Application of Catalysts in the Conversion of Biomass and Its Derivatives
Abstract
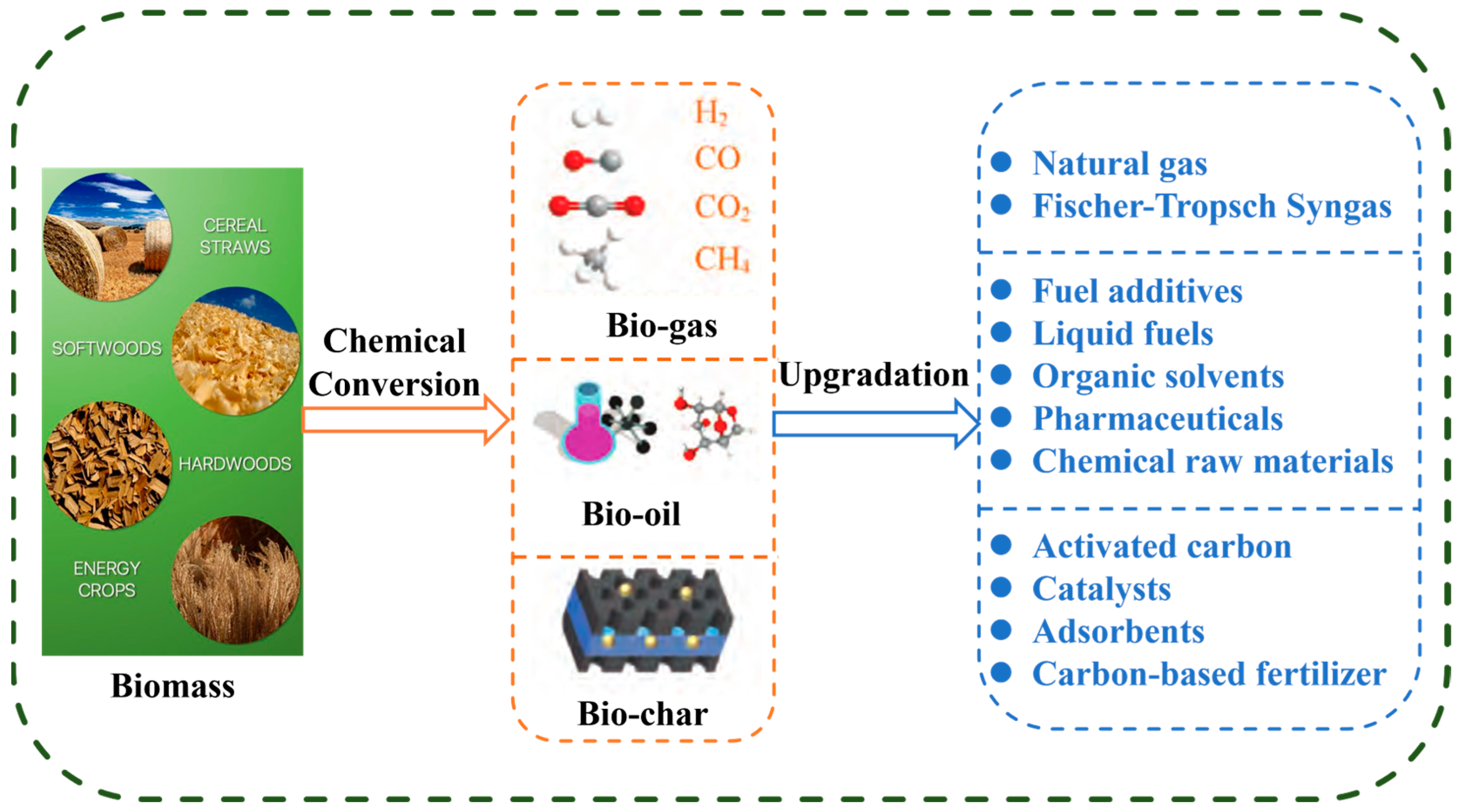
:1. Introduction
2. Application of Catalysts in Biomass Conversion
2.1. Application of Bio-Char Catalysts
2.2. Application of Catalysts in Bio-Oil
2.3. Application of Catalysts in Biomass Syngas
3. Application of Catalysts in the Preparation of High-Value-Added Chemicals from Biomass and Its Derivatives
3.1. Application of Catalysts in the Preparation of 5-HMF and Its Derivatives from Biomass
3.2. Application of Catalysts in the Preparation of LA and Its Derivatives from Biomass
4. Prospects and Challenges of Catalysts in Biomass Conversion
- (1)
- Thorough study of the reaction mechanisms and kinetics of catalysts in biomass conversion. People should adopt advanced scientific research methods and technologies to further systematically deepen and elucidate the key theoretical research of catalysts in each step of biomass conversion processes, especially in the study of efficient catalytic mechanisms for specific biomass conversion products and high-value-added chemical components, in order to better guide practical applications with theory.
