Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME)
Abstract
1. Introduction
2. Results
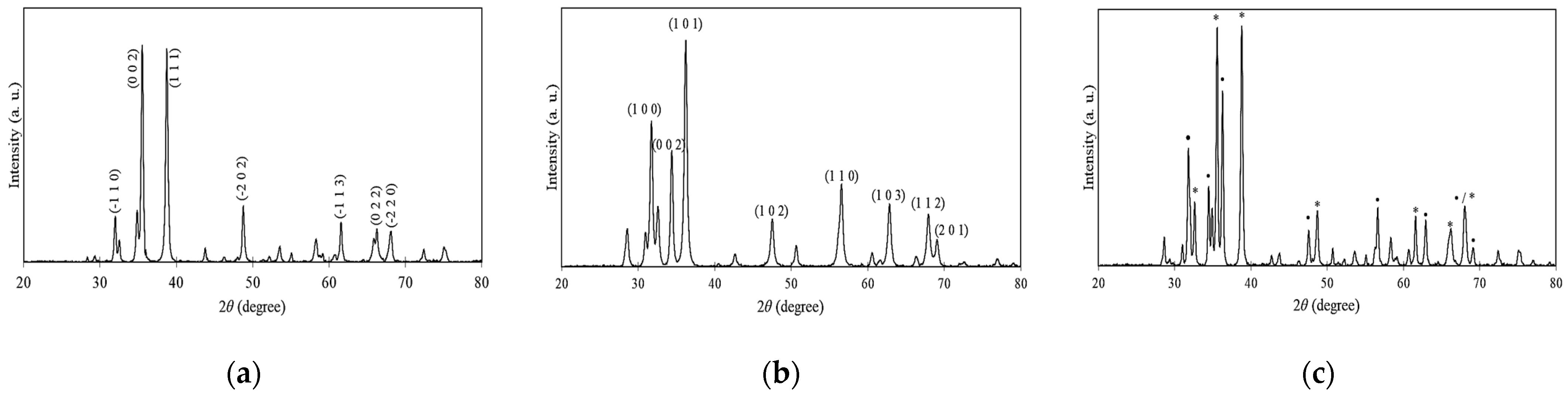
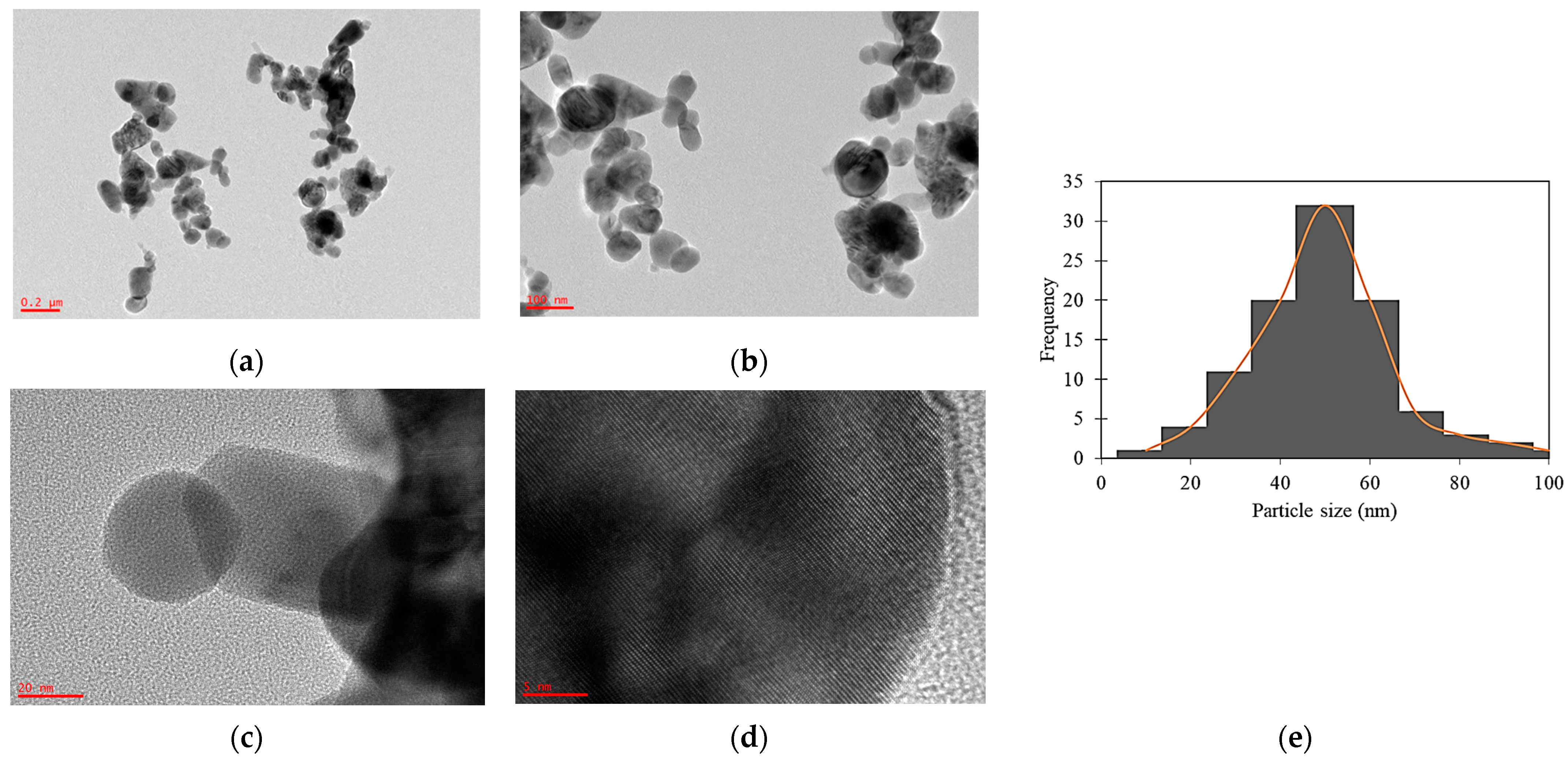
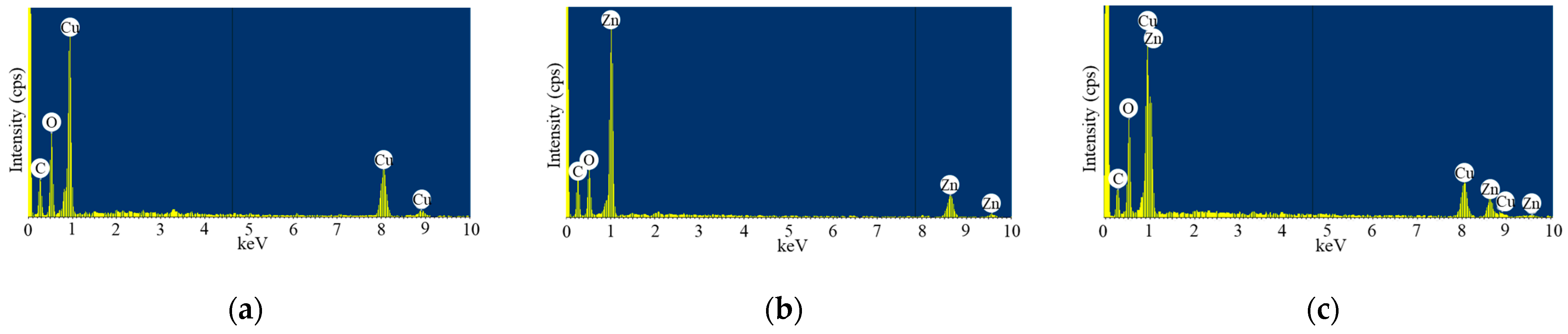
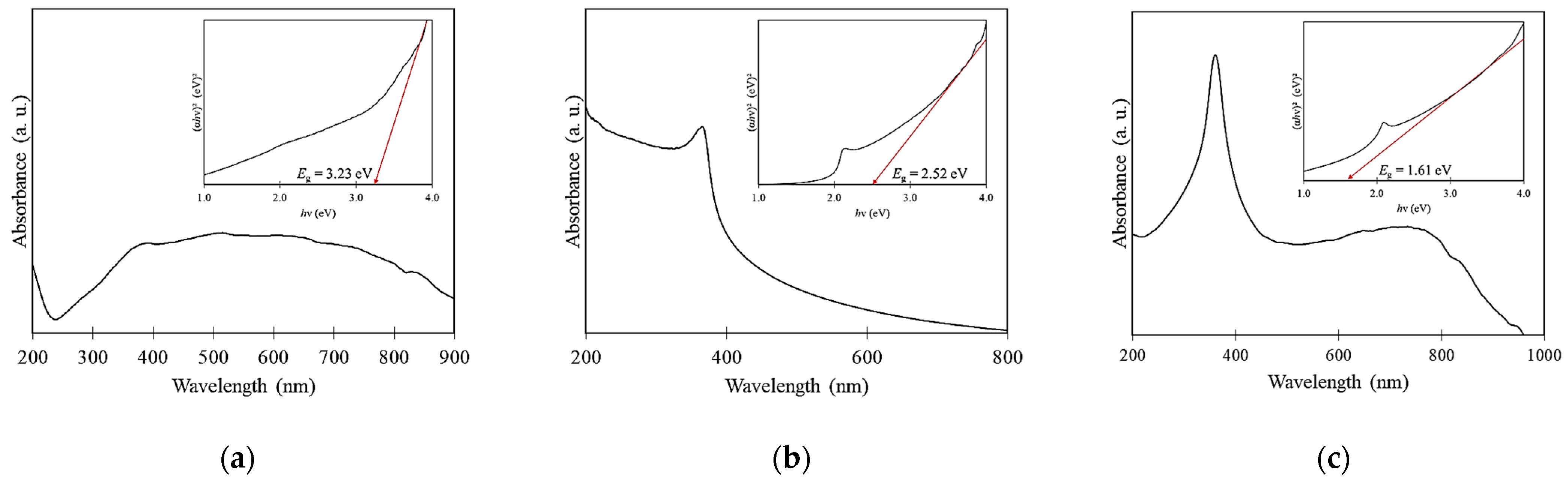
2.1. Physicochemical Properties
2.2. COD Removal Efficiency
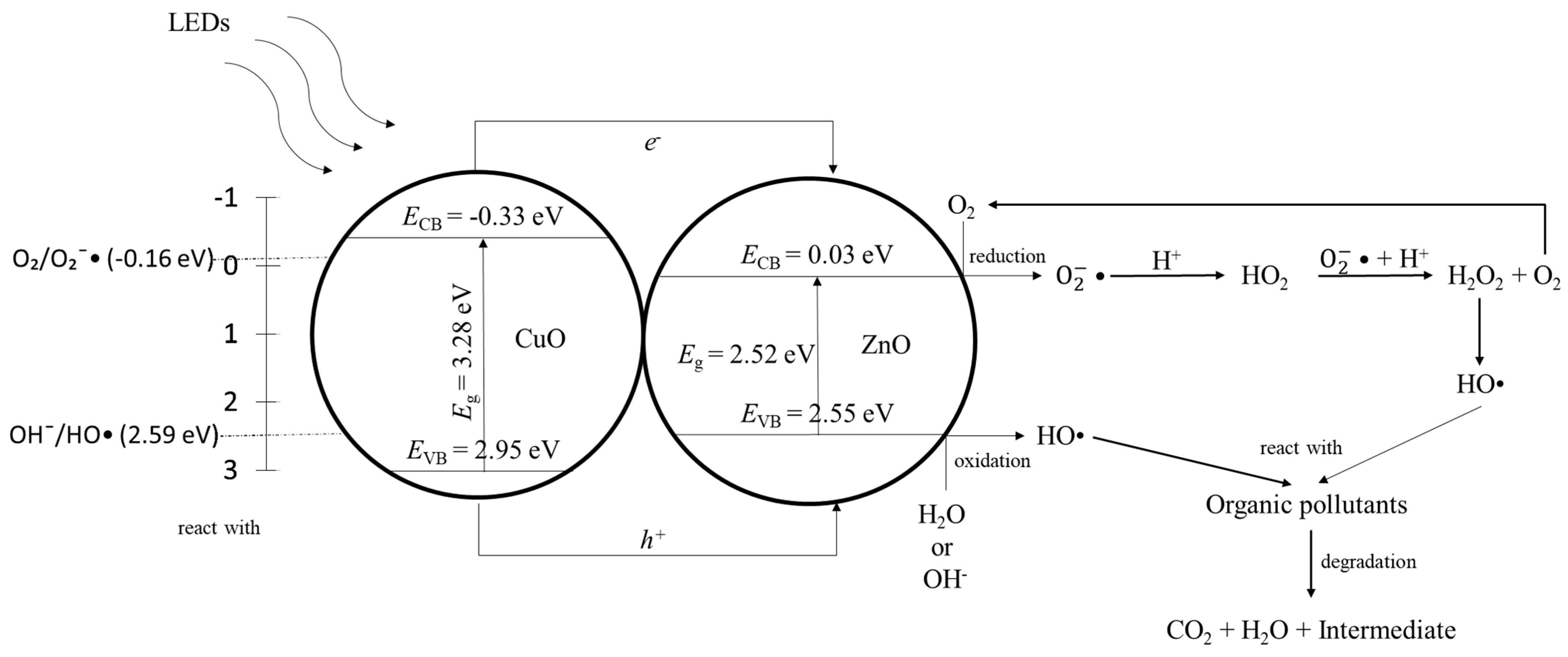
3. Discussion
4. Materials and Methods
4.1. Preparation of Mangosteen-Leaf Aqueous Extract
4.2. Synthesis of CuO NPs, ZnO NPs and CuO-ZnO NCs
4.3. Characterization of CuO NPs, ZnO NPs and CuO-ZnO NCs
4.4. Photocatalytic Performance
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igwe, J.C.; Onyegbado, C.C. A Review of palm oil mill effluent (POME) water treatment. Glob. J. Env. Res. 2007, 1, 54–62. [Google Scholar]
- Mahmod, S.S.; Takriff, M.S.; AL-Rajabi, M.M.; Abdul, P.M.; Gunny, A.A.N.; Silvamany, H.; Jahim, J.M. Water reclamation from palm oil mill effluent (POME): Recent technologies, by-product recovery, and challenges. J. Water. Process. Eng. 2023, 52, 103488–103502. [Google Scholar] [CrossRef]
- Sidik, D.A.B.; Hairom, N.H.H.; Rozman, A.I.; Johari, M.J.S.; Muhammad, A. The photocatalytic activity of green zinc oxide nanoparticles in the treatment of aerobically palm oil mill effluent. J. Sci. Technol. 2023, 15, 7–15. [Google Scholar] [CrossRef]
- Agustina, L.; Suprihatin, S.; Romli, M.; Suryadarma, P. Processing of palm mill oil effluent using photocatalytic: A literature review. J. Ecol. Eng. 2021, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, S.S.; Arisht, S.N.; Jahim, J.M.; Takriff, M.S.; Tan, J.P.; Luthfi, A.A.I.; Abdul, P.M. Enhancement of biohydrogen production from palm oil mill effluent (POME): A review. Int. J. Hydrogen Energy 2021, 47, 40637–40655. [Google Scholar] [CrossRef]
- Puasa, N.A.; Hairom, N.H.H.; Dzinun, H.; Madon, R.H.; Ahmad, N.S.; Sidik, D.A.B.; Azmi, A.A.A.R. Photocatalytic degradation of palm oil mill secondary effluent in presence of zinc oxide nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100413–100418. [Google Scholar] [CrossRef]
- Ratnasari, A.; Zaidi, N.S.; Syafiuddin, A.; Boopathy, R.; Kueh, A.B.H.; Amalia, R.; Prasetyo, D.D. Prospective biodegradation of organic and nitrogenous pollutants from palm oil mill effluent by acidophilic bacteria and archaea. Bioresour. Technol. Rep. 2021, 15, 100809–100819. [Google Scholar] [CrossRef]
- Saputera, W.H.; Amri, A.F.; Mukti, R.R.; Suendo, V.; Devianto, H.; Sasongko, D. Photocatalytic degradation of palm oil mill effluent (POME) waste using BiVO4 based catalysts. Molecules 2021, 26, 6225. [Google Scholar] [CrossRef] [PubMed]
