A Kinetic Model for Catalytic N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions in a Fluidized CREC Riser Simulator
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
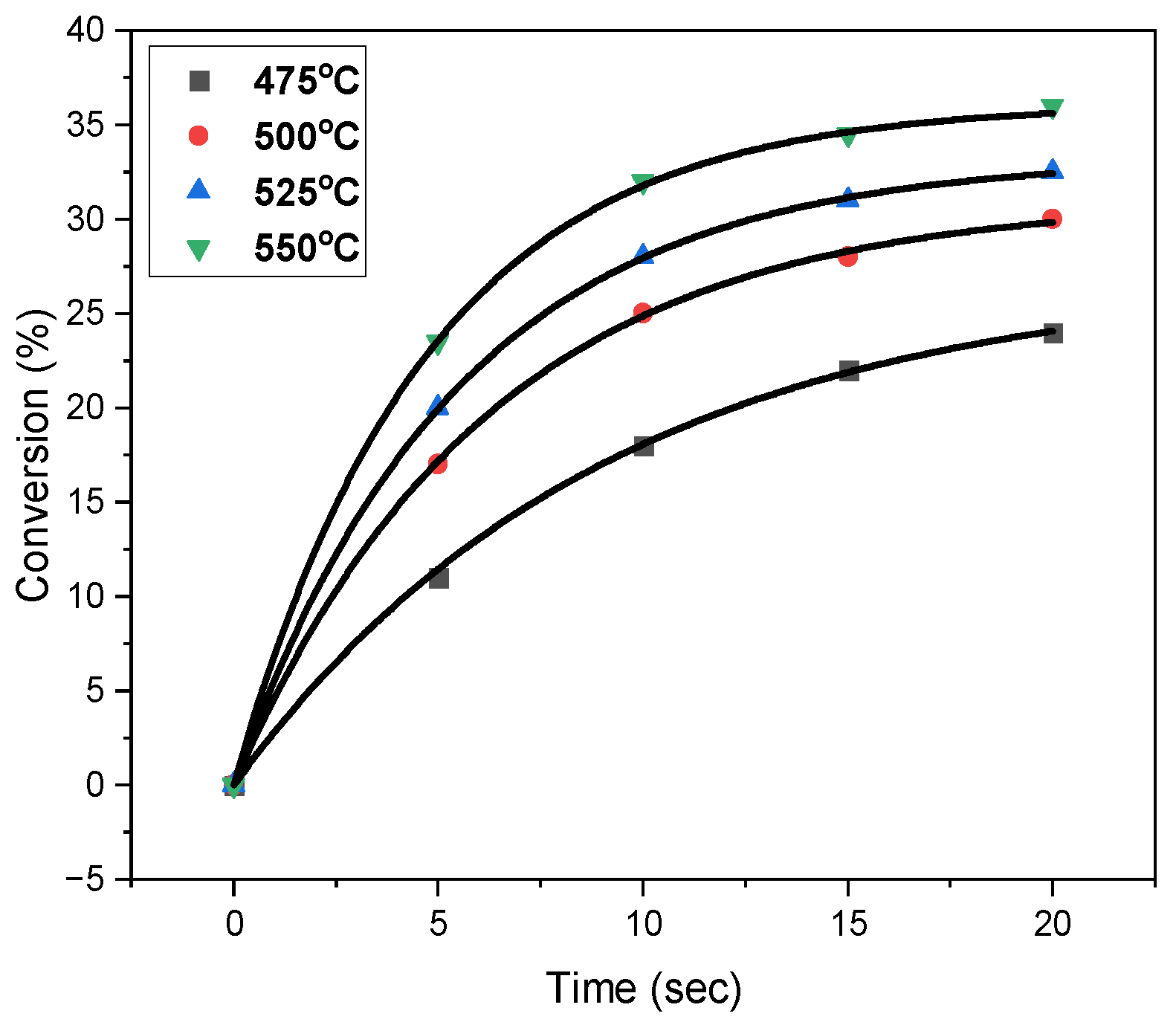
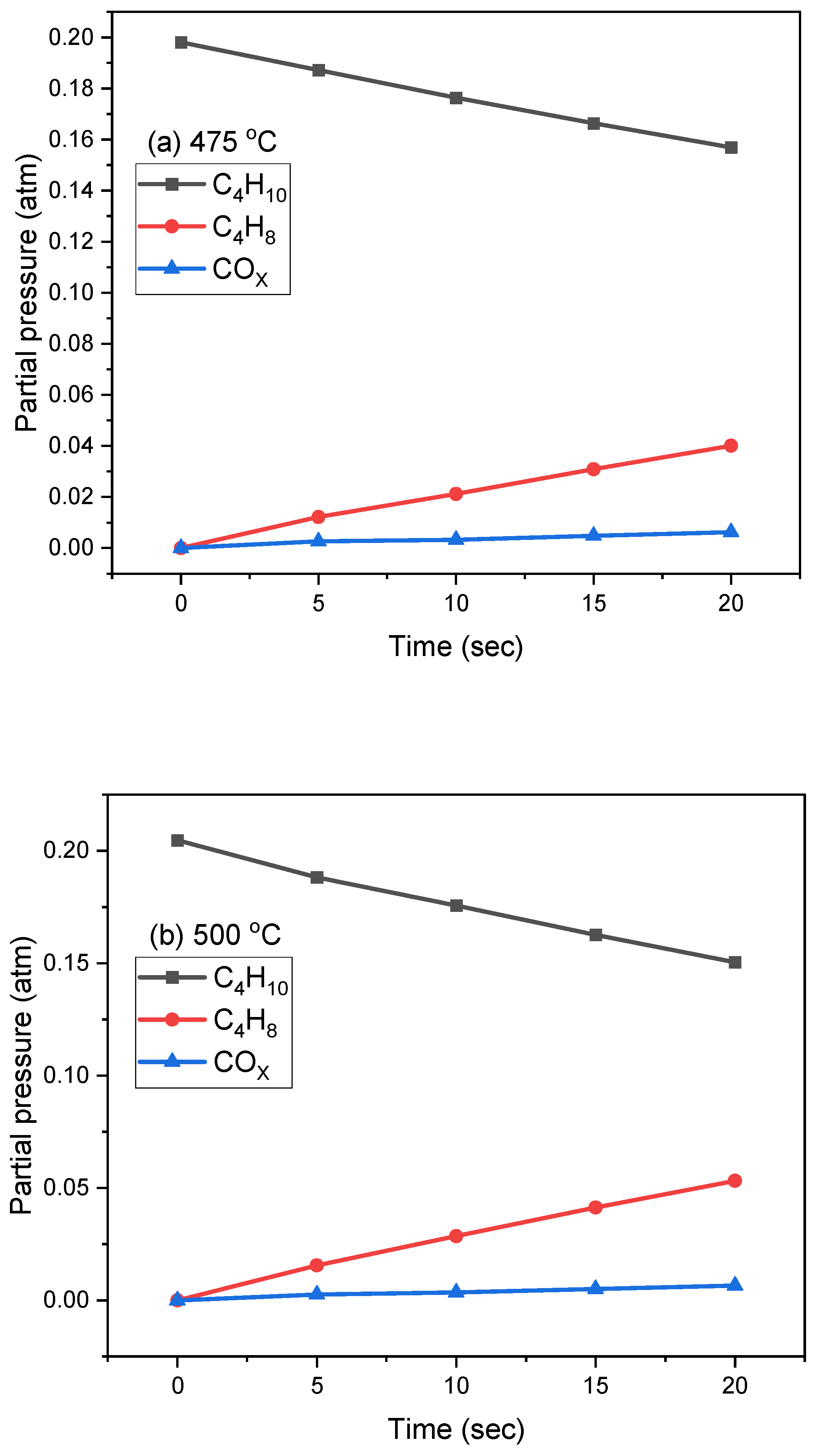
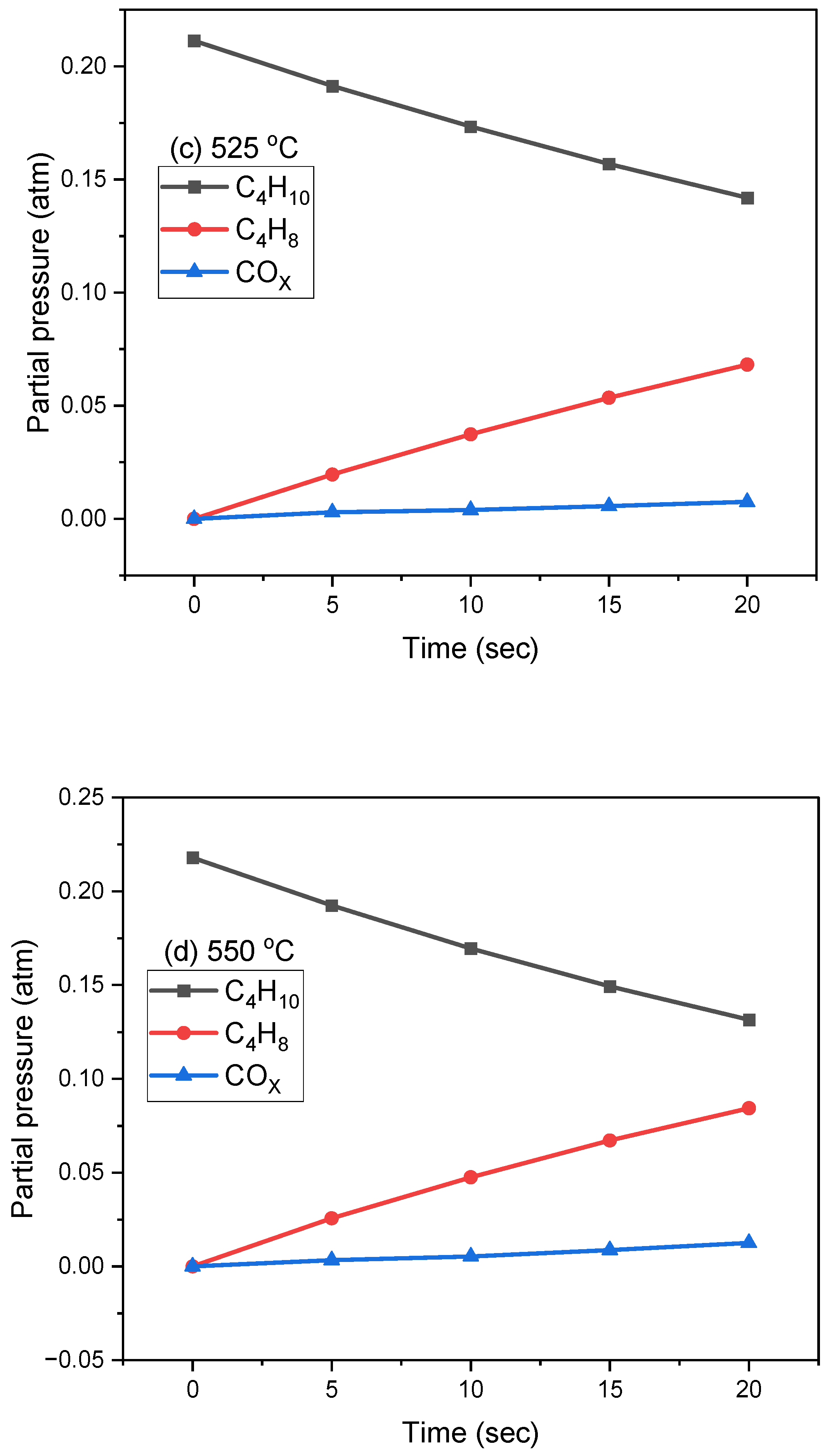