- (2)
- Focusing on the catalytic efficiency, deactivation, regeneration, and recycling of catalysts in the biomass conversion process. Adopting new technologies and concepts to develop high-performance green catalysts with high selectivity, good stability, long service life, low cost, and environmental friendliness.
- (3)
- Optimizing and constructing the reaction conditions and production processes for catalysts in biomass conversion. Adopting scientific and systematic methods to construct safe, stable, economical, efficient, and green biomass conversion processes, reasonably optimizing and adjusting different reaction conditions, and achieving their synergistic effect on the catalytic conversion of biomass.
- (4)
- Exploring the application of catalysts in biomass conversion by combining structure-reactivity relationships on the basis of molecular simulation and machine learning. Through new technologies such as molecular simulation and machine learning, we can scientifically integrate experimental and computational data, where, combined with structure reactivity relationships, efficient catalysts are directly screened and specifically designed and their technical and economic performance is reasonably evaluated.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Theerthagiri, J.; Karuppasamy, K.; Park, J.; Rahamathulla, N.; Kumari, M.L.A.; Souza, M.K.R.; Cardoso, E.S.F.; Murthy, A.P.; Maia, G.; Kim, H.S.; et al. Electrochemical conversion of biomass-derived aldehydes into fine chemicals and hydrogen: A review. Environ. Chem. Lett. 2023, 21, 1555–1583. [Google Scholar] [CrossRef]
- Babu, S.; Jojo, L.; James, A.; Melethil, K.; Thomas, B. Metal-based catalysis for biomass and renewables valorization-current status. Tetrahedron Green Chem 2023, 2, 100018. [Google Scholar] [CrossRef]
- Guo, L.Y.; Li, J.F.; Lu, Z.W.; Zhang, J.; He, C.T. Biomass-Derived Carbon-Based Multicomponent Integration Catalysts for Electrochemical Water Splitting. ChemSusChem 2023, 16, e202300214. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lin, N.; Li, Y.; Xue, J.; Li, F.; Wei, L.; Yu, M.; Zha, X.; Li, W. Research on the application of catalytic materials in biomass pyrolysis. J. Anal. Appl. Pyrolysis. 2024, 177, 106321. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.N.; Parmon, V.N. Use of Microalgae Biomass to Synthesize Marketable Products: 2. Modern Approaches to Integrated Biorefinery of Microalgae Biomass. Catal. Industry 2024, 16, 69–76. [Google Scholar] [CrossRef]
- Wu, G.; Shen, C.; Liu, S. Research progress on the preparation and application of biomass derived methyl levulinate. Green Chem. 2021, 23, 9254–9282. [Google Scholar] [CrossRef]
- Delmas, G.H.; Banoub, J.H.; Delmas, M. Lignocellulosic biomass refining: A review promoting a method to produce sustainable hydrogen, fuels, and products. Waste Biomass Valorization 2022, 13, 2477–2491. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Priya, B.; Chinthala, R. Valorization of biomass-derived furans over molecular catalysts. Tetrahedron Green Chem 2023, 1, 100008. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S. Lignocellulosic biomass-based pyrolysis: A comprehensivereview. Chemosphere 2022, 286, 131824. [Google Scholar]
- Wang, S.; Li, Z.; Bai, X.; Yi, W.; Fu, P. Catalytic pyrolysis of lignin in a cascade dual-catalyst system of modified red mud and HZSM-5 for aromatic hydrocarbonproduction. Bioresour. Technol. 2019, 278, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; A Kozinski, J.; K Dalai, A. Lignocellulosic biomass: A review of conversion technologies and fuel products. Curr. Biochem. Eng. 2016, 3, 24–36. [Google Scholar] [CrossRef]
