Efficient Methylene Blue Degradation by Activation of Peroxymonosulfate over Co(II) and/or Fe(II) Impregnated Montmorillonites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Samples
2.2. Degradation Performance of MB by Montmorillonites/PMS
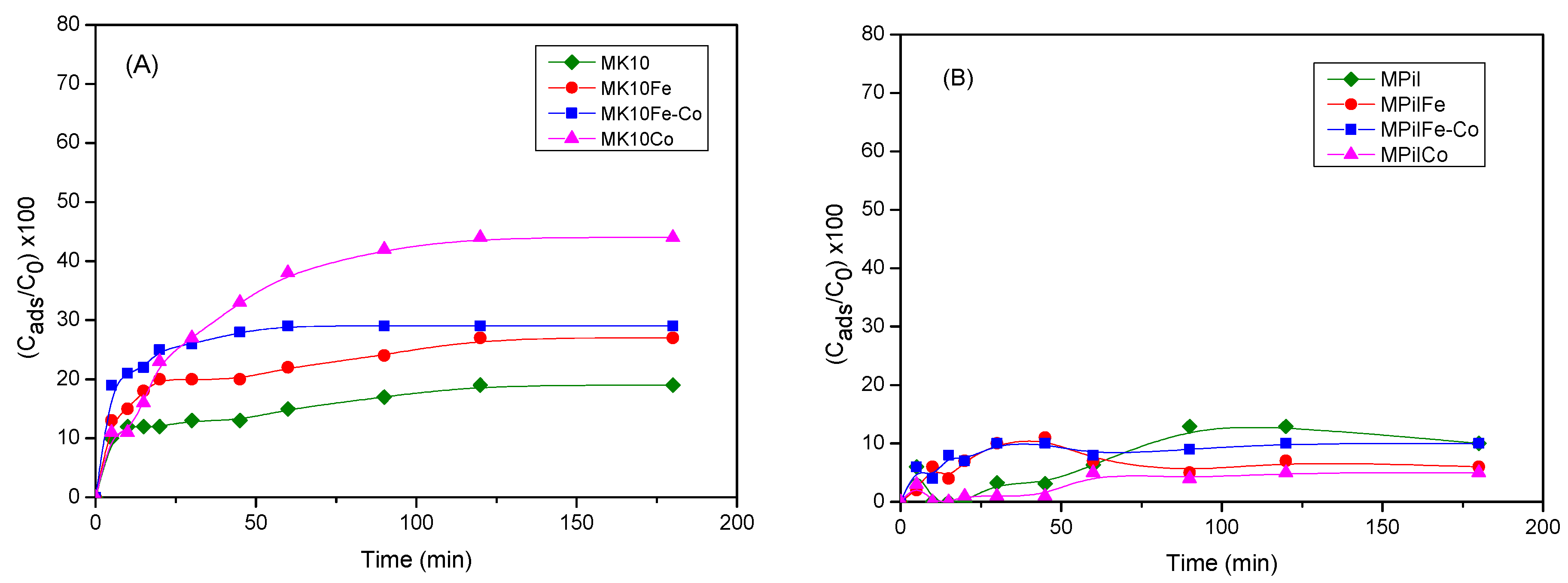
2.2.1. Adsorption of MB on Montmorillonites
2.2.2. Influence of Catalyst Dosage and Dye Concentration
2.2.3. Dominant Reactive Species
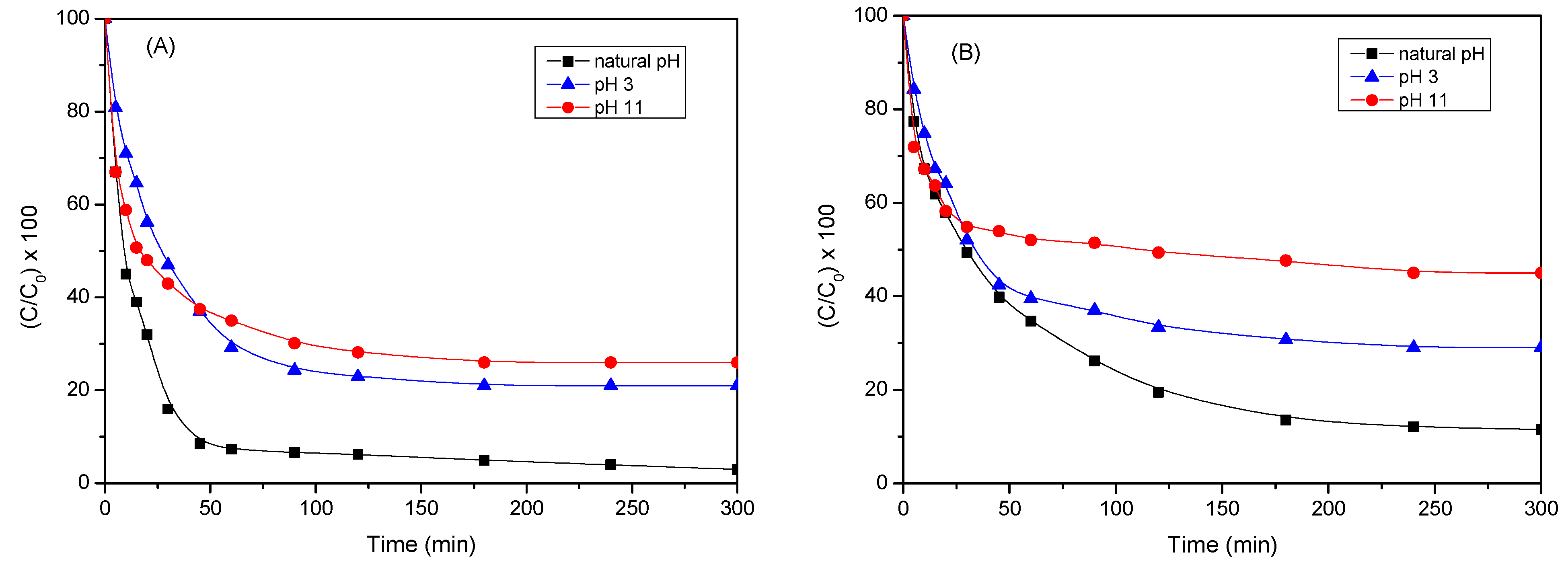
2.2.4. Influence of pH of Reaction
2.2.5. Leaching of Metals and Recyclability of Catalysts
3. Materials and Methods
3.1. Reactants and Materials
3.2. Preparation of the Fe-Co Impregnated Montmorillonites
3.3. Characterization of Samples
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poyatos, J.M.; Munio, M.M.; Almecija, M.C.; Torres, J.C.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Durairaj, A.; Sakthivel, T.; Obadiah, A.; Vasanthkumar, S. Enhanced photocatalytic activity of transition metal ions doped g–C3N4 nanosheet activated by PMS for organic pollutant degradation. J. Mater. Sci. Mater. Electron. 2018, 29, 8201–8209. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef]
- Verma, S.; Nakamura, S.; Sillanpää, M. Application of UV-C LED activated PMS for the degradation of anatoxin-a. Chem. Eng. J. 2016, 284, 122–129. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, F.; Liu, C.; Chen, N.; Zhou, D.; Fang, G.; Gao, J. Reductive Hexachloroethane Degradation by S2O8•– with Thermal Activation of Persulfate under Anaerobic Conditions. Environ. Sci. Technol. 2018, 52, 8548–8557. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, S.; Wang, J.; Zhao, X.; Zhang, L.; Tang, A.; Dong, X.; Fu, L.; Yang, H. Efficient activation of peroxymonosulfate by iron-containing mesoporous silica catalysts derived from iron tailings for degradation of organic pollutants. Chem. Eng. J. 2022, 446, 137044. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res. 2009, 43, 2513–2521. [Google Scholar] [CrossRef]
