Advanced PtCo Catalysts Based on Platinum Acetate Blue for the Preferential CO Oxidation in H2-Rich Mixture
Abstract
:1. Introduction
2. Results
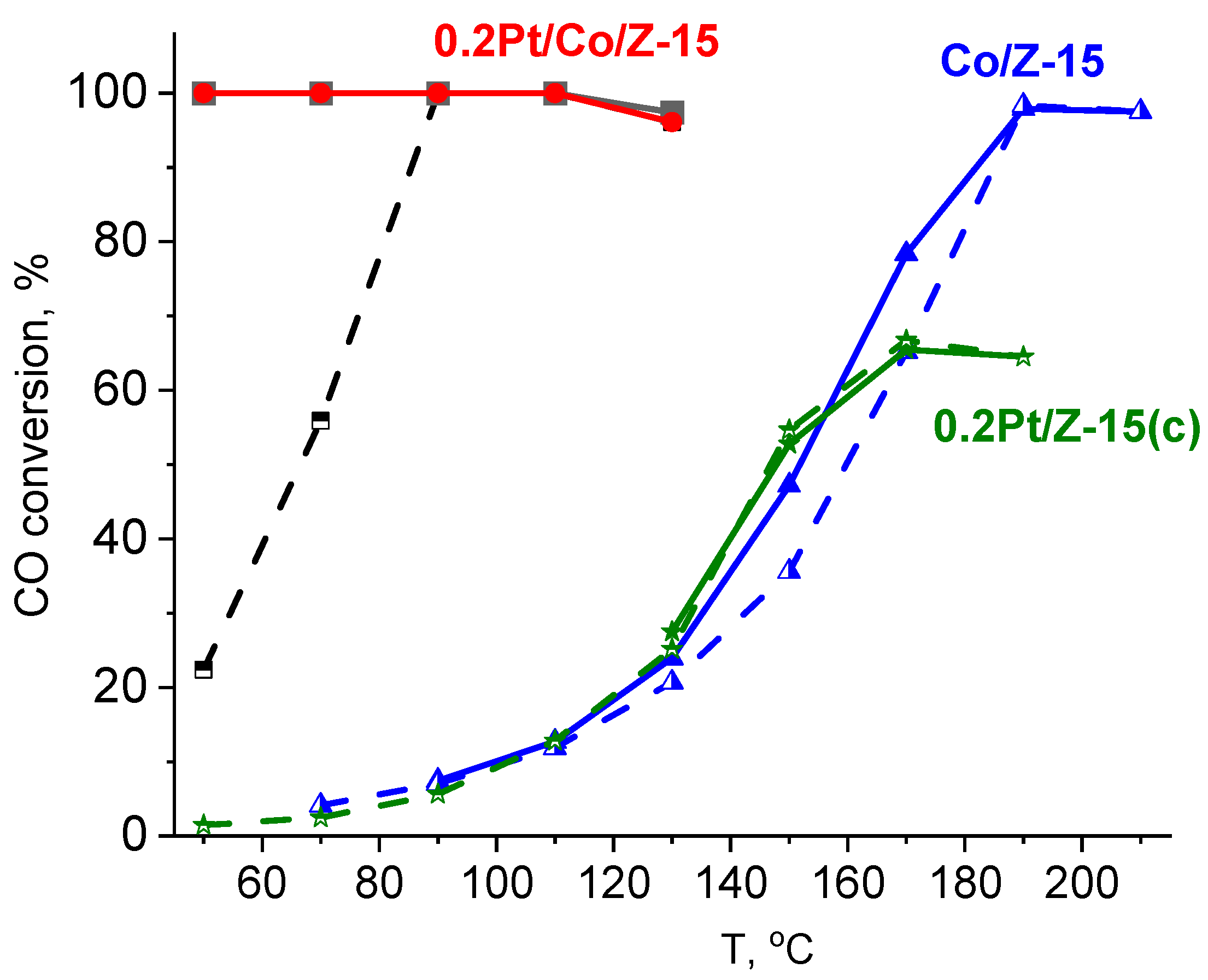
2.1. Catalytic Performance in the Preferential CO Oxidation in H2-Rich Mixture
2.1.1. Pt-Modified Zeolites
2.1.2. Bimetallic PtCo Catalysts
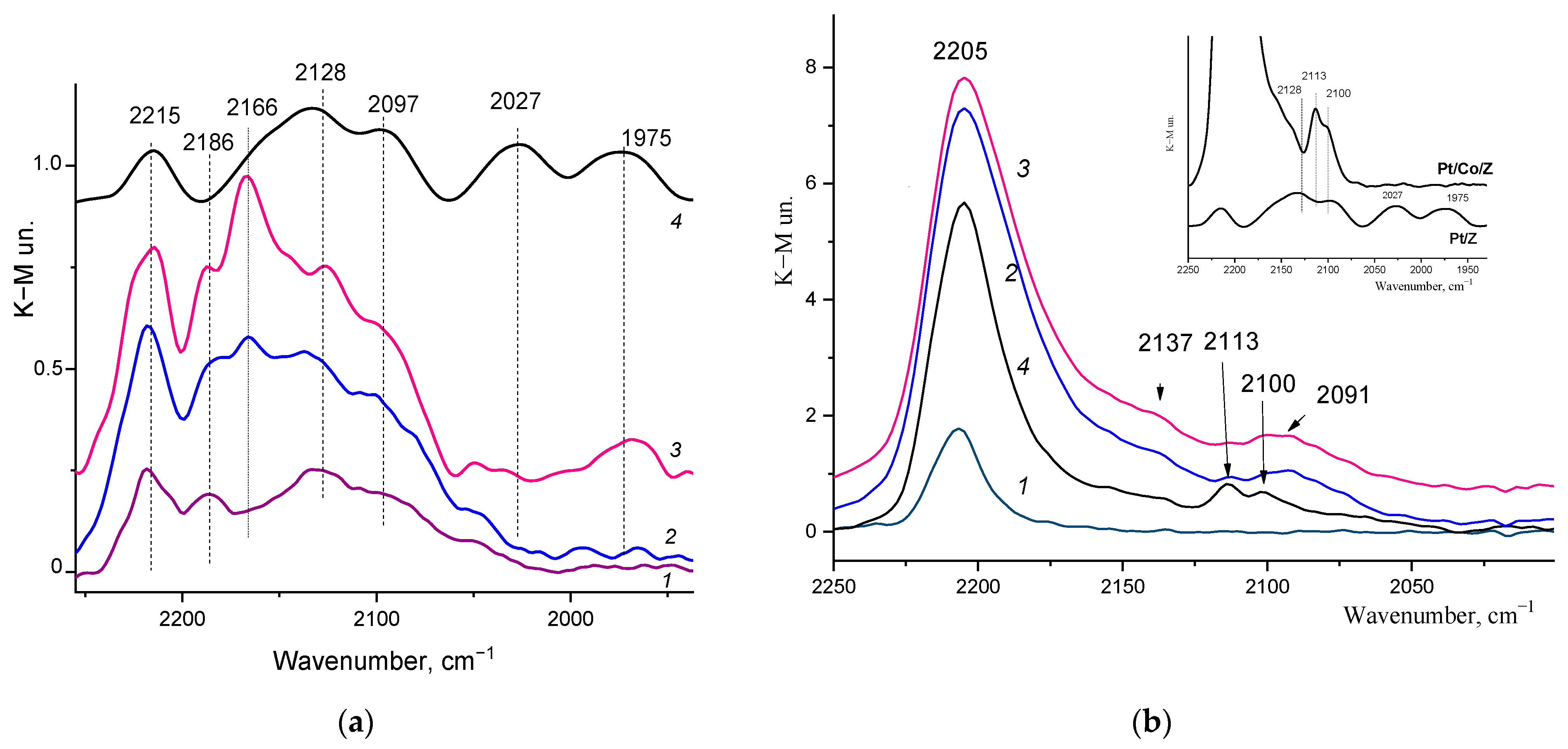
2.2. DRIFT Spectroscopy of the Adsorbed CO
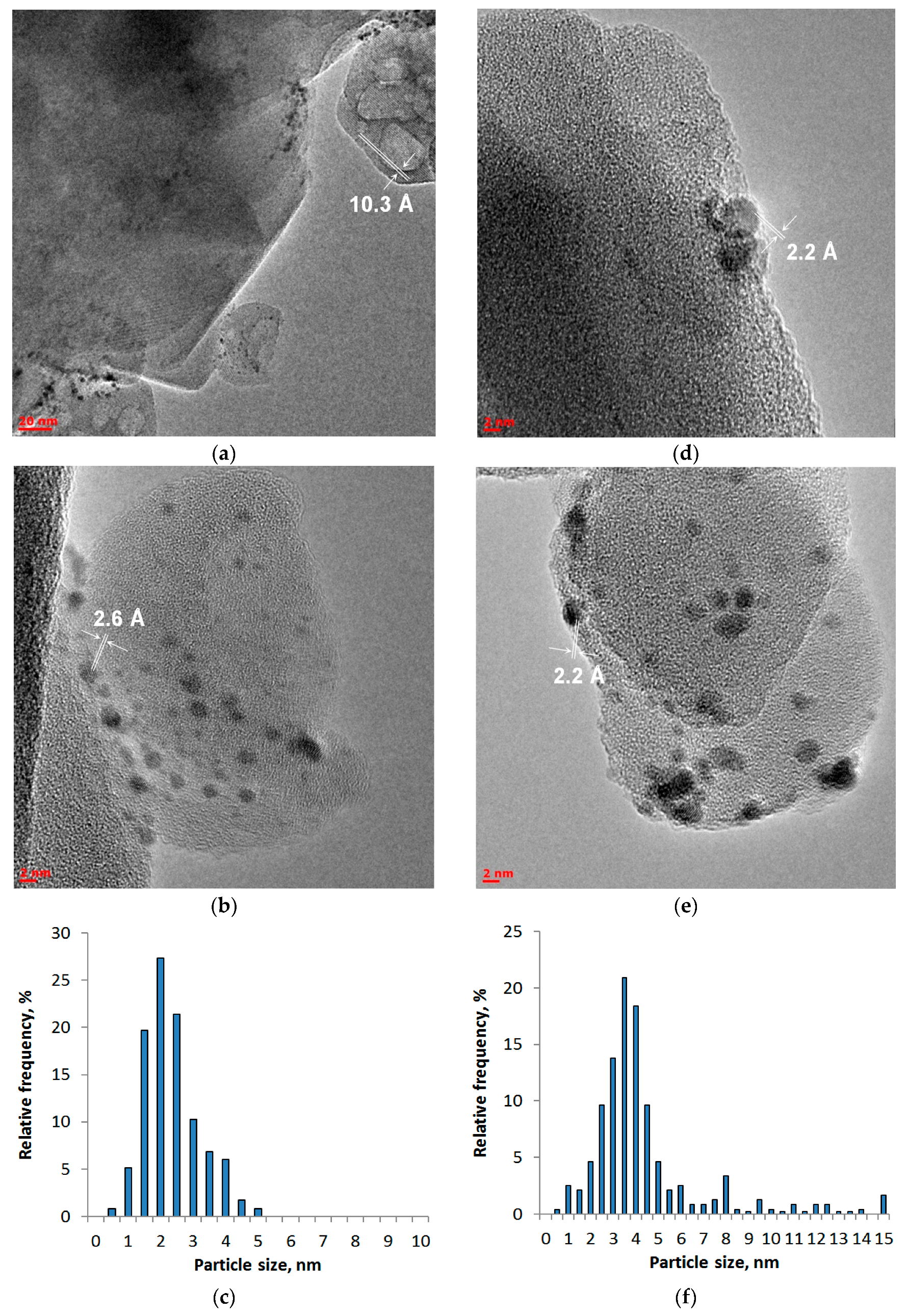
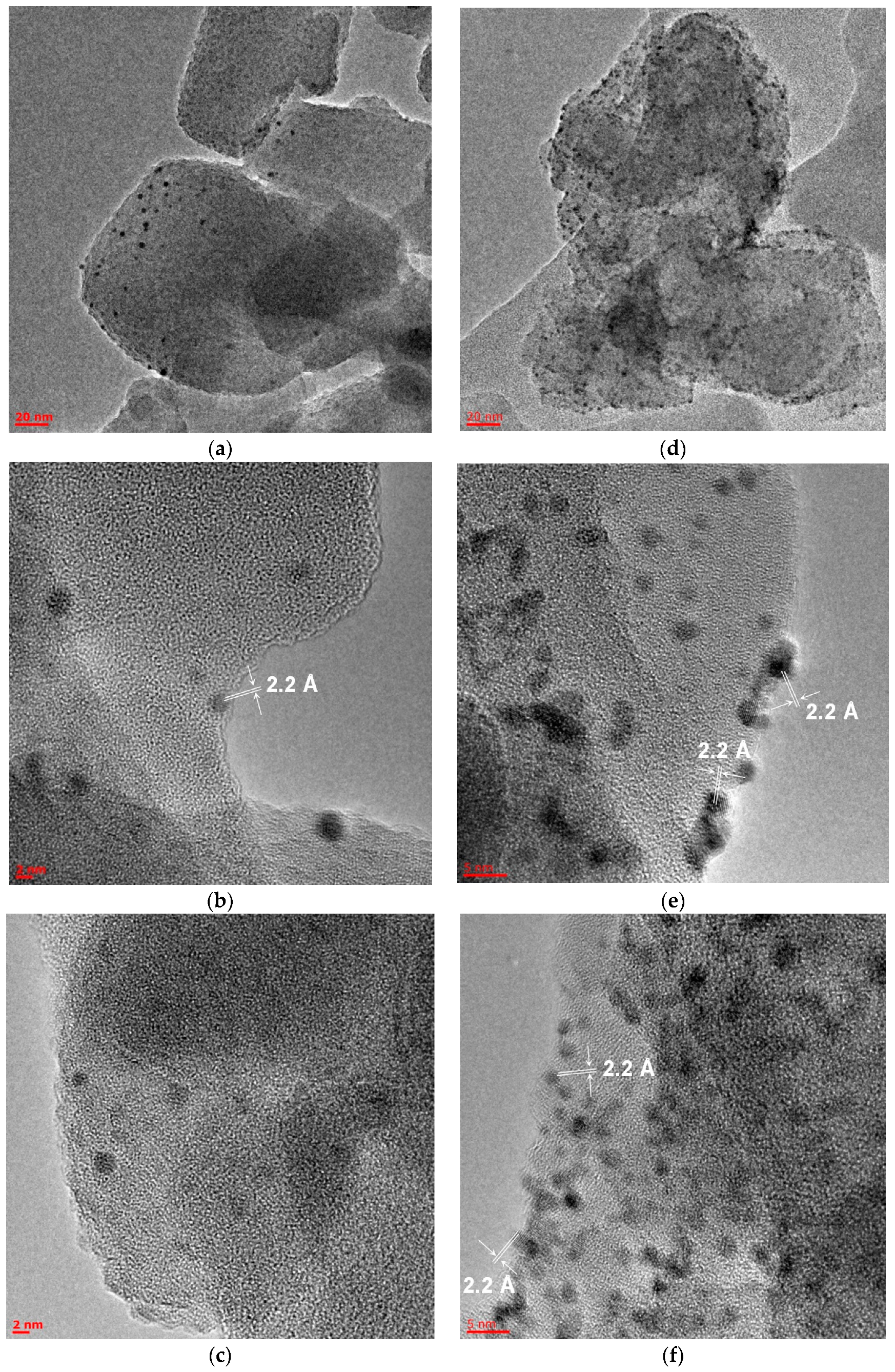
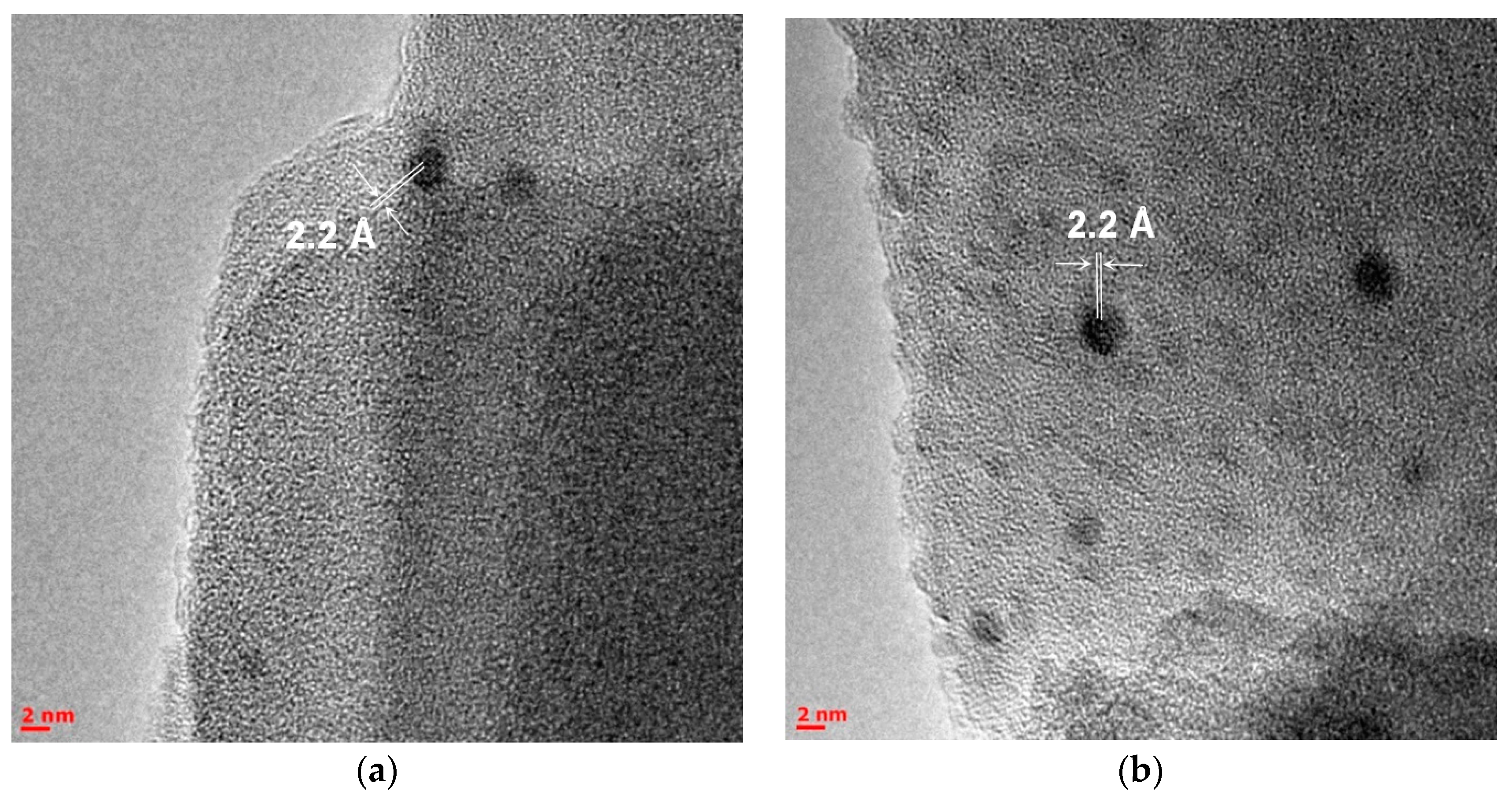
2.3. Microscopic Studies
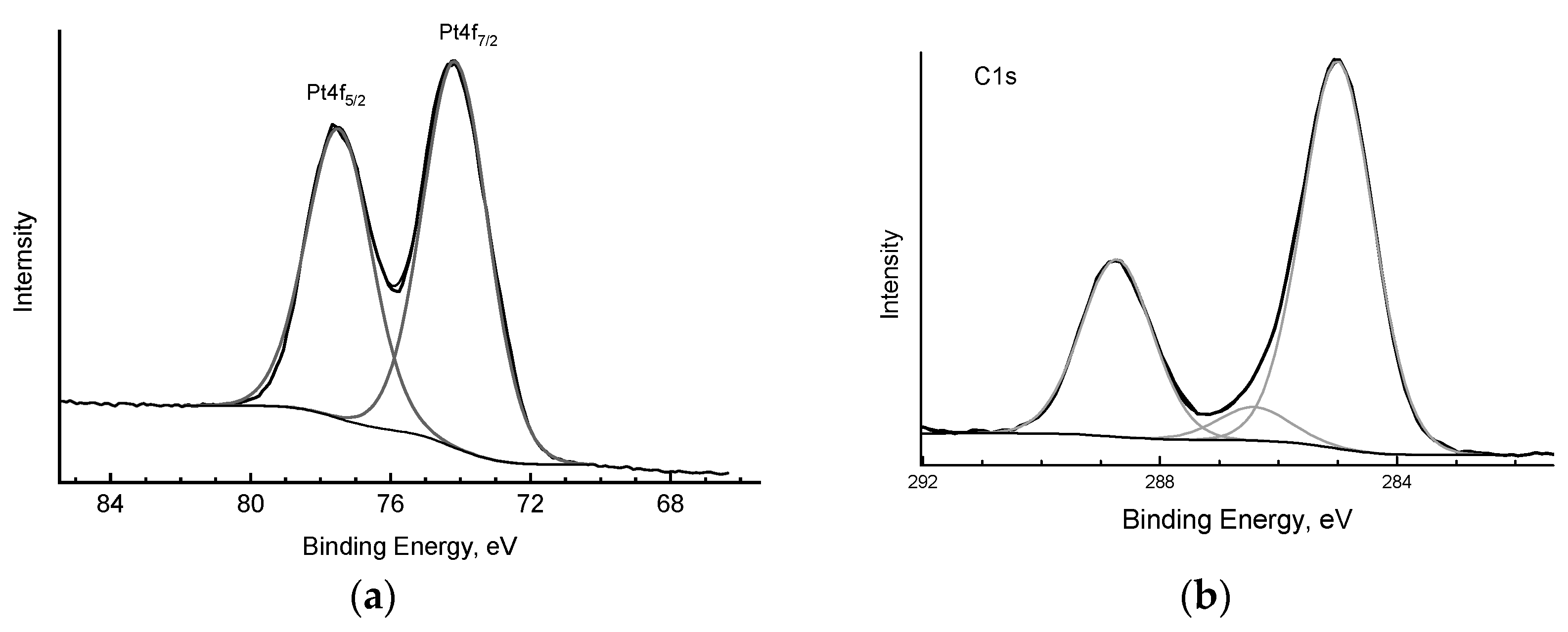
2.4. XPS Studies
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, S.; Gutmann, T.; Buntkowsky, G.; Paul, S.; Thiele, G.; Sievers, H.; Bäumer, M.; Kunz, S. Insights into the reaction mechanism and particle size effects of CO oxidation over supported Pt nanoparticle catalysts. J. Catal. 2019, 377, 662–672. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- McClure, S.M.; Goodman, D.W. New insights into catalytic CO oxidation on Pt-group metals at elevated pressures. Chem. Phys. Lett. 2009, 469, 1–13. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2-rich stream. Catal. Today 2009, 139, 280–290. [Google Scholar] [CrossRef]
- Liu, J.; Hensley, A.J.R.; Giannakakis, G.; Therrien, A.J.; Sukkar, A.; Schilling, A.C.; Groden, K.; Ulumuddin, N.; Hannagan, R.T.; Ouyang, M.; et al. Developing single-site Pt catalysts for the preferential oxidation of CO: A surface science and first principles-guided approach. Appl. Catal. B-Environ. 2021, 284, 119716. [Google Scholar] [CrossRef]
- Du, X.; Lang, Y.; Cao, K.; Yang, J.; Cai, Y.; Shan, B.; Chen, R. Bifunctionally faceted Pt/Ru nanoparticles for preferential oxidation of CO in H2. J. Catal. 2021, 396, 148–156. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Yavarinasab, A.; Siavashi, M.; Matian, M.; Van herle, J. Progress in the proton exchange membrane fuel cells (PEMFCs) water/thermal management: From theory to the current challenges and real-time fault diagnosis methods. Energy Rev. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Lee, S.; Yang, S.; Liu, J.; Giannakakis, G.; Li, M.; Ouyang, M.; Wang, D.; Sykes, E.C.H.; et al. High-loading single Pt atom sites [Pt-O(OH)x] catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 2020, 6, eaba3809. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J. Design of efficient noble metal single atom and cluster catalysts toward low-temperature preferential oxidation of CO in H2. Int. J. Hydrogen Energy 2023, 48, 24788–24808. [Google Scholar] [CrossRef]
