Seeding as a Decisive Tool for Increasing Space-Time-Yields in the Preparation of High-Quality Cu/ZnO/ZrO2 Catalysts
Abstract
:1. Introduction
- Catalyst Preparation
- Aging Kinetics
- Objectives
2. Results and Discussion
2.1. Influence of Seeding on Aging Kinetics and Space-Time-Yield
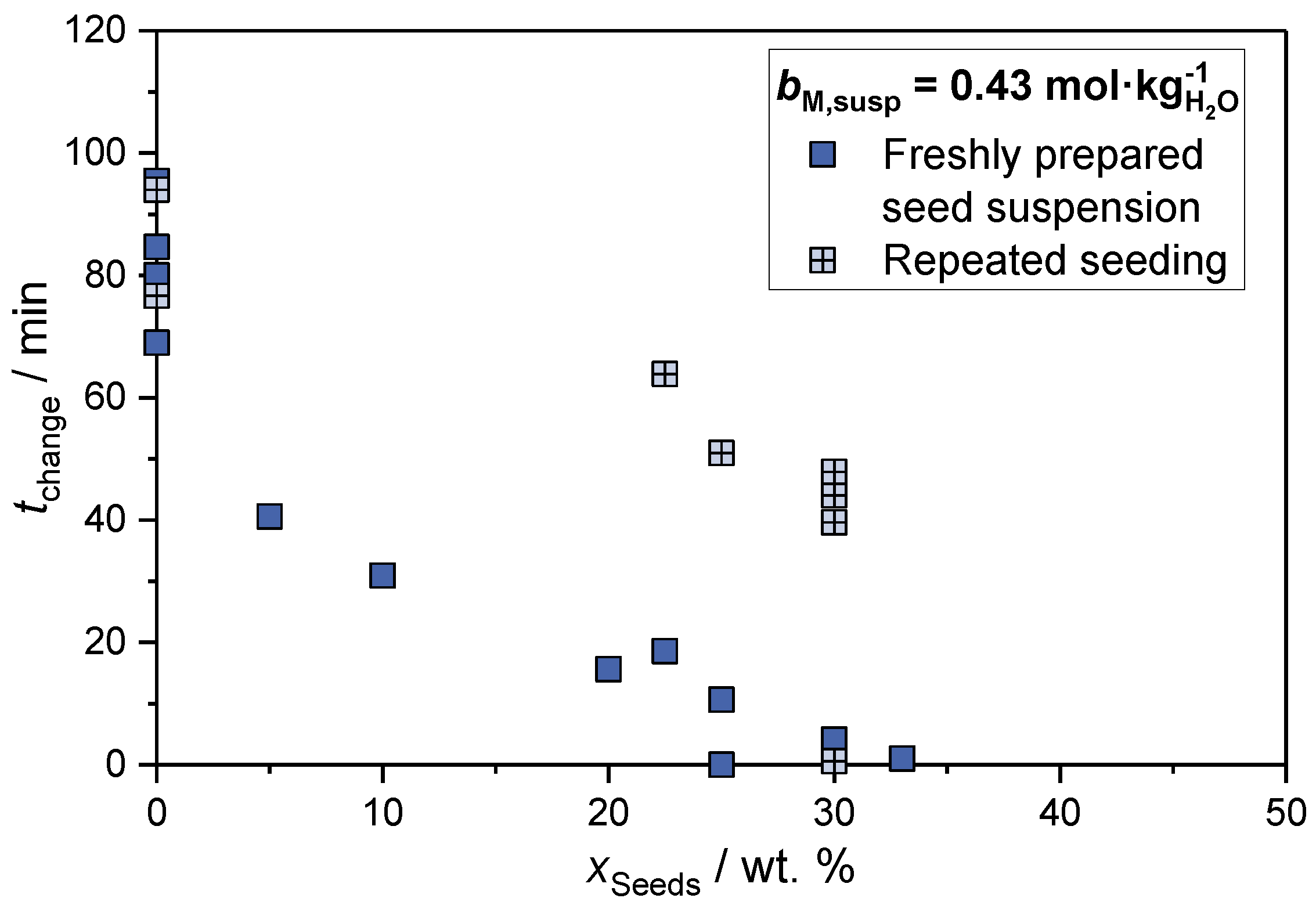
2.1.1. Seed Mass Fraction
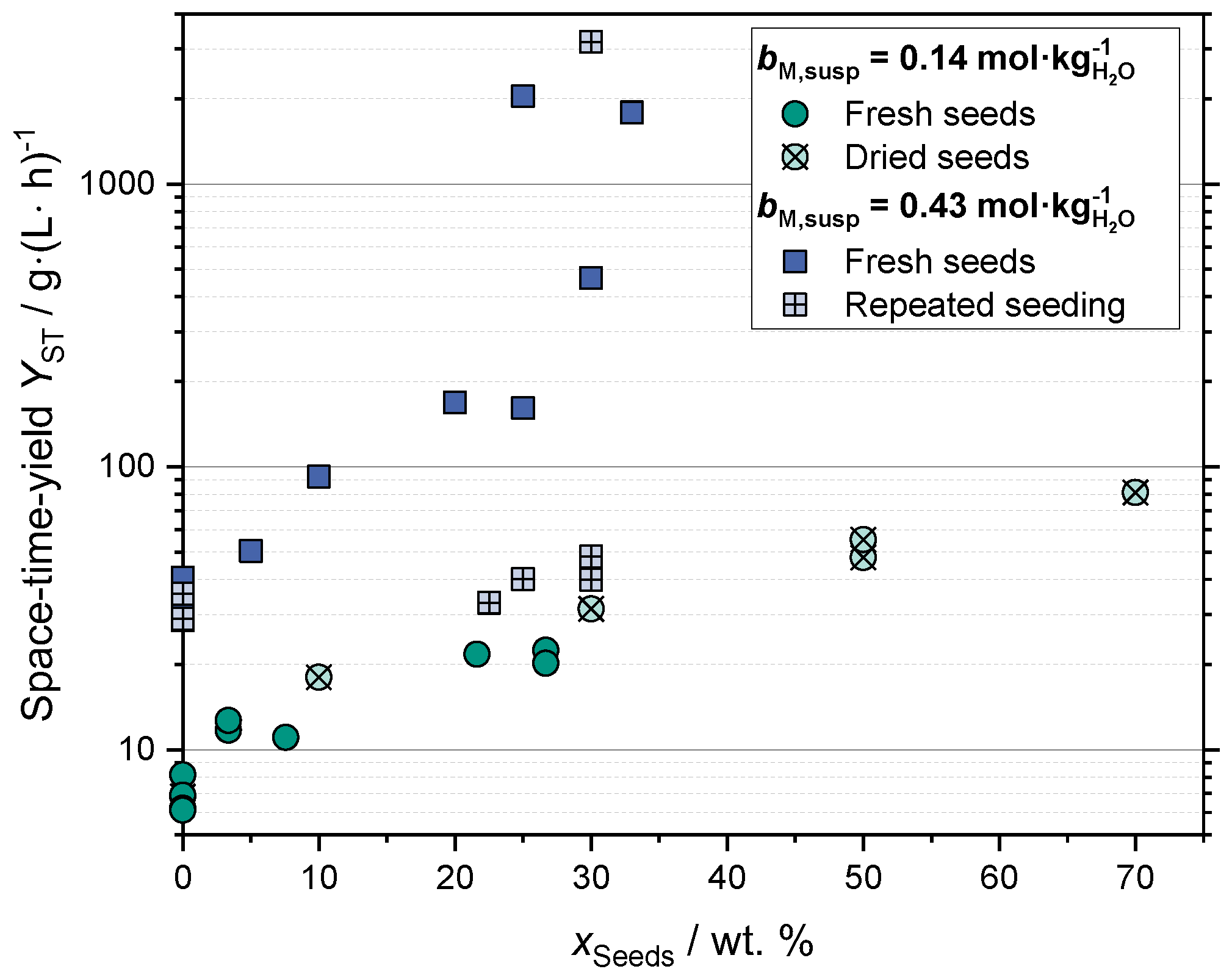
2.1.2. Space-Time Yield
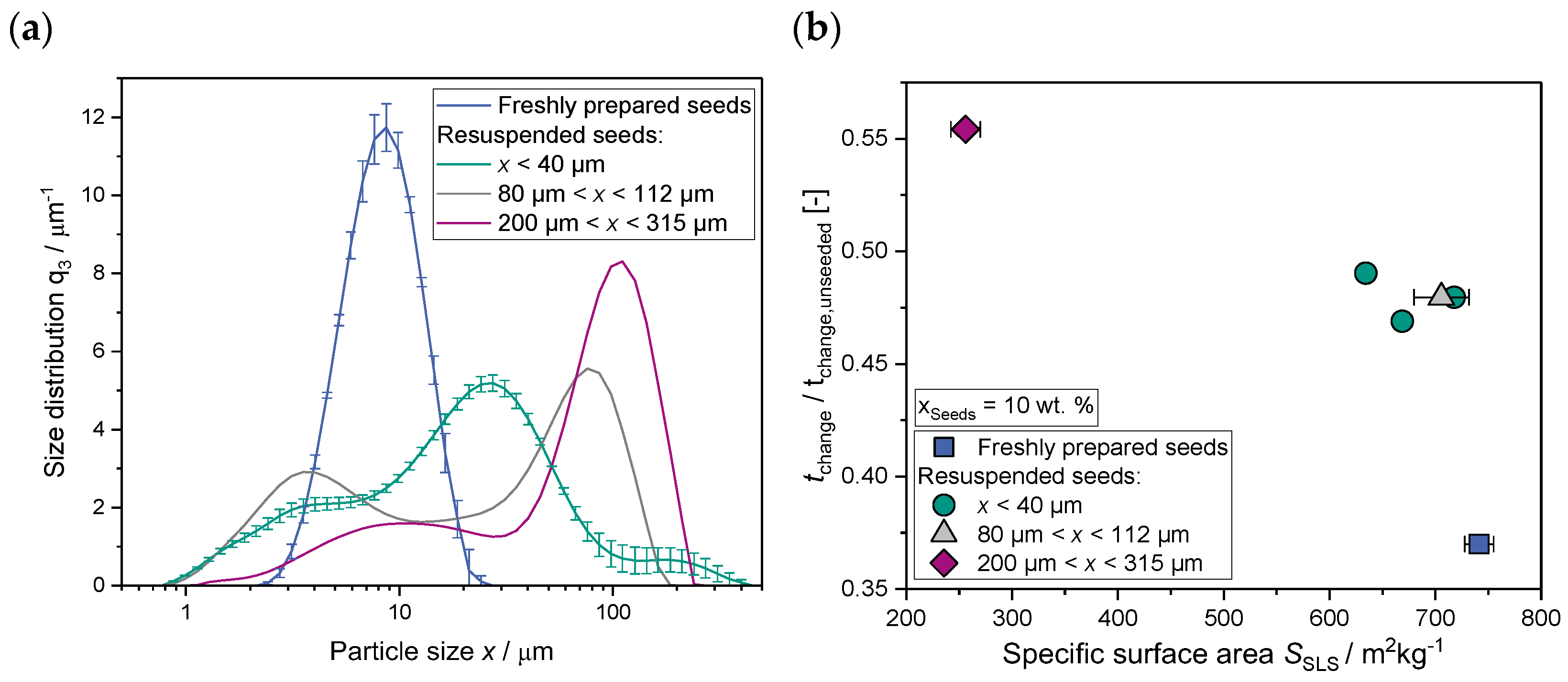
