finardi, S.; et al. Composition and emission of VOC from biogas produced by illegally managed waste landfills in Giugliano (Campania, Italy) and potential impact on the local population. Sci. Total Environ. 2018, 640, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Cheng, S.; Zhu, J.; Zhang, X.; Cheng, L.; Wang, K. Determining an optimal control strategy for anthropogenic VOC emissions in China based on source emissions and reactivity. J. Environ. Sci. 2024, 136, 248–260. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Sharp, B.; Gu, Y.; Xu, S.-C.; Nie, J.; Long, R.-Y.; Wu, M.-F. Climate co-benefits of VOC control policies in China based on a cross-scale approach. J. Environ. Manag. 2023, 345, 118692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhu, Q.; Duan, W.; Wan, S.; Guo, T.; Li, H.; Feng, H.; Du, W.; Gu, J. Thermodynamic design and experimental study of a condensation recovery system for VOCs. Appl. Therm. Eng. 2024, 236, 121822. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P. VOCs absorption from gas streams using deep eutectic solvents—A review. J. Hazard. Mater. 2023, 448, 130957. [Google Scholar] [CrossRef]
- Hou, M.; Ren, H.; Cheng, W.; Li, L.; Zhang, S.; Chen, Y.; Yu, C.; Li, F.; Tian, S.; Deng, Z. Development of a headspace-gas chromatography/mass spectrometry method based on matrix-matched calibration for evaluating VOC content, characterization, source, and risk in RO membrane. Polym. Test. 2022, 107, 107474. [Google Scholar] [CrossRef]
