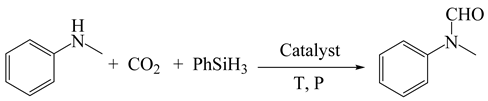Abstract
The reduction of CO2 is an important method to produce chemicals such as methanol, formic acid, formaldehyde, etc. In general, the reduction of CO2 is carried out at high temperatures and pressures with precious metals as catalysts, which is not favorable for industrial procedures. Thus, it will be very useful if researchers can find cost-effective catalysts for industrial application in CO2 reduction. In this work, commercially available ethylenediaminetetraacetic acid (EDTA) was tested as a cheap, non-toxic, and recyclable catalyst to initiate the N-carbonylation reaction of CO2 with amines. After screening various reaction parameters, including temperature, pressure, time, solvent, and reducing agent, the optimal reaction conditions were obtained: 80 °C, 2 MPa, 6 h, 50 mmol% catalyst dosage, 1 mL DMSO, and 1:1 molar ratio of amine to reducing agent. Notably, further studies confirmed that EDTA could also be effective for N-formylation even under ambient conditions (0.1 MPa and room temperature). The suitability of the catalyst for 26 kinds of substrates (including aliphatic amines, aromatic amines, and alicyclic amines) and its reusability were also investigated, with satisfactory results. Scale-up research has been performed effectively with a high conversion of amine (83%) to obtain the mono-formylated product selectively. Finally, the mechanism of the reaction between amine and CO2 has been proposed via control experiments and compared with results in the literature.
1. Introduction
Global warming not only raises sea levels and contaminates freshwater reserves in coastal areas but also threatens food production by reducing the accuracy of precipitation forecasts through climate variability and affecting the spread of pests and diseases from tropical to polar regions [1]. CO2 accounts for more than 80% of greenhouse gases, which are the main cause of global warming [2]. Researchers have therefore proposed carbon capture and storage (CCS) or carbon sequestration [3,4] to reduce atmospheric CO2 levels. However, CO2 can also be used as a cheap, non-toxic, and abundant C1 source to produce value-added chemicals such as oxazolidinones [5], cyclic carbonates [6,7,8], and carboxylic acids [9,10] via the construction of C-N, C-O, and C-C bonds. In addition to producing oxazolidinones, the construction of C-N bonds can be used in N-formylation reactions of amines to produce formamides, which are important solvents and chemical intermediates. Formylation of amines has also been used to protect the amino group in organic reactions [11]. Previously, the synthesis of formamide required coupling reagents such as chloral, formaldehyde, and formic acid or the use of CO or CO2 as a carbon source to synthesize formamide [12,13,14].
In the formylation reaction of amine and CO2, hydroborane [15], phenylsilane [16,17,18], and hydrogen gas [19,20,21] are used as reducing agents. The activation of H2 usually takes place at high temperatures and pressures and requires precious metals [22,23,24,25,26,27] such as Ru, Ir, Rh, and Au as catalysts, while hydroborane is sensitive to air and water, thus limiting its application in the formylation of amines [28]. Air- and water-tolerant phenylsilane is moderately reactive and does not require as harsh reaction conditions as H2. Therefore, using phenylsilane as a reducing agent in the N-formylation of CO2 with amines has been widely studied.
The catalytic system required for the reduction of CO2 commonly uses metal catalysis or organocatalysis. Cesium carbonate [29] was used as a catalyst to selectively obtain N-methylated products and N-formylation products by adjusting the temperature, the amount of catalyst, the type of phenylsilane, and the amount of CO2. However, due to the strong basicity and water sensitivity of cesium carbonate, large-scale industrial production is not feasible. Therefore, the amine N-formylation reaction catalyzed by the “cesium effect” is also limited. The N-formylation of aliphatic amines, aromatic amines, and alicyclic amines with CO2 can be catalyzed at room temperature using intermolecular synergy between Zn(salen) and tetrabutylammonium bromide (TBAB) [30]. Further, by synthesizing bifunctional Zn(salen) catalysts with imidazole-based ionic liquids, the same catalytic effect as the previous two-component catalysts can be achieved, which are easily recovered and reused. However, due to the complicated preparation process and instability of catalysts, metal catalysts are limited in their application to this reaction. Thus, organocatalyses have been widely investigated. The first organocatalyst used in the N-formylation reaction of amines with CO2 was 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) [31], and in subsequent studies, N-heterocyclic carbenes (NHCs) [32], imidazolium-based ionic liquids [33], 1,3,2-diazaphosphorus(NHP-H) [34], dihydrolevoglucosenone with biomass derivatives [35], dimethyl sulfoxide (DMSO) [36], DMF [37,38,39], betaine [40], anomalous N-heterocyclic carbines (αNHC) [41], acetate-based ionic liquids ([n-Bu4N]OAc) [42], tetrabutylphosphonium succinimides ([P4444][Suc]) [43], and alkanolamine [44] were also used in the N-formylation reaction of CO2 with amines.
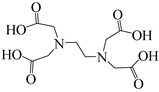
In the research on the reduction of CO2 and its fixation into formamide, although many efforts and great progress have been made, as mentioned above, the reduction of CO2 is generally carried out at high temperatures and pressures with precious metals as catalysts, which is not favorable for industrial procedures. Thus, it is still a big challenge to find economical catalysts for industrial application in CO2 reduction. Building on the previous work mentioned above and considering that oxygen and nitrogen atoms in ethylenediaminetetraacetic acid (EDTA) have unpaired lone pairs of electrons, which can interact with hydrosilane to form a five-centered silicon that can facilitate CO2 insertion and transfer of hydrogen atom to form silyl formate intermediates, this paper tests commercially available EDTA as a cheap, non-toxic, and recyclable catalyst to promote the N-carbonylation reaction of CO2 with amines. A complex synthesis process is not required for EDTA, and the catalyst can be recycled after the reaction. In this way, the industrial production cost is greatly reduced, pollution to the environment is decreased, and the requirements of green chemistry are fulfilled.
2. Results and Discussions
2.1. Screening of Optimal Reaction Conditions for N-Formylation Reaction of CO2 with Amines
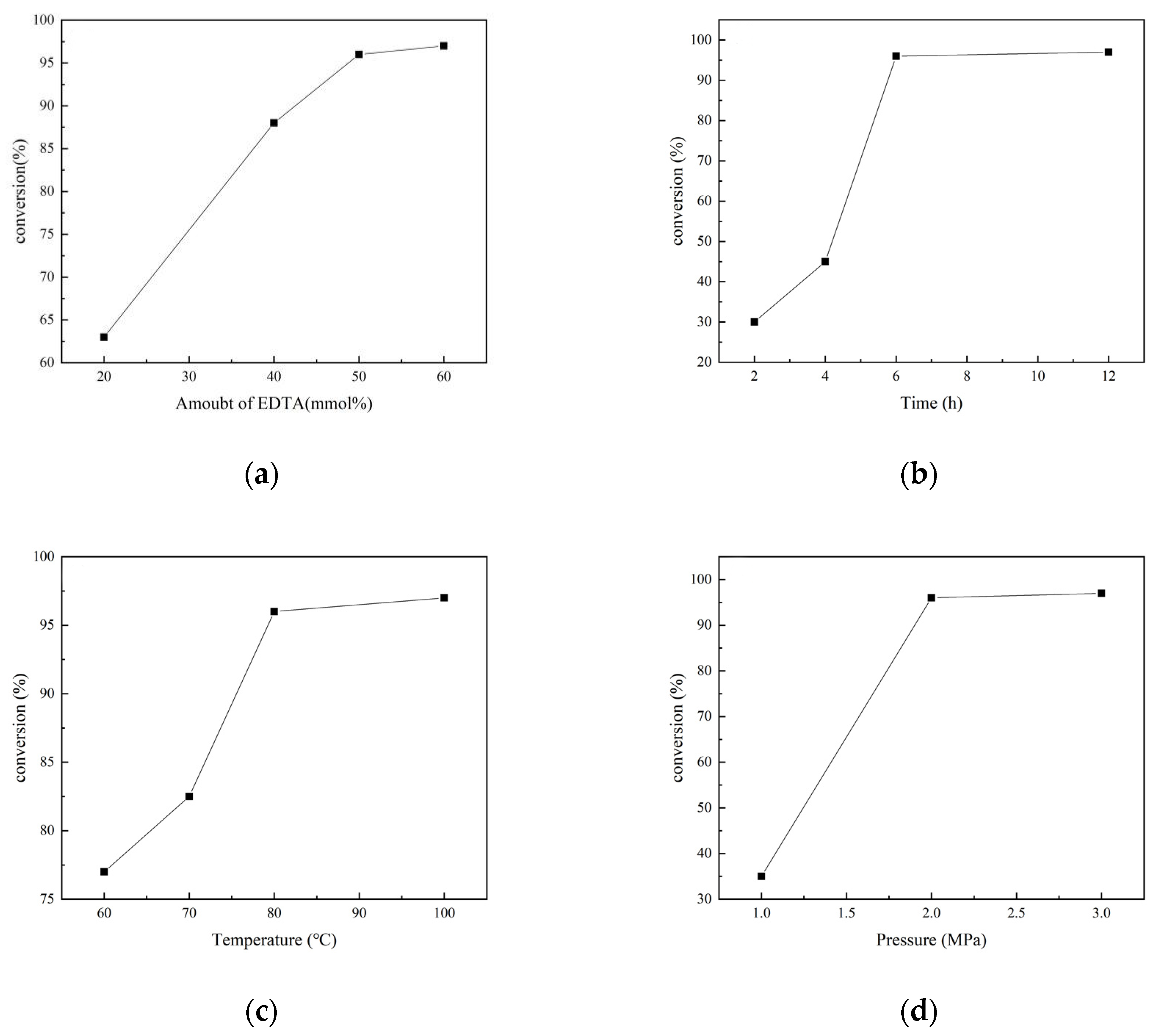
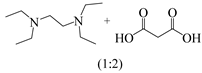
N-formylation of CO2 with N-methylaniline was chosen as a template reaction with EDTA as a catalyst under solvent-free conditions. The EDTA dosage had a great influence on the catalytic reaction, and when it rose from 20% to 40%, the yield of N-methylformylaniline increased from 63 mol% to 88 mol%. A further increase in the EDTA dosage to 50 mmol% increased the yield to 96%. When the EDTA dosage reached 60 mol%, the yield was almost unchanged (Figure 1a). Therefore, the EDTA dosage was set at 50 mmol% for subsequent investigation.
