Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies
Abstract
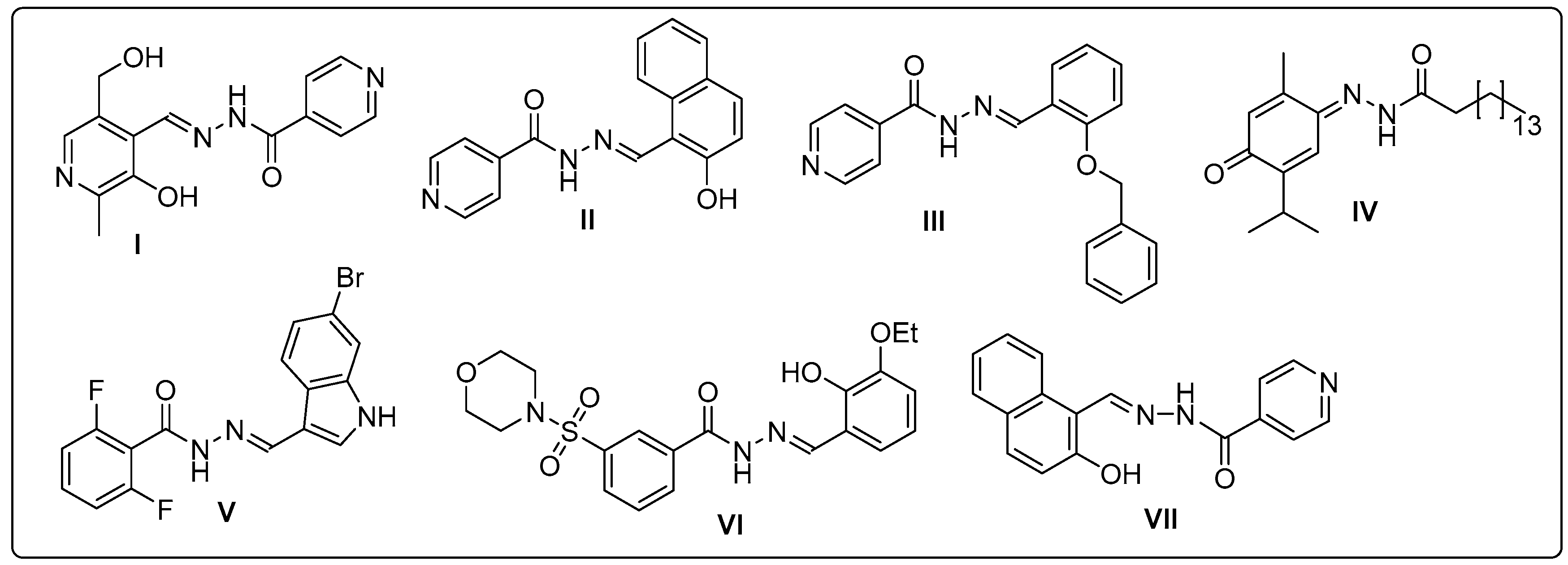
:1. Introduction
2. Results and Discussion
2.1. Antitumor Study
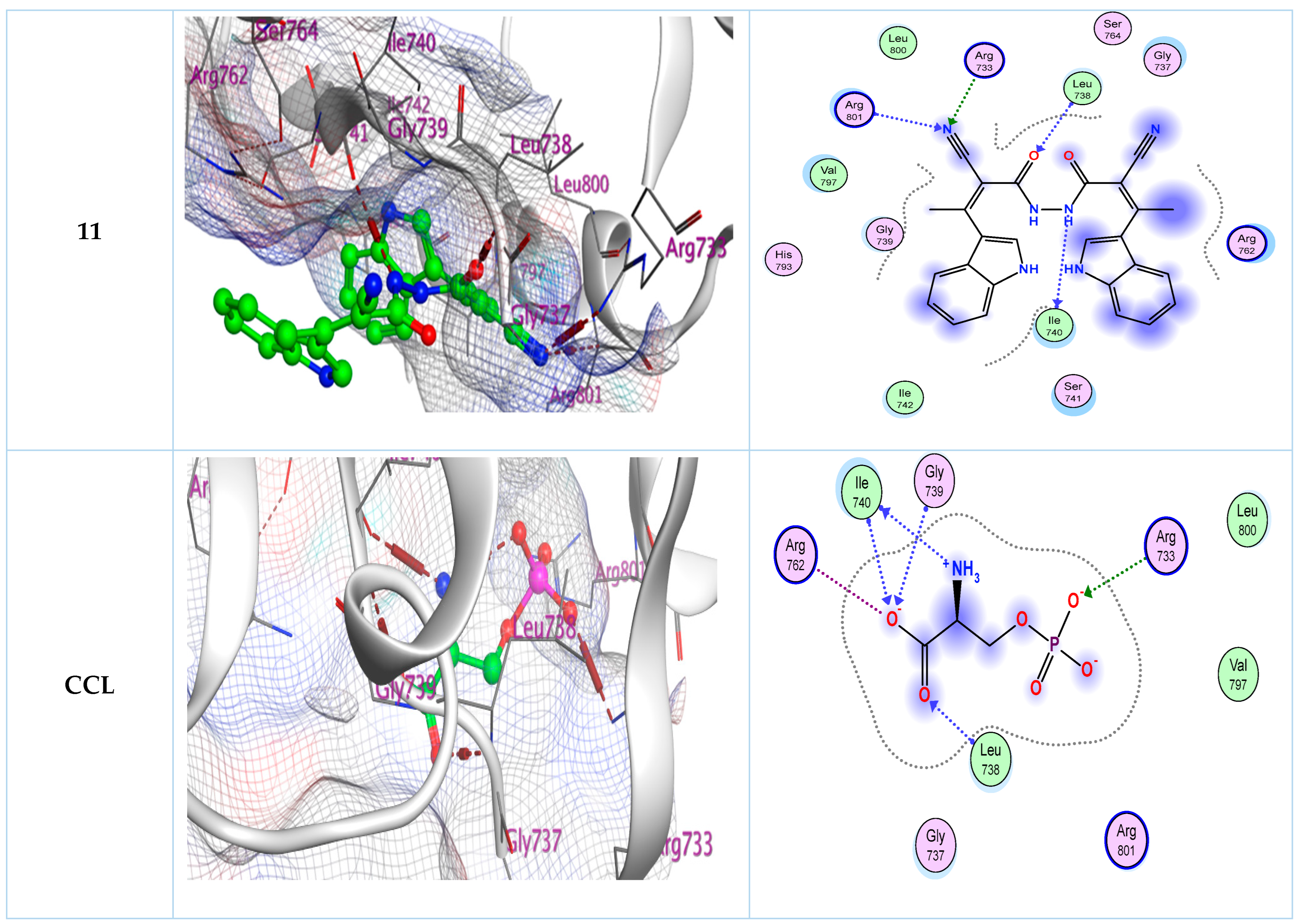
2.2. Molecular Docking Study
2.3. In Silico Pharmacokinetic Profile (ADMET)
3. Experimental
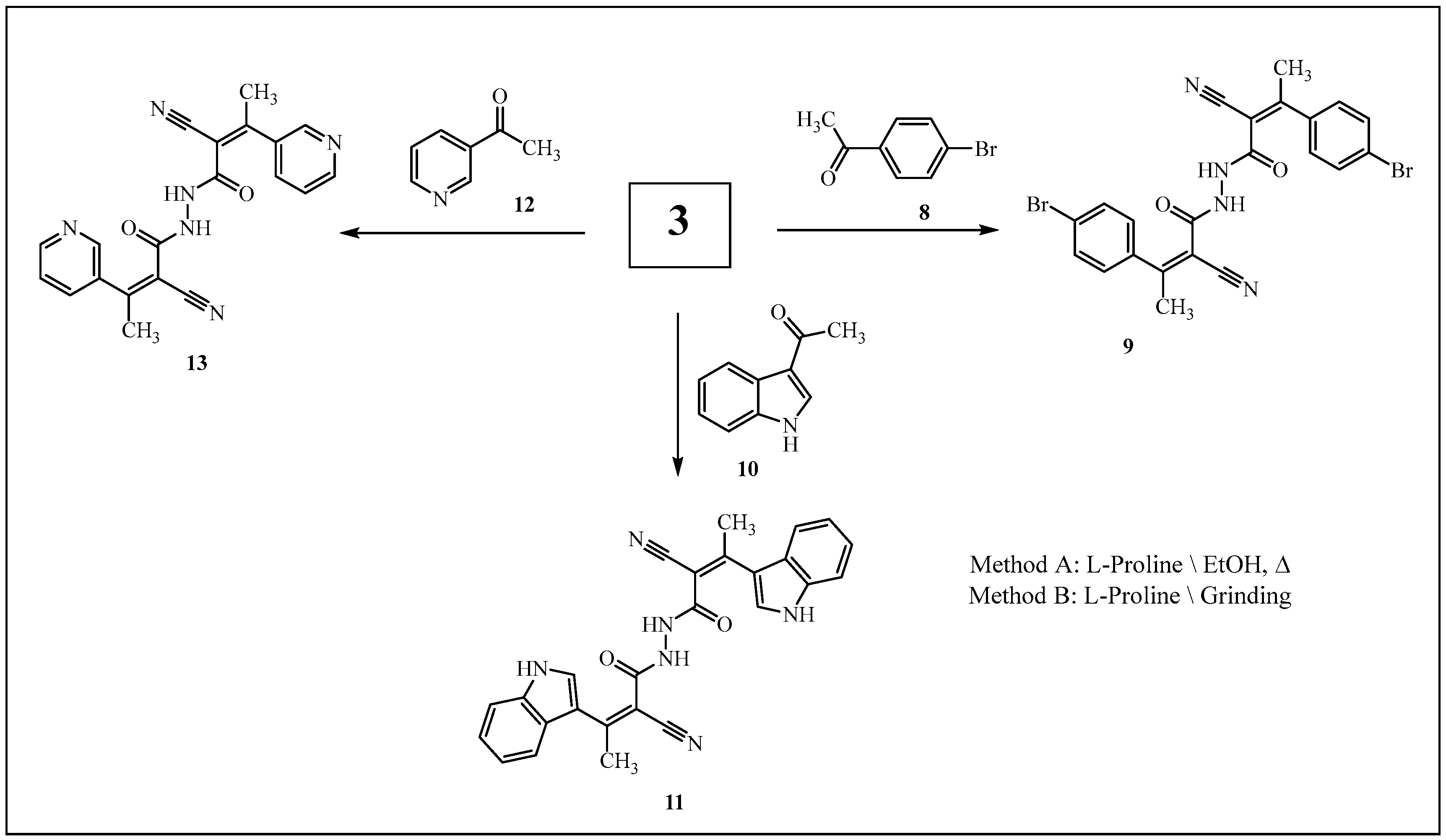
3.1. Synthesis of 2-cyano-N’-(2-cyanoacetyl)acetohydrazide (3)
3.2. Reaction of 3 with Aromatic (or Heteroarmatic) Carbonyl Compounds 4a–e, 6a,b, 8, 10, and 12
3.2.1. Method A
3.2.2. Method B
3.3. Cytotoxicity Assay
3.4. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, Z.; Qu, W.; Yang, R.; Lin, X.; Yan, S.; Lin, J. An environmentally benign, mild, and catalyst-free reaction of quinones with heterocyclic ketene aminals in ethanol: Site-selective synthesis of rarely fused [1,2-a]indolone derivatives via an unexpected anti-Nenitzescu strategy. Green Chem. 2014, 16, 4359–4370. [Google Scholar] [CrossRef]
- Kiyani, H.; Ghorbani, F. Expeditious Green Synthesis of 3,4-disubstituted isoxazole-5(4H)-ones Catalyzed by Nano-MgO. Res. Chem. Intermed. 2016, 42, 6831–6844. [Google Scholar] [CrossRef]
- Kiyani, H.; Darbandi, H.; Mosallanezhad, A.; Ghorbani, F. 2-Hydroxy-5-sulfobenzoic acid: An efficient organocatalyst for the three-component synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. Res. Chem. Intermed. 2015, 41, 7561–7579. [Google Scholar] [CrossRef]
- Brahmachari, G.; Banerjee, B. Facile and One-Pot Access to Diverse and Densely Functionalized 2-Amino-3-cyano-4H-pyrans and Pyran-Annulated Heterocyclic Scaffolds via an Eco-Friendly Multicomponent Reaction at Room Temperature Using Urea as a Novel Organo-Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 411–422. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Hassan, S.A. Green Synthesis and Molecular Docking of Thiazolyl-thiazole Derivatives as Potential Cytotoxic Agents. Mini-Rev. Med. Chem. 2017, 17, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Badrey, M.G.; Arafa, W.A.A. DABCO-catalyzed green synthesis of thiazole and 1,3-thiazine derivatives linked to benzofuran. Heterocycles 2016, 92, 1450–1461. [Google Scholar]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green synthesis, molecular docking and anticancer activity of novel 1,4-dihydropyridine-3,5-dicarbohydrazones under grind-stone chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar]