- Mu, Y.; Williams, P.T. Recent advances in the abatement of volatile organic compounds (VOCs) and chlorinated-VOCs by non-thermal plasma technology: A review. Chemosphere 2022, 308, 136481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, J.; Zhang, R.; Ma, M.; Li, X.; Gong, P. Recent advances in different catalysts for synergistic removal of NOx and VOCs: A minor review. J. Environ. Chem. Eng. 2024, 12, 111764. [Google Scholar] [CrossRef]
- Boycheva, S.; Szegedi, Á.; Lázár, K.; Popov, C.; Popova, M. Advanced high-iron coal fly ash zeolites for low-carbon emission catalytic combustion of VOCs. Catal. Today 2023, 418, 114109. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Cocuzza, C.; Sartoretti, E.; Novara, C.; Giorgis, F.; Bensaid, S.; Russo, N.; Fino, D.; Piumetti, M. Copper-manganese oxide catalysts prepared by solution combustion synthesis for total oxidation of VOCs. Catal. Today 2023, 423, 114292. [Google Scholar] [CrossRef]
- González-Cobos, J.; Mylonoyannis, B.; Chai, G.; Zhang, W.; Tian, C.; Kaddouri, A.; Gil, S. Low-temperature gas-phase toluene catalytic combustion over modified CoCr2O4 spinel catalysts: Effect of Co/Cr content and calcination temperature. Appl. Catal. A Gen. 2023, 657, 119162. [Google Scholar] [CrossRef]
- Weng, C.-H.; Liao, C.-Y.; Tzeng, J.-H.; Chen, Y.-C.; Anotai, J.; Lin, Y.-T. Constructing oxygen vacancy induced Fe-Mn-Cu mixed oxides for efficient catalytic combustion of ethylene. Appl. Surf. Sci. 2023, 631, 157555. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, B.; Li, Z.; Zhang, X.; Liu, R.; Yun, J. Amorphous-microcrystal combined manganese oxides for efficiently catalytic combustion of VOCs. Mol. Catal. 2020, 489, 110920. [Google Scholar] [CrossRef]