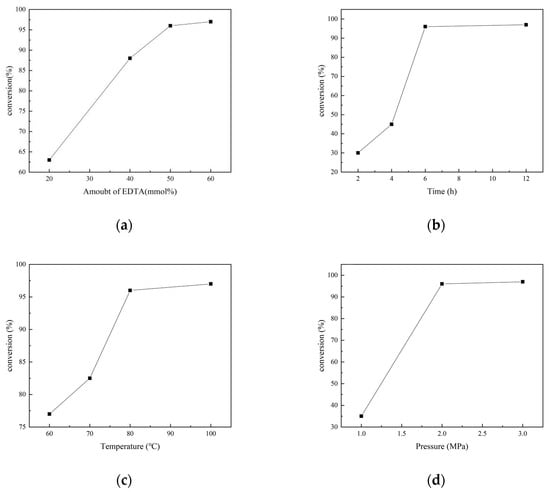
Figure 1.
Effects of reaction conditions on the conversion of N-methylaniline: (a) amount of EDTA; (b) effect of reaction time; (c) effect of temperature; (d) effect of CO2 pressure.
The yield of N-methylformylaniline was also positively correlated with the reaction time, and the yield reached 96% at a reaction time of 6 h. Further prolongation of the reaction time had little effect on the yield (Figure 1b). A similar trend was observed when the temperature was changed. Increasing the temperature from 60 °C to 80 °C increased the yield from 76% to 96%, while further increases in temperature contributed little to the yield (Figure 1c).
The effect of CO2 pressure on the catalytic reaction is shown in Figure 1d; when the pressure was increased from 1 MPa to 2 MPa, the yield of N-methylformylaniline increased from 35% to 96%. After that, there was no significant yield increase when the pressure of CO2 continued to increase. Through the above study, the optimal conditions for the N-methylformylation reaction of amines catalyzed by EDTA were screened as follows: 50 mmol% EDTA, reaction time of 6 h, reaction temperature of 80 °C, and CO2 pressure of 2 MPa.
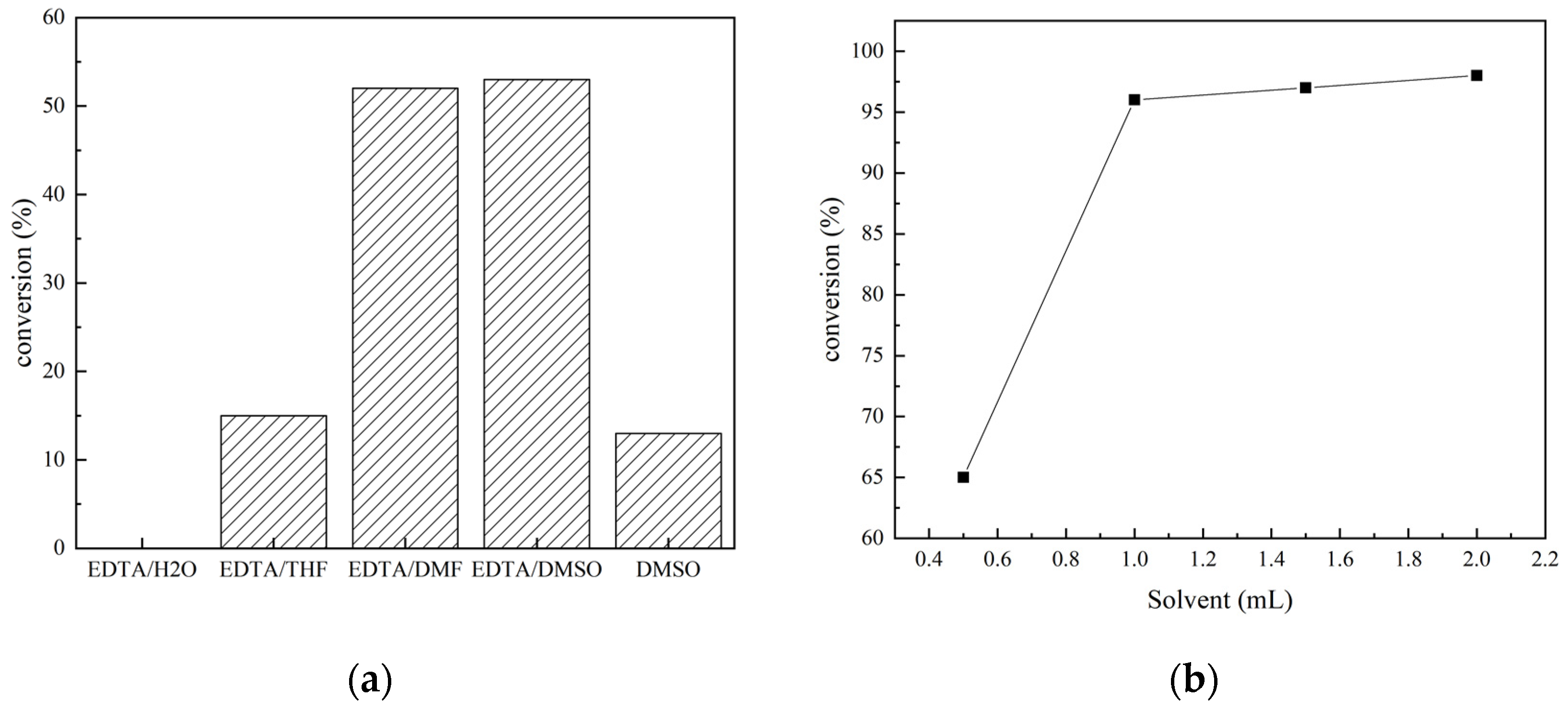
Under solvent-free conditions, EDTA is insoluble in N-methylaniline, the reaction system is solid–liquid dual-phase, and the catalytic efficiency is not high. Thus, it is necessary to add a solvent to make the reaction system homogeneous. Different solvents such as water, THF, DMF, and DMSO were employed. The yields of N-methylformylaniline with 1 mL of the different solvents under set conditions (20 mmol% EDTA, 5.0 mmol of N-methylaniline, 5.0 mmol PhSiH3, 1 MPa CO2, and 60 °C for 24 h) are shown in Figure 2a. The yield of N-methylformylaniline increased from 15% to 52% and 53% with DMF and DMSO as solvents, respectively. It is noteworthy that N-methylformylaniline was obtained in 13% yield when DMSO alone was used without EDTA, proving that DMSO also has a certain catalytic effect, as reported previously [31]. The amount of the solvent further influenced the concentration of the reactants. When the amount of DMSO was increased from 0.5 mL to 1 mL, the yield of N-methylformylaniline rose from 65% to 96% due to the enhanced solubility. Once EDTA was fully dissolved in DMSO and the activation of the Si-H bond in phenylsilane by EDTA was enhanced, the yield increased. Further increasing the amount of solvent to 2 mL led to a slight change in the yield (Figure 2b). Therefore, in the N-carbonylation reaction of CO2 and amines catalyzed by EDTA, DMSO was chosen as the solvent, with a dosage of 1 mL.
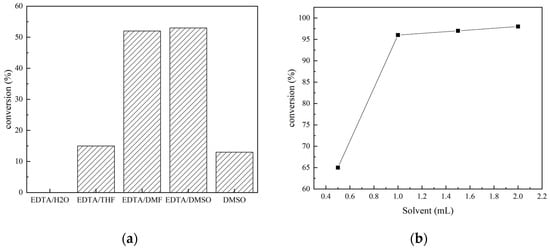
Figure 2.
(a) Effect of different solvents on the conversion of N-methylaniline; (b) effect of DMSO dosage on the conversion of N-methylaniline.
The choice of reducing agent has a great influence on the products of the reaction of CO2 with amines [29,33,42,45]. The yields of N-methylformylaniline obtained using 1 equiv. of different hydrosilanes as reducing agents were investigated at 80 °C, 2 MPa CO2, 1 mL DMSO, and 50 mmol% EDTA, as shown in Table 1. The use of diphenylhydrosilane (Table 1, entry 3), dimethylphenylsilane (Table 1, entry 4), triethoxyhydrosilane (Table 1, entry 2), and dimethylaminomethylborane (Table 1, entry 5) did not produce detectable N-methylformylaniline. Only the use of phenylsilane as a reducing agent completed the reaction of CO2 with amines to produce N-methylformylaniline with high yield (Table 1, entry 1).

Table 1.
Effect of different hydrosilanes on the formylation reaction of carbon dioxide and N-methylaniline.