- Mariño, F.; Descorme, C.; Duprez, D. Noble metal catalysts for the preferential oxidation of carbon monoxide in the presence of hydrogen (PROX). Appl. Catal. B-Environ. 2004, 54, 59–66. [Google Scholar] [CrossRef]
- Zlotea, C.; Oumellal, Y.; Provost, K.; Morfin, F.; Piccolo, L. Role of hydrogen absorption in supported Pd nanocatalysts during CO-PROX: Insights from operando X-ray absorption spectroscopy. Appl. Catal. B-Environ. 2018, 237, 1059–1065. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Li, D.; Adogwa, A.; Huang, S.; Yang, M.; Yang, J.; Jin, Q. Reaction-driven evolutions of Pt states over Pt-CeO2 catalysts during CO oxidation. Appl. Catal. B-Environ. 2023, 330, 122662. [Google Scholar] [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Stonkus, O.A.; Stadnichenko, A.I.; Romanenko, A.V.; Boronin, A.I. The role of ionic and cluster active centers of Pt/CeO2 catalysts in CO oxidation. Experimental study and mathematical modeling. Chem. Eng. Sci. 2023, 267, 118328. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Hu, Y.; Tian, M. Catalytic oxidation of CO over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A-Gen. 2021, 622, 118218. [Google Scholar] [CrossRef]
- Jing, P.; Gong, X.; Liu, B.; Zhang, J. Recent advances in synergistic effect promoted catalysts for preferential oxidation of carbon monoxide. Catal. Sci. Technol. 2020, 10, 919–934. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, J.; Zhang, Y.; Pan, C.; Dong, Y.; Zhu, Y. Metal-support interaction for heterogeneous catalysis: From nanoparticles to single atoms. Mater. Today Nano 2020, 12, 100093. [Google Scholar] [CrossRef]
- Lagunova, V.; Filatov, E.; Plyusnin, P.; Kostin, G.; Urlukov, A.; Potemkin, D.; Korenev, S. Metal-oxide catalysts for CO TOX and PROX processes in the Pt-Cr/Mo/W systems. Int. J. Hydrogen Energy 2023, 48, 25133–25143. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Cao, K.; Lang, Y.; Chu, S.; Shan, B.; Chen, R. Highly dispersed Pt studded on CoOx nanoclusters for CO preferential oxidation in H2. J. Mater. Chem. A 2020, 8, 10180–10187. [Google Scholar] [CrossRef]
- Nguyen, T.-S.; Morfin, F.; Aouine, M.; Bosselet, F.; Roussert, J.-L.; Piccolo, L. Trends in the CO oxidation and PROX performances of the platinum-group metals supported on ceria. Catal. Today 2015, 253, 106–114. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Li, L.; Lin, Q.; Wei, H.; Liu, J.; Zhang, T. Ferric oxide-supported Pt subnano clusters for preferential oxidation of CO in H2-rich gas at room temperature. ACS Catal. 2014, 4, 2113–2117. [Google Scholar] [CrossRef]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhao, Y.; Chen, M.; Wan, H. Effect of dispersion on catalytic performance of supported Pt catalysts for CO oxidation. Chin. J. Catal. 2012, 33, 1901–1905. [Google Scholar] [CrossRef]