- Kumar, S.; Sharma, M. A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction. Green Chem. 2011, 13, 2017–2020. [Google Scholar] [CrossRef]
- Pasha, M.A.; Nizam, A. Amberlite IR-120 catalyzed, microwave-assisted rapid synthesis of 2-aryl-benzimidazoles. J. Saudi Chem. Soc. 2012, 16, 237–240. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Padmini, N.; Bhuvanesh, N.S.P. Chemodivergent, One-Pot, Multi-Component Synthesis of Pyrroles and Tetrahydropyridines under Solvent- and Catalyst-Free Conditions Using the Grinding Method. ACS Comb. Sci. 2016, 18, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1047. [Google Scholar] [CrossRef] [PubMed]
- Dolotko, J.W.; Wiench, K.W.; Dennis, K.W.; Pecharsky, V.K.; Balema, V.P. Mechanically induced reactions in organic solids: Liquid eutectics or solid-state processes? New J. Chem. 2010, 34, 25–28. [Google Scholar] [CrossRef]
- Brahmachari, G.; Das, S. L-Proline catalyzed multicomponent one-pot synthesis of gem-diheteroarylmethane derivatives using facile grinding operation under solvent-free conditions at room temperature. RSC Adv. 2014, 4, 7380. [Google Scholar] [CrossRef]
- Edrees, M.M.; Abu-Melha, S.A.; Saad, M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-Friendly synthesis, characterization and biological evaluation of some novel pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, H.R.M.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo[1,2-b]phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M.; Abolibda, T.Z.; Hassan, S.A.; Abdalla, M.M. Green synthesis, molecular docking and pharmacological evaluation of new triazolo-thiadiazepinylcoumarine derivatives as sedative-hypnotic scaffold. J. Heterocycl. Chem. 2020, 57, 1034–1043. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304. [Google Scholar] [CrossRef]
- Huang, W.; Anwar, S.; Chen, K. Morita–Baylis–Hillman (MBH) reaction derived nitroallylic alcohols, acetates and amines as synthons in organocatalysis and heterocycle synthesis. Chem. Rec. 2017, 17, 363–381. [Google Scholar] [CrossRef]
- Amarante, G.W.; Coelho, F. Quim. p-Sulfonic acid calix[4]arene as a new reusable organocatalyst for the transesterification of vegetable oil. Nova 2009, 32, 469. [Google Scholar]
