One-Pot Synthesis of Acidic Mesoporous Activated Carbon Obtained from Yerba Mate Twigs as Suitable Catalyst for the Production of Levulinic Ester Biofuel Additives
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Methods
3.1. General Remarks
3.2. Factorial Experimental Desing 2k
3.3. Yerba Mate Activated Carbon Preparation
3.4. Physicochemical Characterization
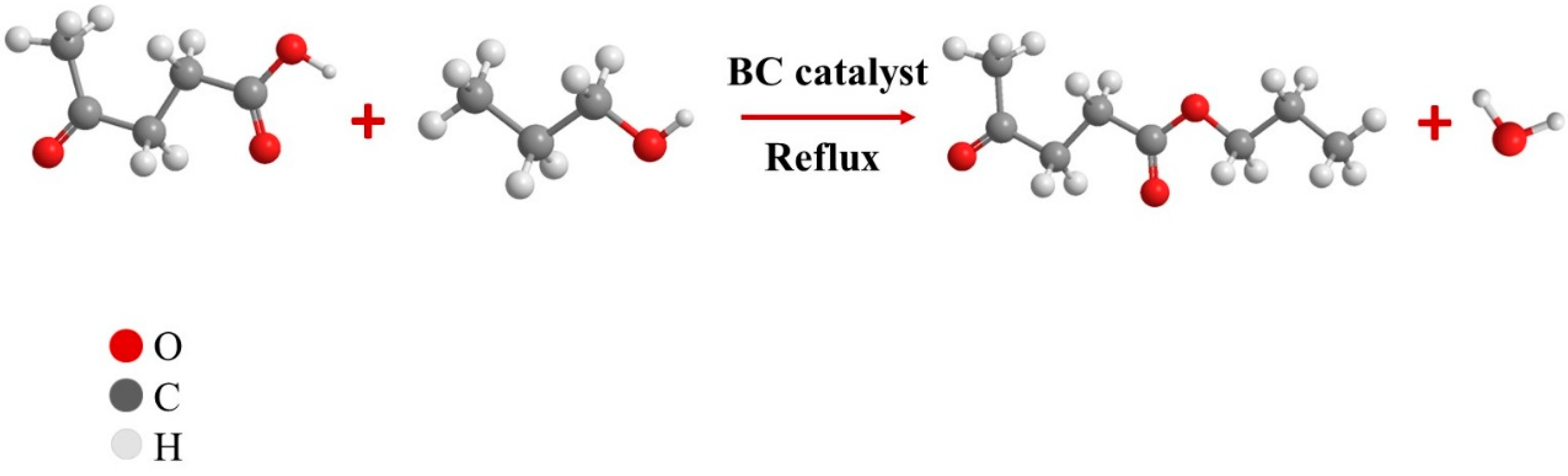
3.5. Catalytic Test
3.6. Catalytic Reuse
3.7. Mass Spectrum of Synthesized Alkyl Levulinates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shan, J.; Wang, Q.; Hao, H.; Guo, H. Critical Review on the Synthesis of Levulinate Esters from Biomass-Based Feedstocks and Their Application. Ind. Eng. Chem. Res. 2023, 62, 17135–17147. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Pei, M.; Chen, Y.; Zhang, L.; Li, L.; Chen, Q.; Tian, Y.; Xie, H. Cellulose Levulinate Ester as a Robust Building Block for the Synthesis of Fully Biobased Functional Cellulose Esters. Int. J. Biol. Macromol. 2023, 246, 125654. [Google Scholar] [CrossRef]
- Pasquale, G.; Vázquez, P.; Romanelli, G.; Baronetti, G. Catalytic Upgrading of Levulinic Acid to Ethyl Levulinate Using Reusable Silica-Included Wells-Dawson Heteropolyacid as Catalyst. Catal. Commun. 2012, 18, 115–120. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, Y.; Liang, X.; Hao, J.; Xu, G.; Chen, B.; He, C.; Jiao, Y.; Chang, C. Theoretical and Experimental Study of 5-Ethoxymethylfurfural and Ethyl Levulinate Production from Cellulose. Chem. Eng. J. 2024, 480, 148093. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zainol, M.M.; Samion, M.A.; Azlan, M.A.; Asmadi, M.; Mohamad Daud, A.R.; Saad, I.; Mohd Nor Azman, N.A.N. Synthesis of Ethyl Levulinate over Sulfonated Lignin-Based Carbon Catalyst as a Fuel Additive to Biodiesel-Diesel Blends towards Engine Emissions. J. Clean. Prod. 2023, 418, 138101. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Z.; Li, Y.; Li, Z.; He, X.; Zhu, J. Performance of a Diesel Engine with Ethyl Levulinate-Diesel Blends: A Study Using Grey Relational Analysis. Bioresources 2013, 8, 2696–2707. [Google Scholar] [CrossRef]