- Ben Hammouda, S.; Zhao, F.; Safaei, Z.; Ramasamy, D.L.; Doshi, B.; Sillanpaa, M. Sulfate radical-mediated degradation and mineralization of bisphenol F in neutral medium by the novel magnetic Sr2CoFeO6 double perovskite oxide catalyzed peroxymonosulfate: Influence of co-existing chemicals and UV irradiation. Appl. Catal. B Environ. 2018, 233, 99–111. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, Y.-C.; Lin, T.-Y.; Yang, H. Lanthanum cobaltite perovskite supported on zirconia as an efficient heterogeneous catalyst for activating Oxone in water. J. Colloid Interface Sci. 2017, 497, 325–332. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Chen, C.; Lu, L.; Li, B.; Hu, W.; Kumar, A.; Muddassir, M. Two new uncommon 3D cobalt-based metal organic frameworks: Temperature induced syntheses and enhanced photocatalytic properties against aromatic dyes. Dye. Pigment. 2021, 187, 109068. [Google Scholar] [CrossRef]
- Sarkar, A.; Mushahary, N.; Basumatary, F.; Das, B.; Basumatary, S.F.; Venkatesan, K.; Selvaraj, M.; Rokhum, S.L.; Basumatary, S. Efficiency of montmorillonite-based materials as adsorbents in dye removal for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112519. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Dong, X.; Sun, Z.; Duan, X.; Ren, B.; Zheng, S.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Wu, J.; Cagnetta, G.; Wang, B.; Cui, Y.; Deng, S.; Wang, Y.; Huang, J.; Yu, G. Efficient degradation of carbamazepine by organo-montmorillonite supported nCoFe2O4-activated peroxymonosulfate process. Chem. Eng. J. 2019, 368, 824–836. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.; Cheng, H. Rational design of kaolinite-based photocatalytic materials for environment decontamination. Appl. Clay Sci. 2021, 208, 106098. [Google Scholar] [CrossRef]
- Huang, W.J.; Liu, J.H.; She, Q.M.; Zhong, J.Q.; Christidis, G.E.; Zhou, C.H. Recent advances in engineering montmorillonite into catalysts and related catalysis. Catal. Rev. 2023, 65, 929–985. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Gupta, V.; Ali, I.; Mohan, D. Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents. J. Colloid Interface Sci. 2003, 265, 257–264. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants Ten Years after the Stockholm Convention–Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Joseph, A.; Vellayan, K.; González, B.; Vicente, M.A.; Gil, A. Effective degradation of methylene blue in aqueous solution using Pd-supported Cu-doped Ti-pillared montmorillonite catalyst. Appl. Clay Sci. 2019, 168, 7–10. [Google Scholar] [CrossRef]
- Chu, Y.; Tan, X.; Shen, Z.; Liu, P.; Han, N.; Kang, J.; Duan, X.; Wang, S.; Liu, L.; Liu, S. Efficient removal of organic and bacterial pollutants by Ag-La0.8Ca0.2Fe0.94O3-δ perovskite via catalytic peroxymonosulfate activation. J. Hazard. Mater. 2018, 356, 53–60. [Google Scholar] [CrossRef]
- Su, C.; Duan, X.; Miao, J.; Zhong, Y.; Zhou, W.; Wang, S.; Shao, Z. Mixed Conducting Perovskite Materials as Superior Catalysts for Fast Aqueous-Phase Advanced Oxidation: A Mechanistic Study. ACS Catal. 2017, 7, 388–397. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, D.; Ma, C.; Wang, G.; Dong, X.; Zhang, X. Activation of peroxymonosulfate by CoFe2O4 loaded on metal-organic framework for the degradation of organic dye. Chemosphere 2020, 241, 125021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, S.; Du, J.; Tang, J.; Zhou, Q. Cu@Co-MOFs as a novel catalyst of peroxymonosulfate for the efficient removal of methylene blue. RSC Adv. 2019, 9, 9410–9420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ning, S.; Zhu, H.; Wang, X.; Yin, X.; Fujita, T.; Wei, Y. Novel NbCo-MOF as an advanced peroxymonosulfate catalyst for organic pollutants removal: Growth, performance and mechanism study. Chemosphere 2022, 288, 132600. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, R.; Khazaei, M.; Godini, K.; Azarian, G.; Latifi, Z.; Javadimanesh, L.; Zolghadr Nasab, H. Degradation and mineralization of methylene blue dye by peroxymonosulfate/Mn3O4 nanoparticles using central composite design: Kinetic study. Inorg. Chem. Commun. 2021, 127, 108501. [Google Scholar] [CrossRef]
- Dung, N.T.; Thu, T.V.; Van Nguyen, T.; Thuy, B.M.; Hatsukano, M.; Higashimine, K.; Maenosono, S.; Zhong, Z. Catalytic activation of peroxymonosulfate with manganese cobaltite nanoparticles for the degradation of organic dyes. RSC Adv. 2020, 10, 3775–3788. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Shang, W.; Wang, M.; Zhang, J.; Sun, F.; Li, M.; Li, X. Singlet oxygen dominated core-shell Co nanoparticle to synergistically degrade methylene blue through efficient activation of peroxymonosulfate. Sep. Purif. Technol. 2023, 308, 122849. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lua, S.-K.; Dong, Z.; Lim, T.-T. Performance of magnetic activated carbon composite as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes. J. Hazard. Mater. 2015, 284, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tadé, M.O.; Wang, S. Reduced Graphene Oxide for Catalytic Oxidation of Aqueous Organic Pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef]