- Mohamed, Z.; Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. The preferential oxidation of CO in hydrogen rich streams over platinum doped nickel oxide catalysts. Appl. Catal. B-Environ. 2016, 180, 687–697. [Google Scholar] [CrossRef]
- Niu, T.; Zhao, W.W.; Liu, G.L.; Cao, A.; Zhang, L.H.; Liu, Y. The graphene-meso-macroporous SiO2 supported Pt-Ni alloy nanocatalyst for preferential oxidation of CO in H2-rich gases. Int. J. Hydrogen Energy 2014, 39, 18929–18939. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhang, N.; Zheng, J.; Zheng, Y.; Li, Y.; Zhong, C.-J.; Chen, B.H. Construction of ultrafine and stable PtFe nano-alloy with ultra-low Pt loading for complete removal of CO in PROX at room temperature. Appl. Catal. B-Environ. 2016, 180, 237–245. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhang, L.; Liu, Y. Aluminumphosphate molecular sieves supported Pt–Co catalysts for the preferential oxidation of CO in H2-rich gases. Appl. Catal. B-Environ. 2013, 136–137, 48–55. [Google Scholar] [CrossRef]
- Lukashuk, L.; Föttinger, K.; Kolar, E.; Rameshan, C.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Yigit, N.; Li, H.; McDermott, E.; et al. Operando XAS and NAP-XPS studies of preferential CO oxidation on Co3O4 and CeO2-Co3O4 catalysts. J. Catal. 2016, 344, 1–15. [Google Scholar] [CrossRef]
- Shilina, M.I.; Rostovshchikova, T.N.; Nikolaev, S.A.; Udalova, O.V. Polynuclear Co-oxo cations in the catalytic oxidation of CO on Co-modified ZSM-5 zeolites. Mater. Chem. Phys. 2019, 223, 287–298. [Google Scholar] [CrossRef]
- Eurov, D.A.; Rostovshchikova, T.N.; Shilina, M.I.; Kirilenko, D.A.; Tomkovich, M.V.; Yagovkina, M.A.; Udalova, O.V.; Kaplin, I.Y.; Ivanin, I.A.; Kurdyukov, D.A. Cobalt oxide decorated porous silica particles: Structure and activity relationship in the catalytic oxidation of carbon monoxide. Appl. Surf. Sci. 2022, 579, 152121. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Tu, S.-T.; Yan, J.; Wang, Z. Catalytic performance and characterization of Al2O3-supported Pt–Co catalyst coatings for preferential CO oxidation in a micro-reactor. Appl. Catal. A-Gen. 2010, 387, 215–223. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Lin, H.; Yuan, Y. Carbon nanotube-supported Pt-Co bimetallic catalysts for preferential oxidation of CO in a H2-rich stream with CO2 and H2O vapor. J. Power Sources 2012, 202, 200–208. [Google Scholar] [CrossRef]