- Moreno-Román, E.J.; Can, F.; Meille, V.; Guilhaume, N.; González-Cobos, J.; Gil, S. MnOx catalysts supported on SBA-15 and MCM-41 silicas for a competitive VOCs mixture oxidation: In-situ DRIFTS investigations. Appl. Catal. B Environ. 2024, 344, 123613. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.; Zheng, J.; Zuo, S. Studies of sulfur poisoning process via ammonium sulfate on MnO2/γ-Al2O3 catalyst for catalytic combustion of toluene. Appl. Catal. B Environ. 2021, 298, 120595. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Gao, B. Synergistic mechanism of Cu-Mn-Ce oxides in mesoporous ceramic base catalyst for VOCs microwave catalytic combustion. Chem. Eng. J. 2022, 429, 132302. [Google Scholar] [CrossRef]
- Ahn, C.-W.; You, Y.-W.; Heo, I.; Hong, J.S.; Jeon, J.-K.; Ko, Y.-D.; Kim, Y.; Park, H.; Suh, J.-K. Catalytic combustion of volatile organic compound over spherical-shaped copper–manganese oxide. J. Ind. Eng. Chem. 2017, 47, 439–445. [Google Scholar] [CrossRef]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu–Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeguchi, T.; Kikuchi, R.; Eguchi, K. Influence of preparation method and additive for Cu–Mn spinel oxide catalyst on water gas shift reaction of reformed fuels. Appl. Catal. A Gen. 2005, 279, 59–66. [Google Scholar] [CrossRef]
- Li, J.-R.; Zhang, W.-P.; Li, C.; Xiao, H.; He, C. Insight into the catalytic performance and reaction routes for toluene total oxidation over facilely prepared Mn-Cu bimetallic oxide catalysts. Appl. Surf. Sci. 2021, 550, 149179. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Q.; Chen, Z.; Li, L.; Jian, Y.; Albilali, R.; He, C.; Ma, M. Efficient selective combustion of n-butylamine on hierarchical Cu-Mn/SAPO-34 catalysts: The effect of mesoporosity and acidity. Appl. Catal. A Gen. 2023, 665, 119354. [Google Scholar] [CrossRef]