- Fan, K.; Mao, W.; Qu, H. Study on government subsidy in a two-level supply chain of direct-fired biomass power generation based on contract coordination. J. Ind. Manag. Optim. 2023, 19, 2436–2451. [Google Scholar] [CrossRef]
- Meena, M.K.; Anand, S.; Ojha, D.K. Interdependency of pyrolysis and combustion: A case study for lignocellulosic biomass. J. Therm. Anal. Calorim. 2023, 148, 5509–5519. [Google Scholar] [CrossRef]
- Pati, S.; De, S.; Chowdhury, R. Exploring the hybrid route of bio-ethanol production via biomass co-gasification and syngas fermentation from wheat straw and sugarcane bagasse: Model development and multi-objective optimization. J. Clean. Product. 2023, 395, 136441. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Sirohi, R. Critical Review of Biochemical Pathways to Transformation of Waste and Biomass into Bioenergy. Bioresour. Technol. 2023, 372, 128679. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar]
- Chen, J.Y.; Xiao, Y.; Guo, F.S.; Li, K.M.; Huang, Y.B.; Lu, Q. Single-Atom Metal Catalysts for Catalytic Chemical Conversion of Biomass to Chemicals and Fuels. ACS Catal. 2024, 14, 5198–5226. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y. Research perspectives for catalytic valorization of biomass. J. Energy Chem. 2023, 78, 102–104. [Google Scholar] [CrossRef]
- Nzediegwu, E.; Dumont, M.J. Chemo-catalytic transformation of cellulose and cellulosic-derived waste materials into platform chemicals. Waste Biomass Valorization 2021, 12, 2825–2851. [Google Scholar]
- Peng, L.; Wang, M.; Li, H. Tert-Butanol intervention enables chemoselective conversion of xylose to furfuryl alcohol over heteropolyacids. Green Chem. 2020, 22, 5656–5665. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Shetty, A.; Molahalli, V.; Sharma, A. Biomass-derived carbon materials in heterogeneous catalysis: A step towards sustainable future. Catalysts 2022, 13, 20. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, X.; Jiang, H. Transition metals-catalyzed amination of biomass feedstocks for sustainable construction of N-heterocycles. Coord. Chem. Rev. 2024, 502, 215622. [Google Scholar] [CrossRef]
- Lodhi, A.; Maheria, K.C. Zeolite-catalysed esterification of biomass-derived acids into high-value esters products: Towards sustainable chemistry. Catal. Commun. 2024, 187, 106883. [Google Scholar] [CrossRef]
- Auta, M.; Ern, L.M.; Hameed, B.H. Fixed-bed catalytic and non-catalytic empty fruit bunch biomass pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 67–72. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Choi, I.G.; Choi, J.W. Catalytic effects of magnesium on the characteristics of fast pyrolysis products–Bio-oil, bio-char, and non-condensed pyrolytic gas fractions. J. Anal. Appl. Pyrolysis 2015, 113, 27–34. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Kim, C.S.; Ellis, N. Microwave-assisted catalytic pyrolysis of switchgrass for improving bio-oil and biochar properties. Bioresour. Technol. 2016, 201, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Effect of oxide catalysts on the properties of bio-oil from in-situ catalytic pyrolysis of palm empty fruit bunch fiber. J. Environ. Manag. 2019, 247, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Yang, H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.T.; Zhang, L.; Li, Q. Catalytic pyrolysis of poplar wood over transition metal oxides: Correlation of catalytic behaviors with physiochemical properties of the oxides. Biomass Bioenergy 2019, 124, 125–141. [Google Scholar] [CrossRef]