- Nuñez, N.E.; Bideberripe, H.P.; Mizrahi, M.; Ramallo-López, J.M.; Casella, M.L.; Siri, G.J. CO selective oxidation using Co-promoted Pt/γ-Al2O3 catalysts. Int. J. Hydrogen Energy 2016, 41, 19005–19013. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Alotaibi, M.T.; El-Bahy, S.M. CO oxidation and 4-nitrophenol reduction over ceria-promoted platinum nanoparticles impregnated with ZSM-5 zeolite. J. Rare Earths 2022, 40, 1247–1254. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, W.; Wei, J.; Zhang, J.; Zhao, S.; Tang, X.; Yi, H. What is the role of Mn/Al/Pt for ZSM-11 being a good catalytic oxidation catalyst? J. Environ. Chem. Eng. 2023, 11, 110822. [Google Scholar] [CrossRef]
- Park, D.C.; Moon, S.; Song, J.H.; Kim, H.; Lee, E.; Lim, Y.H.; Kim, D.H. Widening the operating window of Pt/ZSM-5 catalysts for efficient NOx removal in H2-SCR: Insights from thermal aging. Catal. Today 2024, 425, 114318. [Google Scholar] [CrossRef]
- Wang, X.-F.; Liu, C.-F.; He, L.-C.; Li, B.; Lu, J.-Q.; Luo, M.-F.; Chen, J. Unveiling geometric and electronic effects of Pt species on water-tolerant Pt/ZSM-5 catalyst for propane oxidation. Appl. Catal. A-Gen. 2023, 655, 119108. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yu, H.; Wang, X. The functions of Pt located at different positions of HZSM-5 in H2-SCR Chem. Eng. J. 2019, 355, 470–477. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, J.; Yang, H.; Shang, S.; Zhang, A.; Song, C.; Guo, X. Synergetic and efficient alkylation of benzene with ethane over Pt/ZSM-5 nanosheet bifunctional catalysts to ethylbenzene. Fuel 2023, 342, 127764. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Xu, L.-L.; Ouyang, L.-R.; Huang, L.-L.; Jia, A.-P.; Wang, Y.; Lu, J.-Q. Tandem c-cl cleavage and oxidation over Pt/ZSM-5 catalysts for CH2Cl2 deep oxidation: Acidity—Redox synergy, rate—Limiting step, and catalyst stability. Chem. Eng. J. 2023, 476, 146551. [Google Scholar] [CrossRef]
- Gao, M.; Gong, Z.; Weng, X.; Shang, W.; Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Methane combustion over palladium catalyst within the confined space of MFI zeolite. Chin. J. Catal. 2021, 42, 1689–1699. [Google Scholar] [CrossRef]
- Friberg, I.; Clark, A.H.; Ho, P.H.; Sadokhina, N.; Smales, G.J.; Woo, J.; Auvray, X.; Ferri, D.; Nachtegaal, M.; Kröcher, O.; et al. Structure and performance of zeolite supported Pd for complete methane oxidation. Catal. Today 2021, 382, 3–12. [Google Scholar] [CrossRef]