- Unlu, D.; Ilgen, O.; Hilmioglu, N.D. Biodiesel Additive Ethyl Levulinate Synthesis by Catalytic Membrane: SO4-2/ZrO2 Loaded Hydroxyethyl Cellulose. Chem. Eng. J. 2016, 302, 260–268. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. A Review on Catalytic Synthesis of Energy Rich Fuel Additive Levulinate Compounds from Biomass Derived Levulinic Acid. Fuel Process. Technol. 2020, 197, 106213. [Google Scholar] [CrossRef]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of Levulinic Acid with Ethanol Catalyzed by Sulfonated Carbon Catalysts: Promotional Effects of Additional Functional Groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Popova, M.; Shestakova, P.; Lazarova, H.; Dimitrov, M.; Kovacheva, D.; Szegedi, A.; Mali, G.; Dasireddy, V.; Likozar, B.; Wilde, N.; et al. Efficient Solid Acid Catalysts Based on Sulfated Tin Oxides for Liquid Phase Esterification of Levulinic Acid with Ethanol. Appl. Catal. A Gen. 2018, 560, 119–131. [Google Scholar] [CrossRef]
- Kothe, V.; Melfi, D.T.; dos Santos, K.C.; Corazza, M.L.; Ramos, L.P. Thermodynamic Analysis, Experimental and Kinetic Modeling of Levulinic Acid Esterification with Ethanol at Supercritical Conditions. Fuel 2020, 260. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Rossano, C.; Cogliano, T.; Vitiello, R.; Leveneur, S.; Di Serio, M. Kinetic Study of Amberlite IR120 Catalyzed Acid Esterification of Levulinic Acid with Ethanol: From Batch to Continuous Operation. Chem. Eng. J. 2020, 401, 126126. [Google Scholar] [CrossRef]
- Mthembu, L.D.; Lokhat, D.; Deenadayalu, N. Esterification of Levulinic Acid to Ethyl Levulinate: Optimization of Process Conditions Using Commercial Levulinic Acid and Extension to the Use of Levulinic Acid Derived from Depithed Sugarcane Bagasse. Biomass Convers. Biorefin. 2023, 13, 3113–3122. [Google Scholar] [CrossRef]