- Dong, Q.; Li, H.; Niu, M. Microwave pyrolysis of moso bamboo for syngas production and bio-oil upgrading over bamboo-based biochar catalyst. Bioresour. Technol. 2018, 266, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Dong, Y.; Dong, K. Role of biochar-based catalysts in microwave-induced biomass pyrolysis: Structural properties and modification with Fe-series metals. Fuel 2023, 341, 127769. [Google Scholar] [CrossRef]
- Li, X.; Fu, H.; Shao, S. Synthesis of hierarchical HZSM-5 utilizing natural cellulose as templates for promoted production of aromatic hydrocarbons in the catalytic pyrolysis of biomass. Fuel Process. Technol. 2023, 248, 107815. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Naqvi, M.; Inayat, A. Impact of layered and delaminated zeolites on catalytic fast pyrolysis of microalgae using fixed-bed reactor and Py-GC/MS. J. Anal. Appl. Pyrolysis 2021, 155, 105025. [Google Scholar] [CrossRef]
- Gai, D.; Shi, J.; Cui, X. Catalytic performance and mechanism of A-site vacancy deficient perovskite catalyst over tar cracking during biomass pyrolysis. J. Clean. Prod. 2023, 405, 136876. [Google Scholar] [CrossRef]
- Hubble, A.H.; Childs, B.A.; Pecchi, M. Role of in situ (in contact with biomass) and ex situ (in contact with pyrolysis vapors) transition metal catalysts on pyrolysis of cherry pits. Fuel 2023, 352, 129062. [Google Scholar] [CrossRef]
- Pagano, M.; Hernando, H.; Cueto, J. Assessing the changes in thermal and catalytic pyrolysis of lignocellulose when shifting from batch to continuous biomass feeding modes. Catal. Today 2024, 426, 114399. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X. Ligin-derived graphene-like ultrathin carbon materials for ORR catalysis. J. Phys. Conf. Ser. 2024, 2749, 012003. [Google Scholar]
- Kong, L.; Wang, J.; Dong, K. Catalytic pyrolysis characteristics of polystyrene by biomass char-supported nanocatalysts. J. Anal. Appl. Pyrolysis 2024, 179, 106511. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, W.; Wang, D. Controlling the growth of ZnNiMoSx on biomass-derived porous carbon materials for deep hydrodesulfurization. Appl. Catal. A General 2023, 660, 119188. [Google Scholar] [CrossRef]
- Lu, D.; Yang, Q.; Chen, Z. High-efficiency phenol removal by novel biomass-based alginate composite hydrogel. Chem. Phys. Lett. 2023, 826, 140676. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Hitam, C.N.C. Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: A review. Environ. Chem. Lett. 2020, 18, 1625–1648. [Google Scholar] [CrossRef]
- Hoang, A.T. 2-Methylfuran (MF) as a potential biofuel: A thorough review on the production pathway from biomass, combustion progress, and application in engines. Renew. Sustain. Energy Rev. 2021, 148, 111265. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Sarker, M. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Zhang, C.; Hu, X.; Guo, H. Pyrolysis of poplar, cellulose and lignin: Effects of acidity and alkalinity of the metal oxide catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Shao, S. Preparation of composite HZSM-5 catalyst by green template and catalytic the pyrolysis of biomass to produce aromatics. Renew. Energy 2023, 206, 506–513. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P. Catalytic pyrolysis of pine needles with nickel doped gamma-alumina: Reaction kinetics, mechanism, thermodynamics and products analysis. J. Clean. Prod. 2021, 286, 124930. [Google Scholar] [CrossRef]