- Saputera, W.H.; Amri, A.F.; Daiyan, R.; Sasongko, D. Photocatalytic technology for palm oil mill effluent (POME) wastewater treatment: Current progress and future perspective. Materials 2021, 14, 2846. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Danquah, M.K.; Zhang, J.; Chiong, T.; Takeo, M.; Jeevanandam, J. Novel concepts for graphene-based nanomaterials synthesis for phenol removal from palm oil mill effluent (POME). Materials 2023, 16, 4379. [Google Scholar] [CrossRef]
- Aqilah Mohd Razali, N.; Norharyati Wan Salleh, W.; Rosman, N.; Hafiza Ismail, N.; Zu Nurain Ahmad, S.; Aziz, F.; Jye, L.W.; Fauzi Ismail, A. Palm oil mill effluent treatment using tungsten trioxide: Adsorption and photocatalytic degradation. Mater. Today Proc. 2020, 42, 22–27. [Google Scholar] [CrossRef]
- Osman, N.A.; Ujang, F.A.; Roslan, A.M.; Ibrahim, M.F.; Hassan, M.A. The effect of palm oil mill effluent final discharge on the characteristics of Pennisetum purpureum. Sci. Rep. 2020, 10, 6613–6622. [Google Scholar] [CrossRef] [PubMed]
- Septiningrum, F.; Yuwono, A.H.; Maulana, F.A.; Nurhidayah, E.; Dhaneswara, D.; Sofyan, N.; Hermansyah, H.; Purwanto, W.W. Mangosteen pericarp extract mediated synthesis of Ag/TiO2 nanocomposite and its application on organic pollutant degradation by adsorption-photocatalytic activity. Curr. Res. Green. Sustain. Chem. 2024, 8, 100394–100404. [Google Scholar] [CrossRef]
- Dien, N.D.; Ha, P.T.T.; Vu, X.H.; Trang, T.T.; Giang, T.D.T.; Dung, N.T. Developing efficient CuO nanoplate/ZnO nanoparticle hybrid photocatalysts for methylene blue degradation under visible light. R. Soc. Chem. Adv. 2023, 13, 24505–24518. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, C.; Jaballah, R.; Mahdhi, N.; Boukhachem, A.; Amlouk, M. CuO-ZnO nanocomposites-based thin films: Characterization, physical properties and sunlight photocatalytic degradation of organic pollutants. J. Alloys Compd. 2023, 968, 172252–172266. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Lam, M.K.; Ayoub, M.; Cheng, C.K.; Lim, J.W.; Yusup, S.; Tang, Y.; Bai, J. Holistic process evaluation of non-conventional palm oil mill effluent (POME) treatment technologies: A conceptual and comparative review. J. Hazard. Mater. 2021, 409, 124964–124996. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, S.S.; Hosamane, S.N.; Patil, C.; Yaragatti, A.; Hukerikar, A.; Patil, S.; Chachadi, P. ZnO-CuO nanocomposites: Synthesis, characterization and antibacterial activity. J. Phys. Conf. Ser. 2020, 1706, 012018–012023. [Google Scholar] [CrossRef]