- Sahu, P.; Sakthivel, A. Zeolite-β Based Molecular Sieves: A Potential Catalyst for Esterification of Biomass Derived Model Compound Levulinic Acid. Mater. Sci. Energy Technol. 2021, 4, 307–316. [Google Scholar] [CrossRef]
- Badia, J.H.; Ramírez, E.; Soto, R.; Bringué, R.; Tejero, J.; Cunill, F. Optimization and Green Metrics Analysis of the Liquid-Phase Synthesis of Sec-Butyl Levulinate by Esterification of Levulinic Acid with 1-Butene over Ion-Exchange Resins. Fuel Process. Technol. 2021, 220. [Google Scholar] [CrossRef]
- Ristiana, D.D.; Suyanta, S.; Nuryono, N. Sulfonic Acid-Functionalized Silica with Controlled Hydrophobicity as an Effective Catalyst for Esterification of Levulinic Acid. Mater. Today Commun. 2022, 32, 103953. [Google Scholar] [CrossRef]
- Bakhtiari, B.; Najafi Chermahini, A.; Babaei, Z. Design of an Acidic Sulfonated Mesoporous Carbon Catalyst for the Synthesis of Butyl Levulinate from Levulinic Acid. Environ. Prog. Sustain. Energy 2021, 40, 1–13. [Google Scholar] [CrossRef]
- Veluturla, S.; Kottam, N.; Vandana, P.; Shetty, A.; Shankar, V. Process Optimisation and Kinetic Study for Esterification of Levulinic Acid and N-Butanol Using Sulfonated Titania–Zirconia Heterogeneous Catalyst. J. Chem. Technol. Biotechnol. 2024, 99, 626–636. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Nelson Appaturi, J.; Andas, J.; Ma, Y.K.; Lee Phoon, B.; Muazu Batagarawa, S.; Khoerunnisa, F.; Hazwan Hussin, M.; Ng, E.P. Recent Advances in Heterogeneous Catalysts for the Synthesis of Alkyl Levulinate Biofuel Additives from Renewable Levulinic Acid: A Comprehensive Review. Fuel 2022, 323, 124362. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zainol, M.M.; Zainuddin, K.R.; Rosmadi, H.A.; Asmadi, M.; Rahman, N.A.; Amin, N.A.S. A Review on Alkyl Levulinates Synthesis from Renewable Levulinic Acid Using Various Modified Carbon-Based Catalysts. Malays. J. Chem. 2022, 24, 264–282. [Google Scholar] [CrossRef]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of Levulinic Acid and Alkyl Levulinates into Biofuels and High-Value Chemicals. Green. Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Zhang, Q.; Li, C.; Wu, H. Current Approaches to Alkyl Levulinates via Efficient Valorization of Biomass Derivatives. Front. Chem. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Budarin, V.; Luque, R.; Macquarrie, D.J.; Clark, J.H. Towards a Bio-Based Industry: Benign Catalytic Esterifications of Succinic Acid in the Presence of Water. Chem. A Eur. J. 2007, 13, 6914–6919. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Pasquale, G.A.; Rengifo-Herrera, J.A.; Romanelli, G.P.; Pizzio, L.R. Mesoporous Activated Carbon from Sunflower Shells Modified with Sulfonic Acid Groups as Solid Acid Catalyst for Itaconic Acid Esterification. Catal. Today 2021, 372, 51–58. [Google Scholar] [CrossRef]
- Urruchua, F.C.; Fernández, M.A.; Jaworski, M.; Mendoza Zelis, P.; Olivelli, M.S.; Montes, M.L. Yerba Mate Waste: Transformation to Magnetic Composites for the Adsorption of Chemically Diverse Pollutants. J. Environ. Chem. Eng. 2023, 11, 110824. [Google Scholar] [CrossRef]
- Jerez, F.; Ramos, P.B.; Córdoba, V.E.; Ponce, M.F.; Acosta, G.G.; Bavio, M.A. Yerba Mate: From Waste to Activated Carbon for Supercapacitors. J. Environ. Manag. 2023, 330, 117158. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, E.; Nunell, G.; Cukierman, A.L.; Bonelli, P. Agroindustrial Waste Conversion into Ultramicroporous Activated Carbons for Greenhouse Gases Adsorption-Based Processes. Bioresour. Technol. Rep. 2022, 18, 101008. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar Production and Applications in Soil Fertility and Carbon Sequestration-a Sustainable Solution to Crop-Residue Burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Chimienti, M.E.; Pizzio, L.R.; Cáceres, C.V.; Blanco, M.N. Tungstophosphoric and Tungstosilicic Acids on Carbon as Acidic Catalysts. Appl. Catal. A Gen. 2001, 208, 7–19. [Google Scholar] [CrossRef]