- Stowe, G.N.; Janda, K.D. Diels-Alder reaction conducted within the parameters of aqueous organocatalysis: Still just smoke and mirrors. Tetrahedron Lett. 2011, 52, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Mecadon, H.; Rumum, M.; Kharbangar, I.; Laloo, B.M.; Kharkongor, I.; Rajbangshi, M.; Myrboh, B. L-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2, 4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in water. Tetrahedron Lett. 2011, 52, 3228. [Google Scholar] [CrossRef]
- Hernández, J.G.; García-López, V.; Juaristi, E. Solvent-free asymmetric aldol reaction organocatalyzed by (S)-proline-containing thiodipeptides under ball-milling conditions. Tetrahedron 2012, 68, 92–97. [Google Scholar] [CrossRef]
- Yarhosseini, M.; Javanshir, S.; Dekamin, M.G.; Farhadnia, M. Tetraethylammonium 2-(carbamoyl)benzoate as a bifunctional organocatalyst for one-pot synthesis of Hantzsch 1,4-dihydropyridine and polyhydroquinoline derivatives. Monatsh. Chem. 2016, 147, 1779–1787. [Google Scholar] [CrossRef]
- Schettini, R.; De Riccardis, F.; Sala, G.D.; Izzo, I. Enantioselective Alkylation of Amino Acid Derivatives Promoted by Cyclic Peptoids under Phase-Transfer Conditions. J. Org. Chem. 2016, 81, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Harakeh, S.M. Bioactive 2(1H)-Pyrazinones and Diketopiperazine Alkaloids from a Tunicate-Derived Actinomycete Streptomyces sp. Molecules 2016, 21, 1116. [Google Scholar] [CrossRef] [PubMed]
- Schettini, R.; Costabile, C.; Della Sala, G.; Buirey, J.; Tosolini, M.; Tecilla, P.; Vaccaro, M.C.; Bruno, I.; De Riccardis, F.; Izzo, I. Tuning the biomimetic performances of 4-hydroxyproline-containing cyclic peptoids. Org. Biomol. Chem. 2018, 16, 6708–6717. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal cancer genetics, incidence and risk factors: In search for targeted therapies. Cancer Manag. Res. 2020, 12, 9869–9882. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Rejhova, A.; Opattova, A.; Cumova, A.; Slíva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Alam, W.; Bouferraa, Y.; Haibe, Y.; Mukherji, D.; Shamseddine, A. Management of colorectal cancer in the era of COVID-19: Challenges and suggestions. Sci. Prog. 2021, 104, 00368504211010626. [Google Scholar] [CrossRef]