2.2. Oxidative Dehydrogenation of N-Butane in the CREC Riser Simulator
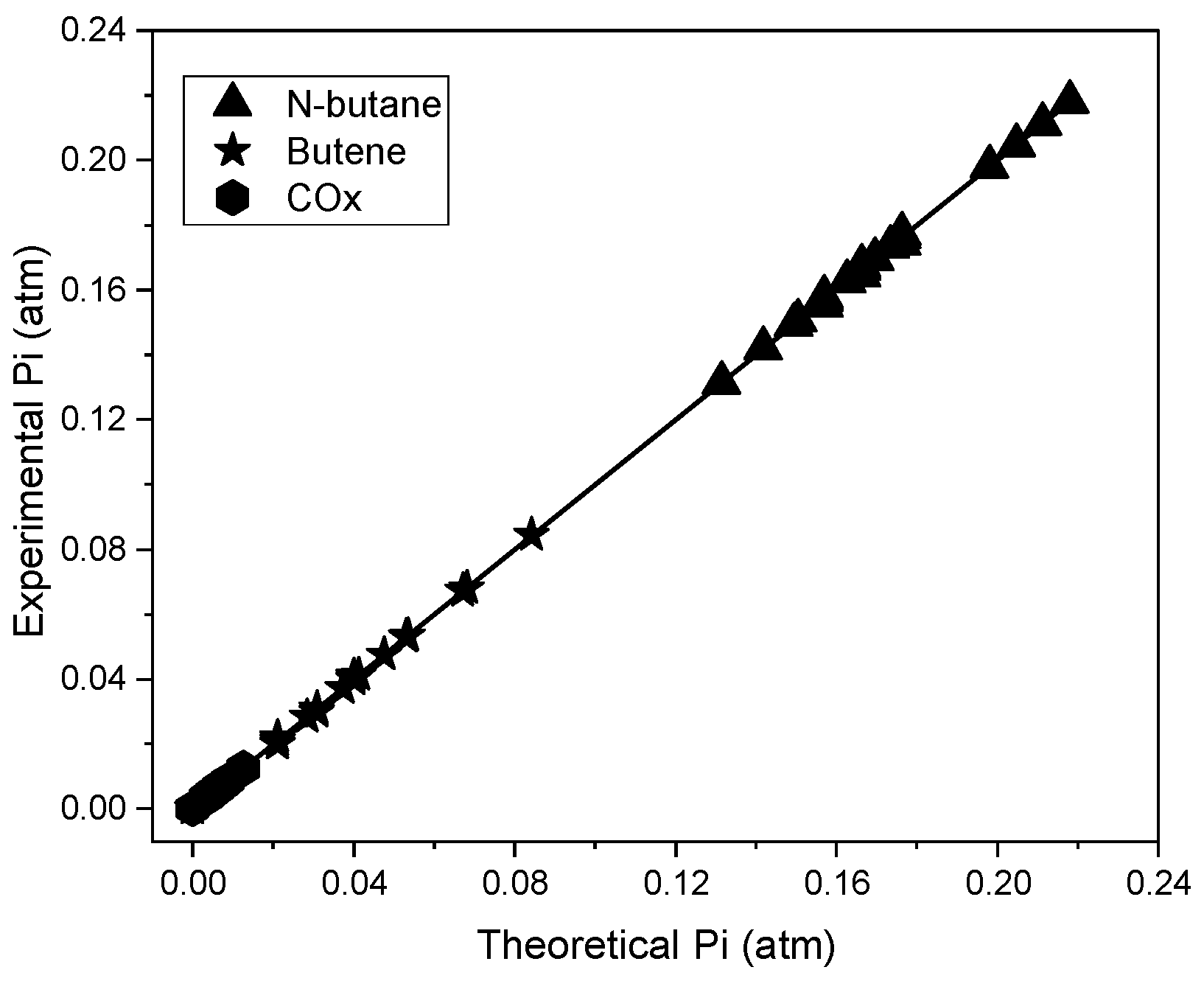
2.3. Kinetic Parameters Estimation
2.4. Adsorption Constants
2.5. Intrinsic Kinetic Parameters
3. Experimental Methods
3.1. Catalyst Synthesis and Characterization
3.2. Oxidative Dehydrogenation of N-Butane Using Catalyst Lattice Oxygen