2.1.3. Seed Surface Area
2.2. Catalyst Properties and Performance
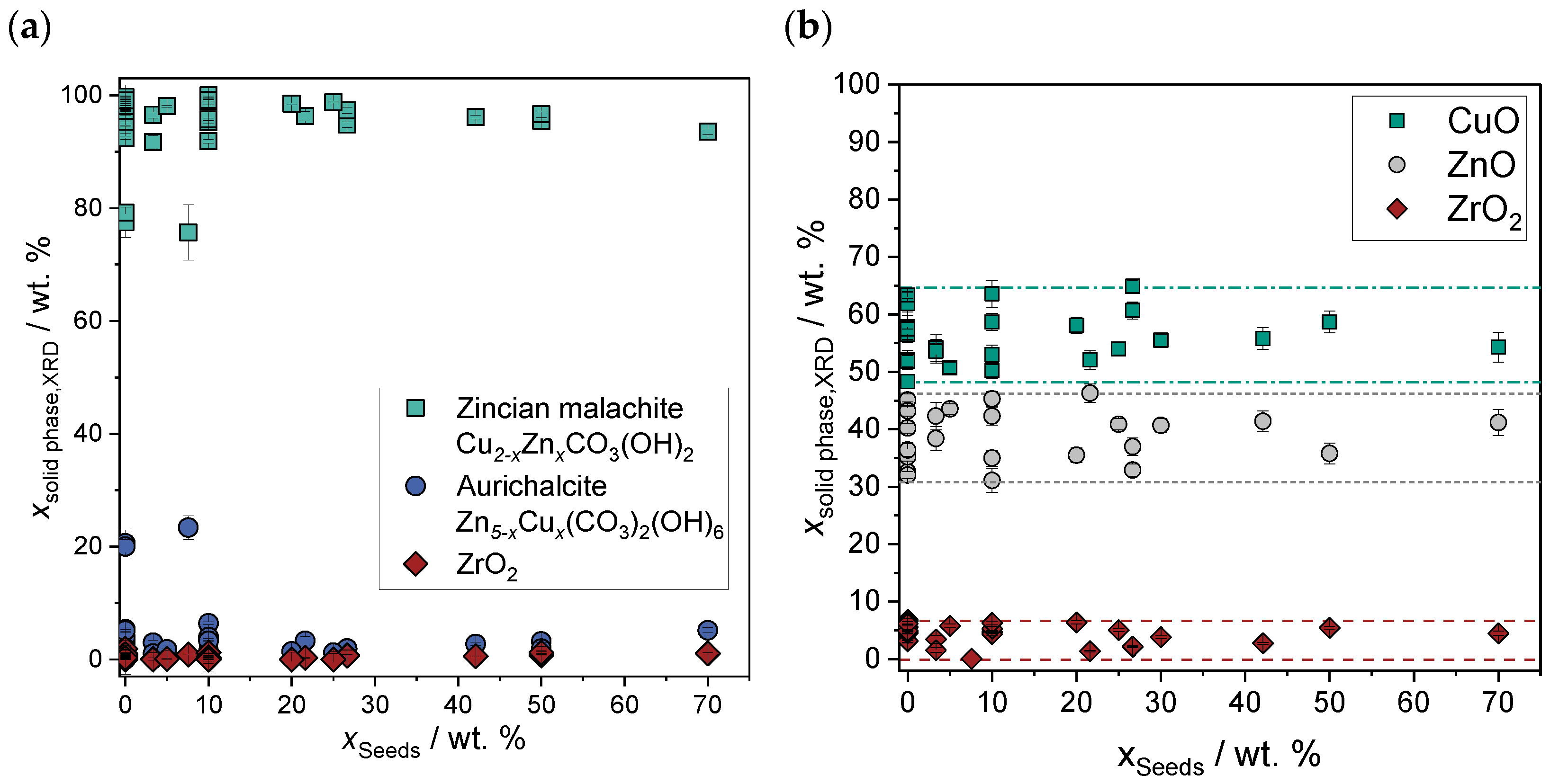
2.2.1. Physicochemical Properties of the Precatalyst
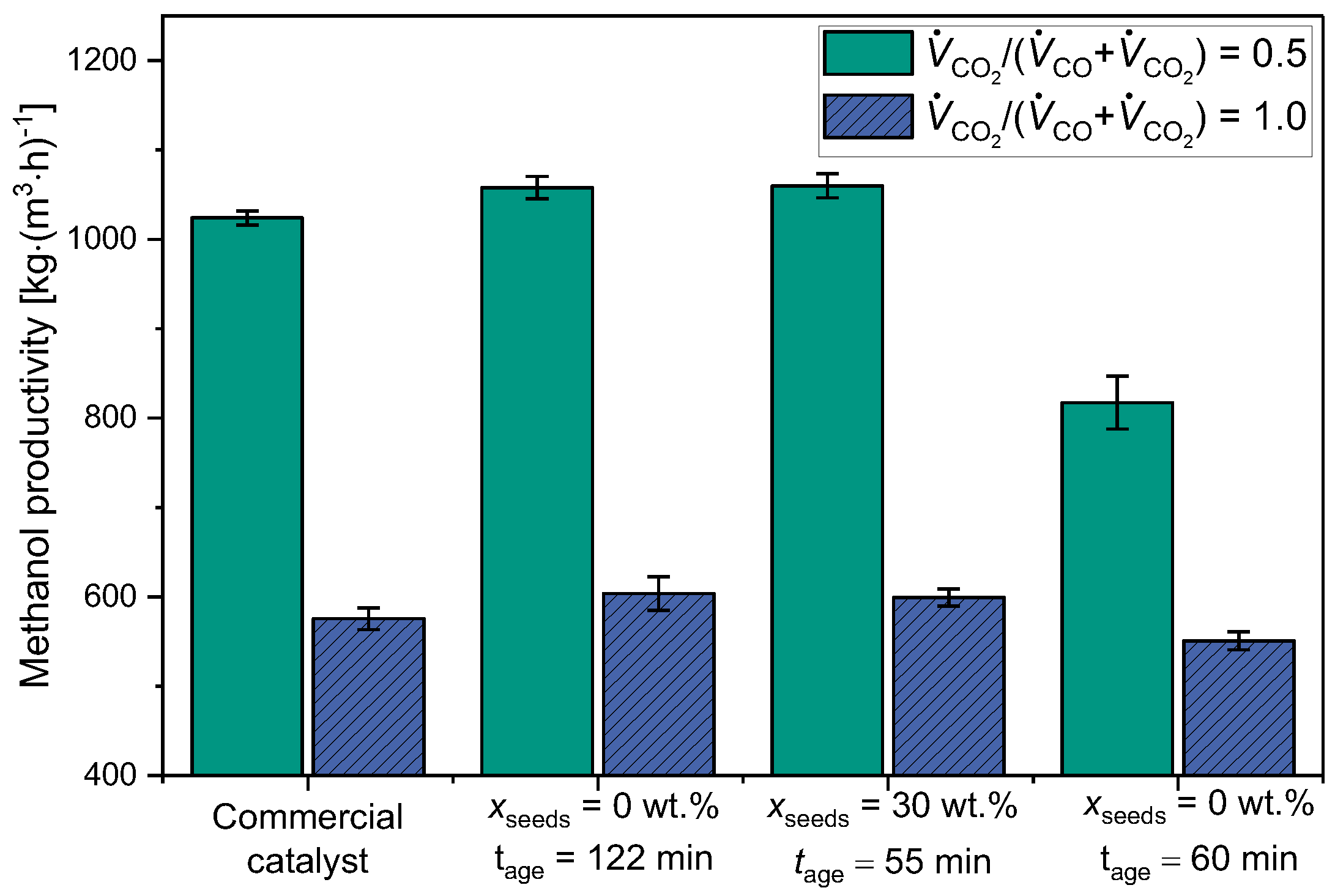
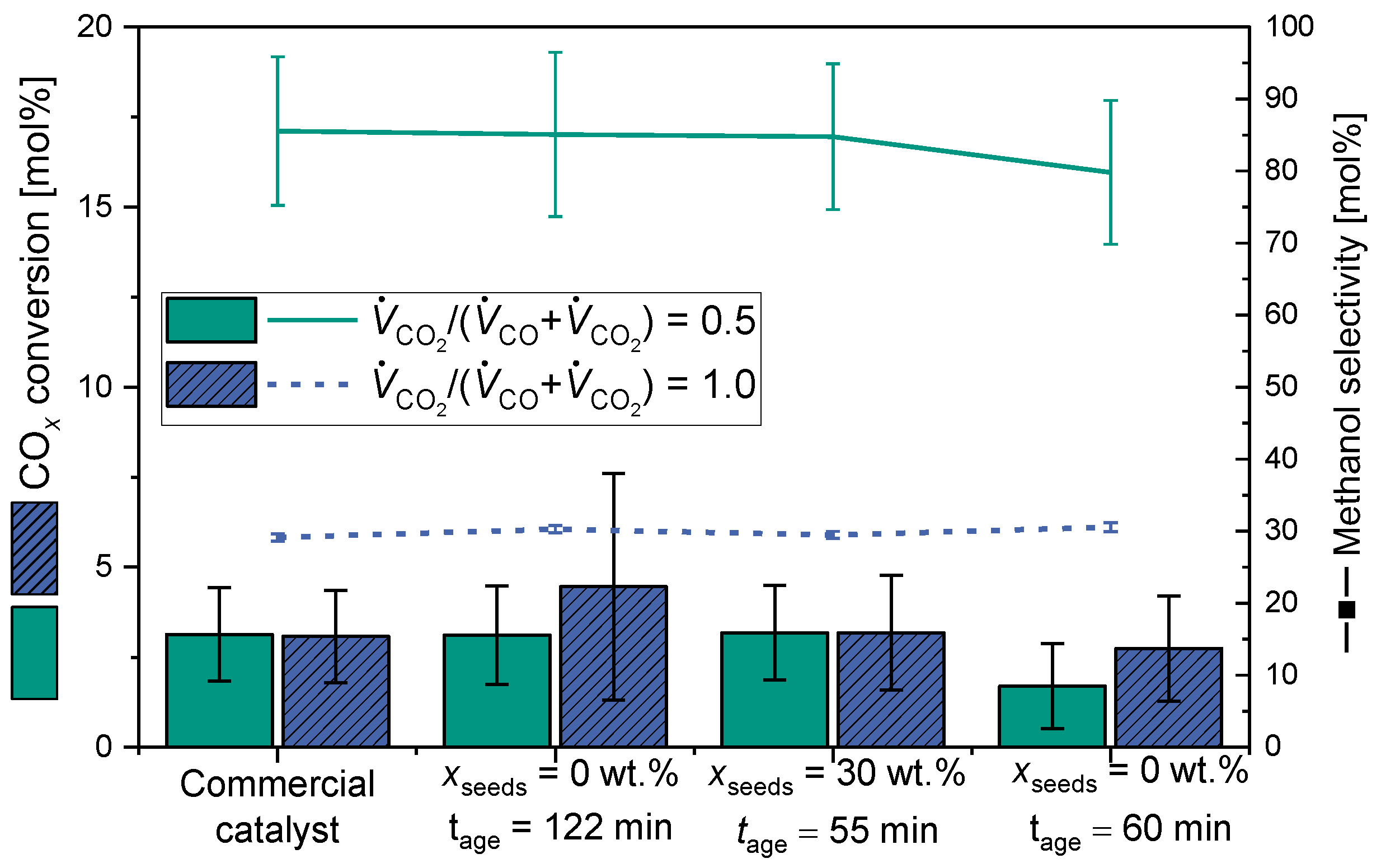
2.2.2. Comparison of Catalytic Behavior
3. Materials and Methods
3.1. Reactants and Intermediates
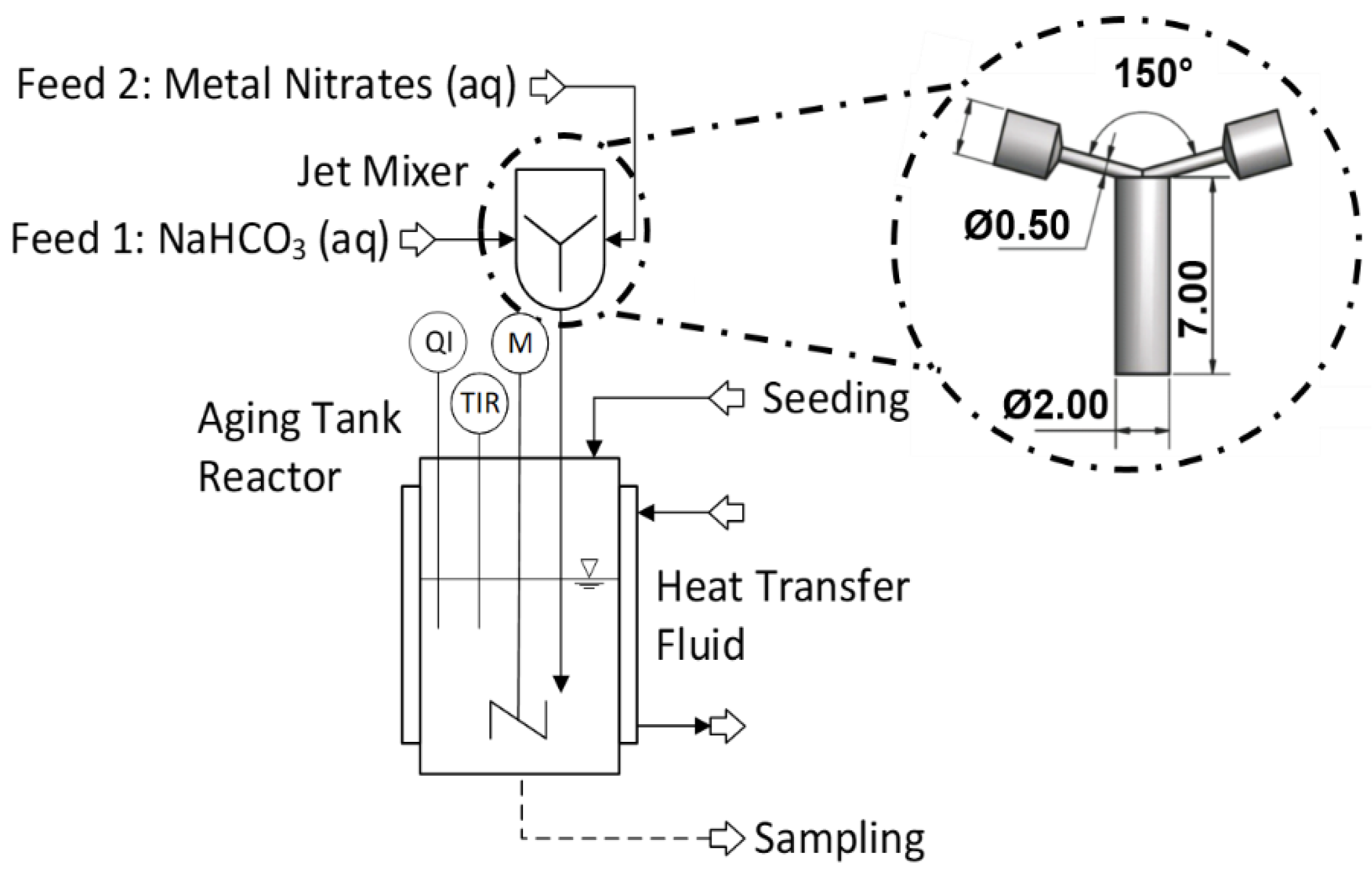
3.2. Experimental Setup and Procedure
3.3. Analytics
3.4. Methanol Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielen, D.; Dolan, G. Innovation Outlook: Renewable Methanol; Irena and Methanol Institute, Ed.; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; ISBN 978-92-9260-320-5. [Google Scholar]
- Production of Methanol Worldwide from 2017 to 2022. Available online: https://www.statista.com/statistics/1323406/methanol-production-worldwide/ (accessed on 20 March 2024).