- Fang, F.Q.; Guo, H.S.; Zhang, J.; Ban, L.Y.; Liu, J.W.; Yu, P.Y. Anti-cancer effects of 2-oxoquinoline derivatives on the HCT116 and LoVo human colon cancer cell lines. Mol. Med. Rep. 2015, 12, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Bornstein, J.C.; Nurgali, K. Anti-colorectal cancer chemotherapy-induced diarrhoea: Current treatments and side effects. Int. J. Clin. Med. 2014, 5, 393–406. [Google Scholar] [CrossRef]
- Ismail, T.; Donati-Zeppa, S.; Akhtar, S.; Turrini, E.; Layla, A.; Sestili, P.; Fimognari, C. Coffee in cancer chemoprevention: An updated review. Expert Opin. Drug Metab. Toxicol. 2020, 17, 69–85. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, Ş.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Raghav, N. Biological activities of hydrazones: A review. Int. J. Pharm. Pharm. Sci. 2011, 3, 26–32. [Google Scholar]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat Ali, M.; Mumtaz Alam, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar]
- Corcé, V.; Gouin, S.G.; Renaud, S.; Gaboriau, F.; Deniaud, D. Recent advances in cancer treatment by iron chelators. Bioorg. Med. Chem. Lett. 2016, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.S.; Parumasivam, T.; Jumaat, F.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- Wirries, A.; Breyer, A.; Quint, K.; Schobert, R.; Ocker, M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Exp. Ther. Med. 2010, 1, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing powerhouse in colon cancer cells by hydrazide−hydrazone-based small molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gutierrez, E.; Kovacevic, E.; Saletta, F.; Obeidy, P.; Suryo Rahmanto, Y.; Richardson, D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Peng, J.-D.; Zhou, W.; Qiao, H.; Deng, X.; Li, Z.-H.; Li, J.-D.; Fu, Y.-D.; Li, S.; Sun, K.; et al. Potent hydrazone derivatives targeting esophageal cancer cells. Eur. J. Med. Chem. 2018, 148, 359–371. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Jang, G.R.; Harris, R.Z.; Lau, D.T. Pharmacokinetics and its role in small molecule drug discovery research. Med. Res. Rev. 2001, 21, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Eddershaw, P.J.; Beresford, A.P.; Bayliss, M.K. ADME/PK as part of a rational approach to drug discovery. Drug Discov. Today 2000, 5, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdalla, M.A.; Abdelaziz, M.; Serag, N. Eco-friendly one-pot synthesis and antiviral evaluation of pyrazolyl pyrazolines of medicinal interest. Turk. J. Chem. 2016, 40, 484–498. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Farag, B.; Al-Hussain, S.A.; Zaki, M.E.A.; Mohamed, M.A. Green Synthesis of Hydrazono-Thiazolones Using Vitamin B1 and Their Antibacterial Implications. Green Chem. Lett. Rev. 2024, 17, 2380746. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and biological activities of 7-arylazo-7H-pyrazolo[5,1-c][1,2,4]triazolo-6(5H)-ones and 7-arylhydrazono-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. J. Chin. Chem. Soc. 2005, 52, 987–994. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Gomha, S.M.; Shawali, A.S.; Abdelhamid, O.A. Convenient method for the synthesis of various fused heterocycles via the utility of 4-acetyl-5-methyl-1-phenyl-pyrazole as a precursor. Turk. J. Chem. 2014, 38, 865–879. [Google Scholar] [CrossRef]
- Gomha, S.M.; El-Sayed, A.A.A.; Alrehaily, A.; Elbadawy, H.M.; Farag, B.; Al-Shahri, A.A.; Alsenani, S.R.; Abdelgawad, F.E.; Zaki, M.E.A. Synthesis, molecular docking, in silico study, and evaluation of bis-thiazole-based curcumin derivatives as potential antimicrobial agents. Res. Chem. 2024, 7, 101504. [Google Scholar] [CrossRef]
- Kurdi, A.A.; Morad, M.; Abumelha, H.M.; Alkhamis, K.; Aljohani, M.M.; Mersal, G.A.M.; El-Metwaly, N. Synthesis and characterization of novel VO(II) complexes from cyanoacetohydrazide-based ligands; Hirshfeld crystals, DFT study and biodiesel catalytic synthesis from waste frying oil. J. Mol. Struct. 2021, 1240, 130603. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Hassan, S.A. Green route synthesis and molecular docking of azines using cellulose sulfuric acid under microwave irradiation. Crystals 2023, 13, 260. [Google Scholar] [CrossRef]