2.2. Reusability of the Catalyst
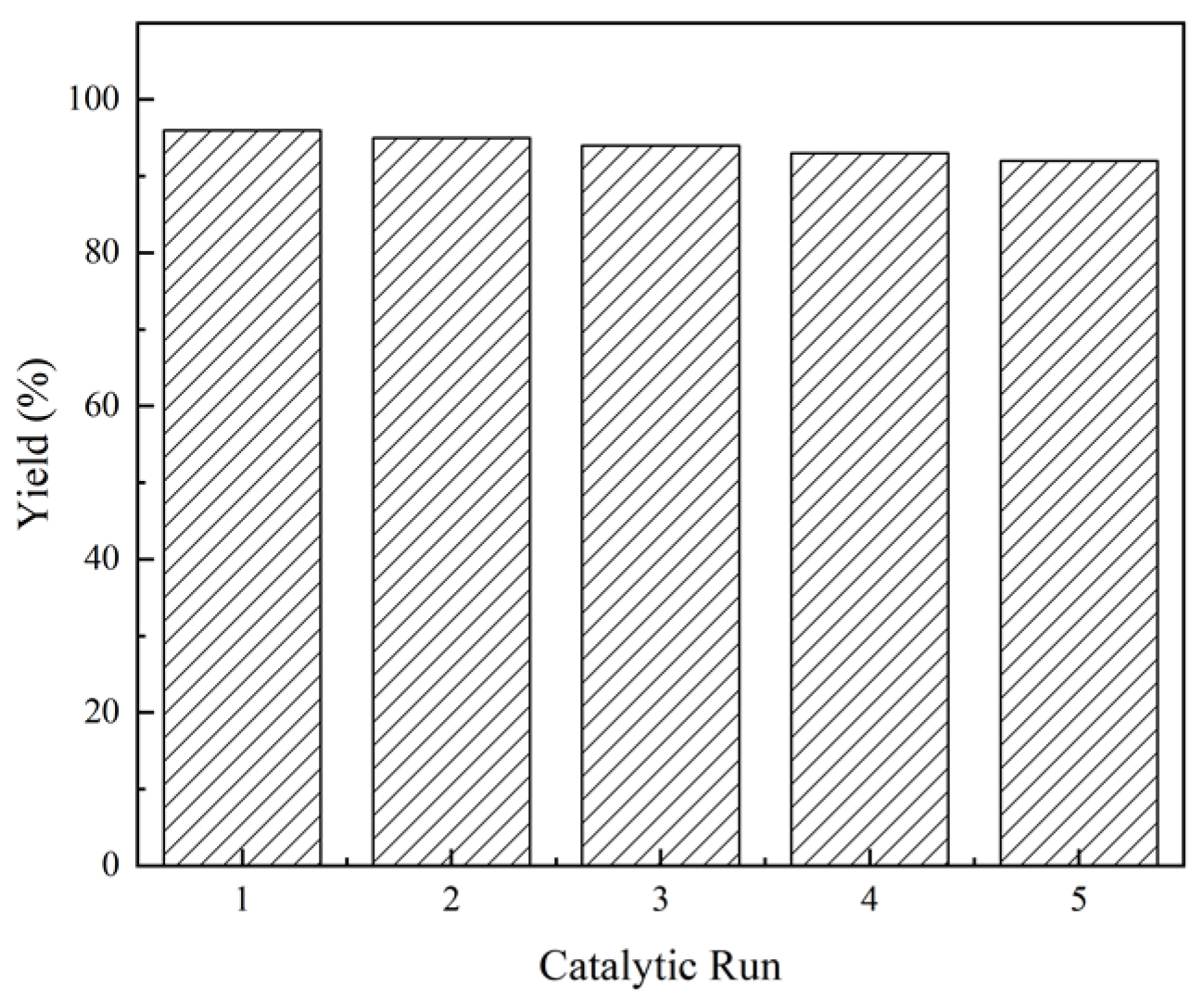
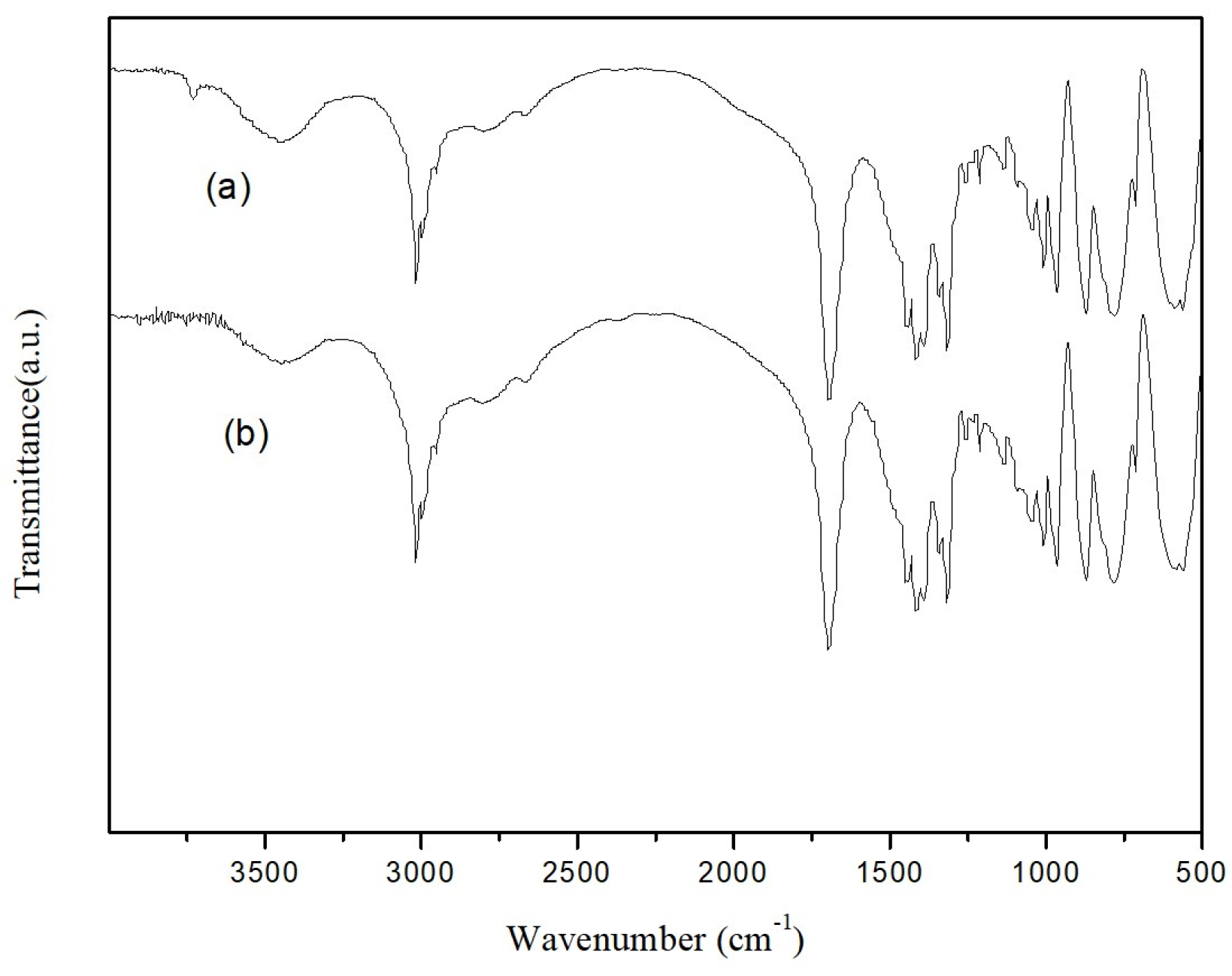
The recyclability of catalysts is another important factor for industrial applications, in addition to the price. The recyclability of EDTA as a catalyst for the N-formylation reaction of CO2 with N-methylaniline was tested under optimal reaction conditions (N-methylaniline 5 mmol, EDTA 50 mmol%, PhSiH3 5 mmol, DMSO 1 mL, 80 °C, 2 MPa, 6 h), and the results are shown in Figure 3. The catalyst was precipitated by adding trichloromethane after completion of the reaction, collected via simple filtration, and used directly. As indicated in Figure 3, the activity of EDTA remained stable in multiple experiment cycles (about 30 h), and the yield of N-methylformylaniline was still above 90% even after repeated use for five times. The infrared spectra of EDTA before and after five cycles of use were also measured (Figure 4). The absorption peaks at 3423, 1690, and 1300 cm−1 correspond to O-H, C=O, and C-O stretching vibrations of the carboxylic acid moiety, respectively. The C-H stretching vibration of EDTA is at 2900 cm−1 and the C-N stretching vibration is at 1360 cm−1. These results show that the structure of EDTA remained stable before and after the reaction.
Figure 3.
Experiment on the recyclability of EDTA as a catalyst.
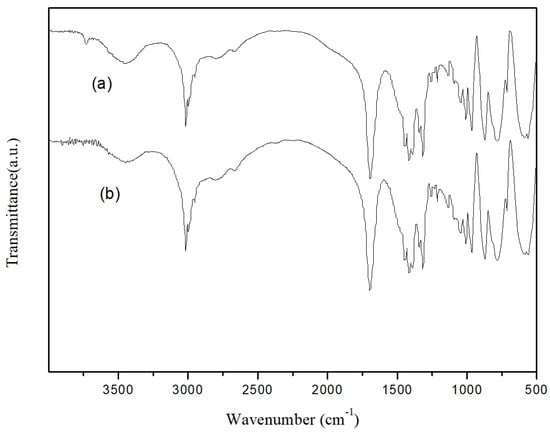
Figure 4.
Comparison of IR spectra of EDTA before and after 5 repeated catalytic cycles: (a) fresh EDTA; (b) EDTA after 5 times of reuse.
