Synthesis of Propiolic and Butynedioic Acids via Carboxylation of CaC2 by CO2 under Mild Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Butynedioic Acid
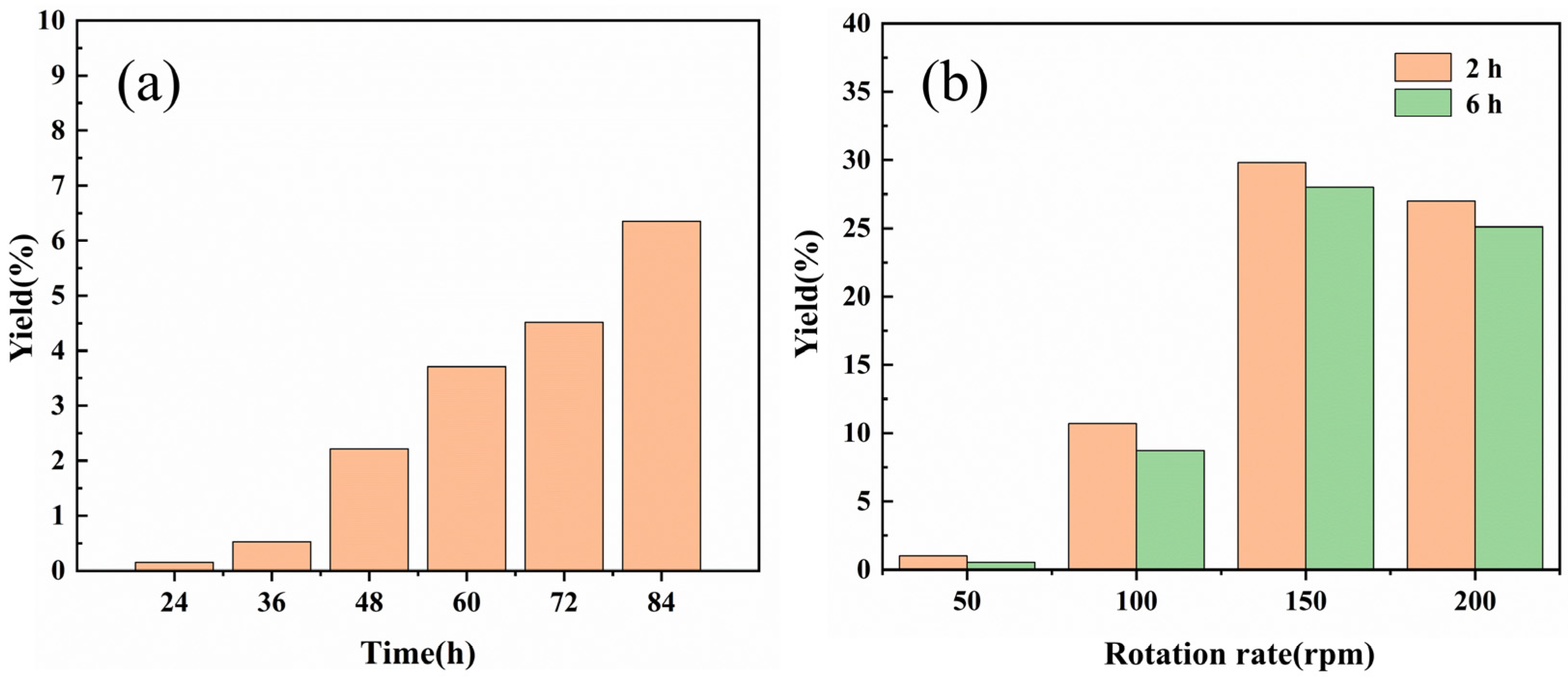
2.1.1. Thermal Reaction Yield versus Time
2.1.2. Ultrasonic Intensification of the Reaction
2.1.3. Mechanochemical Intensification of the Reaction
2.2. Preparation of Propiolicic Acid
2.2.1. The Reaction Intensification by Ultrasound and Mechanochemistry
2.2.2. The Reaction Yield at Different Mechanochemical Conditions
2.2.3. Influence of Hydrogen Donor and Solvents on the Reaction
3. Materials and Methods
3.1. Materials and Equipment
3.2. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, K.; Razzaq, R.; Hu, Y.; Ding, K. Homogeneous Reduction of Carbon Dioxide with Hydrogen. Top. Curr. Chem. 2017, 375, 23. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent Advances in CO2 Hydrogenation to Value-Added Products-Current Challenges and Future Directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the Art and Perspectives in Heterogeneous Catalysis of CO2 Hydrogenation to Methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Hu, X.-M.; Daasbjerg, K. Molecular Catalyst Converts Carbon Dioxide to Methanol. Nature 2019, 575, 598–599. [Google Scholar] [CrossRef]
- Chang, X.; Hondo, E.; Hu, Q.; Yu, W.; Ali, K.M.; Nyako, A.; Xing, C.; Yang, Z.; Lu, P. Effect of Acidity and Oxygen Vacancy in Mn Loaded SAPO-34 on CO2 Hydrogenation to Light Olefin. Fuel 2023, 353, 129160. [Google Scholar] [CrossRef]
- Bhat, G.A.; Darensbourg, D.J. Coordination Complexes as Catalysts for the Coupling Reactions of Oxiranes and Carbon Dioxide. Coord. Chem. Rev. 2023, 492, 215277. [Google Scholar] [CrossRef]
- Poland, S.J.; Darensbourg, D.J. A Quest for Polycarbonates Provided via Sustainable Epoxide/CO2 Copolymerization Processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y. The Direct Carboxylation of Terminal Alkynes with Carbon Dioxide. Green Chem. 2011, 13, 1275–1279. [Google Scholar] [CrossRef]
- Qiao, C.; Cao, Y.; He, L.N. Transition Metal-catalyzed Carboxylation of Terminal Alkynes with CO2. Mini-Rev. Org. Chem. 2018, 15, 283–290. [Google Scholar] [CrossRef]
- Arndt, M.; Risto, E.; Krause, T.; Gooßen, L.J. C-H Carboxylation of Terminal Alkynes Catalyzed by Low Loadings of Silver(i)/DMSO at Ambient CO2 Pressure. ChemCatChem 2012, 4, 484–487. [Google Scholar] [CrossRef]
- Gaillard, S.; Slawin, A.M.Z.; Nolan, S.P. A N-Heterocyclic Carbene Gold Hydroxide Complex: A Golden Synthon. Chem. Commun. 2010, 46, 2742–2744. [Google Scholar] [CrossRef]
- Manjolinho, F.; Arndt, M.; Gooßen, K.; Gooßen, L.J. Catalytic C-H Carboxylation of Terminal Alkynes with Carbon Dioxide. ACS Catal. 2012, 2, 2014–2021. [Google Scholar] [CrossRef]