- Basit, R.A.; Abbasi, Z.; Hafeez, M.; Ahmad, P.; Khan, J.; Khandaker, M.U.; Al-Mugren, K.S.; Khalid, A. Successive photocatalytic degradation of methylene blue by ZnO, CuO and ZnO/CuO synthesized from Coriandrum sativum plant extract via green synthesis technique. Crystals 2023, 13, 281. [Google Scholar] [CrossRef]
- Li, H.; Ma, W.; Zeng, X.; Liu, S.; Xiao, L.; Fang, Z.; Feng, Y.; Yang, M.; Zhu, H.; Yang, Y.; et al. ZnO/CuO Piezoelectric nanocatalysts for the degradation of organic pollutants. ACS. Appl. Nano. Mater. 2023, 6, 21113–21122. [Google Scholar] [CrossRef]
- Mubeen, K.; Irshad, A.; Safeen, A.; Aziz, U.; Safeen, K.; Ghani, T.; Khan, K.; Ali, Z.; ul Haq, I.; Shah, A. Band structure tuning of ZnO/CuO composites for enhanced photocatalytic activity. J. Saudi. Chem. Soc. 2023, 27, 101639–101651. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, Y.N.N.; Tran, X.T.; Nguyen, T.T.T.; Tran, T.V. Green synthesis of CuO, ZnO and CuO/ZnO nanoparticles using Annona glabra leaf extract for antioxidant, antibacterial and photocatalytic activities. J. Environ. Chem. Eng. 2023, 11, 111003–111016. [Google Scholar] [CrossRef]
- Takele, E.; Bogale, R.F.; Shumi, G.; Kenasa, G. Green synthesis, characterization, and antibacterial activity of CuO/ZnO nanocomposite using Zingiber officinale rhizome extract. J. Chem. 2023, 2023, 20233481389. [Google Scholar] [CrossRef]
- Vindhya, P.S.; Kavitha, V.T. Effect of cobalt doping on antimicrobial, antioxidant and photocatalytic activities of CuO nanoparticles. Mater. Sci. Eng. B. 2023, 289, 116258–116270. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Haitosa, H.H.; Chen, X.; Wu, Y.-N. Recent progress on plant extract-mediated biosynthesis of ZnO-based nanocatalysts for environmental remediation: Challenges and future outlooks. Adv. Colloid. Interface Sci. 2023, 317, 102931–102951. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules 2022, 27, 3206. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Goddati, M.; Lee, J.; Sabir, F.K. Green synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega 2022, 7, 30908–30919. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.H.; Jalani, N.F.; Mamat, R.; Astimar, A.A. A review on the development of palm oil mill effluent (POME) final discharge polishing treatments. J. Oil Palm Res. 2017, 29, 528–540. [Google Scholar] [CrossRef]