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Beck, A.; Newton, M.A.; van de Water, L.G.A.; van Bokhoven, J.A. The Enigma of Methanol Synthesis by Cu/ZnO/Al2O3-Based Catalysts. Chem. Rev. 2024, 124, 4543–4678. [Google Scholar] [CrossRef]
- Pacchioni, G. From CO2 to Methanol on Cu/ZnO/Al2O3 Industrial Catalyst. What Do We Know about the Active Phase and the Reaction Mechanism? ACS Catal. 2024, 14, 2730–2745. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, Characterization and Activity Pattern of Cu–ZnO/ZrO2 Catalysts in the Hydrogenation of Carbon Dioxide to Methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Sternberg, A.; Bardow, A. Life Cycle Assessment of Power-to-Gas: Syngas vs. Methane. ACS Sustain. Chem. Eng. 2016, 4, 4156–4165. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, T.; Nguyen, D.D.; Cheng, C.K.; Lam, S.S.; Xia, C.; Nadda, A.K. Integrated Catalytic Insights into Methanol Production: Sustainable Framework for CO2 Conversion. J. Environ. Manag. 2021, 289, 112468. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Methanol Economy and Net Zero Emissions: Critical Analysis of Catalytic Processes, Reactors and Technologies. Green Chem. 2021, 23, 8361–8405. [Google Scholar] [CrossRef]
- Bampaou, M.; Haag, S.; Kyriakides, A.-S.; Panopoulos, K.D.; Seferlis, P. Optimizing Methanol Synthesis Combining Steelworks Off-Gases and Renewable Hydrogen. Renew. Sustain. Energy Rev. 2023, 171, 113035. [Google Scholar] [CrossRef]
- Chinchen, G.C.; Denny, P.J.; Jennings, J.R.; Spencer, M.S.; Waugh, K.C. Synthesis of Methanol. Part 1. Catalysts and Kinetics. Appl. Catal. 1988, 36, 1–65. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Sneeden, R.P.A. Copper-Zinc Oxide-Alumina Methanol Catalysts Revisited. Catal. Today 1987, 2, 1–124. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-State Interactions, Adsorption Sites and Functionality of Cu-ZnO/ZrO2 Catalysts in the CO2 Hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Wild, S.; Polierer, S.; Zevaco, T.A.; Guse, D.; Kind, M.; Pitter, S.; Herrera Delgado, K.; Sauer, J. Direct DME Synthesis on CZZ/H-FER from Variable CO2/CO Syngas Feeds. RSC Adv. 2021, 11, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Słoczyński, J.; Grabowski, R.; Kozłowska, A.; Olszewski, P.; Lachowska, M.; Skrzypek, J.; Stoch, J. Effect of Mg and Mn Oxide Additions on Structural and Adsorptive Properties of Cu/ZnO/ZrO2 Catalysts for the Methanol Synthesis from CO2. Appl. Catal. A Gen. 2003, 249, 129–138. [Google Scholar] [CrossRef]
- Köppel, R.A.; Stöcker, C.; Baiker, A. Copper- and Silver-Zirconia Aerogels: Preparation, Structural Properties and Catalytic Behavior in Methanol Synthesis from Carbon Dioxide. J. Catal. 1998, 179, 515–527. [Google Scholar] [CrossRef]
- Peláez, R.; Bryce, E.; Marín, P.; Ordóñez, S. Catalyst Deactivation in the Direct Synthesis of Dimethyl Ether from Syngas over CuO/ZnO/Al2O3 and γ-Al2O3 Mechanical Mixtures. Fuel Process. Technol. 2018, 179, 378–386. [Google Scholar] [CrossRef]
- Fichtl, M.B.; Schlereth, D.; Jacobsen, N.; Kasatkin, I.; Schumann, J.; Behrens, M.; Schlögl, R.; Hinrichsen, O. Kinetics of Deactivation on Cu/ZnO/Al2O3 Methanol Synthesis Catalysts. Appl. Catal. A Gen. 2015, 502, 262–270. [Google Scholar] [CrossRef]
- Baltes, C.; Vukojevic, S.; Schuth, F. Correlations between Synthesis, Precursor, and Catalyst Structure and Activity of a Large Set of CuO/ZnO/Al2O3 Catalysts for Methanol Synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]
- Choi, Y.; Futagami, K.; Fujitani, T.; Nakamura, J. The Role of ZnO in Cu/ZnO Methanol Synthesis Catalysts—Morphology Effect or Active Site Model? Appl. Catal. A Gen. 2001, 208, 163–167. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The Chemical Modification Seen in the Cu/ZnO Methanol Synthesis Catalysts. Appl. Catal. A Gen. 2000, 191, 111–129. [Google Scholar] [CrossRef]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology-Dependent Interactions of ZnO with Cu Nanoparticles at the Materials’ Interface in Selective Hydrogenation of CO2 to CH3OH. Angew. Chem. Int. Ed. 2011, 50, 2162–2165. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the Promotion of Cu Catalysts by ZnO for Methanol Synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef]
- Behrens, M.; Schlögl, R. How to Prepare a Good Cu/ZnO Catalyst or the Role of Solid State Chemistry for the Synthesis of Nanostructured Catalysts. Z. Anorg. Allg. Chem. 2013, 639, 2683–2695. [Google Scholar] [CrossRef]
- Whittle, D.M.; Mirzaei, A.A.; Hargreaves, J.S.J.; Joyner, R.W.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Co-Precipitated Copper Zinc Oxide Catalysts for Ambient Temperature Carbon Monoxide Oxidation: Effect of Precipitate Ageing on Catalyst Activity. Phys. Chem. Chem. Phys. 2002, 4, 5915–5920. [Google Scholar] [CrossRef]
- Spencer, M.S. The Role of Zinc Oxide in Cu/ZnO Catalysts for Methanol Synthesis and the Water–Gas Shift Reaction. Top. Catal. 1999, 8, 259–266. [Google Scholar] [CrossRef]
- Behrens, M.; Girgsdies, F. Structural Effects of Cu/Zn Substitution in the Malachite-Rosasite System. Z. Anorg. Allg. Chem. 2010, 636, 919–927. [Google Scholar] [CrossRef]
- Zwiener, L.; Girgsdies, F.; Brennecke, D.; Teschner, D.; Machoke, A.G.F.; Schlögl, R.; Frei, E. Evolution of Zincian Malachite Synthesis by Low Temperature Co-Precipitation and Its Catalytic Impact on the Methanol Synthesis. Appl. Catal. B Environ. 2019, 249, 218–226. [Google Scholar] [CrossRef]
- Behrens, M.; Girgsdies, F.; Trunschke, A.; Schlögl, R. Minerals as Model Compounds for Cu/ZnO Catalyst Precursors: Structural and Thermal Properties and IR Spectra of Mineral and Synthetic (Zincian) Malachite, Rosasite and Aurichalcite and a Catalyst Precursor Mixture. Eur. J. Inorg. Chem. 2009, 2009, 1347–1357. [Google Scholar] [CrossRef]