- Abolibda, T.Z.; Albalawi, M.; Abdelaziz, M.R.; Abdel-aziz, H.M.; Gomha, S.M. Synthesis and molecular docking of some novel 3-thiazolyl-coumarins as inhibitors of VEGFR-2 kinase. Molecules 2023, 28, 689. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Saka, Y.; Gervasio, F.L.; Tanaka, H. Structural analysis of phosphorylation-associated interactions of human MCC with Scribble PDZ domains. FEBS J. 2019, 286, 4910–4925. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; El-Gohary, N.S.; El-Messiry, M.A.; Mohamed, N.A.; Gomha, S.M. Mechanochemical synthesis and molecular docking studies of new azines bearing indole as anticancer agents. Molecules 2023, 28, 3869. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Moda, T.L.; Bender, A.; Williams, A.J.; Ekins, S. PK/DB: Database for pharmacokinetic properties and predictive in silico ADME models. Bioinformatics 2008, 24, 2270–2271. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Sun, X.; Zhang, L.; Miao, R.; Zhang, H.; Jiang, H.; Wang, J. ADMET evaluation in drug discovery. 11. PharmacoKinetics Knowledge Base (PKKB): A comprehensive database of pharmacokinetic and toxic properties for drugs. J. Chem. Inf. Model. 2012, 52, 1132–1137. [Google Scholar] [CrossRef]
- Cumming, J.G.; Davis, A.M.; Muresan, S.; Haeberlein, M.; Chen, H. Chemical predictive modelling to improve compound quality. Nat. Rev. Drug Discov. 2013, 12, 948–962. [Google Scholar] [CrossRef]


| Compounds | Thermal Method | Grinding Method | ||
|---|---|---|---|---|
| Time (h) | (%) Yield | Time (min) | (%) Yield | |
| 5a | 5 | 75 | 25 | 90 |
| 5b | 4 | 74 | 27 | 89 |
| 5c | 3 | 79 | 21 | 92 |
| 5d | 4 | 73 | 26 | 91 |
| 5e | 3 | 78 | 20 | 89 |
| 7a | 5 | 72 | 29 | 84 |
| 7b | 5 | 70 | 30 | 85 |
| 9 | 4 | 78 | 27 | 91 |
| 11 | 5 | 77 | 23 | 90 |
| 13 | 4 | 79 | 28 | 90 |
| Entry | Catalyst (mol%) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | 1 | 25 | 25 | 71 |
| 2 | 3 | 25 | 25 | 83 |
| 3 a | 5 | 25 | 25 | 90 |
| 4 | 10 | 25 | 25 | 90 |
| State of Catalyst | New Catalyst | Recycled Catalyst | ||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | ||
| Yield of product 5a | 90 | 87 | 81 | 78 | 74 | 49 |
| Tested Compounds | IC50 Values (μM) | Tested Compounds | IC50 Values (μM) |
|---|---|---|---|
| 5a | 3.8 ± 0.7 | 7b | 37.2 ± 4.2 |
| 5b | 3.2 ± 1.1 | 9 | 9.3 ± 1.7 |
| 5c | 9.3 ± 1.4 | 11 | 2.5 ± 0.81 |
| 5d | 8.5 ± 1.3 | 13 | 3.7 ± 1.0 |
| 5e | 27.0 ± 2.5 | Cisplatin* | 2.43 ± 1.1 |
| 7a | 35.3 ± 3.1 |
| Compounds | Docking Score (kcal/mol) | No. of Hydrogen Bonding | No. of Arene Interaction | Donor Atom | Acceptor Atom |