- Santander, J.A.; Diez, A.; Gutierrez, V. Partial deoxygenation of pyrolysis intermediates toward fossil fuel miscible bioliquids. Environ. Prog. Sustain. Energy 2023, 42, e13945. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, R.; He, Y. Preparation of bio-jet fuels by a controllable integration process: Coupling of biomass fermentation and olefin polymerization. Bioresour. Technol. 2023, 382, 129175. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T. Catalytic pyrolysis of lignocellulosic biomass: A review of variations in process factors and system structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, N.; Ma, Y. Biomass volatiles reforming by integrated pyrolysis and plasma-catalysis system for H2 production: Understanding roles of temperature and catalyst. Energy Convers. Manag. 2023, 288, 117159. [Google Scholar] [CrossRef]
- Singh, P.P.; Jaswal, A.; Singh, A. From Waste to Clean Energy: An Integrated Pyrolysis and Catalytic Steam Reforming Process for Green Hydrogen Production from Agricultural Crop Residues. ACS Sustain. Chem. Eng. 2024, 12, 2058–2069. [Google Scholar] [CrossRef]
- Tang, W.; Cao, J.P.; Yang, F.L. Highly active and stable HF acid modified HZSM-5 supported Ni catalysts for steam reforming of toluene and biomass pyrolysis tar. Energy Convers. Manag. 2020, 212, 112799. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Huang, S. Study on the mechanism of syngas production from catalytic pyrolysis of biomass tar by Ni–Fe catalyst in CO2 atmosphere. Fuel 2023, 335, 126705. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Liu, P. Feasibility assessment of recycling waste aluminum dross as a basic catalyst for biomass pyrolysis to produce hydrogen-rich gas. Int. J. Hydrogen Energy 2023, 48, 36361–36376. [Google Scholar]
- Tong, X.; Zheng, J.; Xue, S. High-Entropy Materials as the Catalysts for Valorization of Biomass and Biomass-Derived Platform Compounds. ACS Sustain. Chem. Eng. 2023, 11, 10203–10218. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Liu, B.; Xiang, S.; Zhao, G. Efficient production of 3-hydroxypropionate from fatty acids feedstock in Escherichia coli. Metab. Eng. 2019, 51, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, L.; Bohn, C.M. Speciation and kinetic study of iron promoted sugar conversion to 5-hydroxymethylfurfural (HMF) and levulinic acid (LA). Org. Chem. Front. 2015, 2, 1388–1396. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, X.; Liu, J. Recent Advances in Zeolites-Catalyzed Biomass Conversion to Hydroxymethylfurfural: The Role of Porosity and Acidity. ChemPlusChem 2024, 89, e202300399. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, L.; Wu, Z. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xu, C.; Shi, W. Selective HMF synthesis from glucose via microwave-assisted metal chloride catalysis. Biomass Bioenergy 2024, 181, 107060. [Google Scholar] [CrossRef]
- Hirano, Y.; Beltramini, J.N.; Mori, A. Microwave-assisted catalytic conversion of glucose to 5-hydroxymethylfurfural using “three dimensional” graphene oxide hybrid catalysts. RSC Adv. 2020, 10, 11727–11736. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Li, B. Fabrication of graphene oxide supported acid–based bifunctional metal–organic frameworks as efficient catalyst for glucose to 5-hydroxymethylfurfural conversion. Energy Technol. 2020, 8, 1901111. [Google Scholar] [CrossRef]