- Perchiazzi, N. Crystal Structure Determination and Rietveld Refinement of Rosasite and Mcguinnessite. In Proceedings of the Ninth European Powder Diffraction Conference, Prague, Czech Republic, 2–5 September 2004; Volume 23, pp. 505–510. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A.; Just, J.; Bridge, P.J. The Synthesis and Composition of Georgeite and Its Reactions to Form Other Secondary Copper(II) Carbonates. Mineral. Mag. 1991, 55, 163–166. [Google Scholar] [CrossRef]
- Pollard, A.M.; Spencer, M.S.; Thomas, R.G.; Williams, P.A.; Holt, J.; Jennings, J.R. Georgeite and Azurite as Precursors in the Preparation of Co-Precipitated Copper/Zinc Oxide Catalysts. Appl. Catal. A Gen. 1992, 85, 1–11. [Google Scholar] [CrossRef]
- Guse, D.; Polierer, S.; Wild, S.; Pitter, S.; Kind, M. Improved Preparation of Cu/Zn-Based Catalysts by Well-Defined Conditions of Co-Precipitation and Aging. Chem. Ing. Tech. 2022, 94, 314–327. [Google Scholar] [CrossRef]
- Bems, B.; Schur, M.; Dassenoy, A.; Junkes, H.; Herein, D.; Schlögl, R. Relations between Synthesis and Microstructural Properties of Copper/Zinc Hydroxycarbonates. Chemistry 2003, 9, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Mota, N.; Guil-Lopez, R.; Pawelec, B.G.; Fierro, J.L.G.; Navarro, R.M. Highly Active Cu/ZnO–Al Catalyst for Methanol Synthesis: Effect of Aging on Its Structure and Activity. RSC Adv. 2018, 8, 20619–20629. [Google Scholar] [CrossRef]
- Angelo, L.; Girleanu, M.; Ersen, O.; Serra, C.; Parkhomenko, K.; Roger, A.-C. Catalyst Synthesis by Continuous Coprecipitation under Micro-Fluidic Conditions: Application to the Preparation of Catalysts for Methanol Synthesis from CO2/H2. Catal. Today 2016, 270, 59–67. [Google Scholar] [CrossRef]
- Behrens, M.; Brennecke, D.; Girgsdies, F.; Kißner, S.; Trunschke, A.; Nasrudin, N.; Zakaria, S.; Idris, N.F.; Hamid, S.B.A.; Kniep, B.; et al. Understanding the Complexity of a Catalyst Synthesis: Co-Precipitation of Mixed Cu,Zn,Al Hydroxycarbonate Precursors for Cu/ZnO/Al2O3 Catalysts Investigated by Titration Experiments. Appl. Catal. A Gen. 2011, 392, 93–102. [Google Scholar] [CrossRef]
- Frei, E.; Schaadt, A.; Ludwig, T.; Hillebrecht, H.; Krossing, I. The Influence of the Precipitation/Ageing Temperature on a Cu/ZnO/ZrO2 Catalyst for Methanol Synthesis from H2 and CO2. ChemCatChem 2014, 6, 1721–1730. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Cheng, K.-P.; Wen, L.-X.; Guo, K.; Chen, J.-F. A Study on the Precipitating and Aging Processes of CuO/ZnO/Al2O3 Catalysts Synthesized in Micro-Impinging Stream Reactors. RSC Adv. 2016, 6, 33611–33621. [Google Scholar] [CrossRef]
- Kaluza, S.; Behrens, M.; Schiefenhövel, N.; Kniep, B.; Fischer, R.; Schlögl, R.; Muhler, M. A Novel Synthesis Route for Cu/ZnO/Al2O3 Catalysts Used in Methanol Synthesis: Combining Continuous Consecutive Precipitation with Continuous Aging of the Precipitate. ChemCatChem 2011, 3, 189–199. [Google Scholar] [CrossRef]
- Polierer, S.; Guse, D.; Wild, S.; Herrera Delgado, K.; Otto, T.N.; Zevaco, T.A.; Kind, M.; Sauer, J.; Studt, F.; Pitter, S. Enhanced Direct Dimethyl Ether Synthesis from CO2-Rich Syngas with Cu/ZnO/ZrO2 Catalysts Prepared by Continuous Co-Precipitation. Catalysts 2020, 10, 816. [Google Scholar] [CrossRef]
- Behrens, M. Meso- and Nano-Structuring of Industrial Cu/ZnO/(Al2O3) Catalysts. J. Catal. 2009, 267, 24–29. [Google Scholar] [CrossRef]
- Schumann, J.; Lunkenbein, T.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Synthesis and Characterisation of a Highly Active Cu/ZnO: Al Catalyst. ChemCatChem 2014, 6, 2889–2897. [Google Scholar] [CrossRef]
- Guse, D.; Warmuth, L.; Kreißig, F.; Pitter, S.; Kind, M. Preparation of Cu/Zn Based Catalyst Precursors—Importance of Thermodynamics and Seeding. In Proceedings of the DGMK-Conference “The Role of Catalysis for the Energy Transition“, Ludwigshafen, Germany, 5–7 October 2022; Volume 2022–2023, pp. 19–39. [Google Scholar] [CrossRef]
- Güldenpfennig, A.; Distaso, M.; Peukert, W. In Situ Investigations on the Amorphous to Crystalline Phase Transformation of Precursors for Methanol Synthesis Catalysts. Chem. Eng. J. 2019, 369, 996–1004. [Google Scholar] [CrossRef]
- Zander, S.; Seidlhofer, B.; Behrens, M. In Situ EDXRD Study of the Chemistry of Aging of Co-Precipitated Mixed Cu,Zn Hydroxycarbonates--Consequences for the Preparation of Cu/ZnO Catalysts. Dalton Trans. 2012, 41, 13413–13422. [Google Scholar] [CrossRef] [PubMed]
- Farahani, B.V.; Rajabi, F.H.; Bahmani, M.; Ghelichkhani, M.; Sahebdelfar, S. Influence of Precipitation Conditions on Precursor Particle Size Distribution and Activity of Cu/ZnO Methanol Synthesis Catalyst. Appl. Catal. Gen. 2014, 482, 237–244. [Google Scholar] [CrossRef]
- Muhamad, E.N.; Irmawati, R.; Taufiq-Yap, Y.H.; Abdullah, A.H.; Kniep, B.L.; Girgsdies, F.; Ressler, T. Comparative Study of Cu/ZnO Catalysts Derived from Different Precursors as a Function of Aging. Catal. Today 2008, 131, 118–124. [Google Scholar] [CrossRef]
- Smith, P.J.; Kondrat, S.A.; Chater, P.A.; Yeo, B.R.; Shaw, G.M.; Lu, L.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Kiely, C.J.; et al. A New Class of Cu/ZnO Catalysts Derived from Zincian Georgeite Precursors Prepared by Co-Precipitation. Chem. Sci. 2017, 8, 2436–2447. [Google Scholar] [CrossRef]
- Sengupta, G.; Das, D.P.; Kundu, M.L.; Dutta, S.; Roy, S.K.; Sahay, R.N.; Mishra, K.K.; Ketchik, S.V. Study of Copper—Zinc Oxide Catalysts, Characterisation of the Coprecipitate and Mixed Oxide. Appl. Catal. 1989, 55, 165–180. [Google Scholar] [CrossRef]