- Hosseinzaei, B.; Hadianfard, M.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Heating Rate and H3PO4 as Catalyst on the Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2022, 168, 105724. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, R.; Li, G.; Hu, C. The Role of H3PO4 in the Preparation of Activated Carbon from NaOH-Treated Rice Husk Residue. RSC Adv. 2015, 5, 32626–32636. [Google Scholar] [CrossRef]
- Abotsi, G.M.K.; Scaroni, A.W. Reaction of Carbons with Ammonia: Effects on the Surface Charge and Molybdenum Adsorption. Carbon 1990, 28, 79–84. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Cánneva, A.; Donadelli, J.A.; Manrique-Holguín, M.; Rengifo-Herrera, J.A.; Pizzio, L.R. Removal of Diclofenac and Ibuprofen on Mesoporous Activated Carbon from Agro-Industrial Wastes Prepared by Optimized Synthesis Employing a Central Composite Design. Biomass Convers. Biorefin. 2023, 13, 13197–13219. [Google Scholar] [CrossRef]
- Chu, G.; Wang, W.; Zhao, J.; Zhou, D. Transformation of Phosphorus Species during Phosphoric Acid-Assisted Pyrolysis of Lignocellulose. Sci. Total Environ. 2023, 866, 161010. [Google Scholar] [CrossRef] [PubMed]
- Moulefera, I.; García-Mateos, F.J.; Benyoucef, A.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Co-Solution of Carbon Precursor and Activating Agent on the Textural Properties of Highly Porous Activated Carbon Obtained by Chemical Activation of Lignin With H3PO4. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic Carbons Activated with Phosphoric—Acid II. Porous Structure. Carbon 2002, 40, 1507–1519. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and Characterization of High Surface Area Activated Carbon from Fox Nut (Euryale Ferox) Shell by Chemical Activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef]