2.3. Application Scope Study: N-Formylation of CO2 with Other Amines Catalyzed by EDTA under High Pressure
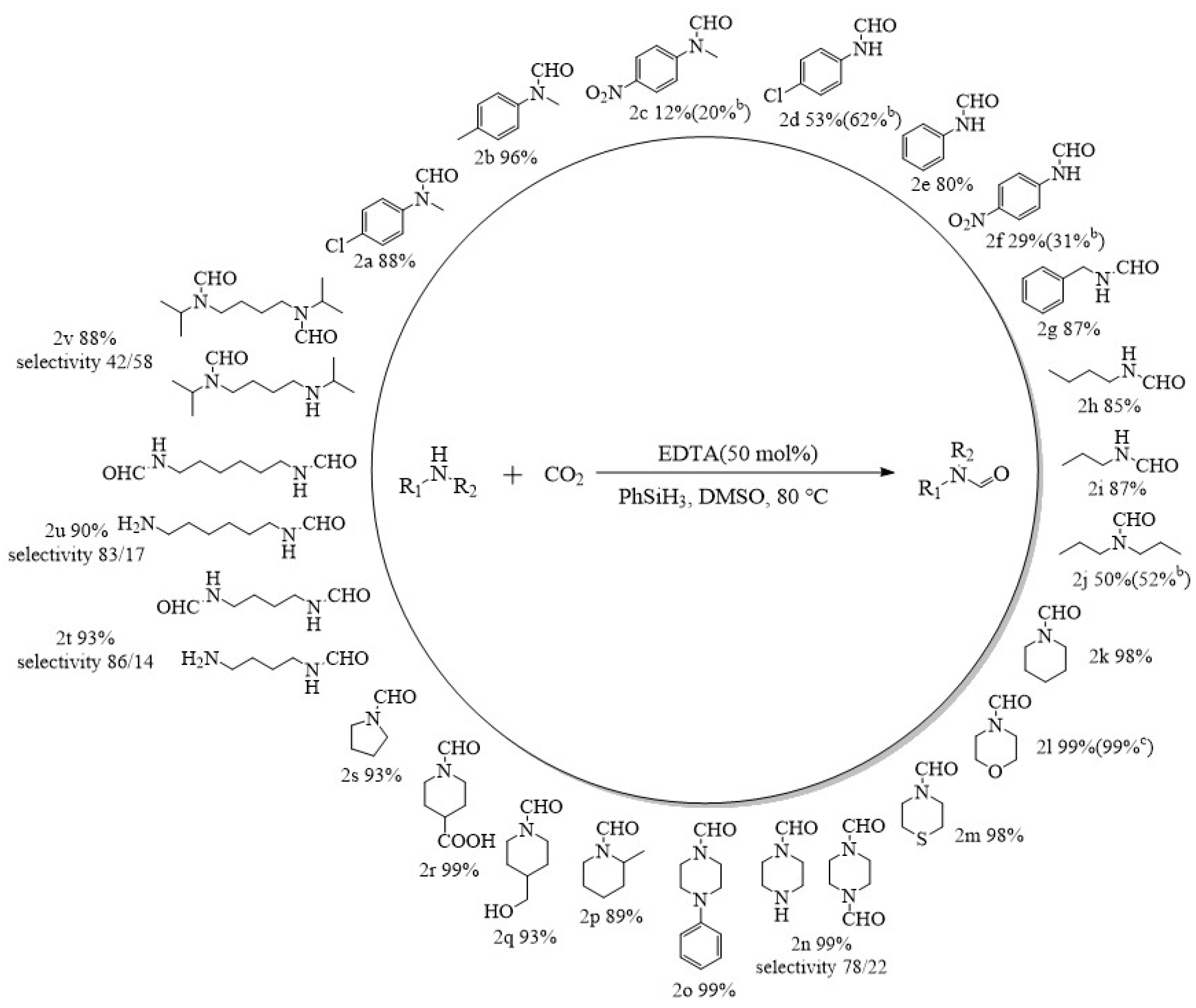
Under the optimal reaction conditions (80 °C, 2 MPa, 6 h, 50 mmol% catalyst dosage), the N-formylation of CO2 with different amines catalyzed by EDTA was investigated to expand its application scope (Figure 5). EDTA can catalyze the N-formylation reactions of most aromatic amines, alicyclic amines, and aliphatic amines with 99% selectivity for the N-carbonylated products. The N-carbonylation products of aromatic amines are related to the substituents on the benzene ring, and in general, a strong electron-withdrawing group (-NO2) on the benzene ring will lead to a lower electron cloud density on N, which reduces the nucleophilicity of the amine attacking silyl formate [33,42,45]. Even if the reaction time of N-methyl-4-nitroaniline and 4-nitroaniline with CO2 was prolonged to 24 h, the yields of N-formylation were only 20% and 31% (2c, 2f), respectively. Similarly, N-methyl-4-chloroaniline and 4-chloroaniline with weaker electron-withdrawing substituents on the aromatic ring gave the formylated products in 88% and 53% yields, respectively, under the same conditions (2a, 2d). Among them, the electron cloud density of N in N-methyl-4-chloroaniline was higher than that in 4-chloroaniline, leading to an increase in nucleophilicity and a corresponding increase in the yield of N-formylation. When the substituent on the benzene ring was an electron-donating substituent, the nucleophilicity of N further increased and the yield obtained from the formylation reaction increased, and the formylated product (2b) was obtained in 96% yield when the formylation reaction was carried out using N-methyl-4-methylaniline as a substrate. When benzylamine was used as a substrate, N-formylbenzylamine (2g) was still obtained in excellent yields. In addition to aromatic amines that gave good yields, the catalytic system also afforded formylated products of alicyclic amines (2k−2s) in 89%−99% yields. For aliphatic amines, the yields were strongly influenced by steric hindrance in the formation of formylated products (2h, 2i), with yields of 87% and 85% when primary amines were used as substrates. The yields dropped to 50% when the substrate was a secondary amine with a large steric hindrance. Moreover, a yield of only 52% (2j) was obtained even when the reaction time was prolonged to 24 h. In addition, EDTA as a catalyst could also achieve bis-formylation of aliphatic diamines with excellent yields and high selectivity (2t–2v), while the selectivity of bis-formylation decreased when N,N′-diispropylbutanediamine was used as a substrate due to the increase in steric hindrance.
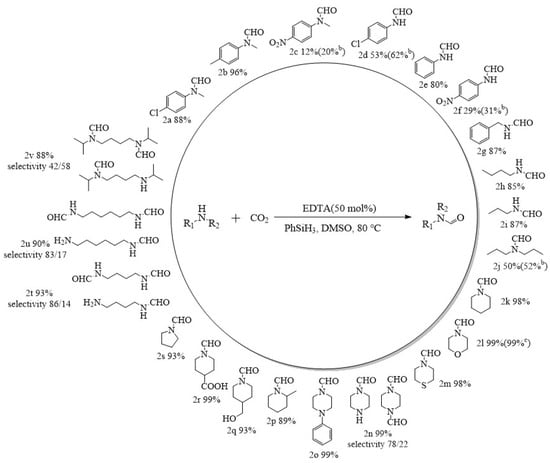
Figure 5.
EDTA-catalyzed formylation reactions of CO2 with various amines under high pressure a. a Reaction conditions: Substrate, 5 mmol; CO2, 2 MPa; PhSiH3, 5 mmol; EDTA, 50 mmol%; DMSO, 1 mL; temperature, 80 °C; time, 6 h. b Reaction conditions: Substrate, 5 mmol; CO2, 2 MPa; PhSiH3, 5 mmol; EDTA, 50 mmol%; DMSO, 1 mL; temperature, 80 °C; time, 24 h. c Reaction conditions: Substrate, 5 mmol; CO2, 2 MPa; PhSiH3, 5 mmol; EDTA, 50 mmol%; DMSO, 1 mL; temperature, 80 °C; time, 1 h. d The conversion and selectivity of the products were detected by 1H NMR (CDCl3, 400 MHz).
2.4. EDTA-Catalyzed Formylation of Different Cyclic Amines and Carbon Dioxide at Room Temperature and Atmospheric Pressure
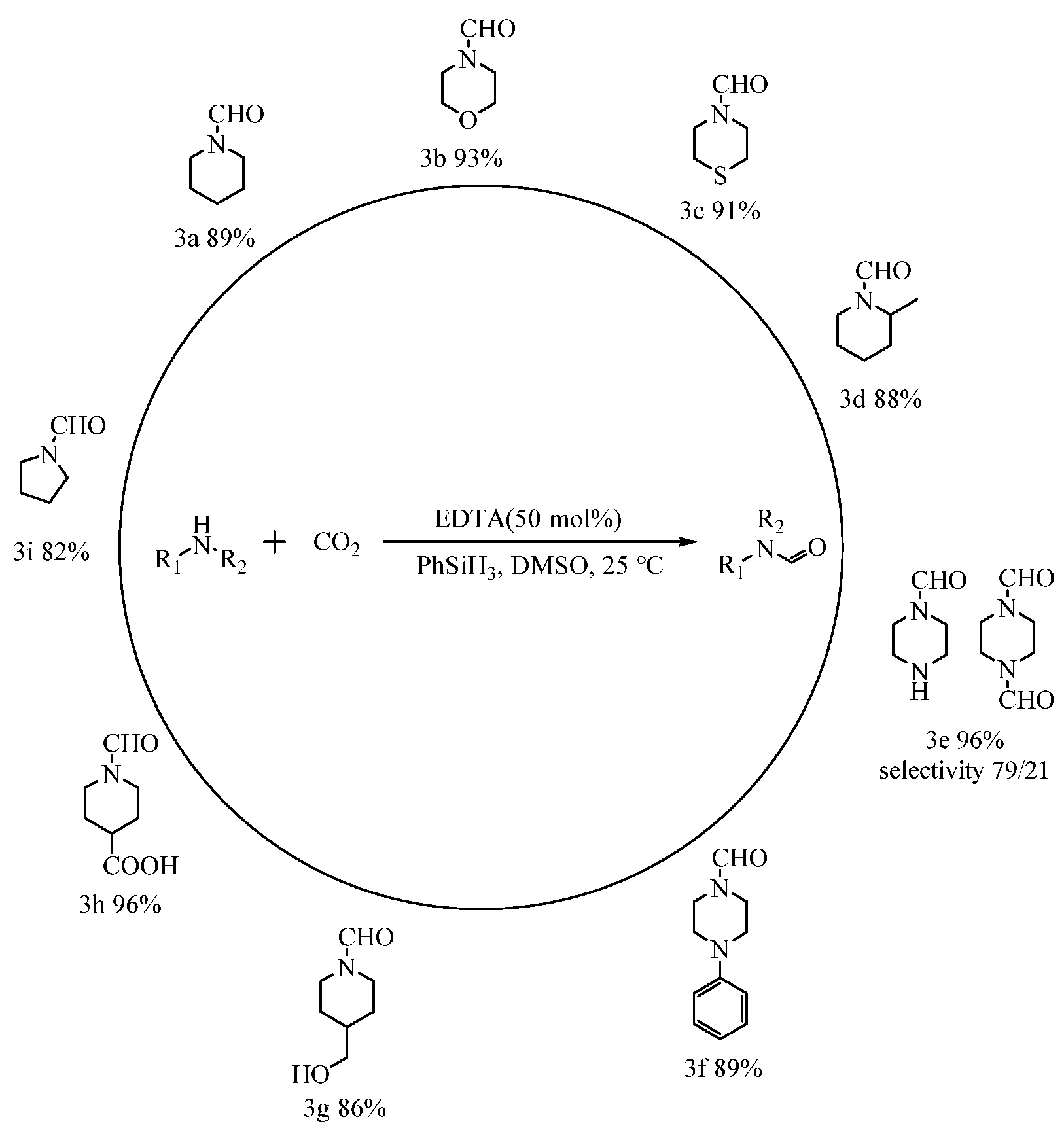
From the previous discussion on amine formylation, it is known that the N-formylation reactions of alicyclic amines performed well, with high activity. Thus, the formylation reactions of CO2 with different alicyclic amines catalyzed by EDTA at atmospheric pressure were further investigated, and the results are shown in Figure 6. Under the set conditions (50 mmol% catalyst dosage, room temperature, 0.1 MPa, and 12 h), piperidine (3a), morpholine (3b), thiomorpholine (3c), 2-methylpiperidine (3d), 4-piperidinemethanol (3g), 1-formylpiperidine-4-carboxylic acid (3h), and N-phenylpiperazine (3f) could still react with CO2 to afford corresponding formylated products in high yields (88%−96%). Notably, when 1,4-diaminopiperazine (3e) was used for the formylation reaction, monoformylated products were obtained with better selectivity. Moreover, not only the six-membered cyclic amine but also the five-membered cyclic tetrahydropyrrole could afford pyrrolidine-1-carbaldehyde (3i) in 82% yield.