- Chuang, K.-H.; Lu, C.-Y.; Wey, M.-Y.; Huang, Y.-N. NO removal by activated carbon-supported copper catalysts prepared by impregnation, polyol, and microwave heated polyol processes. Appl. Catal. A Gen. 2011, 397, 234–240. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Liang, H.; Yang, H.; Yang, Y. Shape-controlled synthesis of manganese oxide nanoplates by a polyol-based precursor route. Mater. Lett. 2010, 64, 891–893. [Google Scholar] [CrossRef]
- dos Santos, T.V.; Pryston, D.B.A.; Assis, G.C.; Meneghetti, M.R.; Meneghetti, S.M.P. Tin, niobium and tin-niobium oxides obtained by the Pechini method using glycerol as a polyol: Synthesis, characterization and use as a catalyst in fructose conversion. Catal. Today 2021, 379, 62–69. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wu, X.; Ye, Q.; Xu, X.; Li, B.; Wang, F. Novel synthesis and shape-dependent catalytic performance of Cu–Mn oxides for CO oxidation. Appl. Surf. Sci. 2017, 403, 335–341. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Wey, M.-Y.; Chen, L.-I. Application of polyol process to prepare AC-supported nanocatalyst for VOC oxidation. Appl. Catal. A Gen. 2007, 325, 163–174. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Yang, Y.; Si, W.; Peng, Y.; Chen, J.; Wang, Y.; Chen, D.; Tian, Z.; Wang, J.; Li, J. Oxygen vacancy engineering on copper-manganese spinel surface for enhancing toluene catalytic combustion: A comparative study of acid treatment and alkali treatment. Appl. Catal. B Environ. 2024, 340, 123142. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Wei, L.; Shi, Z.; Han, L.; Yuan, H.; Li, B.; Dong, L.; Wang, F.; Sun, C. Preparation and Evaluation of Copper–Manganese Oxide as a High-Efficiency Catalyst for CO Oxidation and NO Reduction by CO. J. Phys. Chem. C 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, Y.; Peng, X.; Pan, W. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene. Chem. Eng. J. 2019, 369, 758–765. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Wang, X.; Zhang, X.; Niu, X.; Zhu, Y. Boosting catalytic toluene combustion over Mn doped Co3O4 spinel catalysts: Improved mobility of surface oxygen due to formation of Mn-O-Co bonds. Appl. Surf. Sci. 2022, 590, 153140. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, Z.X.; Liu, R.F.; Chen, J.L.; Xu, B.Q. Rod-like CuMnOx transformed from mixed oxide particles by alkaline hydrothermal treatment as a novel catalyst for catalytic combustion of toluene. Phys. Chem. Chem. Phys. 2016, 18, 22794–22798. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, W.B.; Liu, R.F. Highly efficient copper-doped manganese oxide nanorod catalysts derived from CuMnO hierarchical nanowire for catalytic combustion of VOCs. Catal. Today 2018, 314, 147–153. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Whittle, D.M.; Mirzaei, A.A.; Hargreaves, J.S.J.; Joyner, R.W.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Co-precipitated copper zinc oxide catalysts for ambient temperature carbon monoxide oxidation: Effect of precipitate ageing on catalyst activity. Phys. Chem. Chem. Phys. 2002, 4, 5915–5920. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Han, Y.; Lu, C.; Wan, H.; Xu, Z.; Zheng, S. Enhanced catalytic toluene oxidation by interaction between copper oxide and manganese oxide in Cu-O-Mn/γ-Al2O3 catalysts. Appl. Surf. Sci. 2017, 420, 260–266. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.-K.; Nah, J.W. Property of a highly active bimetallic catalyst based on a supported manganese oxide for the complete oxidation of toluene. Powder Technol. 2014, 266, 292–298. [Google Scholar] [CrossRef]