- García-Sancho, C.; Fúnez-Núñez, I.; Moreno-Tost, R. Beneficial effects of calcium chloride on glucose dehydration to 5-hydroxymethylfurfural in the presence of alumina as catalyst. Appl. Catal. B Environ. 2017, 206, 617–625. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Lin, L. Efficient conversion of glucose into 5-hydroxymethylfurfural by chromium (III) chloride in inexpensive ionic liquid. Ind. Eng. Chem. Res. 2012, 51, 1099–1104. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, Y.; Wang, S. Heterogeneous interface catalysts with electron local exchange toward highly selective oxidation of biomass platform compounds. ACS Catal. 2023, 13, 5665–5677. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Wang, X. Convergent production of 2, 5-furandicarboxylic acid from biomass and CO2. Green Chem. 2019, 21, 2923–2927. [Google Scholar] [CrossRef]
- Pan, W.; Chen, H.; Chen, A. Selective transformation of biomass-derived 5-hydroxymethylfurfural to the building blocks 2, 5-bis (hydroxymethyl) furan on α-Ni (OH)2/SiO2 catalyst without prereduction. React. Kinet. Mech. Catal 2024, 137, 1635–1649. [Google Scholar] [CrossRef]
- Meng, Y.; Jian, Y.; Li, J. Surface-active site engineering: Synergy of photo-and supermolecular catalysis in hydrogen transfer enables biomass upgrading and H2 evolution. Chem. Eng. J. 2023, 452, 139477. [Google Scholar] [CrossRef]
- Ratrey, G.; Solanki, B.S.; Kamble, S.P. Highly Efficient Chemoselective Hydrogenation of 5-HMF to BHMF over Reusable Bimetallic Pd-Ir/C Catalyst. ChemistrySelect 2022, 7, e202200456. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, J.; Zhang, Q. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran over Co/UiO-66-NH2. Catal. Lett. 2022, 152, 361–371. [Google Scholar] [CrossRef]
- Kar, S.; Zhou, Q.Q.; Ben-David, Y. Catalytic furfural/5-hydroxymethyl furfural oxidation to furoic acid/furan-2, 5-dicarboxylic acid with H2 production using alkaline water as the formal oxidant. J. Am. Chem. Soc. 2022, 144, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Pachapur, V.L.; Castillo, M.V.; Saini, R. Integrated biorefinery approach for utilization of wood waste into levulinic acid and 2-Phenylethanol production under mild treatment conditions. J. Biotechnol. 2024, 389, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gundekari, S.; Kalusulingam, R.; Varkolu, M. Preparation of 4, 4-bis (5-methylthiophen-2-yl) pentanoic acid from biomass-based functional molecules using solid acid catalysts. Catal. Commun. 2024, 187, 106906. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Jiang, J. Catalytic valorization of biomass carbohydrates into levulinic acid/ester by using bifunctional catalysts. Renew. Energy 2024, 221, 119851. [Google Scholar] [CrossRef]
- Zhang, R.; He, S.; Wang, F. A porous B/L acid zeolite-based catalyst regulates and controls the degradation of biomass carbohydrates to produce levulinic acid/esters. Chem. Phys. 2024, 584, 112344. [Google Scholar] [CrossRef]
- Xu, X.; Liang, B.; Zhu, Y. Direct and efficient conversion of cellulose to levulinic acid catalyzed by carbon foam-supported heteropolyacid with Brønsted–Lewis dual-acidic sites. Bioresource Technology: Biomass, Bioenergy, Biowastes, Conversion Technologies, Biotransformations. Prod. Technol. 2023, 387, 129600. [Google Scholar]