- Andani, R.; Fajrina, A.; Asra, R.; Eriadi, A. Antibacterial activity test of mangosteen plants (Garcinia mangostana L.): A review. Asian J. Pharm. Res. Dev. 2021, 9, 164–171. [Google Scholar] [CrossRef]
- Diniatik, D.; Anggraeni, R.S. Antibacterial (Staphylococcus aureus and Escherichia coli) and antifungal (Saccharomyces cerevisiae) activity assay on nanoemulsion formulation of ethanol extract of mangosteen leaves (Garcinia mangostana L.) as fruit preservative. J. Food Pharm. Sci. 2021, 9, 351–365. [Google Scholar] [CrossRef]
- Jassim, A.M.N.; Shafy, G.M.; Mohammed, M.T.; Farhan, S.A.; Noori, O.M. Antioxidant, anti-inflammatory and wound healing of biosynthetic gold nanoparticles using mangosteen (G. mangostona). Iraqi J. Ind. Res. 2021, 8, 59–74. [Google Scholar] [CrossRef]
- Tran, V.A.; Vo, T.-T.T.; Nguyen, M.-N.T.; Dat, N.D.; Doan, V.-D.; Nguyen, T.-Q.; Vu, Q.H.; Le, V.T.; Tong, T.D. Novel α-mangostin derivatives from mangosteen (Garcinia mangostana L.) peel extract with antioxidant and anticancer potential. J. Chem. 2021, 2021, 9985604. [Google Scholar] [CrossRef]
- Jaithon, T.; Atichakaro, T.; Phonphoem, W.; T-Thienprasert, J.; Sreewongchai, T.; T-Thienprasert, N.P. Potential usage of biosynthesized zinc oxide nanoparticles from mangosteen peel ethanol extract to inhibit Xanthomonas oryzae and promote rice growth. Heliyon 2024, 10, e24076–e24085. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectrometry, 5th ed.; Nelson Education Ltd.: Stamford, CT, USA, 2015; pp. 1–690. [Google Scholar]
- Suresh, S.; Gunasekaran, S.; Srinivasan, S. Vibrational spectra (FT-IR, FT-Raman), frontier molecular orbital, first hyperpolarizability, NBO analysis and thermodynamics properties of Piroxicam by HF and DFT methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 447–459. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.C.; Mudau, F.N.; Maaza, M. Effect of optimized precursor concentration, temperature, and doping on optical properties of ZnO nanoparticles synthesized via a green route using bush tea (Athrixia phylicoides DC.) leaf extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef]
- Khan, M.I.; Fatima, N.; Shakil, M.; Tahir, M.B.; Riaz, K.N.; Rafique, M.; Iqbal, T.; Mahmood, K. Investigation of in vitro antibacterial and seed germination properties of green synthesized pure and nickel doped ZnO nanoparticles. Phys. B Phys. Condens. Matter. 2021, 601, 412563. [Google Scholar] [CrossRef]