- Jastrzbski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared Spectroscopy of Different Phosphates Structures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ingall, E. Polyphosphates as a Source of Enhanced P Fluxes in Marine Sediments Overlain by Anoxic Waters: Evidence from 31P NMR. Geochem. Trans. 2005, 6, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Barberot, C.; Moniot, A.; Allart-Simon, I.; Malleret, L.; Yegorova, T.; Laronze-Cochard, M.; Bentaher, A.; Médebielle, M.; Bouillon, J.P.; Hénon, E.; et al. Synthesis and Biological Evaluation of Pyridazinone Derivatives as Potential Anti-Inflammatory Agents. Eur. J. Med. Chem. 2018, 146, 139–146. [Google Scholar] [CrossRef]
- Pithadia, D.; Patel, A. Anchored Silicotungstic Acid: Study of the Synthesis of Biofuel Additive from Levulinic Acid & Effect of Supports. Catal. Today 2024, 433, 114666. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Zhao, W.; Wen, S.; Xiang, Y.; Liu, X. A New Sulfonic Acid-Functionalized Organic Polymer Catalyst for the Synthesis of Biomass-Derived Alkyl Levulinates. Catal. Lett. 2020, 150, 3553–3560. [Google Scholar] [CrossRef]
- Manikandan, K.; Cheralathan, K.K. Heteropoly Acid Supported on Silicalite–1 Possesing Intracrystalline Nanovoids Prepared Using Biomass—An Efficient and Recyclable Catalyst for Esterification of Levulinic Acid. Appl. Catal. A Gen. 2017, 547, 237–247. [Google Scholar] [CrossRef]
- Enumula, S.S.; Gurram, V.R.B.; Chada, R.R.; Burri, D.R.; Kamaraju, S.R.R. Clean Synthesis of Alkyl Levulinates from Levulinic Acid over One Pot Synthesized WO3-SBA-16 Catalyst; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 426, ISBN 9140271609. [Google Scholar]
- Budarin, V.L.; Clark, J.H.; Henschen, J.; Farmer, T.J.; Macquarrie, D.J.; Mascal, M.; Nagaraja, G.K.; Petchey, T.H.M. Processed Lignin as a Byproduct of the Generation of 5-(Chloromethyl)Furfural from Biomass: A Promising New Mesoporous Material. ChemSusChem 2015, 8, 4172–4179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Li, N.; Qin, Z.; Zheng, X.C. Sulfonated Porous Biomass-Derived Carbon with Superior Recyclability for Synthesizing Ethyl Levulinate Biofuel. Res. Chem. Intermed. 2020, 46, 5325–5343. [Google Scholar] [CrossRef]