- Kaluza, S.; Muhler, M. On the Role of Aging, Washing, and Drying in the Synthesis of Polycrystalline Zinc Oxide by Precipitation: Combining Fast Continuous Mixing, Spray Drying and Freeze Drying to Unravel the Solid-State Transformations of the Precipitate. Catal. Lett. 2009, 129, 287–292. [Google Scholar] [CrossRef]
- Schumann, J.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Cu,Zn-Based Catalysts for Methanol Synthesis: On the Effect of Calcination Conditions and the Part of Residual Carbonates. Appl. Catal. A Gen. 2016, 516, 117–126. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2001; ISBN 978-0-7506-4833-2. [Google Scholar]
- Manuel García-Ruiz, J. Nucleation of Protein Crystals. J. Struct. Biol. 2003, 142, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Myerson, A.S. Handbook of Industrial Crystallization, 2nd ed.; Butterworth-Heinemann: Boston, UK, 2002; ISBN 978-0-08-053351-3. [Google Scholar]
- Beckmann, W. Crystallization: Basic. Concepts and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1-299-15731-9. [Google Scholar]
- Threlfall, T.L.; De’Ath, R.W.; Coles, S.J. Metastable Zone Widths, Conformational Multiplicity, and Seeding. Org. Process Res. Dev. 2013, 17, 578–584. [Google Scholar] [CrossRef]
- Threlfall, T.L.; Coles, S.J. A Perspective on the Growth-Only Zone, the Secondary Nucleation Threshold and Crystal Size Distribution in Solution Crystallisation. CrystEngComm 2016, 18, 369–378. [Google Scholar] [CrossRef]
- Barros Groß, M.; Kind, M. Comparative Study on Seeded and Unseeded Bulk Evaporative Batch Crystallization of Tetragonal Lysozyme. Cryst. Growth Des. 2017, 17, 3491–3501. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, L.; Wang, Z.; Lu, J. Microstructure Characters of Cu/ZnO Catalyst Precipitated inside Microchannel Reactor. J. Mol. Catal. A Chem. 2016, 423, 457–462. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, X.; Ling, C.; Wang, Z.; Lu, J. The Effect of Mixing on Co-Precipitation and Evolution of Microstructure of Cu-ZnO Catalyst. AIChE J. 2018, 124, 123. [Google Scholar] [CrossRef]
- Klokishner, S.; Behrens, M.; Reu, O.; Tzolova-Müller, G.; Girgsdies, F.; Trunschke, A.; Schlögl, R. Cation Ordering in Natural and Synthetic (Cu1−xZnx)2CO3(OH)2 and (Cu1−xZnx)5(CO3)2(OH)6. J. Phys. Chem. A 2011, 115, 9954–9968. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Smith, P.J.; Wells, P.P.; Chater, P.A.; Carter, J.H.; Morgan, D.J.; Fiordaliso, E.M.; Wagner, J.B.; Davies, T.E.; Lu, L.; et al. Stable Amorphous Georgeite as a Precursor to a High-Activity Catalyst. Nature 2016, 531, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Loï Mi Lung-Somarriba, B.; Moscosa-Santillan, M.; Porte, C.; Delacroix, A. Effect of Seeded Surface Area on Crystal Size Distribution in Glycine Batch Cooling Crystallization: A Seeding Methodology. J. Cryst. Growth 2004, 270, 624–632. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, T.; Cheng, Y.; Cohen, Y. Observed Crystallization Induction Time in Seeded Gypsum Crystallization. Ind. Eng. Chem. Res. 2019, 58, 23359–23365. [Google Scholar] [CrossRef]
- Evans, T.W.; Sarofim, A.F.; Margolis, G. Models of Secondary Nucleation Attributable to Crystal-Crystallizer and Crystal-Crystal Collisions. AIChE J. 1974, 20, 959–966. [Google Scholar] [CrossRef]
- Kaysan, G.; Rica, A.; Guthausen, G.; Kind, M. Contact-Mediated Nucleation of Subcooled Droplets in Melt Emulsions: A Microfluidic Approach. Crystals 2021, 11, 1471. [Google Scholar] [CrossRef]
- Zhang, K.; Chanpura, R.A.; Mondal, S.; Wu, C.-H.; Sharma, M.M.; Ayoub, J.A.; Parlar, M. Particle Size Distribution Measurement Techniques and Their Relevance or Irrelevance to Sand Control Design. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26 February 2014. [Google Scholar]
- D’Ans, J.; Lax, E. Taschenbuch Für Chemiker Und Physiker.: Band I: Makroskopische Physikalisch-Chemische Eigenschaften, 3rd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1967. [Google Scholar]
- Waller, D.; Stirling, D.; Stone, F.S.; Spencer, M.S. Copper–Zinc Oxide Catalysts. Activity in Relation to Precursor Structure and Morphology. Faraday Discuss. Chem. Soc. 1989, 87, 107–120. [Google Scholar] [CrossRef]
- Kniep, B.L.; Girgsdies, F.; Ressler, T. Effect of Precipitate Aging on the Microstructural Characteristics of Cu/ZnO Catalysts for Methanol Steam Reforming. J. Catal. 2005, 236, 34–44. [Google Scholar] [CrossRef]
- Frusteri, F.; Cordaro, M.; Cannilla, C.; Bonura, G. Multifunctionality of Cu–ZnO–ZrO2/H-ZSM5 Catalysts for the One-Step CO2-to-DME Hydrogenation Reaction. Appl. Catal. B Environ. 2015, 162, 57–65. [Google Scholar] [CrossRef]
- Warmuth, L.; Steurer, M.; Schild, D.; Zimina, A.; Grunwaldt, J.-D.; Pitter, S. Reversible and Irreversible Structural Changes in Cu/ZnO/ZrO2 Catalysts during Methanol Synthesis. ACS Appl. Mater. Interfaces 2024, 16, 8813–8821. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, K.; Li, H.; Yu, Z. Thermodynamic Analysis of Chemical and Phase Equilibria in CO2 Hydrogenation to Methanol, Dimethyl Ether, and Higher Alcohols. Ind. Eng. Chem. Res. 2018, 57, 4081–4094. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Zetasizer Nano User Manual; Malvern Instruments Ltd.: Malvern, UK, 2013. [Google Scholar]
- Mastersizer User Guide 2024. Available online: https://www.malvernpanalytical.com/de/learn/knowledge-center/user-manuals/man0474en (accessed on 12 June 2024).
- Malvern Panalytical. Malvern Panalytical Selecting an Appropiate Particle Absorption for Laser Diffraction Particle Size Calculations. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/TN101104SelectingParticleAbsorbtionLaserDiffractio (accessed on 12 June 2024).