- Chan, Y.B.; Aminuzzaman, M.; Tey, L.-H.; Win, Y.F.; Watanabe, A.; Djearamame, S.; Akhtaruzzaman, M. Impact of diverse parameters on the physicochemical characteristics of green-synthesized zinc oxide–copper oxide nanocomposites derived from an aqueous extract of Garcinia mangostana L. leaf. Materials 2023, 16, 5421. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tan, L.X.; Yip, F.W.; Eddy Cheah, S.G.; Heng, M.H.; Tey, L.H.; Arullappan, S.; Algethami, N.; Alharthi, S.S.; et al. Synthesis, characterization, and preliminary in vitro antibacterial evaluation of ZnO nanoparticles derived from soursop (Annona muricata L.) leaf extract as a green reducing agent. J. Mater. Res. Technol. 2022, 20, 2931–2941. [Google Scholar] [CrossRef]
- Parapat, R.Y.; Schwarze, M.; Ibrahim, A.; Tasbihi, M.; Schomäcker, R. Efficient preparation of nanocatalysts. Case study: Green synthesis of supported Pt nanoparticles by using microemulsions and mangosteen peel extract. R. Soc. Chem. Adv. 2022, 12, 34346–34358. [Google Scholar] [CrossRef]
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO nanoparticles with high dispersibility based on oriented attachment (OA) process. Nanoscale. Res. Lett. 2019, 14, 210. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of metal and metal oxide nanoparticles. Chem. Bio. Eng. Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Amin, F.; Fozia; Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evid. Based Complement. Altern. Med. 2021, 2021, 589703. [Google Scholar] [CrossRef]
- Phang, Y.-K.; Aminuzzaman, M.; Akhtaruzzaman, M.; Muhammad, G.; Ogawa, S.; Watanabe, A.; Tey, L.-H. Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustainability 2021, 13, 796. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green synthesis of copper oxide (CuO) nanoparticles by Punica granatum peel extract. Mater. Today Proc. 2021, 36, 751–755. [Google Scholar] [CrossRef]
- Park, G.C.; Seo, T.Y.; Park, C.H.; Lim, J.H.; Joo, J. Effects of calcination temperature on morphology, microstructure, and photocatalytic performance of TiO2 mesocrystals. Ind. Eng. Chem. Res. 2017, 56, 8235–8240. [Google Scholar] [CrossRef]
- Habibi, M.H.; Karimi, B. Effect of the annealing temperature on crystalline phase of copper oxide nanoparticle by copper acetate precursor and sol-gel method. J. Therm. Anal. Calorim. 2014, 115, 419–423. [Google Scholar] [CrossRef]