- Klähn, M.; Mathias, G.; Kötting, C.; Nonella, M.; Schlitter, J.; Gerwert, K.; Tavan, P. IR Spectra of Phosphate Ions in Aqueous Solution: Predictions of a DFT/MM Approach Compared with Observations. J. Phys. Chem. A 2004, 108, 6186–6194. [Google Scholar] [CrossRef]
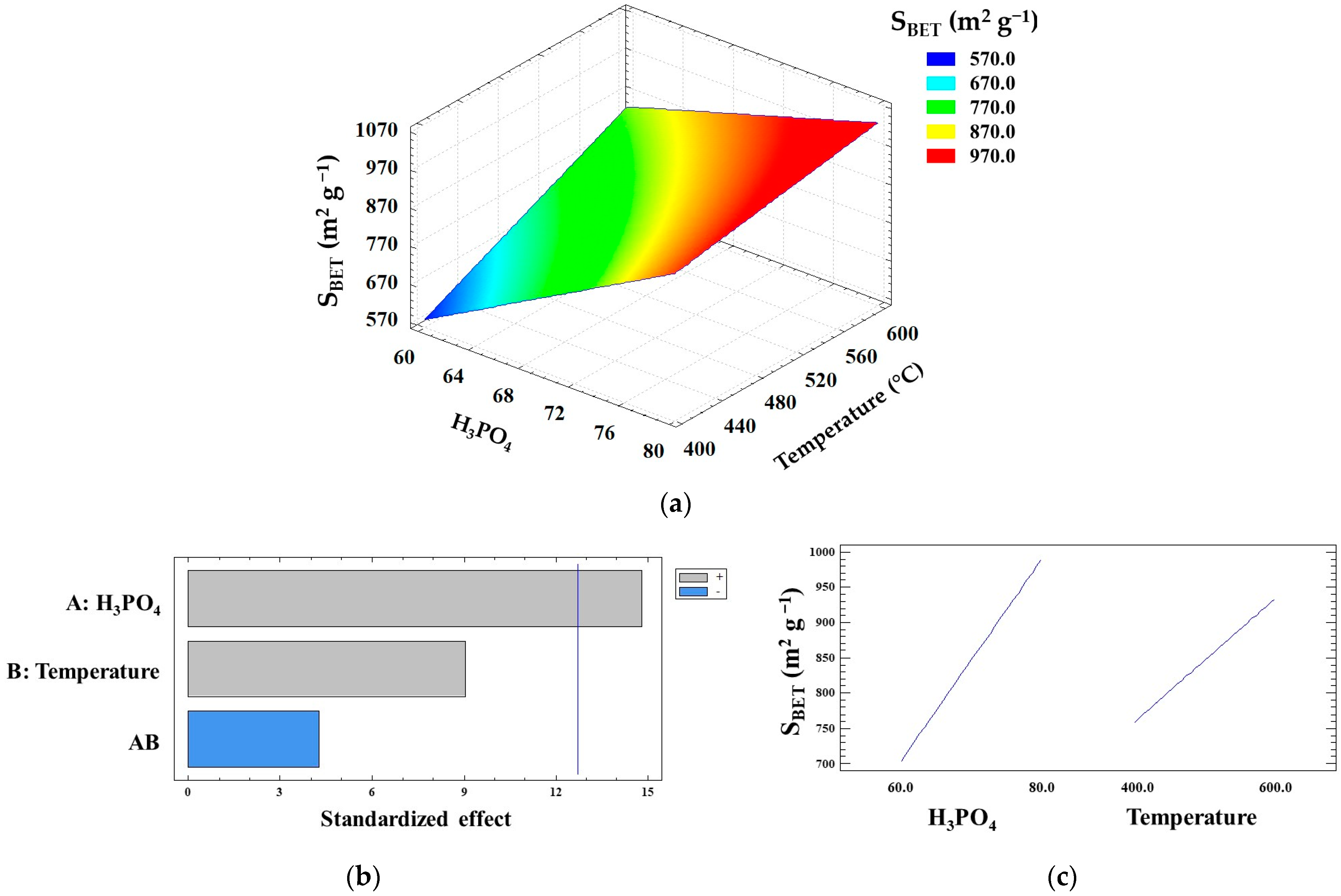

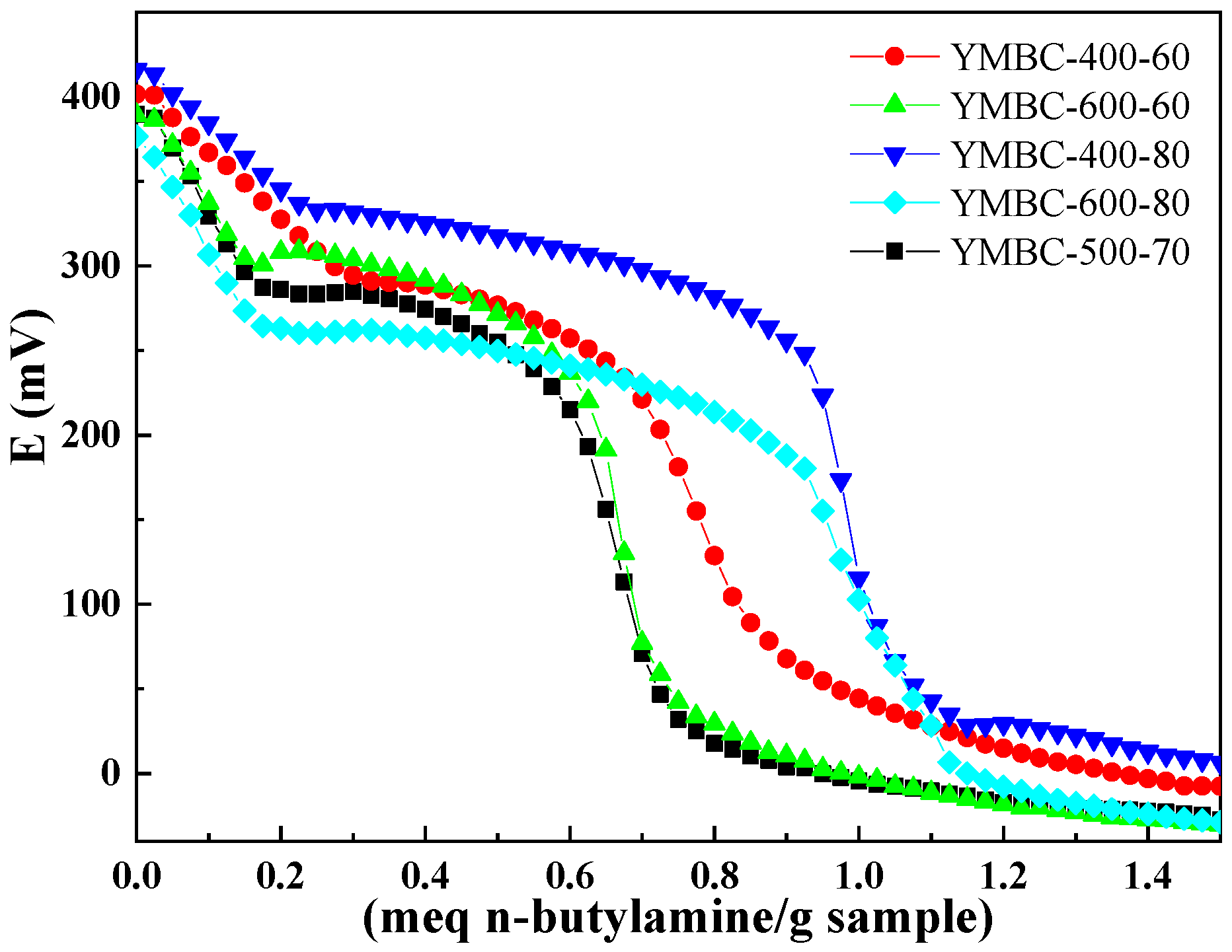
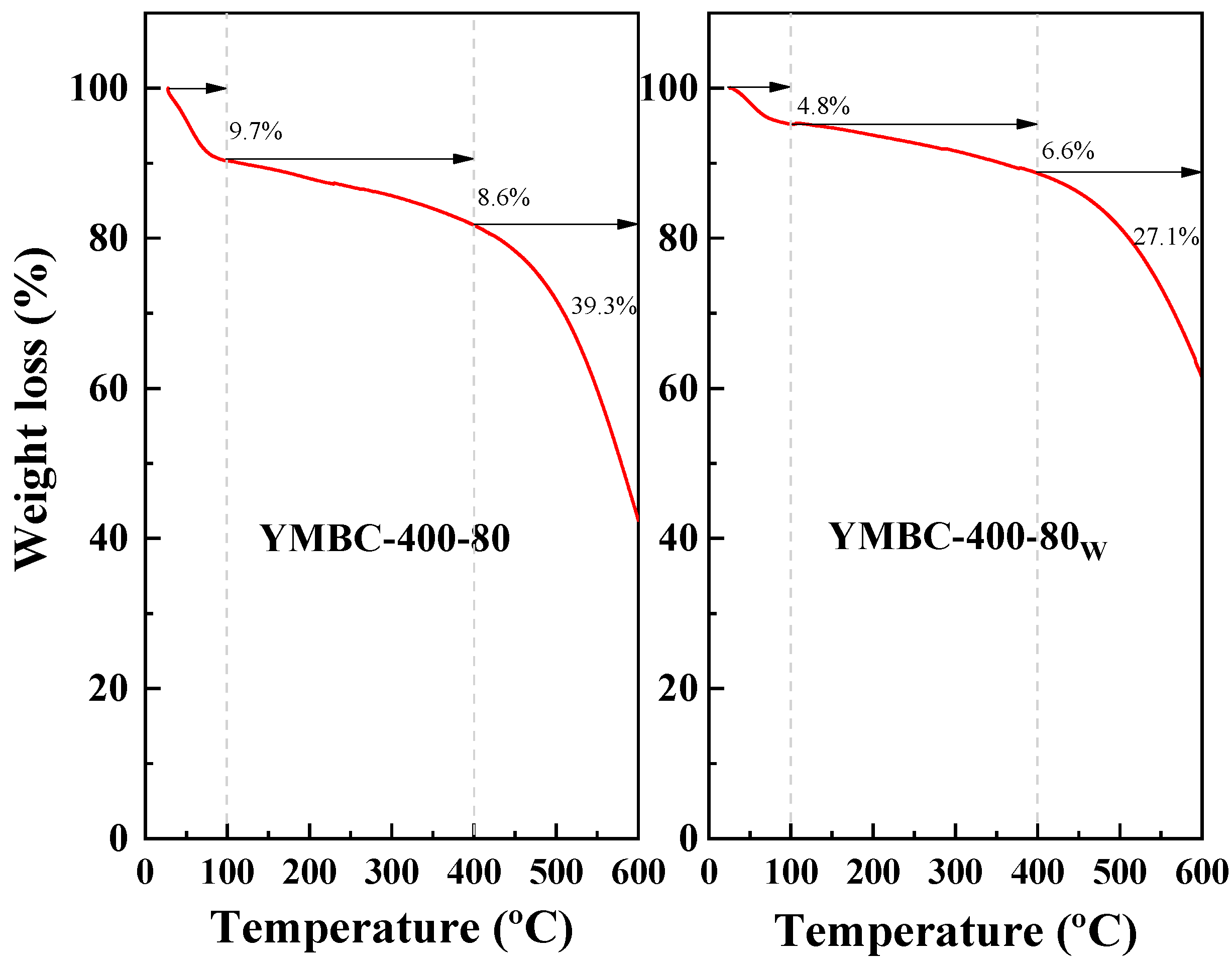


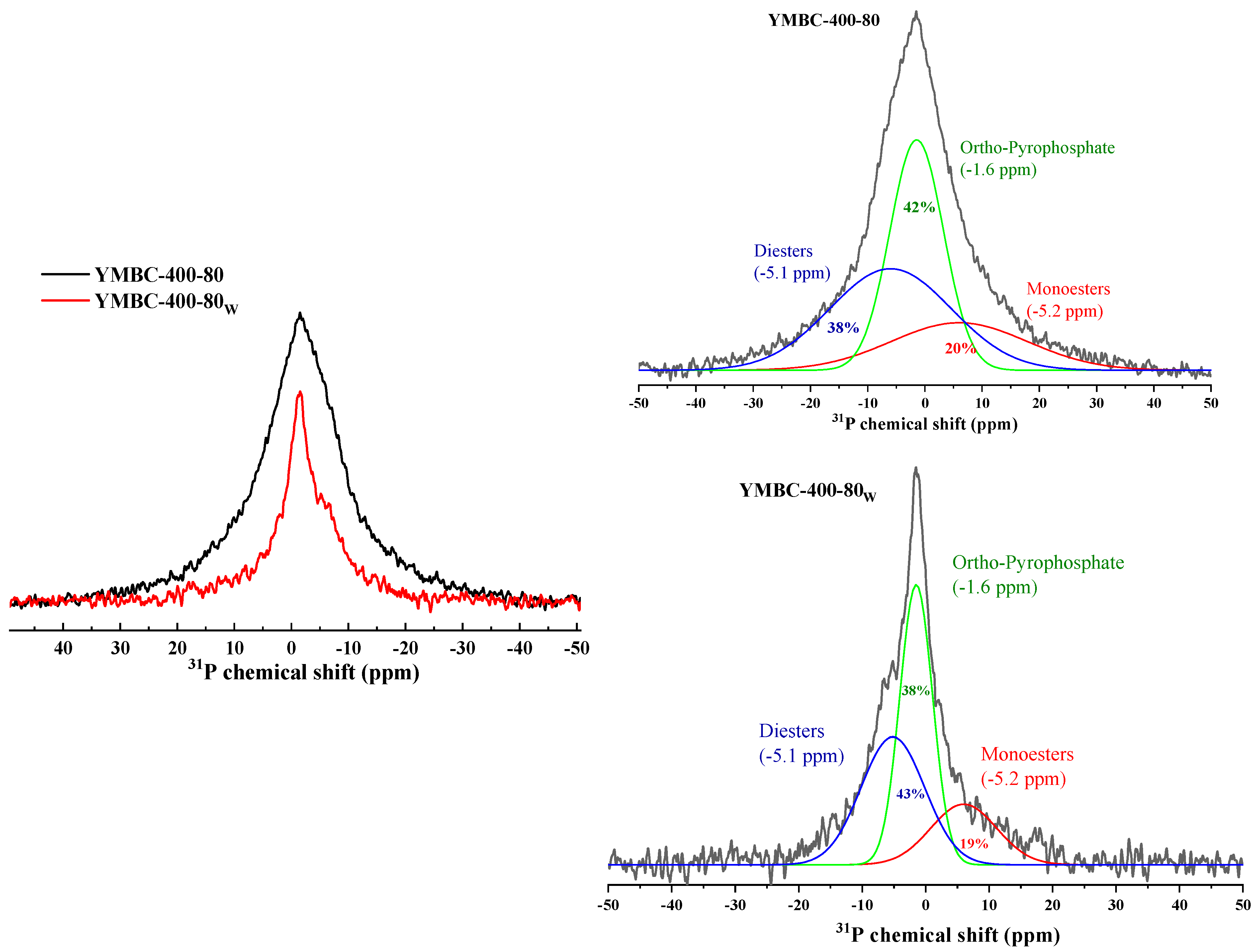
| Factors | Levels | ||||
|---|---|---|---|---|---|
| Low (−) | Central Point (0) | High (+) | |||
| x1: H3PO4:YMT ratio (wt%) | 60 | 70 | 80 | ||
| x2: carbonization temperature (°C) | 400 | 500 | 600 | ||
| Run | Sample Name | x1 | x2 | Response variables | |
| 1 | YMBC-400-60 | 60 | 400 | ||
| 2 | YMBC-400-80 | 80 | 400 | SBET | |
| 3 | YMBC-600-60 | 60 | 600 | ||
| 4 | YMBC-600-80 | 80 | 600 | ||
| 5 | YMBC-500-70 | 70 | 500 | ||
| Experimental Design | Textural Properties | |||||
|---|---|---|---|---|---|---|
| Run | Level | SBET | Smeso | Dp | ||
| Sample name | A Factor | B Factor | m2 g−1 | m2 g−1 | nm | |
| 1 | YMBC-400-60 | − | − | 572 | 485 | 4.2 |
| 2 | YMBC-400-80 | + | − | 828 | 769 | 3.8 |
| 3 | YMBC-600-60 | − | + | 939 | 870 | 3.2 |
| 4 | YMBC-600-80 | + | + | 1031 | 686 | 2.3 |
| 5 | YMBC-500-70 | 0 | 0 | 864 | 707 | 3.6 |
| Factors | Sum of Squares | F-Value | p-Value |
|---|---|---|---|
| A: | 81,225.0 | 219.6 | 0.0429 |
| B: | 30,276.0 | 81.8 | 0.0701 |
| AB | 6724.0 | 18.2 | 0.1466 |
| Total error | 369.8 | ||
| R2 | 99.6 | ||
| Standard error | 19.23 | ||
| Absolute error | 6.8 | ||
| Durbin–Watson | 2.5 |
| Entry | Catalyst | Fresh | 1st Reuse | 2nd Reuse | 3rd Reuse |
|---|---|---|---|---|---|
| 1 | YMBC-400-60NP | 27 | - | - | - |
| 2 | YMBC-400-80NP | 25 | - | - | - |
| 3 | YMBC-600-60NP | 47 | 48 | 46 | - |
| 4 | YMBC-600-80NP | 41 | 40 | 43 | - |
| 5 | YMBC-500-70NP | 49 | 49 | 48 | 47 |