- Azlan, N.S.M.; Yap, C.L.; Tiong, Y.W. The interplay of Bronsted-Lowry and Lewis Acid Sites in Bifunctional Catalyst for the Biomass Conversion to Levulinic Acid. J. Biomim. Biomater. Biomed. Eng. 2023, 61, 71–76. [Google Scholar] [CrossRef]
- Sobuś, N.; Czekaj, I. Catalytic transformation of biomass-derived glucose by one-pot method into levulinic acid over Na-BEA zeolite. Processes 2022, 10, 223. [Google Scholar] [CrossRef]
- Taghavi, S.; Ghedini, E.; Menegazzo, F. CuZSM-5@ HMS composite as an efficient micro-mesoporous catalyst for conversion of sugars into levulinic acid. Catal. Today 2022, 390, 146–161. [Google Scholar] [CrossRef]
- Xu, H.; Hu, D.; Lin, L. MOF-derived bimetallic NiCo nanoalloys for the hydrogenation of biomass-derived levulinic acid to γ-valerolactone. AIChE J. 2023, 69, e17973. [Google Scholar] [CrossRef]
- Yang, J.; Shi, R.; Xu, X. Yolk-shell electron-rich Ru@ hollow pyridinic-N-doped carbon nanospheres with tunable shell thickness and ultrahigh surface area for biomass-derived levulinic acid hydrogenation under mild conditions. Appl. Catal. B Environ. Energy 2024, 355, 124193. [Google Scholar] [CrossRef]
- Zhao, R.; Bae, J.W. Catalytic conversion of biomass-derived levulinic acid to γ-valerolactone over phosphate modified ZrO2/SBA-15. Catal. Today 2024, 435, 114718. [Google Scholar] [CrossRef]
- Martínez, F.K.G.; Martínez, F.A.; Segobia, D.J. Valeric Biofuels from Biomass-Derived γ-Valerolactone: A Critical Overview of Production Processes. ChemPlusChem 2023, 88, e202300381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, C.; Sun, P. Synergistic catalysis for promoting ring-opening hydrogenation of biomass-derived cyclic oxygenates. ACS Catal. 2023, 13, 5170–5193. [Google Scholar] [CrossRef]
- Xiong, Y.; Jia, Y.; Yang, R. Foam-Like Multi-Stage Porous Al-KIT-6-4T (WI) Catalyst for Efficient Catalyzing Biomass-Derived GVL to Butene. Catal. Lett. 2024, 154, 3025–3034. [Google Scholar] [CrossRef]


| Catalyst | Biomass | Operating Conditions | Conversion Products | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| T | HR | SGF | Bio-Char | Bio-Oil | Bio-Gas | |||
| K2CO3 | Empty fruit bunch | 600 °C | 10 °C/min | 200 (N2) mL/min | 24.3 wt% | 28.4 wt% | 46.7 wt% | [25] |
| MgCl2 | Yellow poplar wood | 500 °C | _ | 7.7 wt% | 54.8 wt% | 37.5 wt% | [26] | |
| K3PO4 | Switchgrass | 400 °C | _ | 1500 (N2) mL/min | 33.3 wt% | 42.2 wt% | 24.5 wt% | [27] |
| MgO | Palm empty fruit bunches | 500 °C | 25.8 °C/min | 20–40 (N2) cm3/min | 25.7 wt% | 42.3 wt% | 32.0 wt% | [28] |
| CaO | Cotton stalk | 600 °C | 13 °C/s | 100 (N2) mL/min | 24.8 wt% | 49.7 wt% | 26.4 wt% | [29] |
| Ca(OH)2 | Empty fruit bunch | 600 °C | 10 °C/min | 200 (N2) mL/min | 10.3 wt% | 42.6 wt% | 47.1 wt% | [25] |
| ZnO | Palm empty fruit bunches | 500 °C | 25.8 °C/min | 20–40 (N2) cm3/min | 27 wt% | 44.7 wt% | 28.2 wt% | [28] |
| TiO2 | Poplar wood | 500 °C | _ | 120 (N2) mL/min | 29.7 wt% | 49.8 wt% | 20.1 wt% | [30] |
| Activated carbon | Switchgrass | 400 °C | _ | 1500 (N2) mL/min | 28.7 wt% | 27.5 wt% | 43.8 wt% | [27] |
| Bamboo-based bio-char | Moso bamboo | 700 °C | _ | 100 (N2) mL/min | 18.7 wt% | 17.2 wt% | 64.1 wt% | [31] |
| KOH-activated pine char | Pine sawdust | 450 °C | _ | 300 (N2) mL/min | 28.4 wt% | 17.0 wt% | 38.4 wt% | [32] |
| Natural zeolite (clinoptilolite) | Switchgrass | 400 °C | _ | 1500 (N2) mL/min | 30 wt% | 34.1 wt% | 35.9 wt% | [27] |