- Yusoff, H.M.; Idris, N.H.; Hipul, N.F.; Yusoff, N.F.M.; Izham, N.Z.M.; Bhat, I.U.H. Green synthesis of zinc oxide nanoparticles using black tea extract and its potential as anode material in sodium-ion batteries. Malays. J. Chem. 2020, 22, 43–51. [Google Scholar]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896–e04908. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of copper-zinc oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003–100009. [Google Scholar] [CrossRef]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Enhanced anti-bacterial activities of ZnO nanoparticles and ZnO/CuO nanocomposites synthesized using Vaccinium arctostaphylos L. fruit extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1200–1209. [Google Scholar] [CrossRef]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: Characterization and their application as anti-bacterial agents. J. Mater. Sci. Mater. Electron. 2018, 29, 13596–13605. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Ansari, M.T.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.; Rafatullah, M. Optimization of facile synthesized ZnO/CuO nanophotocatalyst for organic dye degradation by visible light irradiation using response surface methodology. Catalysts 2021, 11, 1509. [Google Scholar] [CrossRef]
- Chan, Y.B.; Selvanathan, V.; Tey, L.-H.; Akhtaruzzaman, M.; Anur, F.H.; Djearamane, S.; Watanabe, A.; Aminuzzaman, M. Effect of calcination temperature on structural, morphological and optical properties of copper oxide nanostructures derived from Garcinia mangostana L. leaf extract. Nanometerials 2022, 12, 3589. [Google Scholar] [CrossRef]
- Sajjad, A.; Bhatti, S.H.; Ali, Z.; Jaffari, G.H.; Khan, N.A.; Rizvi, Z.F.; Zia, M. Photoinduced fabrication of zinc oxide nanoparticles: Transformation of morphological and biological response on light irradiance. ACS. Omega. 2021, 6, 11783–11793. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, D.K.; Chawla, K.; Lai, N.; Lai, C. Synthesis and characterization of CuO nanoparticles by Aloe barbadensis leaves. Quantum J. Eng. Sci. Technol. 2021, 2, 1–9. [Google Scholar]
- You, W.; Ahn, J.C.; Boopathi, V.; Arunkumar, L.; Rupa, E.J.; Akter, R.; Kong, B.M.; Lee, G.S.; Yang, D.C.; Kang, S.C.; et al. Enhanced antiobesity efficacy of tryptophan using the nanoformulation of Dendropanax morbifera extract mediated with ZnO nanoparticle. Materials 2021, 14, 824. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Chong, C.-Y.; Goh, W.-S.; Phang, Y.-K.; Tey, L.-H.; Chee, S.-Y.; Akhtaruzzaman, M. Biosynthesis of NiO nanoparticles using soursop (Annona muricata L.) fruit peel green waste and their photocatalytic performance on crystal violet dye. J. Clust. Sci. 2021, 32, 949–958. [Google Scholar] [CrossRef]