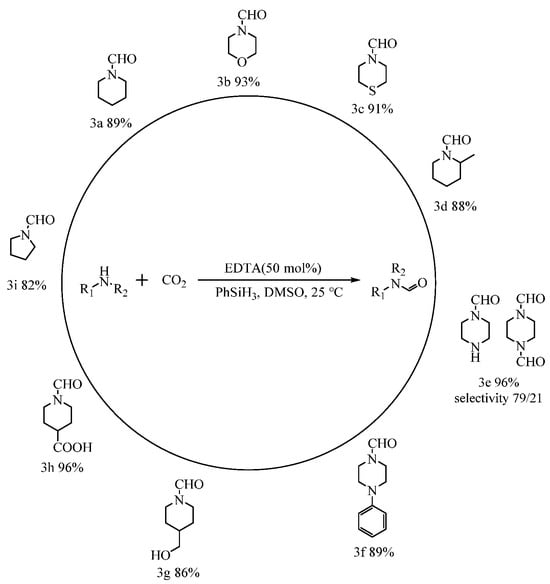
Figure 6.
EDTA-catalyzed formylation reactions of CO2 with various amines at atmospheric pressure a,b. a Reaction conditions: Substrate, 2.5 mmol; CO2, 0.1 MPa; PhSiH3, 2.5 mmol; EDTA, 50 mmol%; DMSO, 2 mL; temperature, 25 °C; time, 12 h. b The conversion and selectivity of the products were detected by 1H NMR (CDCl3, 400 MHz).
2.5. Scale-Up Experiment
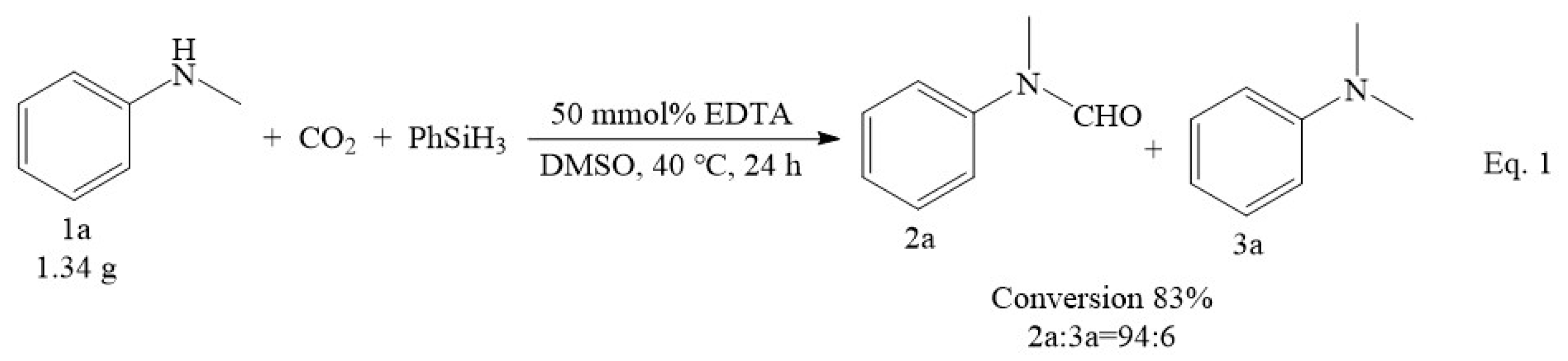
In addition to its broad application for various substrates, the EDTA catalyst system also performed quite well for the N-formylation of CO2 and N-methylaniline on a gram scale (Scheme 1, Equation (1)). In this reaction, N-methyl-N-phenylformamide was obtained with good yield and high selectivity. This confirmed that the present approach has the potential to be applied in scale-up synthesis.
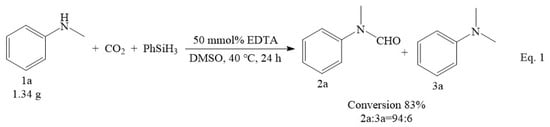
Scheme 1.
Gram-scale study on the formylation of N-methylaniline with CO2.
2.6. Possible Mechanism for the Coupling of Carbon Dioxide and Amines
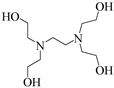
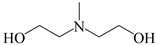
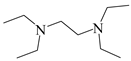
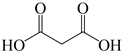
To investigate the coupling mechanism, some other compounds with a similar structure to EDTA were employed as catalysts to catalyze the N-formylation of CO2 with N-methylaniline under the same conditions (Table 2). As seen from Table 2, EDTA had the highest catalytic activity, obtaining the target product in 96% yield (Table 2, entry 1). When using hydrogen-free tetrasodium EDTA as the catalyst, the yield of N-methylformylaniline decreased significantly to 57% (Table 2, entry 2). In the case of using carboxyl-free N,N,N′,N′-tetraethyl ethylenediamine, the yield of N-methylformylaniline decreased further to 48% (Table 2, entry 5). These experimental results suggest that the presence of a carboxyl group with an active hydrogen atom could enhance the catalyst activity. To demonstrate the importance of the presence of carboxyl groups, the reaction of amines with CO2 was catalyzed by N-methyl diethanolamine, in which the carboxyl groups are replaced by active hydrogen, and the N-methylformylaniline yield was only 56% (Table 2, entry 4). With a further increase in the active hydrogen number using N,N,N′,N′-tetrakis(2-hydroxyethyl)ethylene diamine as a catalyst, the product yield decreased to 27% (Table 2, entry 3), which could probably be attributed to the high viscosity of the catalyst system. When using malonic acid, which contains only carboxylic acid groups, as a catalyst, the N-carbonylation product yield was only 59% (Table 2, entry 6), demonstrating that the presence of N has a significant effect on the catalyst activity due to the presence of lone pairs of electrons on the nitrogen atom with certain nucleophilicity for the activation of phenylsilanes. The N-formylation reaction using N,N,N′,N′-tetraethyl ethylene diamine and malonic acid as a two-component catalytic system resulted in 67% yield of N-methylformylaniline (Table 2, entry 7), which is higher than the values obtained in the single-component catalytic systems using malonic acid alone or N,N′,N′-tetraethylethylenediamine alone. All the results demonstrate that the presence of the nitrogen atom and carboxyl groups had a significant effect on the catalytic activity (Table 2, entries 5−7). However, the yield obtained using the two-component catalytic system was still lower than that obtained using EDTA as a single catalyst (Table 2, entries 1 and 7), suggesting that the N-formylation of amines is more susceptible to intramolecular co-catalysis than to intermolecular co-catalysis.

Table 2.
Reaction of amines with CO2 catalyzed by different catalysts.