- Tian, X.; Shan, Y.; Zhang, J.; Yan, Z.; Sun, Y.; Ding, W.; Yu, Y. The study of Pt/zeolites for CO oxidation: Effects of skeleton structure and Si/Al ratio. Catal. Comm. 2023, 178, 106679. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Nikolaev, S.A.; Krotova, I.N.; Maslakov, K.I.; Udalova, O.V.; Gurevich, S.A.; Yavsin, D.A.; Shilina, M.I. ZSM-5 and BEA zeolites modified with Pd nanoparticles by laser electrodispersion. The structure and catalytic activity in CO and CH4 oxidation. Russ. Chem. Bull. 2022, 71, 1179–1193. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Hu, W.; Dai, Q.; Wang, L.; Zhan, W.; Guo, Y.; Cao, X.-M.; Hu, P.; Lu, G. Low-temperature methane combustion over Pd/H-ZSM-5: Active Pd sites with specific electronic properties modulated by acidic sites of H-ZSM-5. ACS Catal. 2016, 6, 8127–8139. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Shi, Y.; Zhou, R. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal. J. Environ. Sci. 2021, 107, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Li, G.; Wang, J.; Shi, Y.; Zhou, R. Promoting effect of acid sites in hierarchical porous Pt/ZSM-5 catalysts for low-temperature removal of VOCs. Appl. Surf. Sci. 2022, 606, 154888. [Google Scholar] [CrossRef]
- Daniel, S.; Monguen, C.K.F.; Ayodele, O.B.; Tian, Z.-Y. Tailored synthesized Pt/ZSM-5 catalysts with excellent water vapor stability for low temperature oxidation of CO and C3H6. J. Environ. Chem. Eng. 2023, 11, 109617. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.-C.; Ouyang, L.; Yang, X.-J.; Mao, W.; Su, J.; Han, Y.-F. Mechanistic study of preferential CO oxidation on a Pt/NaY zeolite catalyst. J. Catal. 2012, 287, 114–123. [Google Scholar] [CrossRef]
- Shilina, M.I.; Krotova, I.N.; Maksimov, S.V.; Maslakov, K.I.; Nikolaev, S.A.; Udalova, O.V.; Gurevich, S.A.; Yavsin, D.A.; Rostovshchikova, T.N. Total and preferential CO oxidation on low-loaded Pt-HZSM-5 zeolites modified using laser electrodispersion. Russ. Chem. Bull. 2023, 72, 1518–1532. [Google Scholar] [CrossRef]
- Sebastian, V.; Irusta, S.; Mallada, R.; Santamaría, J. Selective oxidation of CO in the presence of H2, CO2 and H2O on different zeolite-supported Pt catalysts. Appl. Catal. A-Gen. 2009, 366, 242–251. [Google Scholar] [CrossRef]
- Shilina, M.I.; Krotova, I.N.; Nikolaev, S.A.; Gurevich, S.A.; Yavsin, D.A.; Udalova, O.V.; Rostovshchikova, T.N. Highly effective Pt-Co/ZSM-5 catalysts with low Pt loading for preferential CO oxidation in H2-rich mixture. Hydrogen 2023, 4, 154–173. [Google Scholar] [CrossRef]
- Malwadkar, S.; Bera, P.; Satyanarayana, C.V.V. Low-temperature preferential CO oxidation in a hydrogen-rich stream over Pt-NaY and modified Pt-NaY catalysts for fuel cell application. Fuel Cells 2023, 23, 15–28. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, M.; Cai, D.; Tan, K.B.; Zhan, G. Fabrication of Pt/Co3O4 nanocatalysts based on pollen template for low-temperature CO oxidation. Cat. Commun. 2023, 174, 106597. [Google Scholar] [CrossRef]