- Milovanović, B.; Marinović, S.; Vuković, Z.; Milutinović-Nikolić, A.; Petrović, R.; Banković, P.; Mudrinić, T. The influence of cobalt loading on electrocatalytic performance toward glucose oxidation of pillared montmorillonite-supported cobalt. J. Electroanal. Chem. 2022, 915, 116332. [Google Scholar] [CrossRef]
- Vuković, Z.; Milutinović-Nikolić, A.; Krstić, J.; Abu-Rabi, A.; Novaković, T.; Jovanović, D. The influence of acid treatment on the nanostructure and textural properties of bentonite clays. Mater. Sci. Forum 2005, 494, 339–344. [Google Scholar] [CrossRef]
- Qin, D.; Niu, X.; Qiao, M.; Liu, G.; Li, H.; Meng, Z. Adsorption of ferrous ions onto montmorillonites. Appl. Surf. Sci. 2015, 333, 170–177. [Google Scholar] [CrossRef]
- Tong, D.S.; Wu, C.W.; Adebajo, M.O.; Jin, G.C.; Yu, W.H.; Ji, S.F.; Zhou, C.H. Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl. Clay Sci. 2018, 161, 256–264. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, Y.; Xue, J.; Gao, Y. Adsorption of chromium by functionalized metal organic frameworks from aqueous solution. Environ. Technol. 2021, 42, 1930–1942. [Google Scholar] [CrossRef]
- Mudrinić, T.; Mojović, Z.; Milutinović-Nikolić, A.; Banković, P.; Dojčinović, B.; Vukelić, N.; Jovanović, D. Beneficial effect of Ni in pillared bentonite based electrodes on the electrochemical oxidation of phenol. Electrochim. Acta 2014, 144, 92–99. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Qiu, W.; Wang, F.; Jiang, H.; Chen, M.; Zhang, W.; Ma, J. Catalytic degradation of micropollutant by peroxymonosulfate activation through Fe(III)/Fe(II) cycle confined in the nanoscale interlayer of Fe(III)-saturated montmorillonite. Water Res. 2020, 182, 116030. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Wu, G.; Wei, F.; Li, X.; Chen, H.; Wang, S. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3−xO4) for Fenton-Like reaction in water. J. Hazard. Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef]
- Pan, Y.; Su, H.; Zhu, Y.; Vafaei Molamahmood, H.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef]
- He, P.; Zhu, J.; Chen, Y.; Chen, F.; Zhu, J.; Liu, M.; Zhang, K.; Gan, M. Pyrite-activated persulfate for simultaneous 2,4-DCP oxidation and Cr(VI) reduction. Chem. Eng. J. 2021, 406, 126758. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Hu, X.; Feng, R.; Zhou, M.; Wang, L. Hollow Cu-Co/N-doped carbon spheres derived from ZIFs as an efficient catalyst for peroxymonosulfate activation. Chem. Eng. J. 2020, 397, 125533. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Mohamed, M.M.; Zidan, F.I.; Thabet, M.S. Photo-degradation of acid green dye over Co–ZSM-5 catalysts prepared by incipient wetness impregnation technique. J. Hazard. Mater. 2008, 153, 364–371. [Google Scholar] [CrossRef]


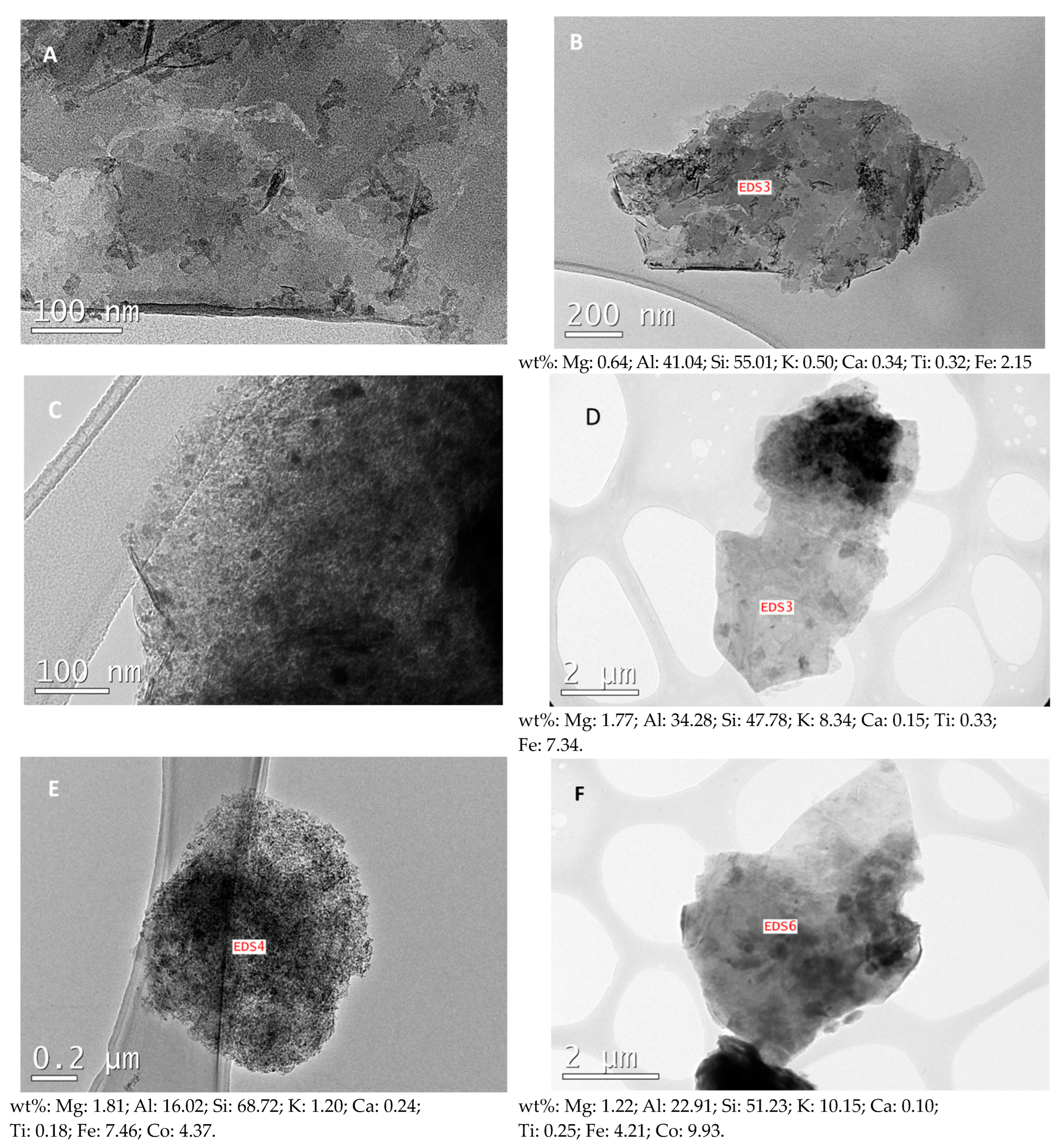

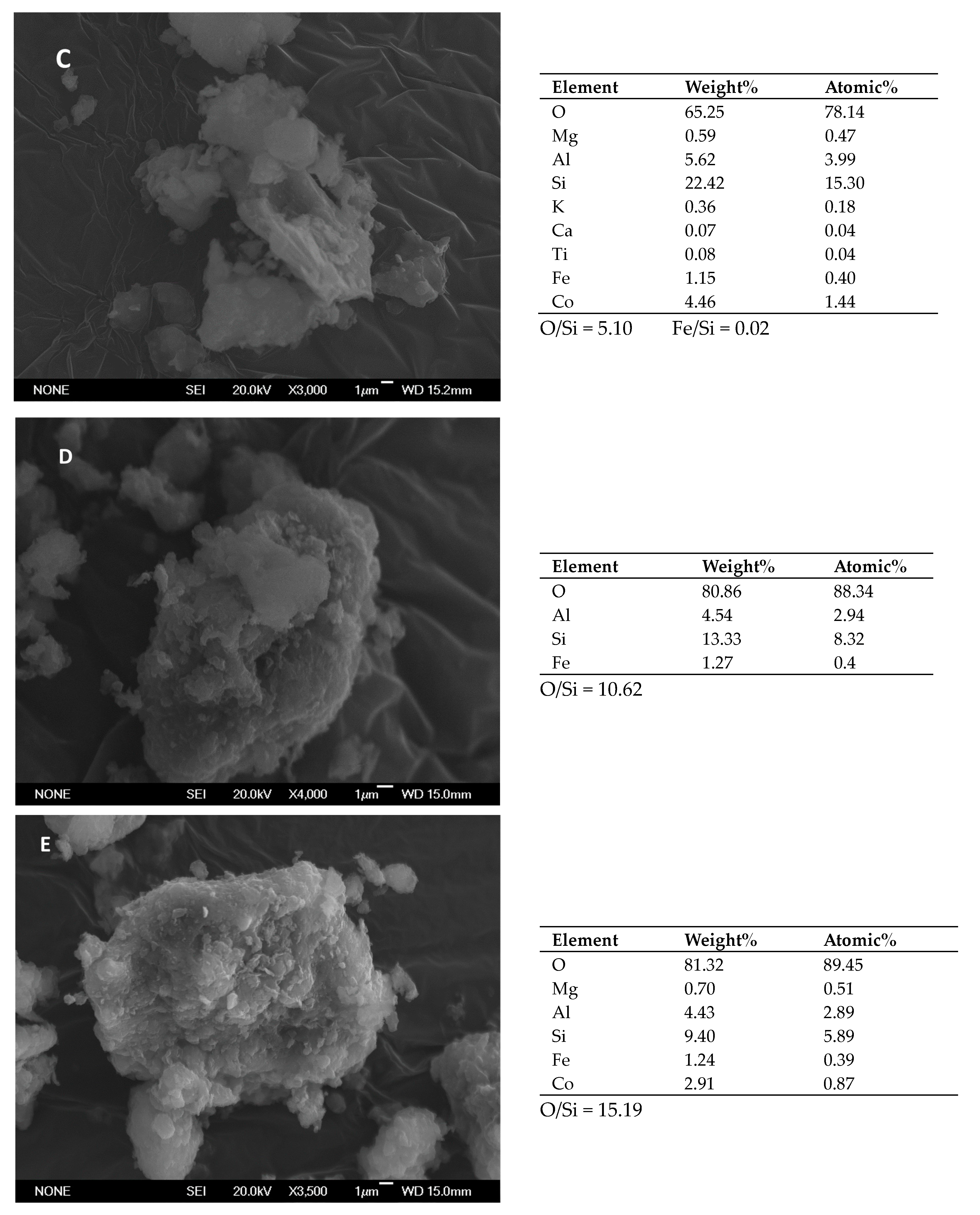






| Catalyst | Fe (wt% ± sd) | Fei (wt% ± sd) | Co (wt% ± sd) | (Fe + Co)i (wt% ± sd) |
|---|---|---|---|---|
| MK10 | 1.54 ± 0.04 | - | - | - |
| MK10Fe | 7.70 ± 0.10 | 6.16 ± 0.10 (7.0) | - | 6.16 ± 0.10 (7.0) |
| MK10Fe-Co | 4.32 ± 0.02 | 2.78 ± 0.04 (3.5) | 3.40 ± 0.10 (3.5) | 6.18 ± 0.10 (7.0) |
| MK10Co | 1.45 ± 0.02 | - | 6.90 ± 0.14 (7.0) | 6.90 ± 0.14 (7.0) |
| MPil | 0.77 ± 0.01 | - | - | - |
| MPilFe | 6.74 ± 0.13 | 5.97 ± 0.13 (7.0) | - | 5.97 ± 0.13 (7.0) |