4. Kinetic Modeling Methods
- ○
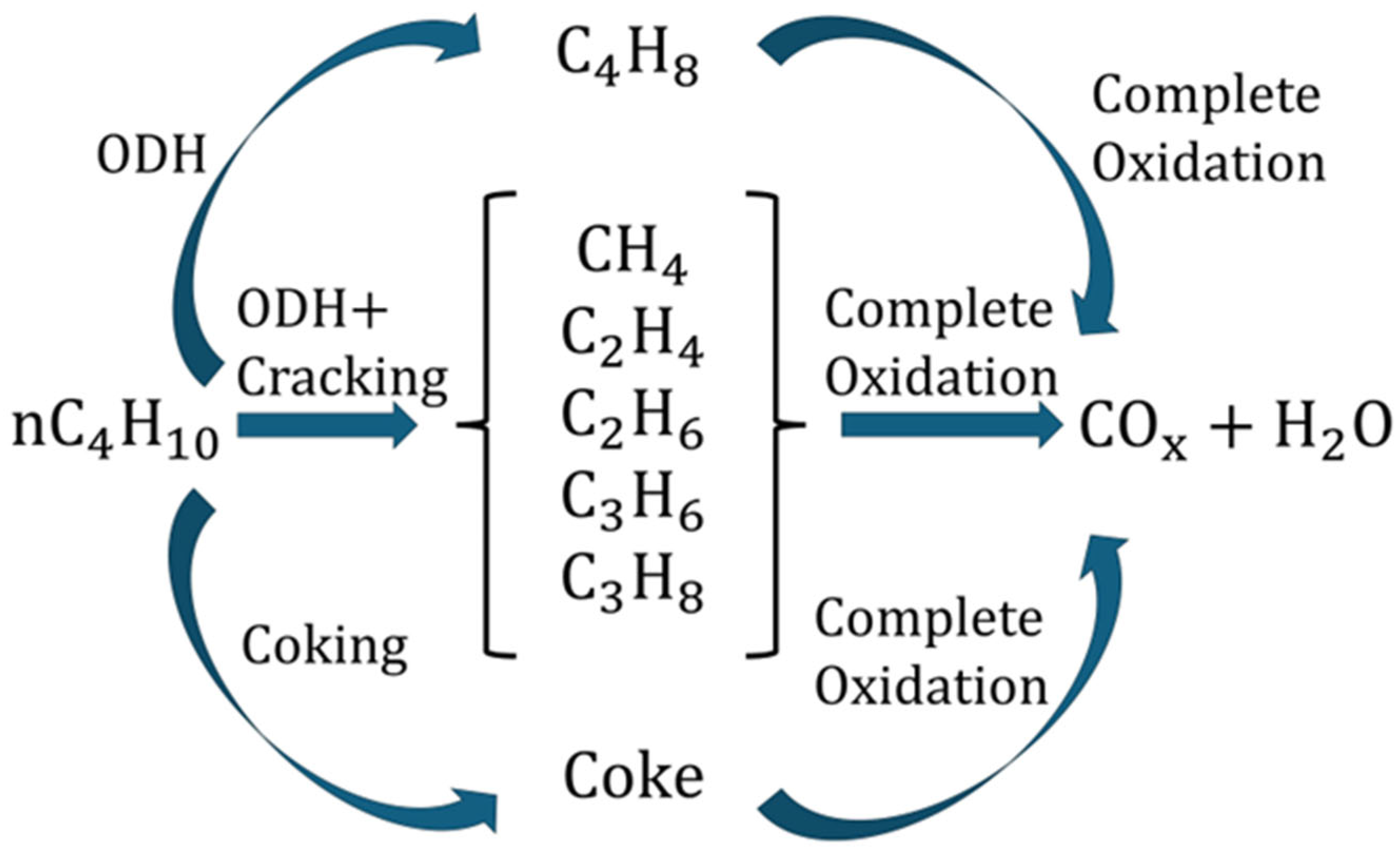
- ODH of n-butane to C4 olefins:
- ○
- Complete oxidation of n-butane:
- ○
- Complete oxidation of C4 olefins:
4.1. Development of the Kinetic Model
- Adsorption of n-butane, on Site-2:C4H10 (g) + [S](s) ←⎯⎯→ C4H10 − [S](s).