- Li, C.; Ke, C.; Han, R.; Fan, G.; Yang, L.; Li, F. The remarkable promotion of in situ formed Pt-cobalt oxide interfacial sites on the carbonyl reduction to allylic alcohols. Mol. Catal. 2018, 455, 78–87. [Google Scholar] [CrossRef]
- Babucci, M.; Guntida, A.; Gates, B.C. Atomically dispersed metals on well-defined supports including zeolites and metal−organic frameworks: Structure, bonding, reactivity, and catalysis. Chem. Rev. 2020, 120, 11956–11985. [Google Scholar] [CrossRef]
- Ivanin, I.A.; Udalova, O.V.; Kaplin, I.Y.; Shilina, M.I. New insights on the Cu-Ce interaction in Cu/Ce catalysts based on ZSM-5 and Beta for the preferential oxidation of carbon monoxide in excess hydrogen. Appl. Surf. Sci. 2024, 655, 159577. [Google Scholar] [CrossRef]
- Cherkashina, N.V.; Kochubey, D.I.; Kanazhevskiy, V.V.; Zaikovskii, V.I.; Ivanov, V.K.; Markov, A.A.; Klyagina, A.P.; Dobrokhotova, Z.V.; Kozitsyna, N.Y.; Baranovsky, I.B.; et al. Platinum acetate blue: Synthesis and characterization. Inorg. Chem. 2014, 53, 8397–8406. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Shilina, M.I.; Udalova, O.V.; Nevskaya, S.M. Synergism in the actions of a transition metal cation and a Lewis acid in the liquid and gas phase catalytic conversion of alkanes over modified ZSM5 zeolites under mild conditions. Kinet. Catal. 2013, 54, 691–702. [Google Scholar] [CrossRef]
- Chakarova, K.; Mihaylov, M.; Hadjiivanov, K. FTIR spectroscopic study of CO adsorption on Pt–H–ZSM-5. Microporous Mesoporous Mater. 2005, 81, 305–312. [Google Scholar] [CrossRef]
- Chakarova, K.; Hadjiivanov, K.; Atanasova, G.; Tenchev, K. Effect of preparation technique on the properties of platinum in NaY zeolite: A study by FTIR spectroscopy of adsorbed CO. J. Mol. Catal. A-Chem. 2007, 264, 270–279. [Google Scholar] [CrossRef]
- Aleksandrov, H.A.; Neyman, K.M.; Hadjiivanov, K.I.; Vayssilov, G.N. Can the state of platinum species be unambiguously determined by the stretching frequency of an adsorbed CO probe molecule? Phys. Chem. Chem. Phys. 2016, 18, 22108–22121. [Google Scholar] [CrossRef] [PubMed]
- DeRita, L.; Dai, S.; Lopez-Zepeda, K.; Pham, N.; Graham, G.W.; Pan, X.; Christopher, P. Catalyst architecture for stable single atom dispersion enables site specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef] [PubMed]
- Ivanin, I.A.; Krotova, I.N.; Udalova, O.V.; Zanaveskin, K.L.; Shilina, M.I. Synergistic catalytic effect of cobalt and cerium in the preferential oxidation of carbon monoxide on modified Co/Ce/ZSM-5 zeolites. Kinet. Catal. 2021, 62, 798–811. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Tsyntsarski, B.; Venkov, T.; Klissurski, D.; Daturi, M.; Saussey, J.; Lavalley, J.-C. FTIR spectroscopic study of CO adsorption on Co–ZSM-5: Evidence of formation of Co+(CO)4 species. Phys. Chem. Chem. Phys. 2003, 5, 1695–1702. [Google Scholar] [CrossRef]