| Entry | Time (h) | Temp. (°C) | Catalyst Amount (mg) | Yield (%) |
|---|---|---|---|---|
| 1 | 6 | 97 | 80 | 49 |
| 2 | 12 | 97 | 80 | 57 |
| 3 | 24 | 97 | 80 | 88 |
| 4 | 48 | 97 | 80 | 94 |
| 5 | 6 | 77 | 80 | 20 |
| 6 | 6 | 87 | 80 | 28 |
| 7 | 6 | 97 | 40 | 37 |
| 8 | 6 | 97 | 120 | 53 |
| Entry | Alcohol | Yield (%) |
|---|---|---|
| 1 | n-Propanol | 49 |
| 2 | n-Butanol | 49 |
| 3 | n-Pentanol | 47 |
| 4 | i-Propanol | 39 |
| 5 | i-Butanol | 45 |
| 6 | t-Butanol | - |
| 7 | Phenol | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvear-Daza, J.J.; Sosa, A.; Ruiz, D.M.; Pasquale, G.A.; Rengifo-Herrera, J.A.; Romanelli, G.P.; Pizzio, L.R. One-Pot Synthesis of Acidic Mesoporous Activated Carbon Obtained from Yerba Mate Twigs as Suitable Catalyst for the Production of Levulinic Ester Biofuel Additives. Catalysts 2024, 14, 522. https://doi.org/10.3390/catal14080522
Alvear-Daza JJ, Sosa A, Ruiz DM, Pasquale GA, Rengifo-Herrera JA, Romanelli GP, Pizzio LR. One-Pot Synthesis of Acidic Mesoporous Activated Carbon Obtained from Yerba Mate Twigs as Suitable Catalyst for the Production of Levulinic Ester Biofuel Additives. Catalysts. 2024; 14(8):522. https://doi.org/10.3390/catal14080522
Chicago/Turabian StyleAlvear-Daza, John J., Alexis Sosa, Diego M. Ruiz, Gustavo A. Pasquale, Julián A. Rengifo-Herrera, Gustavo P. Romanelli, and Luis R. Pizzio. 2024. "One-Pot Synthesis of Acidic Mesoporous Activated Carbon Obtained from Yerba Mate Twigs as Suitable Catalyst for the Production of Levulinic Ester Biofuel Additives" Catalysts 14, no. 8: 522. https://doi.org/10.3390/catal14080522
APA StyleAlvear-Daza, J. J., Sosa, A., Ruiz, D. M., Pasquale, G. A., Rengifo-Herrera, J. A., Romanelli, G. P., & Pizzio, L. R. (2024). One-Pot Synthesis of Acidic Mesoporous Activated Carbon Obtained from Yerba Mate Twigs as Suitable Catalyst for the Production of Levulinic Ester Biofuel Additives. Catalysts, 14(8), 522. https://doi.org/10.3390/catal14080522