- Wild, S.; Lacerda de Oliveira Campos, B.; Zevaco, T.A.; Guse, D.; Kind, M.; Pitter, S.; Herrera Delgado, K.; Sauer, J. Experimental Investigations and Model-Based Optimization of CZZ/H-FER 20 Bed Composition for the Direct Synthesis of DME from CO2-Rich Syngas. React. Chem. Eng. 2022, 7, 943–956. [Google Scholar] [CrossRef]
- Judat, B.; Kind, M. Morphology and Internal Structure of Barium Sulfate—Derivation of a New Growth Mechanism. J. Colloid. Interface Sci. 2004, 269, 341–353. [Google Scholar] [CrossRef]
- Shen, G.-C.; Fujita, S.; Matsumoto, S.; Takezawa, N. Steam Reforming of Methanol on Binary CuZnO Catalysts: Effects of Preparation Condition upon Precursors, Surface Structure and Catalytic Activity. J. Mol. Catal. A Chem. 1997, 124, 123–136. [Google Scholar] [CrossRef]
- Fujita, S.; Satriyo, A.M.; Shen, G.C.; Takezawa, N. Mechanism of the Formation of Precursors for the Cu/ZnO Methanol Synthesis Catalysts by a Coprecipitation Method. Catal. Lett. 1995, 34, 85–92. [Google Scholar] [CrossRef]
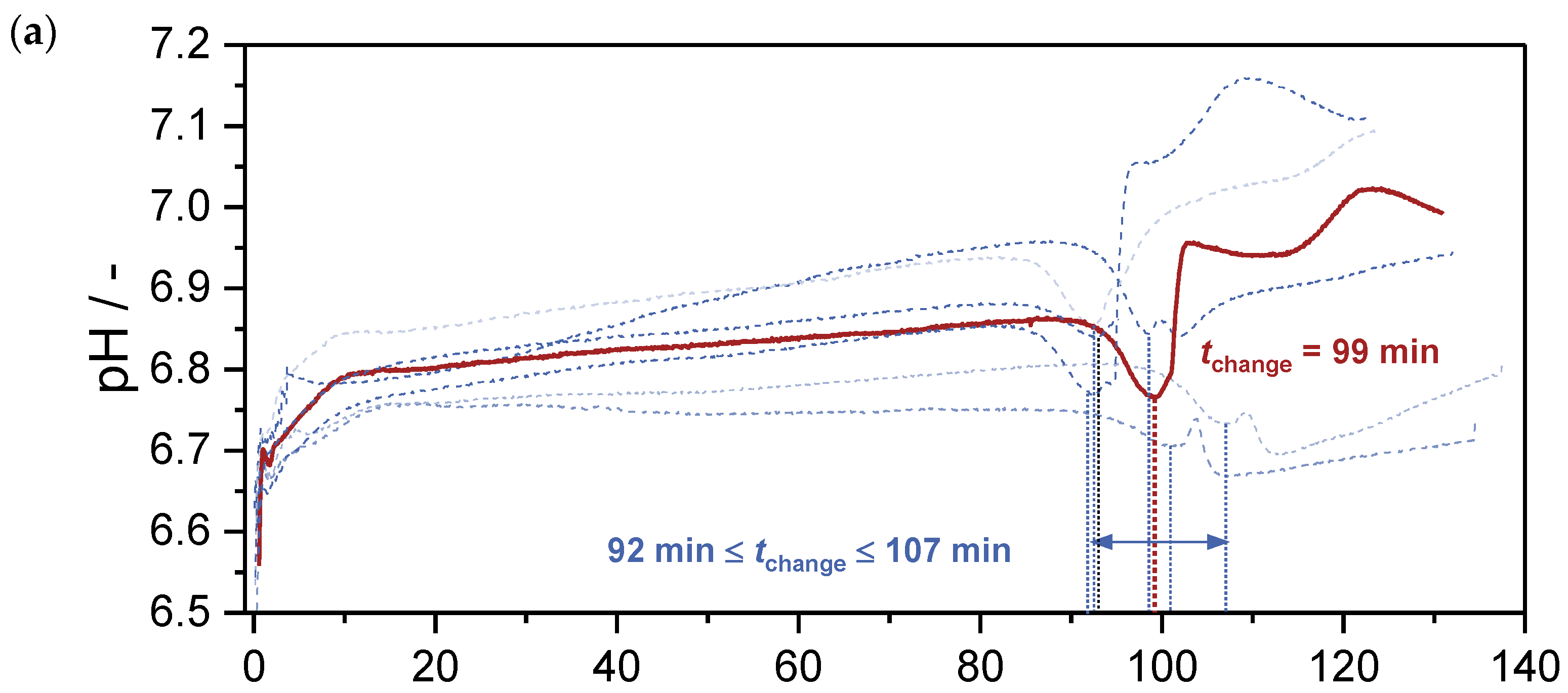
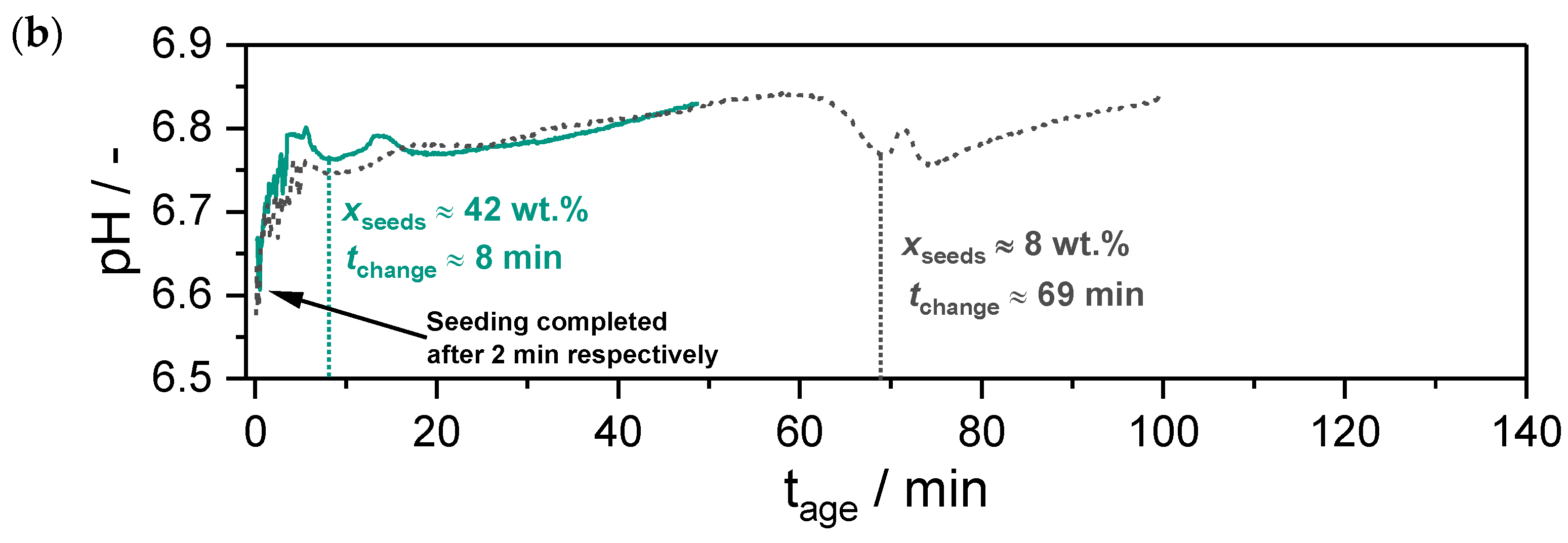
 ) and in zincian malachite (
) and in zincian malachite ( ) as a function of the aging time : (a) a standard aging process without seeding (), based on the work of Guse et al. [46]; (b) a preparation where seeds were added after co-precipitation is completed at ().
) as a function of the aging time : (a) a standard aging process without seeding (), based on the work of Guse et al. [46]; (b) a preparation where seeds were added after co-precipitation is completed at ().
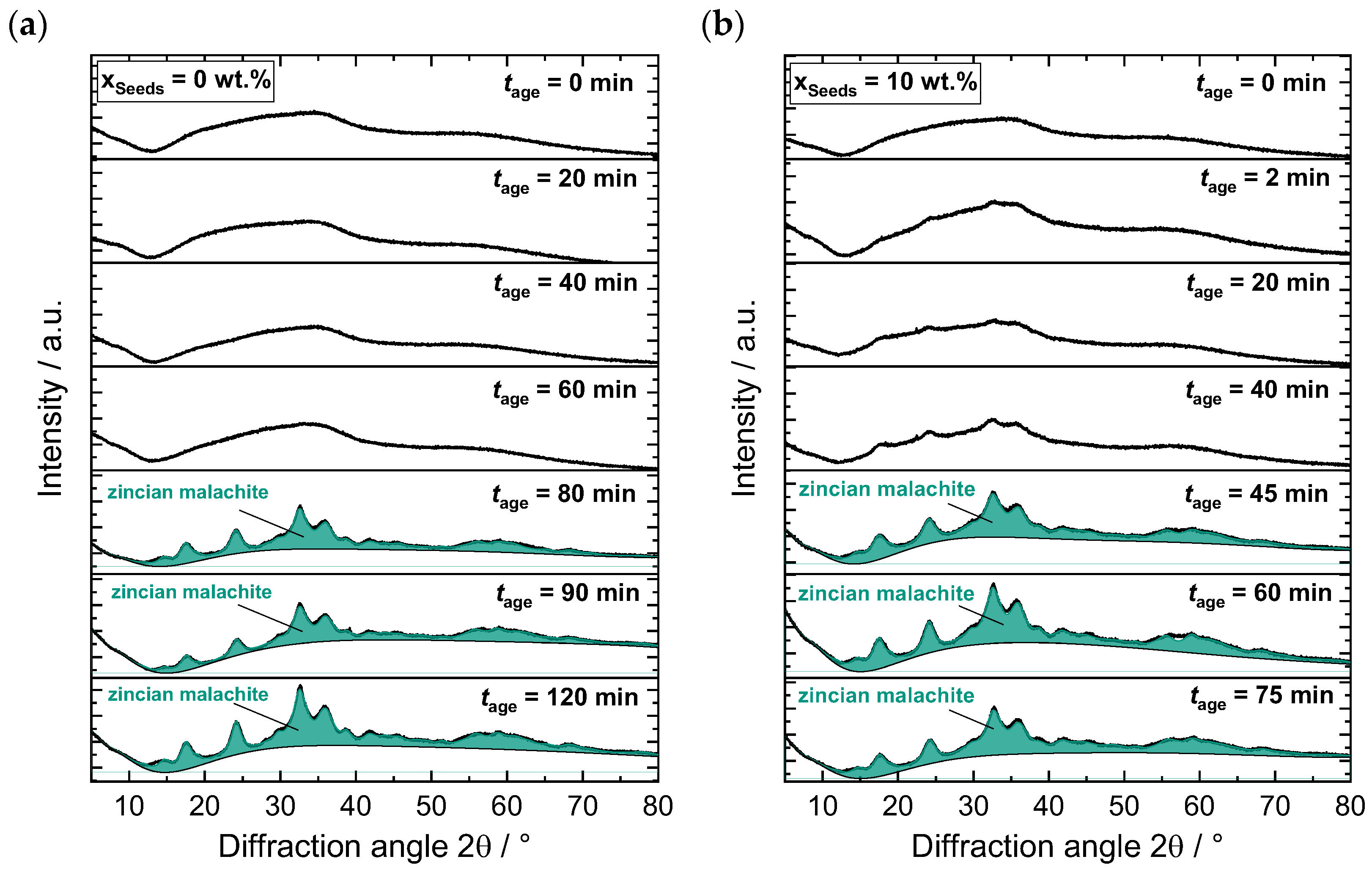
 ) and in zincian malachite (
) and in zincian malachite ( ) as a function of the aging time : (a) a standard aging process without seeding (), based on the work of Guse et al. [46]; (b) a preparation where seeds were added after co-precipitation is completed at ().