| MPilFe-Co | 3.83 ± 0.11 | 3.06 ± 0.11 (3.5) | 3.45 ± 0.07 (3.5) | 6.51 ± 0.11 (7.0) |
| MPilCo | 0.75 ± 0.01 | - | 6.97 ± 0.02 (7.0) | 6.97 ± 0.02 (7.0) |
| Catalyst | SBET (m2/g) | Smic (m2/g) | Vp (cm3/g) | Vmes (cm3/g) | Vmic (cm3/g) | dmeso (nm) |
|---|---|---|---|---|---|---|
| MK10 | 231.7 | 14.2 | 0.360 | 0.340 | 0.006 | 6.5 |
| MK10Fe | 200.1 | - | 0.318 | 0.300 | - | 6.2 |
| MK10Fe-Co | 191.3 | - | 0.315 | 0.296 | - | 6.5 |
| MK10Co | 161.8 | - | 0.289 | 0.271 | - | 6.6 |
| MPil | 175.3 | 119.1 | 0.186 | 0.175 | 0.052 | 12.3 |
| MPilFe | 143.8 | 80.3 | 0.156 | 0.102 | 0.047 | 9.4 |
| MPilFe-Co | 141.9 | 85.0 | 0.170 | 0.107 | 0.037 | 12.1 |
| MPilCo | 164.2 | 99.7 | 0.168 | 0.100 | 0.044 | 10.3 |
| Catalyst | Ccat: 125 mg/L CMB: 0.078 mM | Ccat: 250 mg/L CMB: 0.078 mM | Ccat: 125 mg/L CMB: 0.052 mM | Ccat: 125 mg/L CMB: 0.156 mM |
|---|---|---|---|---|
| MK10 | 0.00632 | 0.01359 | - | - |
| MK10Fe | 0.01021 | 0.03631 | - | - |
| MK10Fe-Co | 0.05349 | 0.14652 | 0.09422 | 0.03417 |
| MK10Co | 0.17178 | - | 0.39670 | 0.03423 |
| MPil | 0.00232 | 0.00820 | - | - |
| MPilFe | 0.00420 | 0.00764 | - | - |
| MPilFe-Co | 0.01619 | 0.03095 | - | - |
| MPilCo | 0.04809 | 0.06588 | 0.10713 | 0.02824 |
| Reference | Catalyst | Catalyst/MB Ratio (mg/L/mg/L) | PMS/MB Molar Ratio | Removal Efficiency |
|---|---|---|---|---|
| [29] | Ag-La0.8Ca0.2Fe0.94O3−δ perovskite | 100 | 12.5 | 90% in 40 min |
| [30] | Mixed conducting perovskite | 5 | 38.3 | 100% in 15 min |
| [31] | CoFe2O4-MOF | 2.5 | 14.5 | 95% in 60 min |
| [32] | Cu@Co-MOF | 3 | 20 | 100% in 30 min |
| [33] | NbCo-MOF | 10 | 15 | 100% in 30 min |
| [34] | Mn3O4 nanoparticles | 20 | 160 | 86% in 20 min |
| [35] | MnCo2O4.5 nanoparticles | 1 | 26 | 100% in 25 min |