- Saito, S.; Nakagawa, S.; Koizumi, T.; Hirayama, K.; Yamamoto, Y. Nickel-Mediated Regio- and Chemoselective Carboxylation of Alkynes in the Presence of Carbon Dioxide. J. Org. Chem. 1999, 64, 3975–3978. [Google Scholar] [CrossRef]
- Münch, A.S.; Katzsch, F.; Weber, E.; Mertens, F.O.R.L. Synthesis, Spectroscopic Characterization and Structural Investigation of a New Symmetrically Trisubstituted Benzene Derivative: 3,3′,3′′-(Benzene-1,3,5-Triyl)Tripropiolic Acid. J. Mol. Struct. 2013, 1043, 103–108. [Google Scholar] [CrossRef]
- Chen, J.-H.; Deng, C.-H.; Fang, S.; Ma, J.-G.; Cheng, P. Binuclear Molybdenum Alkoxide as the Versatile Catalyst for the Conversion of Carbon Dioxide. Green Chem. 2018, 20, 989–996. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, B.; Yao, Y.; Lu, C. Carboxylation of Terminal Alkynes with CO2 Catalyzed by Bis(Amidate) Rare-Earth Metal Amides. Green Chem. 2015, 17, 1675–1682. [Google Scholar] [CrossRef]
- Shi, J.-B.; Bu, Q.; Liu, B.-Y.; Dai, B.; Liu, N. Organocatalytic Strategy for the Fixation of CO2 via Carboxylation of Terminal Alkynes. J. Org. Chem. 2021, 86, 1850–1860. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Werner, G.; Kucherov, F.A.; Ananikov, V.P. Calcium Carbide: A Unique Reagent for Organic Synthesis and Nanotechnology. Chem. Asian J. 2016, 11, 965–976. [Google Scholar] [CrossRef]
- Shao, H.; Lu, Y.; Liang, X.; Li, C. The Catalytic Mechanism of [Bmim]Cl-Transition Metal Catalysts for Hydrochlorination of Acetylene. Catalysts 2024, 14, 93. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, M.; Zhang, H.; Yu, F.; Wang, C.; Dai, B. Activated Carbon Supported Mo-Ti-N Binary Transition Metal Nitride as Catalyst for Acetylene Hydrochlorination. Catalysts 2017, 7, 200. [Google Scholar] [CrossRef]
- Xu, Z.; He, P.; Chen, Y.; Zhu, M.; Wang, X.; Dai, B. Performance Study of Zn-Co-Ni/AC Catalyst in Acetylene Acetylation. Catalysts 2021, 11, 1271. [Google Scholar] [CrossRef]
- He, P.; Huang, L.; Wu, X.; Xu, Z.; Zhu, M.; Wang, X.; Dai, B. A Novel High-Activity Zn-Co Catalyst for Acetylene Acetoxylation. Catalysts 2018, 8, 239. [Google Scholar] [CrossRef]
- Schobert, H. Production of Acetylene and Acetylene-Based Chemicals from Coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Bolm, C.; Hernández, J.G. Mechanosynthesis of Odd-Numbered Tetraaryl[n] Cumulenes. Angew. Chem. Int. Ed. 2019, 58, 12945–12949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, H.; Liu, Z.; Zhong, P.; Zhang, L.; Huang, X.; Cheng, J. The Use of Calcium Carbide in One-Pot Synthesis of Symmetric Diaryl Ethynes. Chem. Commun. 2006, 4826–4828. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Lu, Q.; Wang, Y.; Wang, Y.; Yang, B. Degradation of Organic Wastewater by Hydrodynamic Cavitation Combined with Acoustic Cavitation. Ultrason. Sonochem. 2018, 43, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Hamdaoui, O.; Haddad, B. Acoustic Cavitation in 1-Butyl-3-Methylimidazolium Bis(Triflluoromethyl-Sulfonyl)Imide Based Ionic Liquid. Ultrason. Sonochem. 2018, 41, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Schreiner, P.R. Direct Exploitation of the Ethynyl Moiety in Calcium Carbide through Sealed Ball Milling. Eur. J. Org. Chem. 2020, 2020, 4339–4346. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Bo, Q.; Xu, S.; Gu, J.; He, X.; Li, C. Green Synthesis of Oxygenic Graphyne with High Electrochemical Performance from Efficient Mechanochemical Degradation of Hazardous Decabromodiphenyl Ether. Green Chem. 2024, 26, 6190–6199. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Xu, X.; Meng, H.; Lu, Y.; Li, C. Mechanochemical Synthesis of Oxygenated Alkynyl Carbon Materials with Excellent Hg(II) Adsorption Performance from CaC2 and Carbonates. Green Energy Environ. 2023, 8, 275–282. [Google Scholar] [CrossRef]
- Xia, L.; Lu, Y.; Meng, H.; Li, C. Preparation of C-MOx Nanocomposite for Efficient Adsorption of Heavy Metal Ions via Mechanochemical Reaction of CaC2 and Transition Metal Oxides. J. Hazard Mater. 2020, 393, 122487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Xu, X.; Gu, J.; He, X.; Meng, H.; Lu, Y.; Li, C. Converting CO2 into an Oxygenated Alkynyl Carbon Material with High Electrochemical Performance through a Mechanochemical Reaction with CaC2. ACS Sustain. Chem. Eng. 2021, 9, 9221–9229. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, H.; Zang, X.; Meng, H.; Fan, H.; Lu, Y.; Li, C. Experimental and Molecular Insights on the Regulatory Effects of Solvent on CaC2 Reaction Activity. AIChE J. 2024, e18511. [Google Scholar] [CrossRef]
| Serial Number | Catalyst AgNO3/g | Complexing Agent PPh3/g | Additive Na2CO3/g | Mole Ratio (CaC2:Na2CO3) | Reaction Time/h | Yield/% |
|---|---|---|---|---|---|---|
| 1 | 0.100 | 0.154 | 1.24 | 1:1 | 6 | 1.0 |
| 2 | 0.100 | 0.154 | 1.24 | 1:1 | 8 | 4.7 |
| 3 | 0.100 | 0.154 | 1.24 | 1:1 | 12 | 13.3 |
| 4 | 0.100 | 0.154 | — | — | 12 | 2.1 |
| 5 | 0.100 | — | 1.24 | 1:1 | 12 | 1.9 |
| 6 | 0.100 | — | — | — | 12 | 1.6 |
| Serial Number | Catalyst AgNO3/g | Complexing Agent PPh3/g | Additive Na2CO3/g | Rotation Rate/rpm | Reaction Time/h | Yield/% |
|---|---|---|---|---|---|---|
| 1 | 0.030 | 0.046 | 0.37 | 50 | 6 | 0.5 |
| 2 | 0.030 | 0.046 | 0.37 | 100 | 6 | 8.7 |
| 3 | 0.030 | 0.046 | 0.37 | 150 | 6 | 28.0 |
| 4 | 0.030 | 0.046 | 0.37 | 200 | 6 | 25.1 |
| 5 | 0.030 | 0.046 | 0.37 | 100 | 2 | 10.7 |