- Reaction between adsorbed n-butane in Site 2 and lattice oxygen in Site1-[V2O50] with C4-olefin formation:C4H10 − [S](s) + [V2O5-O] (s) → C4H8 − [S](s) + H2O(g) + [V2O5R](s).
- Desorption of C4-olefin formed in Site 2:C4H8 − [S](s) ←⎯⎯→ C4H8 (g) + [S](s).
- Reaction between adsorbed n-butane in Site 2 and lattice oxygen [V2O50] in Site 1 with formation of COx:C4H10 − [S](s) + (5 + 4x) [V2O5-O-](s) → 4COx(g) + 5H2O(g) + (5 + 4x) [V2O5R](s) + [S](s)
- Reaction between adsorbed butene in Site 2 and lattice oxygen [V2O50] in Site 1 with formation of COx.C4H8 − [S](s) + (4 + 4x) [V2O5-O-] (s) → 4COx(g) + 4H2O(g) + (4 + 4x) [V2O5R](s) + [S](s).
- Site 1 reoxidation with gas-phase oxygen during catalyst regeneration:[V2O5R] (s) +0.5 O2 (g) → [V2 O5-O-](s).
4.2. Kinetic Modeling in the Riser Simulator Reactor
5. Conclusions
- (a)
- A reaction kinetics for n-butane ODH, using a 5 wt%V/MgO−γAl2O3 (1:1) fluidizable catalyst within an oxygen-free atmosphere, can be successfully established using data obtained from a fluidized bed CREC Riser Simulator.
- (b)
- The adsorption and intrinsic kinetic parameters from the postulated Langmuir–Hinshelwood rate model can be determined separately via independent adsorption and reaction experiments.
- (c)
- The evaluated intrinsic kinetic parameters for n-butane ODH (k10, k20, k30, E1, E2, and E3) can be successfully determined using a large degree of freedom (DOF) data set, with satisfactorily reduced 95% confidence intervals and low kinetic parameter cross-correlation.
- (d)
- The derived n-butane ODH kinetics provides a good estimation of n-butane conversions and butene selectivities at various temperatures and contact times. In particular, the postulated kinetics accurately predicts the n-butane conversions, ranging from 25% to 27%, and C4-olefins selectivities, ranging from 84% to 87%, at 10 s with negligible coke formation.
- (e)
- The derived n-butane ODH kinetics provides an excellent tool for riser and downer reactor industrial scale simulations, given that it was derived under operating conditions (temperature, reaction time, partial pressures, catalyst/n-butane weight ratios) close to the ones anticipated for large scale riser and downer units.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| COx | Carbon oxides |
| Edes | Activation energy of desorption (kJ/mol) |
| Ei | Activation energy for “i” species (kJ/mol) |
| kdes | Pre-exponential desorption factor (cm3/gcat.min) |
| ki | Reaction rate constant for “i” species (mol/gcat.s) |
| ki0 | Intrinsic kinetics constant pre-exponential factor for “i” species (mol/gcat.s) |
| Ki | Adsorption constant for for “i” species (atm−1) |
| Ki0 | Adsorption constant pre-exponential factor for “i” species (atm−1) |
| pi | Partial pressure of species “i” (atm) |
| ri | “i” species reaction rate (mol/gcat.s) |
| R | Universal gas constant |
| SBET | Brunauer−Emmet−Teller specific surface area (m2/g) |
| Si | Selectivity for for “i” species (%) |
| Tm | Median temperature in the 475–550 °C range (K) |
| Vm | Volume of monolayer coverage (cm3/gcat) |
| ViA | Species volume adsorbed on the catalyst (cm3/gcat) |
| V2O5 | vanadium oxide species at the reduced state |
| V2O5-O | vanadium oxide species at the oxidized state |
| XC4H10 | N-butane conversion (%) |
| YC4H8 | C4-olefins yield (%) |