|---|---|---|---|---|---|
| 5a | −5.75 | 1 (Gly739) 1 (Ile740) 1 (Arg733) 1 (Gly737) | 1 (π-H) [Gly739] | - | O N |
| 5b | −5.78 | 1 (Ile740) 2 (Gly737) 1 (Gly739) | - | - | O O/N O |
| 5c | −5.61 | 1 (Ile740) | 1 (π-H) [Leu738] 1 (π-Cation) [Arg801] | N | - |
| 5d | −5.72 | 1(Gly739) | 1 (π-H) [Gly739] | - | O |
| 11 | −6.40 | 1 (Ile740) 1 (Leu738) 1 (Arg733) 1 (Arg801) | - | N - - - | - O N N |
| CCL | −4.60 | 2 (Ile740) 1 (Gly739) 1 (Leu738) 1 (Arg733) | - | N/- - - - | -/O O O O |
| Compounds | Absorption | Distribution | Metabolism | Excretion (Log mL/min/kg) | Toxicity |
|---|---|---|---|---|---|
| Intestinal Absorption (Human) Numeric (% Absorbed) | VDss (Log L/kg) | AMES Toxicity (Categorical) (Yes/No) | |||
| 5a | 74.39 | −0.5 | CYP3A4 Substrate and CYP3A4 inhibitor | 0.25 | No |
| 5b | 65.21 | −0.33 | CYP1A2 inhibitor | 0.13 | No |
| 5c | 81.9 | −0.51 | CYP3A4 substrate; CYP1A2, CYP2C19, CYP2C9 and CYP3A4 inhibitor | −0.57 | No |
| 5d | 81.37 | −0.48 | CYP3A4 substrate; CYP2C19, CYP2C9 and CYP3A4 inhibitor | −0.61 | No |
| 11 | 83.02 | −0.04 | CYP2D6, CYP3A4 substrate; CYP1A2, CYP2C19, CYP2C9, and CYP3A4 inhibitor | 0.76 | No |
| Cisplatin * | 92.60 | 0.30 | - | 1.24 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Abolibda, T.Z.; Alruwaili, A.H.; Farag, B.; Boraie, W.E.; Al-Hussain, S.A.; Zaki, M.E.A.; Hussein, A.M. Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts 2024, 14, 489. https://doi.org/10.3390/catal14080489
Gomha SM, Abolibda TZ, Alruwaili AH, Farag B, Boraie WE, Al-Hussain SA, Zaki MEA, Hussein AM. Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts. 2024; 14(8):489. https://doi.org/10.3390/catal14080489
Chicago/Turabian StyleGomha, Sobhi M., Tariq Z. Abolibda, Awatif H. Alruwaili, Basant Farag, Waleed E. Boraie, Sami A. Al-Hussain, Magdi E. A. Zaki, and Ahmed M. Hussein. 2024. "Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies" Catalysts 14, no. 8: 489. https://doi.org/10.3390/catal14080489
APA StyleGomha, S. M., Abolibda, T. Z., Alruwaili, A. H., Farag, B., Boraie, W. E., Al-Hussain, S. A., Zaki, M. E. A., & Hussein, A. M. (2024). Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts, 14(8), 489. https://doi.org/10.3390/catal14080489