- Chupin, C.; van Veen, A.C.; Konduru, M.; Deprés, J.; Mirodatos, C. Identity and location of active species for NO reduction by CH4 over Co-ZSM-5. J. Catal. 2006, 241, 103–114. [Google Scholar] [CrossRef]
- Mitsumi, M.; Murase, T.; Kishida, H.; Yoshinari, T.; Ozawa, Y.; Toriumi, K.; Sonoyama, T.; Kitagawa, H.; Mitani, T. Metallic behavior and periodical valence ordering in a MMX chain compound, Pt2(EtCS2)4I. J. Am. Chem. Soc. 2001, 123, 11179–11192. [Google Scholar] [CrossRef]
- Saveleva, V.A.; Papaefthimiou, V.; Daletou, M.K.; Doh, W.H.; Ulhaq-Bouillet, C.; Diebold, M.; Zafeiratos, S.; Savinova, E.R. Operando near ambient pressure XPS (NAP-XPS) study of the Pt electrochemical oxidation in H2O and H2O/O2 ambients. J. Phys. Chem. C 2016, 120, 15930–15940. [Google Scholar] [CrossRef]
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.A.; Shilina, M.I.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Slavinskay, E.M. Role of surface coverage of alumina with Pt nanoparticles deposited by laser electrodispersion in catalytic CO oxidation. Appl. Surf. Sci. 2021, 536, 147656. [Google Scholar] [CrossRef]
- Shilina, M.; Udalova, O.; Krotova, I.; Ivanin, I.; Boichenko, A. Oxidation of carbon monoxide on Co/Ce-modified ZSM-5 zeolites: Impact of mixed Oxo-Species. ChemCatChem 2020, 12, 2556–2568. [Google Scholar] [CrossRef]
- Xiang, G.; Qiu, Z.; Fei, H.; Liu, Z.; Yin, S.; Wu, Y. Synergistic effect of platinum single atoms and nanoclusters for preferential oxidation of carbon monoxide in hydrogen-rich stream. J. Power Sources 2024, 591, 233873. [Google Scholar] [CrossRef]
- Navas-Cárdenas, C.; Wolf, E.E.; Benito, N.; Gracia, F. Strong electrostatic adsorption of Pt over CoOx/TiO2 dual-support: Effect of synthesis pH on the catalytic activity for the CO oxidation in presence of hydrogen. Int. J. Hydrogen Energy 2023, 48, 24809–24825. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Knözinger, H. Nature and Estimation of Functional Groups on Solid Surfaces. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 39–207. [Google Scholar] [CrossRef]



| Catalyst | Pt, wt% | Si/Al | Calcination Temperature, °C | X150, % | Xmax, % | Tmax, °C |
|---|---|---|---|---|---|---|
| 0.1 Pt/Z-15(a) | 0.1 | 15 | 200 | 18 | 48 | 190 |
| 0.1 Pt/Z-15(b) | 0.1 | 15 | 300 | 9 | 32 | 210 |
| 0.1 Pt/Z-28(a) | 0.1 | 28 | 200 | 15 | 57 | 210 |
| 0.1 Pt/Z-28(b) | 0.1 | 28 | 300 | 10 | 52 | 210 |
| 0.2 Pt/Z-15(a) | 0.2 | 15 | 200 | 19 | 48 | 190 |
| 0.2 Pt/Z-15(c) | 0.2 | 15 | 200 + 300 | 57 | 72 | 170 |