- Matějová, L.; Topka, P.; Jirátová, K.; Šolcová, O. Total oxidation of model volatile organic compounds over some commercial catalysts. Appl. Catal. A Gen. 2012, 443, 40–49. [Google Scholar] [CrossRef]

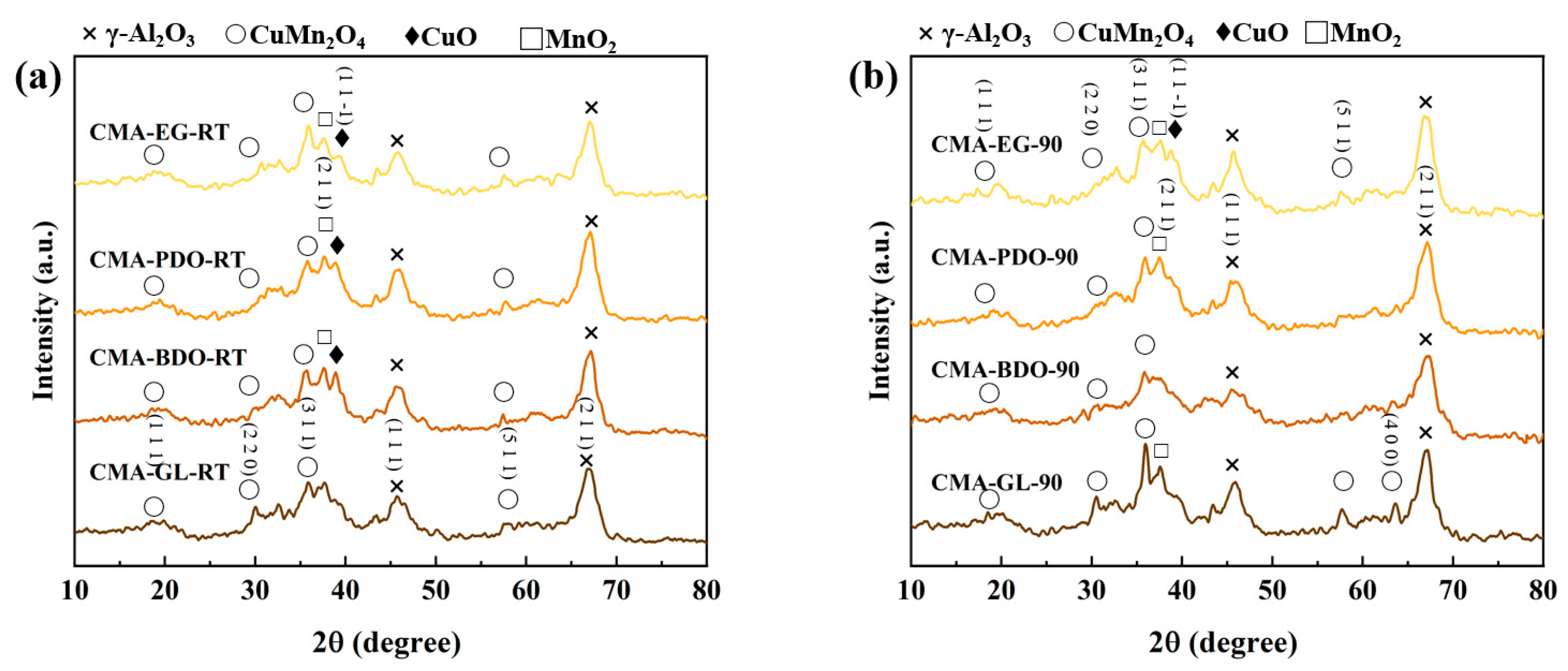

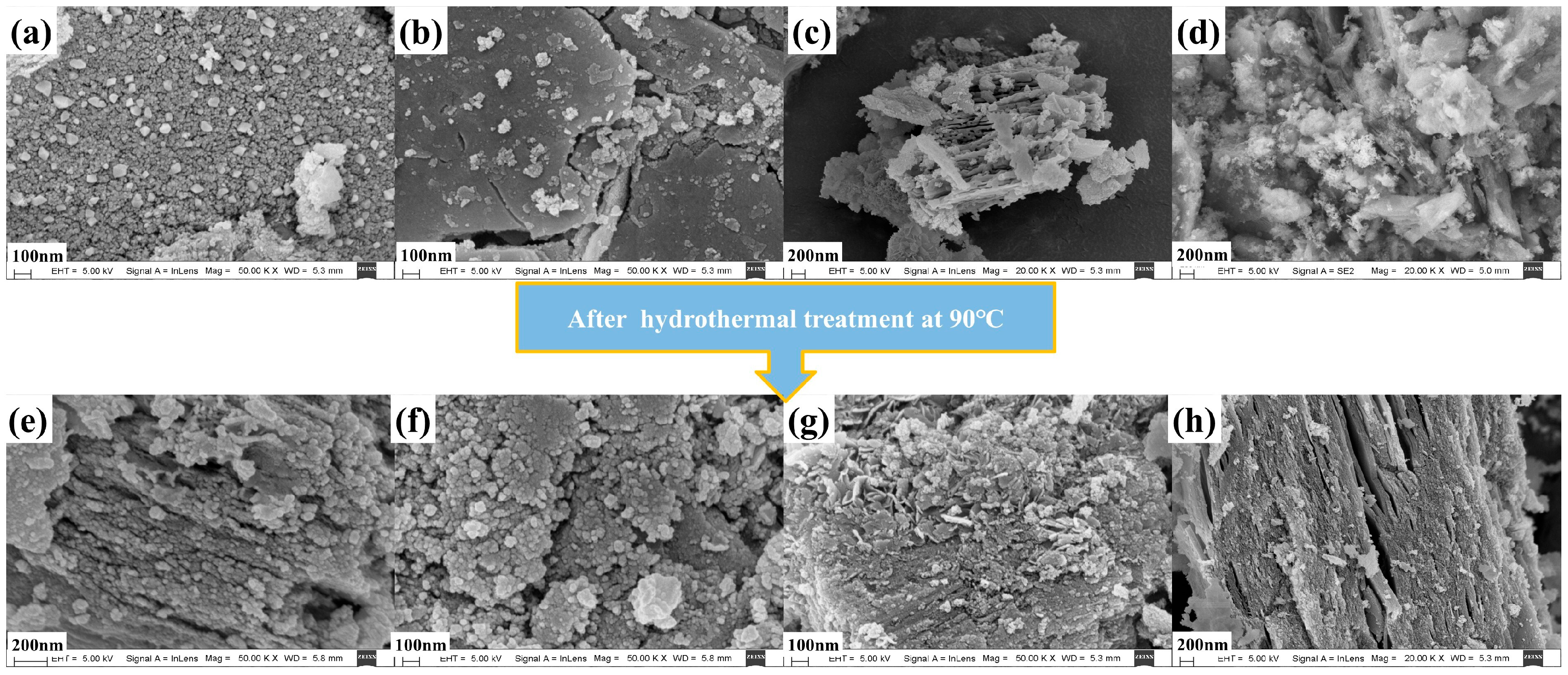
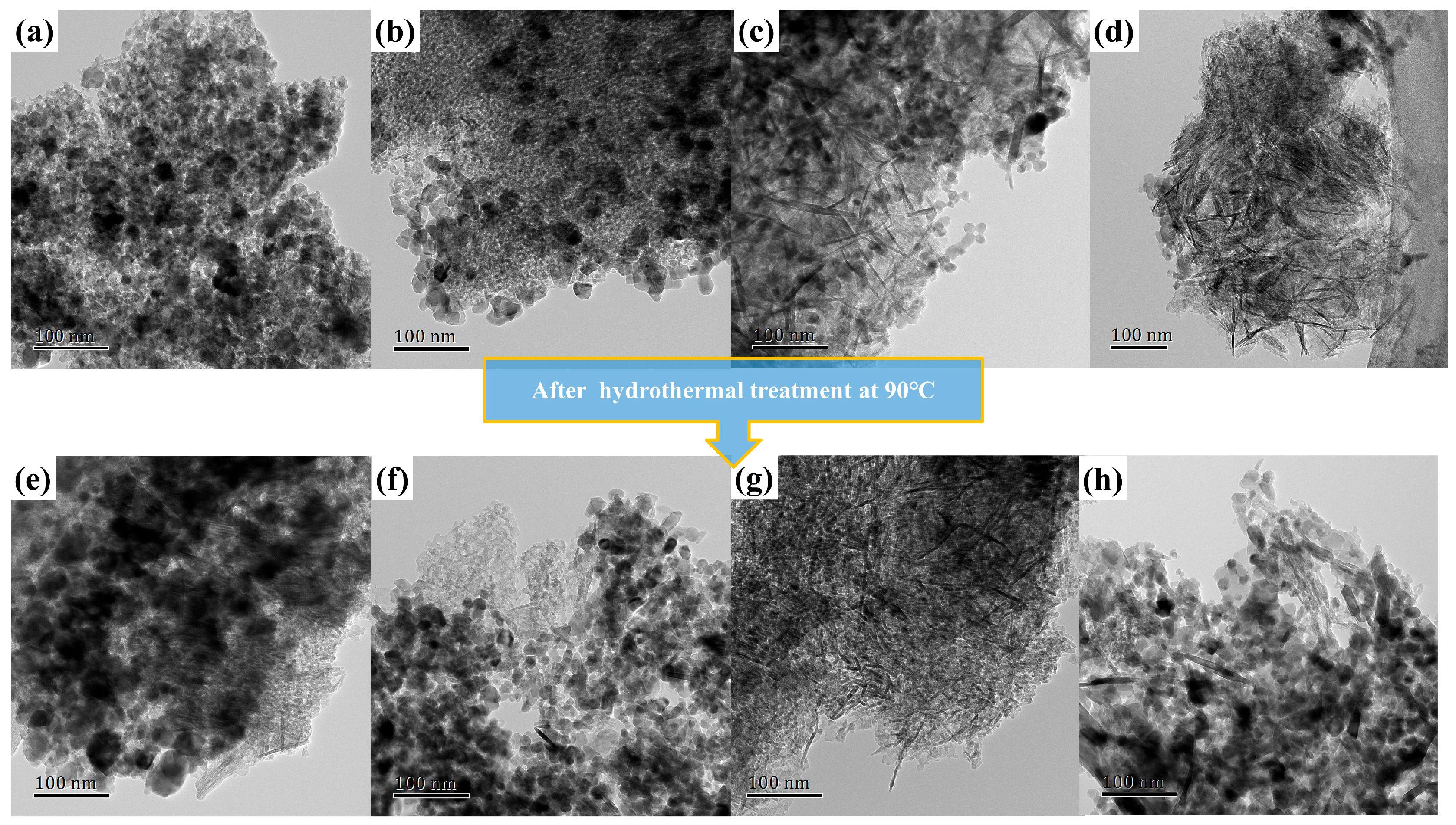
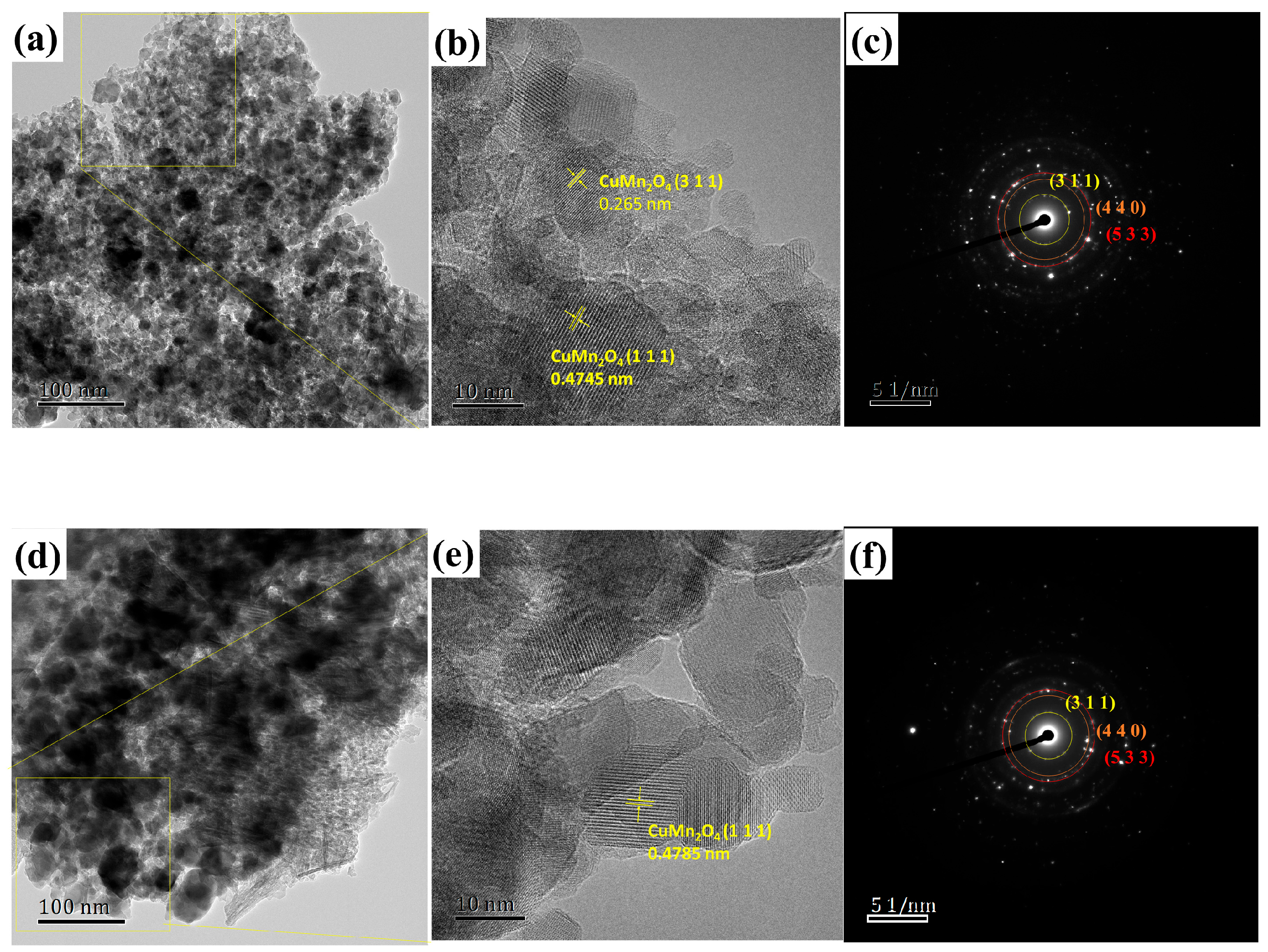




| Catalysts | S aBET (m2·g−1) | V bBJH (mL·g−1) | rave (nm) | Al at% c | O at% c | Mn at % c | Cu at % c |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 257.08 | 0.44 | 15.40 | - | - | - | - |
| CMA-GL-RT | 94.37 | 0.28 | 2.67 | 38.78 | 57.87 | 1.52 | 1.82 |
| CMA-GL-90 | 95.99 | 0.31 | 2.63 | 38.43 | 57.91 | 1.72 | 1.94 |
| CMA-BDO-RT | 106.11 | 0.31 | 2.91 | 37.26 | 58.64 | 2.51 | 1.58 |
| CMA-BDO-90 | 123.03 | 0.37 | 2.78 | 39.19 | 57.76 | 1.51 | 1.53 |
| CMA-PDO-RT | 106.04 | 0.31 | 2.87 | 38.07 | 59.05 | 1.47 | 1.41 |
| CMA-PDO-90 | 108.25 | 0.30 | 2.96 | 37.59 | 58.94 | 1.75 | 1.71 |
| CMA-EG-RT | 106.71 | 0.28 | 2.72 | 37.55 | 59.46 | 1.58 | 1.41 |
| CMA-EG-90 | 107.81 | 0.31 | 2.86 | 38.28 | 58.33 | 1.89 | 1.50 |
| Catalyst | Surface Mn/Cu | O1s | Mn2p | Cu2p | ||
|---|---|---|---|---|---|---|
| Oads/Olatt | Mn2+/% | Mn3+/% | Mn4+/% | Cu2+/Cu+ | ||
| CMA-GL-RT | 0.83 | 0.67 | 80.41 | 5.18 | 14.41 | 5.67 |
| CMA-GL-90 | 0.88 | 2.85 | 37.12 | 51.03 | 11.85 | 0.96 |
| CMA-BDO-RT | 1.58 | - | 54.38 | 15.74 | 29.89 | 5.67 |
| CMA-BDO-90 | 0.98 | 0.49 | 51.91 | 20.02 | 28.07 | 0.27 |
| CMA-PDO-RT | 1.04 | - | 63.21 | 18.85 | 17.94 | 2.33 |