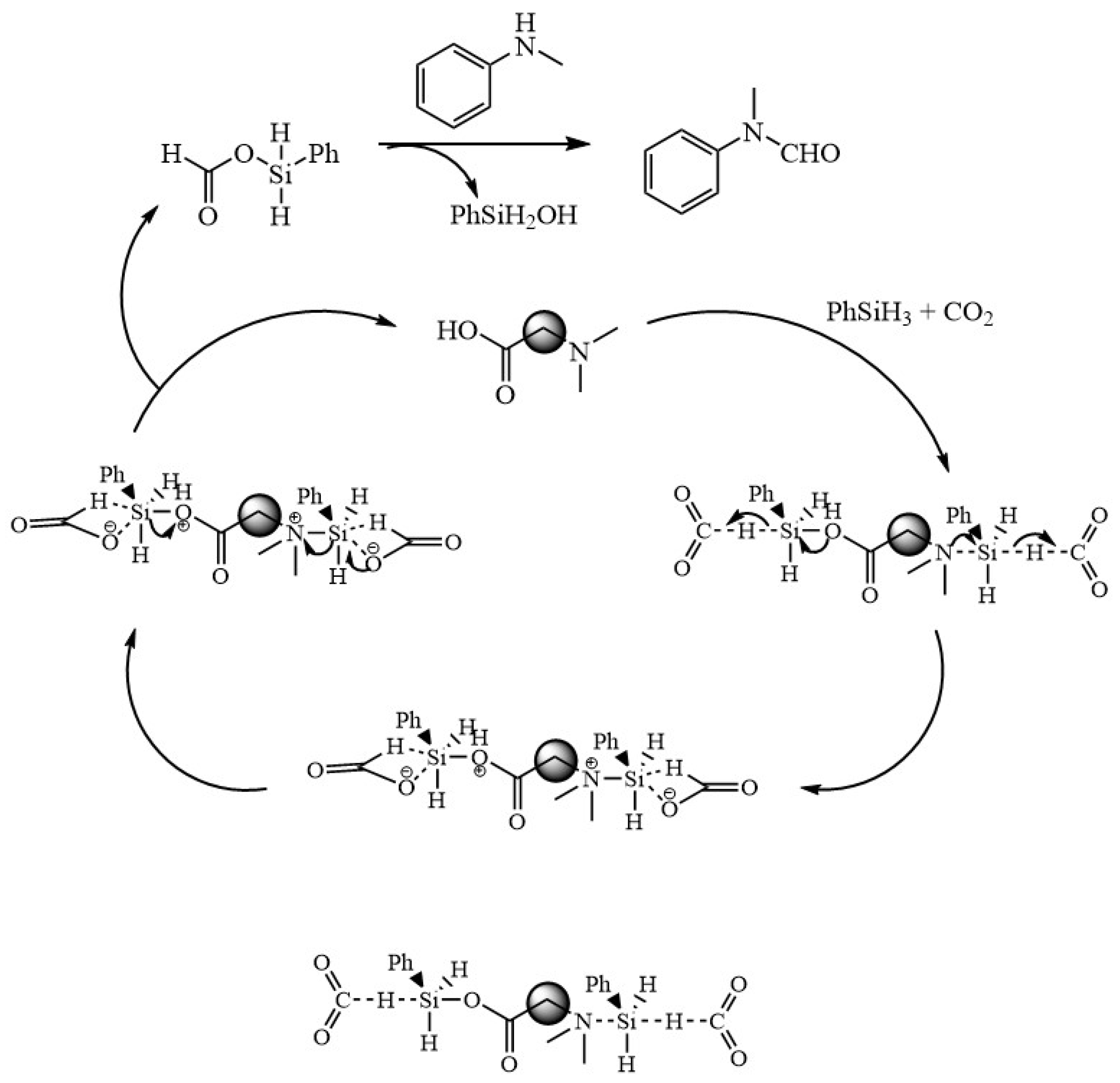
Based on the above findings and combined with reports in the literature [46,47,48,49,50], the mechanism of the formylation reaction of CO2 with amines is proposed and shown in Figure 7. First, N and O with lone pair of electrons in EDTA activate phenylsilane by nucleophilic attack to form pentavalent silica, and then, CO2 inserts into the highly reactive Si-H bond to form a formate silanolate intermediate and regenerates the catalyst. The generated formic acid silanol ester is then nucleophilically attacked by N-methylaniline, and the phenylsilanol is removed to form N-methylformylaniline.
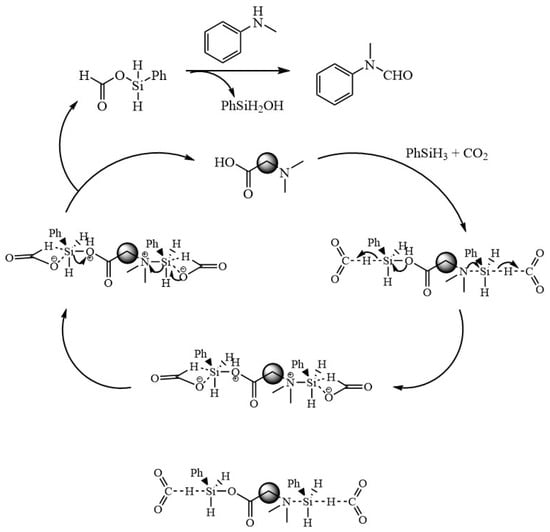
Figure 7.
Possible mechanism of EDTA-catalyzed N-carbonylation reaction of CO2 with amines.
3. Materials and Methods
3.1. Materials and Instruments
N-methyl-p-toluidine (purity ≥ 97%), N-methyl-p-nitroaniline (purity ≥ 97%), N,N′-diisopropylethylamine (purity ≥ 98%), thiomorpholine (purity ≥ 98%), morpholine (purity ≥ 99%), and diphenylsilane (purity ≥ 90%) were purchased from J&K Scientific. Ethylenediaminetetraacetic acid tetrasodium salt (purity ≥ 99%), malonic acid (purity ≥ 98%), N-methylaniline (purity ≥ 98%), 4-chloroaniline (purity ≥ 99%), p-nitroaniline (purity ≥ 97%), N-phenylpiperazine (purity ≥ 98%), tetrahydropyrrole (purity ≥ 99%), piperazine (purity ≥ 98%), piperidine (purity ≥ 99%), phenylsilane (purity ≥ 97%), and triethoxysilane (purity ≥ 98%) were produced by Shanghai Darui Fine Chemicals Co. Ethylene diamine tetraacetic acid (purity ≥ 99%) and 1,6-hexanediamine (purity ≥ 99%) were obtained from Sinopharm Chemical Reagent Co. CO2 (purity ≥ 99.99%) was obtained from Sichuan Tianyi Science and Technology Co. All reagents did not require further purification. Reaction yields and selectivity were determined with a Bruker Al-400 MHz NMR instrument.
3.2. General Procedure of EDTA-Catalyzed N-Formylation of Carbon Dioxide with Amines
High-pressure experiments: A clean and dry 50 mL autoclave was charged with CO2 gas three times; then, quantitative amounts of the catalyst, amine, and hydrosilane were added into the autoclave under the protection of CO2. Next, the autoclave with a stirrer was sealed and placed in an oil bath at a set temperature. Thirty minutes later, CO2 gas was pressurized into the autoclave to a set pressure and stirring was started. The reaction was stopped after reaching the predetermined reaction time, and the reaction kettle was placed into ice water to release the gas slowly. Then, a small amount of the reaction system was taken directly for NMR testing to determine the yield and selectivity of the N-formylated product.
Atmospheric pressure: A desired quantity of solid catalyst was added into a 75 mL Schlenk flask, and the device was sealed and evacuated three times under a N2 atmosphere. After evacuation, the Schlenk flask was kept under vacuum, and then, a CO2 balloon was connected to fill the flask with carbon dioxide, followed by injection of the solvent into the flask using a syringe. If the hydrosilanes and amines were solid at room temperature, they were added together with the catalyst before the N2 and evacuation, and if the hydrosilanes and amines were liquid at room temperature, they were injected into the bottle together with the solvent by syringe after the N2 and evacuation. Afterwards, the vial was sealed again. The reaction was started by preheating the prepared Schlenk flask in a constant-temperature oil bath for 20 min and stopped after reaching the predetermined reaction time.
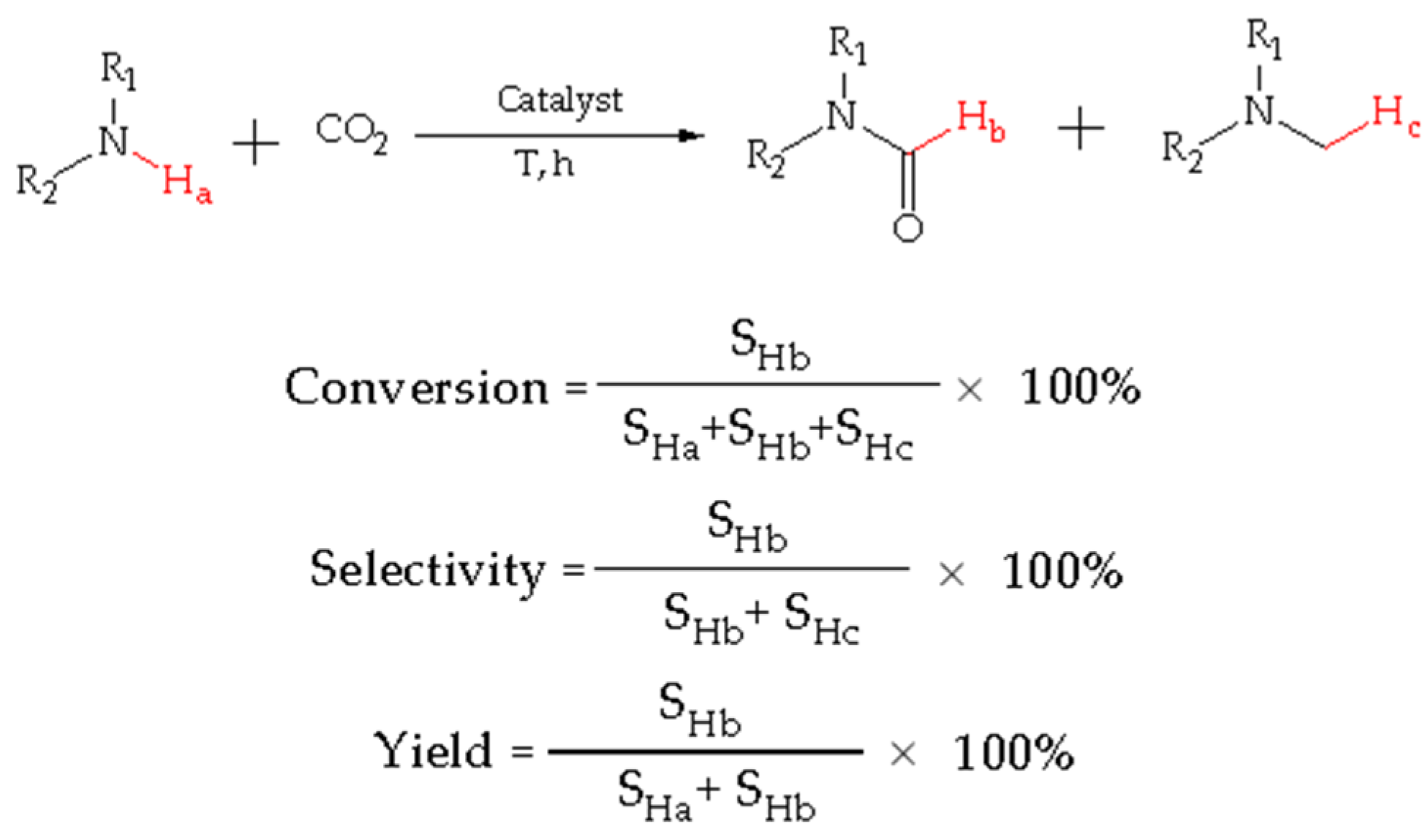
The conversion, yield, and selectivity of products were calculated as indicated in Figure 8: SHa denotes the signal peak area integral of Ha for the reactant amine in 1H NMR spectra, SHb denotes the signal peak area integral of Hb for the product formamide in 1H NMR spectra, and SHc denotes the signal peak area integral of Hc for the by-product methylamine in 1H NMR spectra.