- Ramzan, M.; Obodo, R.M.; Mukhtar, S.; Ilyas, S.Z.; Aziz, F.; Thovhogi, N. Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater. Today Proc. 2021, 36, 576–581. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Shin, S.-W.; Kim, K.; Park, Y. Facile green synthesis of titanium dioxide nanoparticles by upcycling mangosteen (Garcinia mangostana) pericarp extract. Nanoscale Res. Lett. 2022, 17, 40–51. [Google Scholar] [CrossRef]
- Basavalingiah, K.R.; Harishkumar, S.; Udayabhanu; Nagaraju, G.; Rangappa, D. Chikkahanumantharayappa. Highly porous, honeycomb like Ag–ZnO nanomaterials for enhanced photocatalytic and photoluminescence studies: Green synthesis using Azadirachta indica gum. SN Appl. Sci. 2019, 1, 935. [Google Scholar] [CrossRef]
- Mfon, R.E.; Hall, S.R.; Sarua, A. Effect of Ocimum gratissimum plant leaf extract concentration and annealing temperature on the structure and optical properties of synthesized zinc oxide nanoparticles. Educ. J. Sci. Math. Technol. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater. Res. 2020, 24, 11. [Google Scholar] [CrossRef]
- Karthiga, P.; Rajeshkumar, S.; Annadurai, G. Mechanism of larvicidal activity of antimicrobial silver nanoparticles synthesized using Garcinia mangostana bark extract. J. Clust. Sci. 2018, 29, 1233–1241. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. Synthesis and characterization of ZnO/CuO nanocomposite by electrochemical method. Mater. Sci. Semicond. Process. 2017, 57, 173–177. [Google Scholar] [CrossRef]
- Georgia, B.J.; Gheena, S.; Pratibha, R.; Rajesh, K.S.; Kartikeyan, R.; Abilasha, R. Anticancer activity of green synthesized selenium nanoparticles from Garcinia mangostana crude extract against MCF-7 breast cancer cells. J. Popul. Ther. Clin. Pharmacol. 2023, 30, e74–e82. [Google Scholar] [CrossRef]
- Yuan, L.-D.; Deng, H.-X.; Li, S.-S.; Wei, S.-H.; Luo, J.-W. Unified theory of direct or indirect band-gap nature of conventional semiconductors. Phys. Rev. B 2018, 98, 245203. [Google Scholar] [CrossRef]
- Lany, S. Semiconducting transition metal oxides. J. Phys. Condens. Matter. 2015, 27, 283203. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.T.; Ravikumar, R.V.S.S.N. Novel Fe-doped ZnO-CdS nanocomposite with enhanced visible light-driven photocatalytic performance. Mater. Res. Innov. 2020, 25, 215–220. [Google Scholar] [CrossRef]
- Fundamentals of Light-Emitting Diodes (LEDs). Available online: https://zeiss-campus.magnet.fsu.edu/print/lightsources/leds-print.html#:~:text=However%2C most white light diodes,emission at a longer wavelength (accessed on 9 January 2024).