| CMA-PDO-90 | 1.02 | 1.70 | 12.16 | 60.55 | 27.29 | 0.28 |
| CMA-EG-RT | 1.12 | 0.64 | 72.54 | 22.57 | 4.89 | 0.82 |
| CMA-EG-90 | 1.27 | 1.08 | 49.72 | 28.15 | 22.12 | 0.56 |
| Name | Polyols | Hydrothermal Reaction | Molecular Structure |
|---|---|---|---|
| CMA-GL-90 | Glycerol (GL) | + |  |
| CMA-GL-RT | Glycerol (GL) | − | |
| CMA-BDO-90 | 1,4-butylene glycol (BDO) | + |  |
| CMA-BDO-RT | 1,4-butylene glycol (BDO) | − | |
| CMA-PDO-90 | 1,3-propanediol (PDO) | + |  |
| CMA-PDO-RT | 1,3-propanediol (PDO) | − | |
| CMA-EG-90 | Ethylene glycol (EG) | + |  |
| CMA-EG-RT | Ethylene glycol (EG) | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, W.; Xu, C.; Hou, X.; Hu, X. Structural Effect of Cu-Mn/Al2O3 Catalysts on Enhancing Toluene Combustion Performance: Molecular Structure of Polyols and Hydrothermal Treatment. Catalysts 2024, 14, 443. https://doi.org/10.3390/catal14070443
Li J, Chen W, Xu C, Hou X, Hu X. Structural Effect of Cu-Mn/Al2O3 Catalysts on Enhancing Toluene Combustion Performance: Molecular Structure of Polyols and Hydrothermal Treatment. Catalysts. 2024; 14(7):443. https://doi.org/10.3390/catal14070443
Chicago/Turabian StyleLi, Junjie, Wenjing Chen, Chenghua Xu, Xiaoxiao Hou, and Xiaodong Hu. 2024. "Structural Effect of Cu-Mn/Al2O3 Catalysts on Enhancing Toluene Combustion Performance: Molecular Structure of Polyols and Hydrothermal Treatment" Catalysts 14, no. 7: 443. https://doi.org/10.3390/catal14070443
APA StyleLi, J., Chen, W., Xu, C., Hou, X., & Hu, X. (2024). Structural Effect of Cu-Mn/Al2O3 Catalysts on Enhancing Toluene Combustion Performance: Molecular Structure of Polyols and Hydrothermal Treatment. Catalysts, 14(7), 443. https://doi.org/10.3390/catal14070443