| 6 | 0.030 | 0.046 | 0.37 | 150 | 2 | 29.8 |
| 7 | 0.030 | 0.046 | 0.37 | 200 | 2 | 27.0 |
| 8 | 0.030 | 0.046 | — | 200 | 6 | 14.7 |
| 9 | 0.030 | — | 0.37 | 200 | 6 | 11.8 |
| 10 | 0.030 | — | — | 200 | 6 | 6.8 |
| Serial No. | Reaction Condition | Time/h | Propiolic Acid Yield/% |
|---|---|---|---|
| (1) | Thermal reaction, 333 K, 1 MPa CO2, magnetic stir | 24 | 40.2 |
| (2) | Ultrasonic reaction at 298 K, 1 MPa CO2 | 6 | 31.5 |
| (3) | Ball mill at 298 K, 150 rpm, 0.15 MPa CO2, charged every 2 h | 6 | 74.2 |
| Serial Number | Catalyst CuI/g | Phenolic Resin/g | Na2CO3/g | Milling Rate/rpm | Reaction Time/h | Yield/% |
|---|---|---|---|---|---|---|
| 1 | 0.030 | 0.37 | 0.37 | 100 | 2 | 58.5 |
| 2 | 0.030 | 0.37 | 0.37 | 150 | 2 | 74.8 |
| 3 | 0.030 | 0.37 | 0.37 | 200 | 2 | 72.9 |
| 4 | 0.030 | 0.37 | 0.37 | 100 | 6 | 57.7 |
| 5 | 0.030 | 0.37 | 0.37 | 150 | 6 | 74.2 |
| 6 | 0.030 | 0.37 | — | 150 | 2 | 43.5 |
| Solvent | H-Donor | pKa | Proton Donor | Reaction Time/h | Yield/% |
|---|---|---|---|---|---|
| Acetonitrile | Phenolic resin | — | -OH | 2 | 74.8 |
| 6 | 72.9 | ||||
| 2,4-Dimethylphenol | 10.3 | -OH | 4 | 29.8 | |
| 8 | 44.7 | ||||
| Phenol | 10 | -OH | 8 | 41.1 | |
| Imidazole | 14.4 | NH | 8 | 19.8 | |
| NaHCO3 | 10.4 | HCO3− | 2 | 19.7 | |
| 4 | 22.6 | ||||
| 6 | 24.3 | ||||
| 8 | 22 | ||||
| NH4HCO3 | 9.4 | NH4+ | 2 | 15.2 | |
| 4 | 23.9 | ||||
| Acetylene | 25~26 | C≡CH | 2 | 10.8 | |
| 4 | 12.4 | ||||
| t-Butanol | 18~19 | -OH | 4 | 45.9 | |
| 8 | 63.1 | ||||
| Acetone | 19~20 | -CH3 | 8 | 25.7 | |
| CH3CN+FeCl3 | <25 | -CH3 | 8 | 18.4 | |
| Chloroform | Acetylene | 25~26 | C≡CH | 8 | 0.21 |
| t-Butanol | 18~19 | -OH | 8 | 1.05 | |
| [BMIM]Cl | t-Butanol | 18~19 | -OH | 8 | 39.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.-M.; Zang, X.; Lu, Y.; Meng, H.; Li, C. Synthesis of Propiolic and Butynedioic Acids via Carboxylation of CaC2 by CO2 under Mild Conditions. Catalysts 2024, 14, 467. https://doi.org/10.3390/catal14070467
Zhao X-M, Zang X, Lu Y, Meng H, Li C. Synthesis of Propiolic and Butynedioic Acids via Carboxylation of CaC2 by CO2 under Mild Conditions. Catalysts. 2024; 14(7):467. https://doi.org/10.3390/catal14070467
Chicago/Turabian StyleZhao, Xiao-Min, Xiaoteng Zang, Yingzhou Lu, Hong Meng, and Chunxi Li. 2024. "Synthesis of Propiolic and Butynedioic Acids via Carboxylation of CaC2 by CO2 under Mild Conditions" Catalysts 14, no. 7: 467. https://doi.org/10.3390/catal14070467
APA StyleZhao, X.-M., Zang, X., Lu, Y., Meng, H., & Li, C. (2024). Synthesis of Propiolic and Butynedioic Acids via Carboxylation of CaC2 by CO2 under Mild Conditions. Catalysts, 14(7), 467. https://doi.org/10.3390/catal14070467