| Greek Symbols | |
| β | Degree of reduction of catalyst |
| λ | Decay constant (−) |
| θ | Surface coverage of adsorbed species |
| φ | Degree of oxidation of the catalyst |
| ΔHi | Heat of adsorption (kJ/mol) |
| Abbreviations | |
| CREC | Chemical Reactor Engineering Centre |
| FID | Flame Ionization Detector |
| FTIR | Fourier Transform Infrared Spectroscopy |
| LRS | Laser Raman Spectroscopy |
| BODH | N-butane Oxidative Dehydrogenation |
| TCD | Thermal Conductivity Detector |
| TPD | Temperature-Programmed Desorption |
| TPO | Temperature-Programmed Oxidation |
| TPR | Temperature-Programmed Reduction |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
Appendix A
Appendix A.1. CREC Riser Simulator—Equipment Description
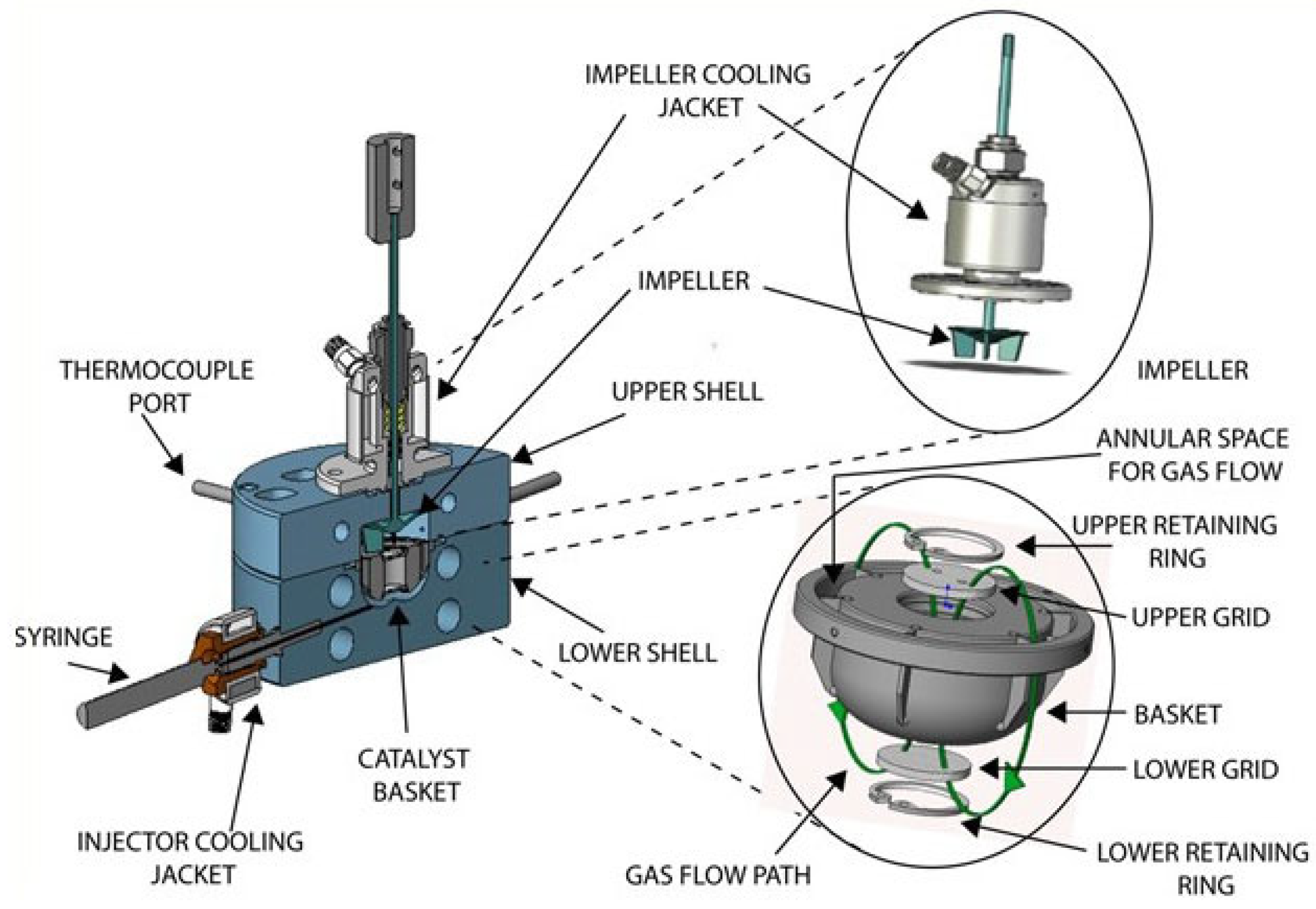
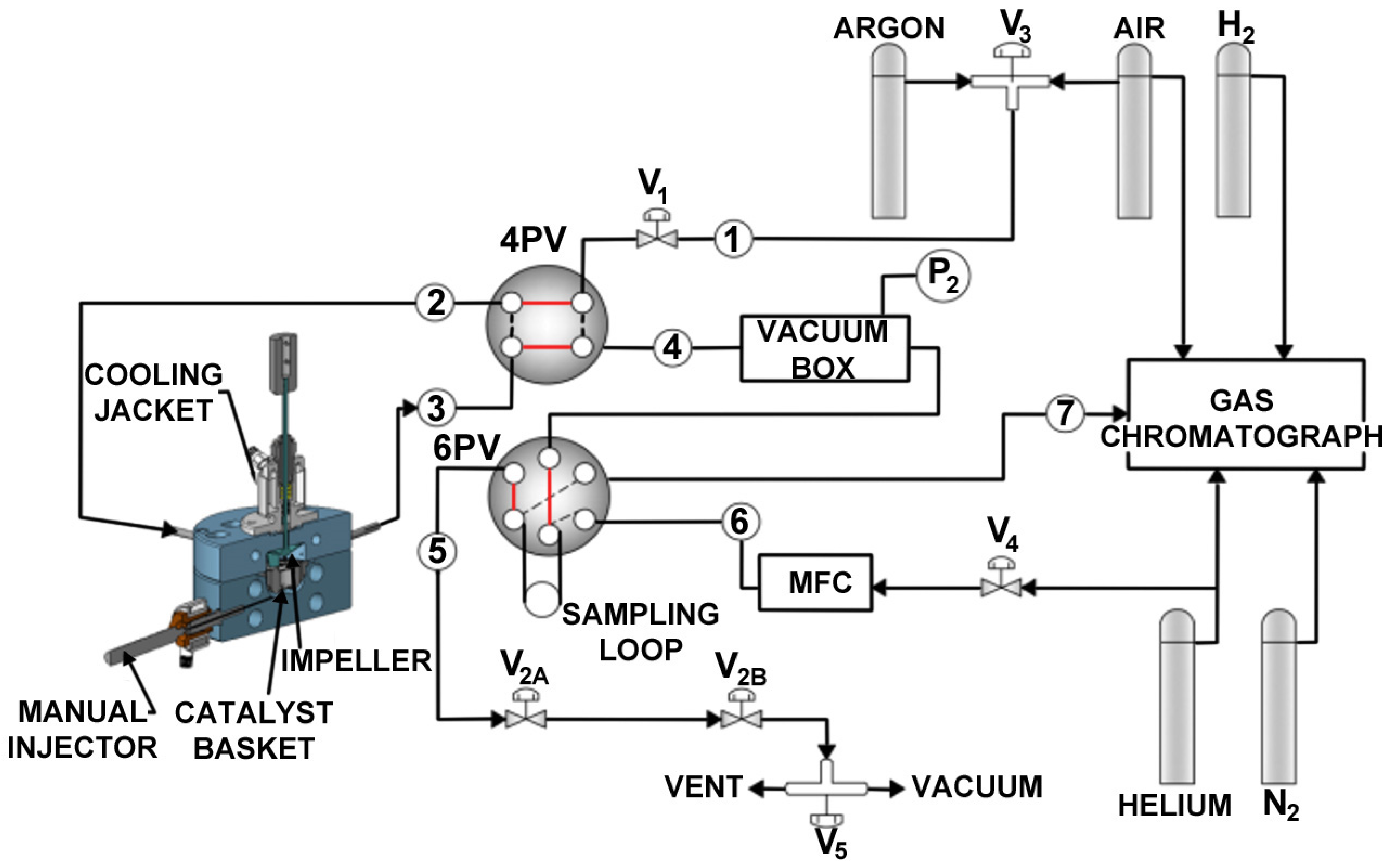

Appendix A.2. Experimental Procedures in the CREC Riser Simulator
References
- Gambo, Y.; Adamu, S.; Tanimu, G.; Abdullahi, I.M.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. CO2-mediated oxidative dehydrogenation of light alkanes to olefins: Advances and perspectives in catalyst design and process improvement. Appl. Catalsis A Gen. 2021, 623, 118273. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Corolla, N.; Eldred, T.; Bose, A.; Gao, W.; Li, F. Alkali metal halide–coated perovskite redox catalysts for anaerobic oxidative dehydrogenation of n-butane. Sci. Adv. 2022, 8, eabo7343. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Lloret, M.; Slot, T.K.; van Leest, N.P.; de Bruin, B.; Sloof, W.G.; Batyrev, E.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Rothenberg, G.; Shiju, N.R. The Role of vacancies in a Ti2CTx MXene-derived catalyst for butane oxidative dehydrogenation. ChemCatChem 2022, 14, 7. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A review on production of light olefins via fluid catalytic cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Akah, A.; Williams, J.; Ghrami, M. An Overview of light olefins production via steam enhanced catalytic cracking. Cataysisl Surv. Asia 2019, 23, 265–276. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, J.; Wei, X.; Liu, L. Increased light olefin production by sequential dehydrogenation and cracking reactions. Catalysts 2022, 12, 1457. [Google Scholar] [CrossRef]
- Luongo, G.; Donat, F.; Bork, A.H.; Willinger, E.; Landuyt, A.; Müller, C.R. Highly selective oxidative dehydrogenation of ethane to ethylene via chemical looping with oxygen uncoupling through structural engineering of the oxygen carrier. Adv. Energy Mater. 2022, 12, 2200405. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Neal, L.M.; Haribal, V.P.; Li, F. Intensified ethylene production via chemical looping through an exergetically efficient redox scheme. iScience 2019, 19, 894–904. [Google Scholar] [CrossRef]
- Watanabe, R.; Suganuma, H.; Yoda, Y.; Karasawa, F.; Verma, P.; Fukuhara, C. Deactivation of an Fe-based catalyst in the dehydrogenation of light alkanes under H2S co-feeding: A case study. Appl. Catal. A Gen. 2024, 683, 119848. [Google Scholar] [CrossRef]
- Gambo, Y.; Adamu, S.; Abdulrasheed, A.A.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. Catalyst design and tuning for oxidative dehydrogenation of propane—A review. Appl. Catal. A Gen. 2021, 609, 117914. [Google Scholar] [CrossRef]