) as a function of the aging time : (a) a standard aging process without seeding (), based on the work of Guse et al. [46]; (b) a preparation where seeds were added after co-precipitation is completed at ().
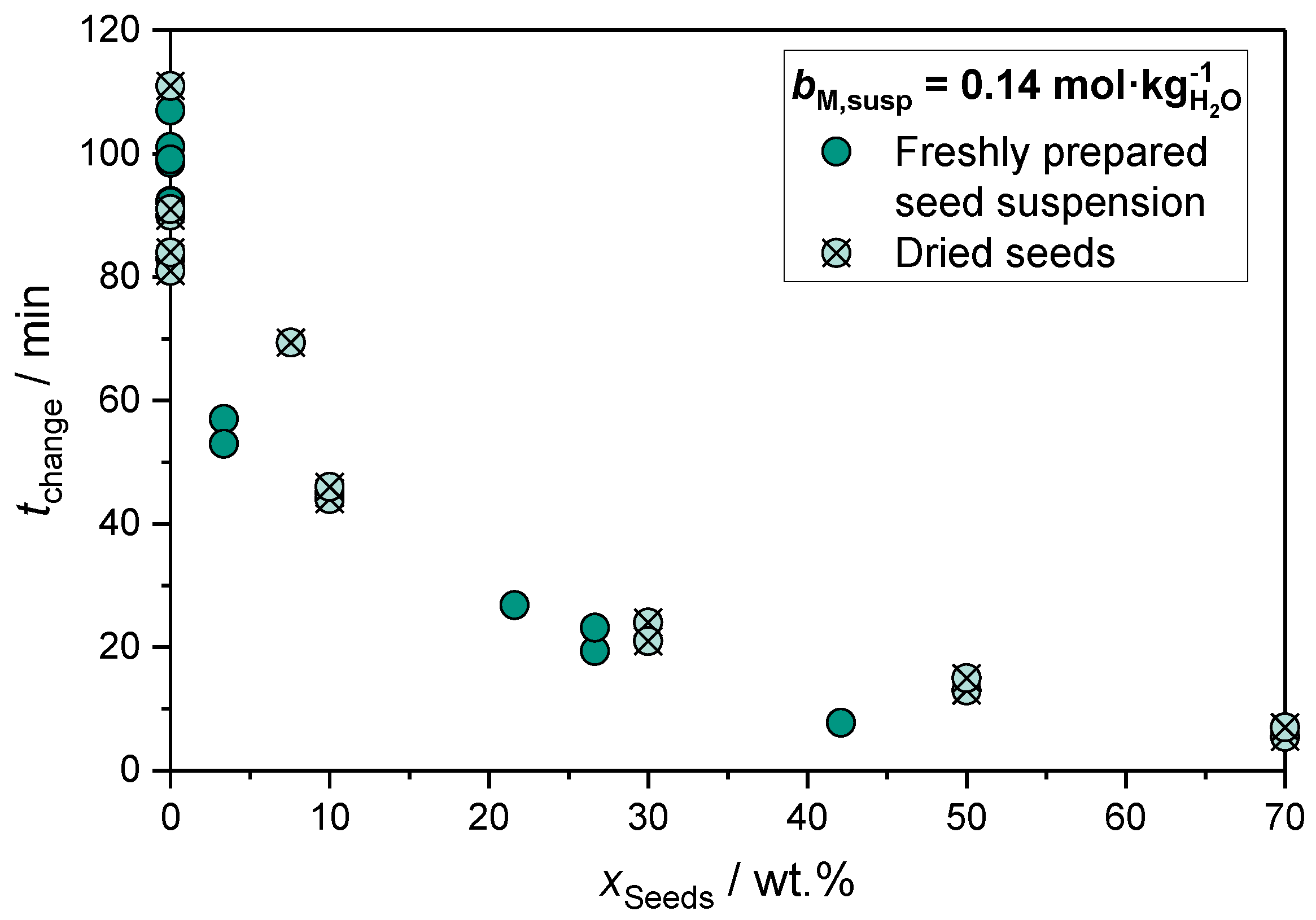


[min] | [mol%] | [mol%] | [mol%] | [nm] | [nm] | [m2g−1] | [m2g−1] | |
|---|---|---|---|---|---|---|---|---|
| 122 ± 6 | 63.2 ± 0.8 | 28.4 ± 0.9 | 8.4 ± 0.4 | 3 ± 1 | 10 ± 1 | 122 ± 4 | 68 | |
| 0 | 60 | 65.7 | 24.0 | 9.1 | 9 ± 0 | 31 | 69 | 46 |
| 3 | 85 ± 2 | 59.1 | 32.1 | 8.7 | 3 ± 0 | 9 ± 0 | 123 ± 1 | - * |
| 10 | 78 ± 1 | 65.3 | 26.8 | 7.9 | 4 ± 0 | - * | - * | - * |
| 27 | 51 ± 2 | 64.3 | 28.4 | 7.2 | 2 ± 0 | 11 | 120 | - * |
| 30 | 55 ± 2 | 64.4 | 27.1 | 8.5 | 3 ± 0 | 9 | 148 | 66 |
| 50 | 14 ± 1 | 64.1 | 26.8 | 9.0 | 4 ± 0 | - * | - * | - * |
| 70 | 7 ± 1 | 64.3 | 26.8 | 7.9 | 4 ± 0 | 11 | 126 | - * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guse, D.; Warmuth, L.; Herfet, M.; Adolf, K.; Zevaco, T.A.; Pitter, S.; Kind, M. Seeding as a Decisive Tool for Increasing Space-Time-Yields in the Preparation of High-Quality Cu/ZnO/ZrO2 Catalysts. Catalysts 2024, 14, 517. https://doi.org/10.3390/catal14080517
Guse D, Warmuth L, Herfet M, Adolf K, Zevaco TA, Pitter S, Kind M. Seeding as a Decisive Tool for Increasing Space-Time-Yields in the Preparation of High-Quality Cu/ZnO/ZrO2 Catalysts. Catalysts. 2024; 14(8):517. https://doi.org/10.3390/catal14080517
Chicago/Turabian StyleGuse, David, Lucas Warmuth, Moritz Herfet, Katharina Adolf, Thomas A. Zevaco, Stephan Pitter, and Matthias Kind. 2024. "Seeding as a Decisive Tool for Increasing Space-Time-Yields in the Preparation of High-Quality Cu/ZnO/ZrO2 Catalysts" Catalysts 14, no. 8: 517. https://doi.org/10.3390/catal14080517
APA StyleGuse, D., Warmuth, L., Herfet, M., Adolf, K., Zevaco, T. A., Pitter, S., & Kind, M. (2024). Seeding as a Decisive Tool for Increasing Space-Time-Yields in the Preparation of High-Quality Cu/ZnO/ZrO2 Catalysts. Catalysts, 14(8), 517. https://doi.org/10.3390/catal14080517