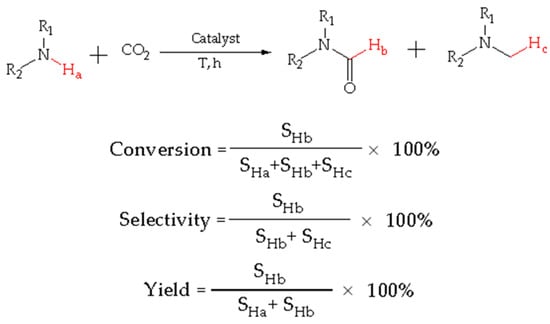
Figure 8.
Calculations for the conversion, yield, and selectivity of products.
The sample solution for NMR was a mixed solution containing incompletely reacted amine, formamide, and methylamine. Thus, the conversion is equal to the signal peak area of the product formamide (SHb) divided by the sum of the signal peak area integrals of the reactants amine, formamide, and methylamine (SHa + SHb + SHc) multiplied by 100%. It is worthy to note that there are three Hcs, but only one Hc should be calculated.
Selectivity was calculated as the formamide signal peak area integral (SHb) divided by the sum of the formamide and methylamine signal peak area integrals (SHb + SHc) multiplied by 100%.
The yield of formamide was calculated as the formamide signal peak area integral (SHb) divided by the sum of the amine and formamide signal peak area integrals (SHa + SHb) multiplied by 100%.
4. Conclusions
In this paper, cheap and stable EDTA was successfully applied as a recyclable, eco-friendly catalyst to the reaction of CO2 and amines to produce N-carbonylation products. The optimum conditions for the N-carbonylation reaction of CO2 and amines were determined through systematic studies of the catalytic system as follows: reaction temperature of 80 °C, CO2 pressure of 2 MPa, reaction time of 6 h, 50 mmol% EDTA, 1 equiv. of PhSiH3, and 1 mL DMSO. Under the optimal conditions, the catalytic system afforded decent to high activity and excellent product selectivity, with the selectivity for N-formylation products reaching 99%. The results show that the catalytic system is suitable for a variety of aromatic and aliphatic amines. Among them, the N-carbonylation reaction of alicyclic amines was very active, and the yield of N-carbonylation products could reach 96% even at room temperature and under atmospheric pressure. EDTA could be precipitated by simply adding trichloromethane after the reaction, and the catalyst activity remained stable after five cycles. The scale-up experiment confirmed that EDTA has potential application for industrial production.
In summary, this study not only confirmed that EDTA has good catalytic applicability in forming products through reactions of CO2 with most aromatic amines, alicyclic amines, and aliphatic amines, with excellent yields, but also validated the stability of EDTA in this reduction system through catalyst recycling experiments. Therefore, it is highly expected that the N-carbonylation reaction of CO2 with amines can be catalyzed using cheap and stable EDTA in the large-scale industrial production of CO2-based value-added chemicals.
Author Contributions
Conceptualization, Q.Z. and X.Y.; methodology, Q.Z.; software, Q.Z.; validation, Q.Z., Y.C., and X.Y.; formal analysis, Q.Z. and J.H.; investigation, X.Y.; resources, Q.Z. and Q.J.; data curation, Q.Z.; writing—original draft preparation, Q.Z.; writing—review and editing, H.-J.Y. and C.-Y.G.; visualization, Q.Z.; supervision, H.-J.Y. and C.-Y.G.; project administration, C.-Y.G.; funding acquisition, H.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number 51073175) and the Fund for Academic Innovation Teams of South-Central Minzu University (No. XTZ24016).
Data Availability Statement
All data are shown in the main text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nema, P.; Nema, S.; Roy, P.J. An overview of global climate changing in current scenario and mitigation action. Renew. Sustain. Energy Rev. 2012, 16, 2329–2336. [Google Scholar] [CrossRef]
- Saptal, V.B.; Bhanage, B.M. N-Heterocyclic olefins as robust organocatalyst for the chemical conversion of carbon dioxide to value-added chemicals. ChemSusChem 2016, 9, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Simsa, R.E.H.; Rognerb, H.; Gregoryc, K. Carbon emission and mitigation cost comparisons between fossil fuel, nuclear and renewable energy resources for electricity generation. Energy Policy 2003, 31, 1315–1326. [Google Scholar] [CrossRef]
- Zahasky, C.; Krevor, S. Global geologic carbon storage requirements of climate change mitigation scenarios. Energy Environ. Sci. 2020, 13, 1561–1567. [Google Scholar] [CrossRef]
- Chen, J.M.; Qi, L.; Zhang, L.L.; Li, L.J.; Hou, C.Y.; Li, W.; Wang, L.J. Copper/DTBP-promoted oxyselenation of propargylic amines with diselenides and CO2: Synthesis of selenyl 2-oxazolidinones. J. Org. Chem. 2020, 85, 10924–10933. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jayakumar, S.; Chen, J.; Tao, L.; Li, H.; Yang, Q.H.; Li, C. Synthesis of bifunctional porphyrin polymers for catalytic conversion of dilute CO2 to cyclic carbonates. ACS Appl. Mater. 2021, 13, 29522–29531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Tu, X.W.; Chen, Y.T.; Han, W.H.; Chen, L.C.; Sun, C.; Zhu, S.X.; Song, Y.J.; Zheng, H. Photothermal catalysis without solvent for fixing CO2 to cyclic carbonate. Mol. Catal. 2023, 538, 112971–112979. [Google Scholar] [CrossRef]
- Wen, Q.; Yuan, X.X.; Zhou, Q.Q.; Yang, H.J.; Jiang, Q.Q.; Hu, J.C.; Guo, C.Y. Solvent-free coupling reaction of carbon dioxide and epoxides catalyzed by quaternary ammonium functionalized schiff base metal complexes under mild conditions. Materials 2023, 16, 1646. [Google Scholar] [CrossRef]
- Ran, C.K.; Niu, Y.N.; Song, L.; Wei, M.K.; Cao, Y.F.; Luo, S.P.; Yu, Y.M.; Liao, L.L.; Yu, D.G. Visible-light photoredox-catalyzed carboxylation of activated C(sp3)-O bonds with CO2. ACS Catal. 2022, 12, 18–24. [Google Scholar] [CrossRef]
- Xiao, H.Z.; Yu, B.; Yan, S.S.; Zhang, W.; Li, X.X.; Bao, Y.; Luo, S.P.; Ye, J.H.; Yu, D.G. Photocatalytic 1,3-dicarboxylation of unactivated alkenes with CO2. Chin. J. Catal. 2023, 50, 222–228. [Google Scholar] [CrossRef]
- Gerack, C.J.; White, L.M. Formylation of amines. Molecules 2014, 19, 7689–7713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zhang, J.; Chen, H.J.; Zhang, Z.X.; Zhang, C.F.; Li, M.R.; Wang, F. Ru/ceria-catalyzed direct formylation of amines and CO to produce formamides. Green Chem. 2017, 19, 88–92. [Google Scholar] [CrossRef]
- Beyazay, T.; Martin, W.F.; Tuysuz, H. Direct synthesis of formamide from CO2 and H2O with nickel–iron nitride heterostructures under mild hydrothermal conditions. J. Am. Chem. Soc. 2023, 145, 19768–19779. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Li, X.Y.; Qiao, C. Transition-metal-free catalysis for the reductive functionalization of CO2 with amines. Synlett 2018, 29, 548–555. [Google Scholar]
- Zou, Q.Z.; Yi, Y.; Zhao, T.X.; Liu, F.; Kang, C.; Hu, X.B. Catalyst-free hierarchical reduction of CO2 with BH3N(C2H5)3 for selective N-methylation and N-formylation of amines. J. CO2 Util. 2021, 50, 101590–101596. [Google Scholar] [CrossRef]
- Li, W.D.; Zhu, D.Y.; Li, G.; Chen, J.; Xia, J.B. Iron-catalyzed selective N-methylation and N-formylation of amines with CO2. Adv. Synth. Catal. 2019, 361, 5098–5104. [Google Scholar] [CrossRef]
- Akhtar, N.; Chauhan, M.; Gupta, P.; Antil, N.; Manna, K. A supported pyridylimine-cobalt catalyst for N-formylation of amines using CO2. Dalton Trans. 2023, 52, 15384–15393. [Google Scholar] [CrossRef]
- Yoo, D.K.; Jhung, S.H. N-formylation of amines with CO2 by using Zr-based metal-organic frameworks: Contribution of defect sites of MOFs to N-formylation. Appl. Catal. A-Gen. 2023, 659, 119170–119177. [Google Scholar] [CrossRef]
- Li, R.P.; Zhao, Y.F.; Wang, H.; Li, D.Y.; Wu, Y.Y.; Zhang, H.Y.; Tang, M.H.; Liu, Z.M. Ionic liquid-promoted formylation of N(sp2)-heteroarenes with CO2/H2 over Pd/C. ACS Sustain. Chem. Eng. 2021, 9, 2507–2514. [Google Scholar] [CrossRef]