- Khan, M.Y.; Adamu, S.; Lucky, R.A.; Razzak, S.A.; Hossain, M.M.; Hossain, M.M. Oxidative dehydrogenation of n-Butane to C4 Olefins using lattice oxygen of VOx/Ce-meso-Al2O3 under gas-phase oxygen-free conditions. Energy Fuels 2020, 34, 7410–7421. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Wu, X.; Surin, I.; Pérez-Ramírez, J.; Hemberger, P.; Bodi, A. Unraveling radical and oxygenate routes in the oxidative dehydrogenation of propane over boron nitride. J. Am. Chem. Soc. 2023, 145, 7910–7917. [Google Scholar] [CrossRef]
- Yan, B.; Li, W.C.; Lu, A.H. Metal-free silicon boride catalyst for oxidative dehydrogenation of light alkanes to olefins with high selectivity and stability. J. Catal. 2019, 369, 296–301. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane oxidative dehydrogenation on vanadium-based catalysts under oxygen-free atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Purdy, S.C.; He, Y.; Huang, Z.; You, R.; Wei, Z.; Meyer, H.M.; Yang, J.; Pan, Y.; et al. Multiple promotional effects of vanadium oxide on boron nitride for oxidative dehydrogenation of propane. JACS Au 2022, 2, 1096–1104. [Google Scholar] [CrossRef]
- McDermott, W.; Venegas, J.; Hermans, I. Selective oxidative cracking of n-Butane to light olefins over hexagonal boron nitride with limited formation of COx. ChemSusChem 2019, 13, 152–158. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Bajec, D.; Likozar, B. Effect of surface oxidation on oxidative propane dehydrogenation over chromia: An Ab initio multiscale kinetic study. ACS Catal. 2021, 11, 11233–11247. [Google Scholar] [CrossRef] [PubMed]
- Kopač, D.; Jurković, D.L.; Likozar, B.; Huš, M. First-principles-based multiscale modelling of nonoxidative butane dehydrogenation on Cr2O3(0001). ACS Catal. 2020, 10, 14732–14746. [Google Scholar] [CrossRef]
- Kazerooni, H.; Towfighi Darian, J.; Mortazavi, Y.; Khadadadi, A.A.; Asadi, R. Titania-supported vanadium oxide synthesis by atomic layer deposition and Its application for low-temperature oxidative dehydrogenation of propane. Catal. Lett. 2020, 150, 2807–2822. [Google Scholar] [CrossRef]
- Xiong, C.; Chen, S.; Yang, P.; Zha, S.; Zhao, Z.J.; Gong, J. Structure-performance relationships for propane dehydrogenation over aluminum supported vanadium oxide. ACS Catal. 2019, 9, 5816–5827. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Z.; Luo, M.; Zhao, Z.; Wang, J.; Xie, Z.; Guo, L. Vanadium-containing dendritic mesoporous silica nanoparticles: Multifunctional catalysts for the oxidative and non-oxidative dehydrogenation of propane to propylene. Microporous Mesoporous Mater. 2019, 282, 133–145. [Google Scholar] [CrossRef]
- Tanimu, G.; Aitani, A.M.; Asaoka, S.; Alasiri, H. Oxidative dehydrogenation of n-butane to butadiene catalyzed by new mesoporous mixed oxides NiO-(beta-Bi2O3)-Bi2SiO5/SBA-15 system. Mol. Catal. 2020, 488, 110893. [Google Scholar] [CrossRef]
- Bin Sulayman, A.; Torres Brauer, N.; de Lasa, H. A Fluidizable catalyst for n-butane oxidative dehydrogenation under oxygen-free reaction conditions. Catalysts 2023, 13, 1462. [Google Scholar] [CrossRef]
- Grabowski, R. Kinetics of oxidative dehydrogenation of C2-C3 alkanes on oxide catalysts. Catal. Rev. Sci. Eng. 2006, 48, 199–268. [Google Scholar] [CrossRef]