| HZSM-5 (Si/Al = 50) | Rape straw | 550 °C | 60 °C/min | 20 (N2) mL/min | 29.3 wt% | 27.5 wt% | 40.7 wt% | [33] |
| Layered zeolites (Si/Al = 35) | Microalgae | 500 °C | 1000 °C/min | _ | 39.2 wt% | 27.1 wt% | 34.3 wt% | [34] |
| LaSrNi0.8Fe0.2O3 | Bamboo sawdust | 700 °C | _ | 300 (N2) mL/min | 17.5 wt% | 42.0 wt% | 40.6 wt% | [35] |
| Al2O3Mn | Cherry pits | 600 °C | 10 °C/min | 100 (N2) mL/min | 26.2 wt% | 12.3 wt% | 48.1 wt% | [36] |
| Catalyst | Reactant | Conversion Products | Operating Conditions | Yield | Reference | |
|---|---|---|---|---|---|---|
| Temperature | Time (h) | |||||
| AlCl3 | Glucose | HMF | 220 W microwave radiation | 0.16 | 74.04% | [66] |
| Reduced-type graphene oxide(rGO) | Glucose | HMF | 200 °C | 0.5 | 7.4% | [67] |
| Ni-rGO | Glucose | HMF | 200 °C | 0.5 | 28.1% | [67] |
| Co-rGO | Glucose | HMF | 200 °C | 0.5 | 17.8% | [67] |
| NiGO-FD | Glucose | HMF | 200 °C | 1 | 95% | [67] |
| UiO-66-SO3H-NH2/PDA@GO | Glucose | HMF | 120 °C | 2 | 55.8% | [68] |
| Υ-Al2O3 | Glucose | HMF | 175 °C | 0.25 | 52% | [69] |
| CrCl3·6H2O | Glucose | HMF | 130 °C | 0.16 | 71.5% | [70] |
| Co3O4/Co2MnO4 | HMF | FDCA | 120 °C | 24 | 98% | [71] |
| Co-SAs/N@C | Furoate | FDCA | 260 °C | 36 | 71.1% | [72] |
| Co-SAs/N@C | HMF | FDCA | 85 °C | 8 | 99.5% | [72] |
| Ni(OH)2/SiO2 | HMF | BHMF | 195 °C | 5 | 98.9% | [73] |
| Ov-WO3@CB[5] | HMF | BHMF | 25 °C | 1 | >99% | [74] |
| Pd-Ir/C | HMF | BHMF | 80 °C | 2.5 | 94.7% | [75] |
| Catalyst | Reactant | Conversion Product | Operating Conditions | Yield | Reference | |
|---|---|---|---|---|---|---|
| Temperature | Time (h) | |||||
| Zr-HY zeolite catalysts | Glucose | LA | 200 °C | 3 | 3.0% | [81] |
| 15-Zr-HY | Glucose | LA | 200 °C | 3 | 5.6% | [81] |
| Carbon foam-supported H5AlW12O40 | Cellulose | LA | 180 °C | 4 | 60.9% | [82] |
| H3PW12O40 | Cellulose | LA | 180 °C | 8 | 8.4% | [82] |
| AlCl3 | Cellulose | LA | 180 °C | 8 | 34.6% | [82] |
| H5AlW12O40 | Cellulose | LA | 180 °C | 8 | 69.6% | [82] |
| Nb2O5 | Palm mesocarp fiber | LA | 180 °C | - | 13.2% | [83] |
| H3PW12O40 | Palm mesocarp fiber | LA | 180 °C | - | 10.5% | [83] |
| HPW-Nb2O5 | Palm mesocarp fiber | LA | 180 °C | - | 16.4% | [83] |
| Na-BEA | Glucose | LA | 200 °C | 5 | 100% | [84] |
| CuZ(60%)@H | Glucose | LA | 200 °C | 5 | 45% | [85] |
| Ni1Co1/C | LA | GVL | 160 °C | 3 | 82.3% | [86] |
| Ru/AC | LA | GVL | 30 °C | 3.5 | 92.5% | [87] |
| Ru@me-HNC | LA | GVL | 30 °C | 3.5 | 98.2% | [87] |
| Ru@tk-HNC | LA | GVL | 30 °C | 3.5 | 98.1% | [87] |
| SBA-15 | LA | GVL | 200 °C | 2 | <5% | [88] |
| ZrP(15)/SBA-15 | LA | GVL | 200 °C | 2 | 61.5% | [88] |
| ZrP(15)/SBA-15 | LA | GVL | 140 °C | 2 | 43.7% | [88] |
| Ov-WO3@CB[5] | LA | GVL | 25 °C | 1 | 99% | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Wei, L.; Wang, J.; Lin, N.; Li, Y.; Li, F.; Zha, X.; Li, W. Application of Catalysts in the Conversion of Biomass and Its Derivatives. Catalysts 2024, 14, 499. https://doi.org/10.3390/catal14080499
Cai J, Wei L, Wang J, Lin N, Li Y, Li F, Zha X, Li W. Application of Catalysts in the Conversion of Biomass and Its Derivatives. Catalysts. 2024; 14(8):499. https://doi.org/10.3390/catal14080499
Chicago/Turabian StyleCai, Jixiang, Lianghuan Wei, Jianguo Wang, Ning Lin, Youwen Li, Feixing Li, Xianghao Zha, and Weizun Li. 2024. "Application of Catalysts in the Conversion of Biomass and Its Derivatives" Catalysts 14, no. 8: 499. https://doi.org/10.3390/catal14080499