- Lai, W.; Jiang, Y.; Liao, H.; Wei, X.; Xu, Z.; Ding, J.; An, N.; Dai, S.; Hou, Z. Multivacant polyoxometalate-stabilizing palladium nanoparticles catalyze the N-formylation of amines with CO2 and H2. New J. Chem. 2024, 48, 11014–11024. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Ji, G.; Sun, Z.; Ma, L.; Li, C.; Du, H.; Yan, L.; Ding, Y. Highly efficient heterogeneous Pd@POPs catalyst for the N-formylation of amine and CO2. Catalysts 2021, 11, 220. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.B.; Zhao, X.Y.; Wang, Z.; Ding, K.L. Highly efficient ruthenium-catalyzed N-formylation of amines with H2 and CO2. Angew. Chem. Int. Ed. 2015, 127, 6186–6189. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Gunasekar, G.H.; Prakash, N.; Jung, K.; Yoon, S. A highly efficient heterogenized iridium complex for the catalytic hydrogenation of carbon dioxide to formate. ChemSusChem 2015, 8, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Toyao, T.; Siddiki, S.M.A.H.; Morita, Y.; Kamachi, T.; Touchy, A.S.; Onodera, W.; Kon, K.; Furukawa, S.; Ariga, H.; Asakura, K.; et al. Rhenium-loaded TiO2: A highly versatile and chemoselective catalyst for the hydrogenation of carboxylic acid derivatives and the N-methylation of amines using H2 and CO2. Chem. Eur. J. 2017, 23, 14848–14859. [Google Scholar] [CrossRef]
- Mitsudome, T.; Urayama, T.; Fujita, S.; Maeno, Z.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. A titanium dioxide supported gold nanoparticle catalyst for the selective N-formylation of functionalized amines with carbon dioxide and hydrogen. ChemCatChem 2017, 9, 3632–3636. [Google Scholar] [CrossRef]
- Bai, P.; Zhao, Y.C.; Li, Y.D. Efficient photocatalytic CO2 N-formylation of amines over Pd/Bi-ZnOx without extra reductant. Catal. Today. 2024, 430, 114551–114561. [Google Scholar] [CrossRef]
- He, Y.; Bai, P.; Yuan, S.B.; Chen, J.F.; Zhao, Y.C.; Li, Y.D. Photocatalytic N-formylation of amines with CO2 over Pt-Bi bimetallic decorated CeO2−x. Mol. Catal. 2024, 559, 114086–114093. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Mei, Q.Q.; Liu, H.Y.; Liu, H.Z.; Zhang, Z.P.; Han, B.X. Copper-catalyzed N-formylation of amines with CO2 under ambient conditions. RSC Adv. 2016, 6, 32370–32373. [Google Scholar] [CrossRef]
- Fang, C.; Lu, C.L.; Liu, M.H.; Zhu, Y.L.; Fu, Y.; Lin, B.L. Selective formylation and methylation of amines using carbon dioxide and hydrosilane catalyzed by alkali-metal carbonates. ACS Catal. 2016, 6, 7876–7881. [Google Scholar] [CrossRef]
- Luo, R.C.; Lin, X.W.; Chen, Y.J.; Zhang, W.Y.; Zhou, X.T.; Ji, H.B. Cooperative catalytic activation of Si-H bonds: CO2-based synthesis of formamides from amines and hydrosilanes under mild conditions. ChemSusChem 2017, 10, 1224–1232. [Google Scholar] [CrossRef]
- Gomes, C.D.N.; Jacquet, O.; Villiers, C.; Thury, P.; Ephritikhine, M.; Cantat, T. A diagonal approach to chemical Recycling of carbon dioxide: Organocatalytic transformation for the reductive functionalization of CO2. Angew. Chem. Int. Ed. 2012, 51, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, O.; Gomes, C.D.N.; Ephritikhine, M.; Cantat, T. Recycling of Carbon and Silicon Wastes: Room Temperature Formylation of N–H Bonds Using Carbon Dioxide and Polymethylhydrosiloxane. J. Am. Chem. Soc. 2012, 134, 2934–2937. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.D.; Zhao, Y.F.; Yu, B.; Yang, Z.Z.; Zhang, H.Y.; Han, B.X.; Gao, X.; Liu, Z.M. Imidazolium-based ionic liquids catalyzed formylation of amines using carbon dioxide and phenylsilane at room temperature. ACS Catal. 2015, 5, 4989–4993. [Google Scholar] [CrossRef]
- Chong, C.C.; Kinjo, R. Hydrophosphination of CO2 and subsequent formate transfer in the 1,3,2-diazaphospholene-catalyzed N-formylation of amines. Angew. Chem. Int. Ed. 2015, 127, 12116–12120. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, D.K.; Xue, Z.M.; Zhang, B.; Mu, T.C. Dihydrolevoglucosenone as a bio-based catalytic solvent for efficient reductive-transformation of CO2 with amines into formamides and benzothiazoles. Chem. Eng. J. 2022, 431, 133397–133405. [Google Scholar] [CrossRef]
- Lu, H.; Xing, Q.; Yue, C.T.; Lei, Z.Q.; Li, F.W. Solvent-promoted catalyst-free N-formylation of amines using carbon dioxide under ambient conditions. Chem. Commun. 2016, 52, 6545–6548. [Google Scholar]
- Niu, H.Y.; Lu, L.J.; Shi, R.Y.; Chiang, C.W.; Lei, A. Catalyst-free N-methylation of amines using CO2. Chem. Commun. 2017, 53, 1148–1151. [Google Scholar] [CrossRef]
- Chen, X.C.; Guo, L.; Shi, G.H.; Zhao, K.C.; Lu, Y.; Liu, Y. Can CO2 be a catalyst? Yes, CO2-catalyzed N-formylation of aliphatic amines with DMF. Mol. Catal. 2022, 528, 112431–112439. [Google Scholar] [CrossRef]
- Chakrabortty, P.; Kumar, S.; Chowdhury, A.; Khan, A.; Bhaumik, A.; Islam, S.M. Ag-nanocatalysts based on porous organic polymers in chemical fixation of CO2 for the N-methylation and N-formylation of amines. ChemCatChem 2024, 16, e202301539. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, X.Y.; Qiao, C.; Fu, H.C.; He, L.N. Betaine Catalysis for Hierarchical Reduction of CO2 with amines and hydrosilane to form formamides, aminals, and methylamines. Angew. Chem. Int. Ed. 2017, 56, 7533–7537. [Google Scholar] [CrossRef]
- Hota, P.K.; Sau, S.C.; Mandal, S.K. Metal-free catalytic formylation of amides using CO2 under ambient conditions. ACS Catal. 2018, 8, 11999–12003. [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, S.S.; Liu, X.F.; Yang, Z.W.; Tan, T.Y.; Yu, A.; He, L.N. Waste recycling: Ionic liquid-catalyzed 4-electron peduction of CO2 with pmines and polymethylhydrosiloxane combining experimental and theoretical study. ACS Sustain. Chem. Eng. 2018, 6, 8130–8135. [Google Scholar] [CrossRef]
- Wen, Q.; Yuan, X.X.; Zhou, Q.Q.; Yang, H.J.; Jiang, Q.Q.; Hu, J.C.; Guo, C.Y. Solvent- and co-catalyst-free cycloaddition of carbon dioxide and epoxides catalyzed by recyclable bifunctional niobium complexes. Materials 2023, 16, 3531. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yuan, X.; Zhou, Q.; Yang, H.J.; Jiang, Q.; Hu, J.; Guo, C.Y. Effcient N-formylation of carbon dioxide and amines with alkanolamine as eco-friendly catalyst under mild conditions. J. CO2 Util. 2023, 69, 102398–102408. [Google Scholar] [CrossRef]
- Lin, W.W.; Cheng, H.Y.; Wu, Q.F.; Zhang, C.; Arai, M.; Zhao, F.Y. Selective N-methylation of N-methylaniline with CO2 and H2 over TiO2-supported PdZn catalyst. ACS Catal. 2020, 10, 3285–3296. [Google Scholar] [CrossRef]
- Hulla, M.; Laurenczy, G.; Dyson, P.J. Mechanistic study of the N-formylation of amines with carbon dioxide and hydrosilanes. ACS Catal. 2018, 8, 10619–10630. [Google Scholar] [CrossRef]
- Liu, X.F.; Qiao, C.; Li, X.Y. DMF-promoted reductive functionalization of CO2 with secondary amines and phenylsilane to methylamines. Pure Appl. Chem. 2018, 90, 1099–1107. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, S.H.; Wang, Y.P. Unsupported nanoporous palladium catalyst for N-formylation of amines using CO2 as a sustainable C1 source. Asian J. Org. Chem. 2022, 11, e202200064. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, H.L.; Yuan, H.K.; Shi, F. Hydroxyl group-regulated active nano-Pd/C catalyst generation via in situ Reduction of Pd(NH3)xCly/C for N-formylation of amines with CO2/H2. ACS Sustain. Chem. Eng. 2017, 5, 5758–5765. [Google Scholar] [CrossRef]
- Gopakumar, A.; Lombardo, L.; Fei, Z.F.; Shyshkanov, S. A polymeric ionic liquid catalyst for the N-formylation and N-methylation of amines using CO2/PhSiH3. J. CO2 Util. 2020, 41, 101240–101245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).