- Cortés, I.; Rubio, O.; Herguido, J.; Menéndez, M. Kinetics under dynamic conditions of the oxidative dehydrogenation of butane with doped V/MgO. Catal. Today 2004, 91, 281–284. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. High propylene selectivity via propane oxidative dehydrogenation using a novel fluidizable catalyst: Kinetic modeling. Ind. Eng. Chem. Res. 2018, 57, 45. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; Hossain, M.M.; de Lasa, H. Kinetic modeling of ethane oxidative dehydrogenation over VOx/Al2O3 catalyst in a fluidized-bed riser simulator. Ind. Eng. Chem. Res. 2013, 52, 5235–5244. [Google Scholar] [CrossRef]
- Elbadawi, A.A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H. Phenomenologically based kinetics of ODH of ethane to ethylene using lattice oxygen of VOx/Al2O3–ZrO2 catalyst. Chem. Eng. Res. Des. 2017, 117, 733–745. [Google Scholar] [CrossRef]
- Lucky, R.A.; Adamu, S.; Khan, M.Y.; Razzak, S.A.; Hossain, M.M. Kinetics of oxidative dehydrogenation of n-butane to C4-Olefins over a VOx/CeO2-γAl2O3 catalyst in gas-phase oxygen-free Conditions. Ind. Eng. Chem. Res. 2020, 59, 17815–17827. [Google Scholar] [CrossRef]
- Dejoz, A.; López Nieto, J.M.; Melo, F.; Vázquez, I. Kinetic study of the oxidation of n-butane on vanadium oxide supported on Al/Mg mixed oxide. Ind. Eng. Chem. Res. 1997, 36, 2588–2596. [Google Scholar] [CrossRef]
- Madaan, N.; Haufe, R.; Shiju, N.R.; Rothenberg, G. Oxidative dehydrogenation of n-butane: Activity and kinetics over VOx/Al2O3 Catalysts. Top. Catal. 2014, 57, 1400–1406. [Google Scholar] [CrossRef]
- Sánchez-García, J.L.; Handy, B.E.; Ávila-Hernández, I.N.; Rodríguez, A.G.; García-Alamilla, R.; Cardenas-Galindo, M.G. Structure, acidity, and redox aspects of VOx/ZrO2/SiO2 catalysts for the n-butane oxidative dehydrogenation. Catalysts 2020, 10, 550. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Hossain, M.M. Kinetics of oxidative dehydrogenation of propane to propylene using lattice oxygen of VOx/CaO/γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 4309–4318. [Google Scholar] [CrossRef]
- de Lasa, H. Multifunctional Riser and Downer Simulator. USA Patent 10,220,363, 3 May 2019. [Google Scholar]
- de Lasa, H. The CREC fluidized riser simulator a unique tool for catalytic process development. Catalysts 2022, 12, 888. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | Dpore (Å) | H2 Uptake (cm3 STP/g) | Reducible V (%) | NH3 Uptake (cm3 STP/g) | Kdes (mmole/gcat.min) | Edes (KJ/mole) |
|---|---|---|---|---|---|---|---|
| γAl2O3 | 208 | 109 | - | - | 6.03 | 1.117 ± 0.78 | 74.43 ± 2.54 |
| 5 wt%V/γAl2O3 | 177 | 105 | 9.22 | 3.02 | 8.22 | 1.151 ± 0.89 | 69.32 ± 2.12 |
| MgO−γAl2O3 (1:1) | 152 | 164 | - | - | 4.34 | 1.615 ± 0.71 | 48.17 ± 2.14 |
| 5 wt% V/MgO−γAl2O3 | 198 | 89 | 12.42 | 3.63 | 5.56 | 1.165 ± 0.96 | 76.97 ± 2.02 |
| Temperature (°C) | Injection | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | ||||
| 475 | 1 | 6.10 | 1.63 | 8.56 | 3.05 | 1.30 | 13.55 | 2.02 | 63.80 | 22.56 | 14.40 |
| 2 | 3.57 | 1.90 | 7.56 | 3.45 | 1.53 | 13.53 | 1.96 | 66.48 | 18.65 | 12.40 | |
| 3 | 2.52 | 2.01 | 7.21 | 3.98 | 1.69 | 13.84 | 1.81 | 66.94 | 17.70 | 11.85 | |
| 4 | 2.22 | 1.97 | 6.79 | 3.92 | 1.78 | 14.57 | 1.65 | 67.11 | 17.22 | 11.55 | |
| 5 | 1.98 | 1.90 | 6.32 | 3.84 | 1.94 | 15.23 | 1.53 | 67.27 | 17.15 | 11.54 | |