| 0.2 Pt/Z-28(a) | 0.2 | 28 | 200 | 17 | 60 | 210 |
| 0.2 Pt/Z-28(c) | 0.2 | 28 | 200 + 300 | 35 | 77 | 190 |
| 0.6 Pt/Z-28(a) | 0.6 | 28 | 200 | 92 | 92 | 150 |
| Catalyst | Pt, wt% | Si/Al | T50 1 | X70 2 | T100 1 | ∆T100 |
|---|---|---|---|---|---|---|
| 0.1 PtCo/Z-15 | 0.1 | 15 | 73 | 97 | 90 | 90–130 |
| 0.2 PtCo/Z-15 | 0.2 | 15 | 66 | 100 | 50 | 50–110 |
| 0.1 PtCo/Z-28 | 0.1 | 28 | 65 | 100 | 70 | 70–110 |
| 0.2 PtCo/Z-28 | 0.2 | 28 | 69 | 100 | 90 | 70–90 |
| Spectrum | Eb, eV | Bond Type | Fraction, at.% |
|---|---|---|---|
| O1s | 532.3 | COO−, O−C | 35.2 |
| C1s | 285.0 | C−C (sp3) | 37.5 |
| 286.4 | C−O | 3.3 | |
| 288.7 | COO− | 17.2 | |
| Pt4f7/2 | 74.2 | Pt3+, Pt4+ | 6.8 |
| Eb, eV | 71.2–71.3 | 72.2–72.4 | 73.5–74.0 | Element Ratio | |
|---|---|---|---|---|---|
| Sample | Conditions | Electronic State, at.% | |||
| Pt0 | Pt2+ | Pt4+ | Pt/(Si + Al) | ||
| 0.2 Pt/Z-15(a) | Initial | 44 | 29 | 27 | 0.004 |
| 0.2 Pt/Z-15(c) | Initial | 74 | 12 | 14 | 0.007 |
| 0.6 Pt/Z-28(a) | Initial | 24 | 50 | 26 | 0.02 |
| Spent | 74 | 20 | 6 | 0.004 | |
| Eb, eV | 71.2 71.3 | 72.2 72.4 | 73.5 74.0 | 781.6 | 779.8 | Element Ratio | |||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Conditions | Electronic State, at.% | |||||||
| Pt0 | Pt2+ | Pt4+ | Co2+ | Co3+ | Pt/(Si + Al) | Co/(Si + Al) | Co/Pt | ||
| 0.2 PtCo/Z-15 | Initial | 42 | 33 | 25 | 74 | 26 | 0.001 | 0.045 | 45 |
| Spent | 65 | 20 | 15 | 74 | 26 | 0.001 | 0.044 | 44 | |
| 0.2 PtCo/Z-28 | Initial | 40 | 28 | 32 | 75 | 25 | 0.001 | 0.093 | 93 |
| Spent | 48 | 28 | 24 | 65 | 35 | 0.001 | 0.089 | 89 | |
| 2.5 Co/Z-15 | Initial | - | - | - | 72 | 28 | - | 0.045 | - |
| 1.7 Co/Z-28 1 | Initial | - | - | - | 83 | 17 | - | 0.025 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilina, M.; Krotova, I.; Nikolaev, S.; Cherkashina, N.; Stolarov, I.; Udalova, O.; Maksimov, S.; Rostovshchikova, T. Advanced PtCo Catalysts Based on Platinum Acetate Blue for the Preferential CO Oxidation in H2-Rich Mixture. Catalysts 2024, 14, 484. https://doi.org/10.3390/catal14080484
Shilina M, Krotova I, Nikolaev S, Cherkashina N, Stolarov I, Udalova O, Maksimov S, Rostovshchikova T. Advanced PtCo Catalysts Based on Platinum Acetate Blue for the Preferential CO Oxidation in H2-Rich Mixture. Catalysts. 2024; 14(8):484. https://doi.org/10.3390/catal14080484
Chicago/Turabian StyleShilina, Marina, Irina Krotova, Sergey Nikolaev, Natalia Cherkashina, Igor Stolarov, Olga Udalova, Sergey Maksimov, and Tatiana Rostovshchikova. 2024. "Advanced PtCo Catalysts Based on Platinum Acetate Blue for the Preferential CO Oxidation in H2-Rich Mixture" Catalysts 14, no. 8: 484. https://doi.org/10.3390/catal14080484