| [36] | Co nanoparticles | 2 | 10.5 | 100% in 10 min |
| [37] | Activated carbon composite | 10 | 104 | 100% in 60 min |
| [38] | CuFe2O4@GO | 10 | 13 | 93% in 30 min |
| [39] | Reduced Graphene oxide | 50 | 100 | 100% in 120 min |
| This work | MK10Co | 5 | 3 | 100% in 20 min |
| This work | MK10Fe-Co | 5 | 3 | 92% in 45 min |
| This work | MK10Co | 7.5 | 3 | 100% in 15 min |
| This work | MK10Fe-Co | 7.5 | 3 | 100% in 45 min |
| This work | MPilCo | 7.5 | 3 | 100% in 45 min |
| This work | MK10Fe-Co | 10 | 3 | 100% in 20 min |
| This work | MPilCo | 10 | 3 | 100% in 60 min |
| This work | MPilFe-Co | 10 | 3 | 85% in 60 min |
| Catalyst | Iron (mg/L) | Iron * (%) | Cobalt (mg/L) | Cobalt * (%) |
|---|---|---|---|---|
| MK10Fe-Co | 0.0093 | 1.74 | 0.573 | 13.48 |
| MPilCo | 0.0023 | 0.24 | 1.156 | 13.27 |
| MK10Co | 0.0032 | 0.2 | 1.154 | 17.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios-Bermúdez, N.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Efficient Methylene Blue Degradation by Activation of Peroxymonosulfate over Co(II) and/or Fe(II) Impregnated Montmorillonites. Catalysts 2024, 14, 479. https://doi.org/10.3390/catal14080479
Barrios-Bermúdez N, Cerpa-Naranjo A, Rojas-Cervantes ML. Efficient Methylene Blue Degradation by Activation of Peroxymonosulfate over Co(II) and/or Fe(II) Impregnated Montmorillonites. Catalysts. 2024; 14(8):479. https://doi.org/10.3390/catal14080479
Chicago/Turabian StyleBarrios-Bermúdez, Niurka, Arisbel Cerpa-Naranjo, and María Luisa Rojas-Cervantes. 2024. "Efficient Methylene Blue Degradation by Activation of Peroxymonosulfate over Co(II) and/or Fe(II) Impregnated Montmorillonites" Catalysts 14, no. 8: 479. https://doi.org/10.3390/catal14080479