| 6 | 1.95 | 1.85 | 5.66 | 3.85 | 2.03 | 15.02 | 1.43 | 68.21 | 17.03 | 11.61 | |
| 500 | 1 | 6.35 | 0.32 | 10.62 | 1.30 | 0.07 | 5.31 | 0.64 | 75.39 | 32.96 | 24.85 |
| 2 | 2.24 | 0.41 | 7.22 | 1.36 | 0.07 | 5.57 | 0.55 | 84.58 | 27.51 | 22.72 | |
| 3 | 1.56 | 0.46 | 6.07 | 1.69 | 0.10 | 6.01 | 0.38 | 85.73 | 26.06 | 21.82 | |
| 4 | 1.42 | 0.52 | 4.62 | 1.91 | 0.10 | 6.06 | 0.32 | 86.84 | 26.10 | 22.20 | |
| 5 | 1.28 | 0.51 | 4.00 | 1.92 | 0.10 | 5.97 | 0.31 | 86.91 | 26.05 | 22.38 | |
| 6 | 1.15 | 0.49 | 3.55 | 1.90 | 0.11 | 5.76 | 0.29 | 87.14 | 25.76 | 22.34 | |
| 525 | 1 | 7.19 | 0.37 | 12.03 | 1.80 | 0.08 | 6.88 | 0.72 | 70.92 | 33.51 | 23.77 |
| 2 | 2.75 | 0.50 | 8.87 | 1.67 | 0.09 | 9.05 | 0.68 | 76.38 | 29.06 | 22.20 | |
| 3 | 2.02 | 0.59 | 7.82 | 2.18 | 0.12 | 9.36 | 0.50 | 77.41 | 28.47 | 22.04 | |
| 4 | 1.90 | 0.70 | 6.20 | 2.57 | 0.13 | 10.10 | 0.43 | 77.97 | 27.69 | 21.59 | |
| 5 | 1.77 | 0.71 | 5.56 | 2.66 | 0.15 | 10.50 | 0.43 | 78.22 | 27.35 | 21.39 | |
| 6 | 1.63 | 0.70 | 5.03 | 2.70 | 0.16 | 10.68 | 0.41 | 78.69 | 27.85 | 21.92 | |
| 550 | 1 | 7.61 | 0.33 | 14.38 | 1.99 | 0.07 | 10.34 | 0.66 | 64.62 | 37.36 | 24.14 |
| 2 | 3.28 | 0.47 | 9.36 | 2.53 | 0.08 | 11.95 | 0.64 | 71.69 | 32.79 | 23.51 | |
| 3 | 2.42 | 0.57 | 8.74 | 2.70 | 0.12 | 12.53 | 0.48 | 72.43 | 31.17 | 22.58 | |
| 4 | 2.14 | 0.66 | 7.64 | 2.81 | 0.12 | 12.98 | 0.40 | 73.24 | 30.84 | 22.59 | |
| 5 | 1.93 | 0.68 | 6.37 | 3.03 | 0.14 | 13.55 | 0.41 | 73.89 | 30.21 | 22.32 | |
| 6 | 1.83 | 0.68 | 5.31 | 3.23 | 0.16 | 13.95 | 0.41 | 74.44 | 29.65 | 22.07 | |
| Parameter (atm−1) | Estimated Value with 95% Confidence Spans | Parameter (KJmol−1) | Estimated Value with 95% Confidence Spans |
|---|---|---|---|
| 0.83 ± 0.03 | 31.2 ± 0.72 | ||
| 0.41 ± 0.012 | 60.8 ± 1.20 | ||
| 0.50 ± 0.018 | 54.0 ± 1.05 |
| Parameters | Value | 95% CI | Correlation Matrix | |||||
| E1 | E2 | E3 | ||||||
| 2.64 × 10−5 | ±2.66 × 10−7 | 1 | ||||||
| 4.87 × 10−7 | ±4.97 × 10−9 | −0.41 | 1 | |||||
| 2.23 × 10−7 | ±4.57 × 10−9 | 0.40 | −0.88 | 1 | ||||
| E1 | 89 | ±8.92 | −0.19 | −0.25 | 0.23 | 1 | ||
| E2 | 42 | ±4.87 | −0.36 | 0.86 | −0.79 | −0.28 | 1 | |
| E3 | 44 | ±5.34 | −0.39 | 0.86 | −0.89 | −0.21 | 0.75 | 1 |
| m | 576 | |||||||
| DOF | 570 | |||||||
| Catalysts | Activation Energies of Formation (KJ/mole) | Reference | ||
|---|---|---|---|---|
| C4-Olefins | Carbon Oxides | |||
| VOx/MgO−γAl2O3 | 89 | 42 a | 44 b | Present Study |
| VOx/CeO2−γAl2O3 | 90.2 | 105.5 a | 81 b | [30] |
| V2O5/Al/Mg | 88.6 | 37.4 a | 45.4 b | [31] |
| VOx/Al2O3 | 70.2 | 65 a | 81.3 b | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Sulayman, A.; de Lasa, H. A Kinetic Model for Catalytic N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions in a Fluidized CREC Riser Simulator. Catalysts 2024, 14, 505. https://doi.org/10.3390/catal14080505
Bin Sulayman A, de Lasa H. A Kinetic Model for Catalytic N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions in a Fluidized CREC Riser Simulator. Catalysts. 2024; 14(8):505. https://doi.org/10.3390/catal14080505
Chicago/Turabian StyleBin Sulayman, Abdulhamid, and Hugo de Lasa. 2024. "A Kinetic Model for Catalytic N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions in a Fluidized CREC Riser Simulator" Catalysts 14, no. 8: 505. https://doi.org/10.3390/catal14080505