- Tan, Y.H.; Goh, P.S.; Lai, G.S.; Lau, W.J.; Ismail, A.F. Treatment of aerobic treated palm oil mill effluent (AT-POME) by using TiO2 photocatalytic process. J. Teknol. 2014, 70, 61–63. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Ahmad, N.L.B.; Sedik, N.B.M.; Long, S.G.H.; Guan, T.M.; Chin, L.Y. Performance of solar photocatalysis and photo-fenton degradation of palm oil mill effluent. Malays. J. Anal. Sci. 2017, 21, 996–1007. [Google Scholar] [CrossRef]
- Chai, H.Y.; Lam, S.M.; Sin, J.C. Green synthesis of ZnO nanoparticles using Hibiscus rosa-sinensis leaves extracts and evaluation of their photocatalytic activities. In Proceedings of the International Symposium on Green and Sustainable Technology (ISGST2019), Perak, Malaysia, 23–26 April 2019. [Google Scholar]
- Lam, S.-M.; Wong, K.-A.; Sin, J.-C. Fabrication of flower-like ZnO micro/nanostructures for photodegradation of pre-treated palm oil mill effluent. IOP Conf. Ser. Earth Environ. Sci. 2018, 112, 012003–012007. [Google Scholar] [CrossRef]
- Chan, Y.B.; Aminuzzaman, M.; Khalilur Rahman, M.; Win, Y.F.; Sultana, S.; Cheah, S.-Y.; Watanabe, A.; Wong, L.S.; Guha, S.M.; Djearamane, S.; et al. Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study. Green Process. Synth. 2024, 13, 20230251–20230270. [Google Scholar] [CrossRef]




| Synthesized Nanomaterials | FT-IR (cm−1) | XRD | FE-SEM | UV-Vis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| v(O–H) | v(C=C) | v(–CH3) | v(C–O–C) | v(M–O) * | Average Crystalline Size (nm) | Dislocation Density (×1014 cm−1) | Micro Strain (×10−4) | Average Particle Size (nm) | Morphology | Energy Bandgap (eV) | |
| CuO NPs | 3393 | 1636 | 1383 | 1094 | 537 | 24.42 | 16.77 | 1.46 | 116.27 | Spherical | 3.23 |
| ZnO NPs | 3458 | 1634 | 1383 | 1113 | 514 | 22.66 | 19.47 | 1.56 | 54.95 | Spherical | 2.52 |
| CuO-ZnO NCs | 3466 | 1633 | 1384 | 1098 | 540 | 29.04 | 11.86 | 1.25 | 82.32 | Spherical | 1.61 |
| Plants | Calcination Temperature (°C) | Energy Bandgap (eV) | Average Crystalline Size (nm) | Average Particle Size (nm) | Morphology | References |
|---|---|---|---|---|---|---|
| Coriandrum sativum (leaf) | 350 | - | 11.30 | 11.00 | Irregular | [18] |
| Annona glabra (leaf) | 500 | 1.32–3.06 | 22.46–27.21 | 16.13–35.19 | Spherical | [21] |
| Zingiber officinale (rhizome) | - | 2.58–2.76 | 18.41–20.50 | - | - | [22] |
| Dovyalis caffra (leaf) | 400 | - | 23.21 | 20.00–32.00 | Spherical | [25] |
| Verbascum sinaiticum (leaf) | 500 | 2.74 | 18.00 | - | Plate | [26] |
| G. mangostana (leaf) | 600 | 1.61 | 29.04 | 82.32 | Spherical | Current study |
| Synthesized Nanomaterials | LEDs | COD Removal Efficiency (Means ± SD) |
|---|---|---|
| CuO NPs | Blue | 39.81 ± 0.016 |
| White | 40.14 ± 0.025 | |
| ZnO NPs | Blue | 43.85 ± 0.010 1 |
| White | 44.02 ± 0.017 1 | |
| CuO-ZnO NPs | Blue | 63.27 ± 0.010 |
| White | 35.77 ± 0.017 |
| Nanomaterials | Conditions | COD Removal Efficiency (%) | References | |||
|---|---|---|---|---|---|---|
| Light Sources | Nanomaterial Loadings (g/L) | POME Volume (mL) | Duration (min) | |||
| CuO | UV light | 0.50 | 300 | 180 | 66.00 | [48] |
| TiO2 | UV light | 5.00 | 250 | 240 | 43.00 | [75] |
| TiO2 | Sunlight | 0.02 | 150 | 300 | 54.30 | [76] |
| ZnO | Sunlight | 0.20 | 150 | 300 | 40.00 | |
| ZnO | UV light | 1.00 | 400 | 240 | 82.00 | [77] |
| ZnO | UV light | 1.00 | 400 | 120 | 96.00 | [78] |
| CuO-ZnO | Blue LED | 0.75 | 200 | 150 | 63.27 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.B.; Aminuzzaman, M.; Win, Y.F.; Djearamane, S.; Wong, L.S.; Guha, S.K.; Almohammadi, H.; Akhtaruzzaman, M.; Tey, L.-H. Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Catalysts 2024, 14, 486. https://doi.org/10.3390/catal14080486
Chan YB, Aminuzzaman M, Win YF, Djearamane S, Wong LS, Guha SK, Almohammadi H, Akhtaruzzaman M, Tey L-H. Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Catalysts. 2024; 14(8):486. https://doi.org/10.3390/catal14080486
Chicago/Turabian StyleChan, Yu Bin, Mohammod Aminuzzaman, Yip Foo Win, Sinouvassane Djearamane, Ling Shing Wong, Samar Kumar Guha, Hamad Almohammadi, Md. Akhtaruzzaman, and Lai-Hock Tey. 2024. "Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME)" Catalysts 14, no. 8: 486. https://doi.org/10.3390/catal14080486
APA StyleChan, Y. B., Aminuzzaman, M., Win, Y. F., Djearamane, S., Wong, L. S., Guha, S. K., Almohammadi, H., Akhtaruzzaman, M., & Tey, L.-H. (2024). Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Catalysts, 14(8), 486. https://doi.org/10.3390/catal14080486






