Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol
Abstract
1. Introduction
2. Results and Discussion
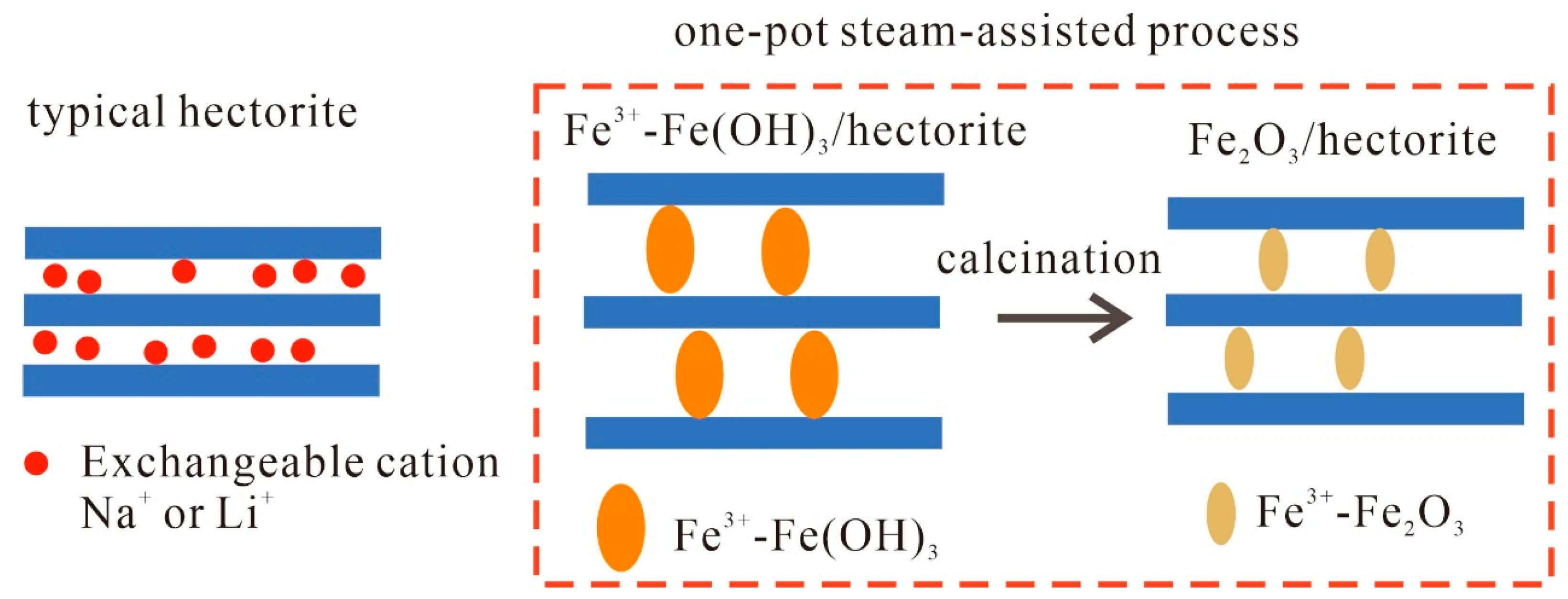
2.1. Steam Synthesis Reaction Mechanism
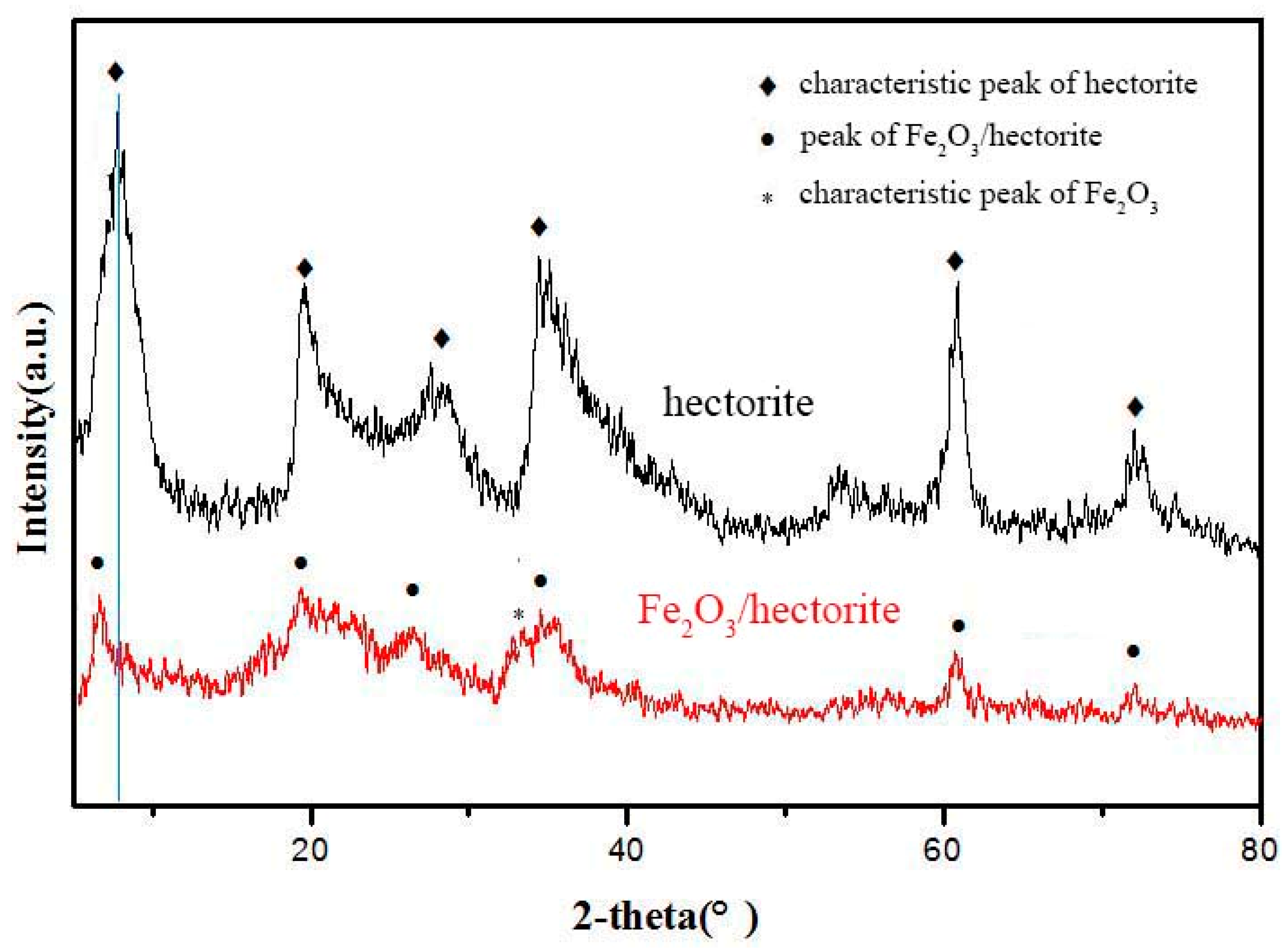
2.2. Characterization
2.3. Optimal Fenton Reaction Conditions
2.4. Stability of the Fe2O3/Hectorite Catalyst
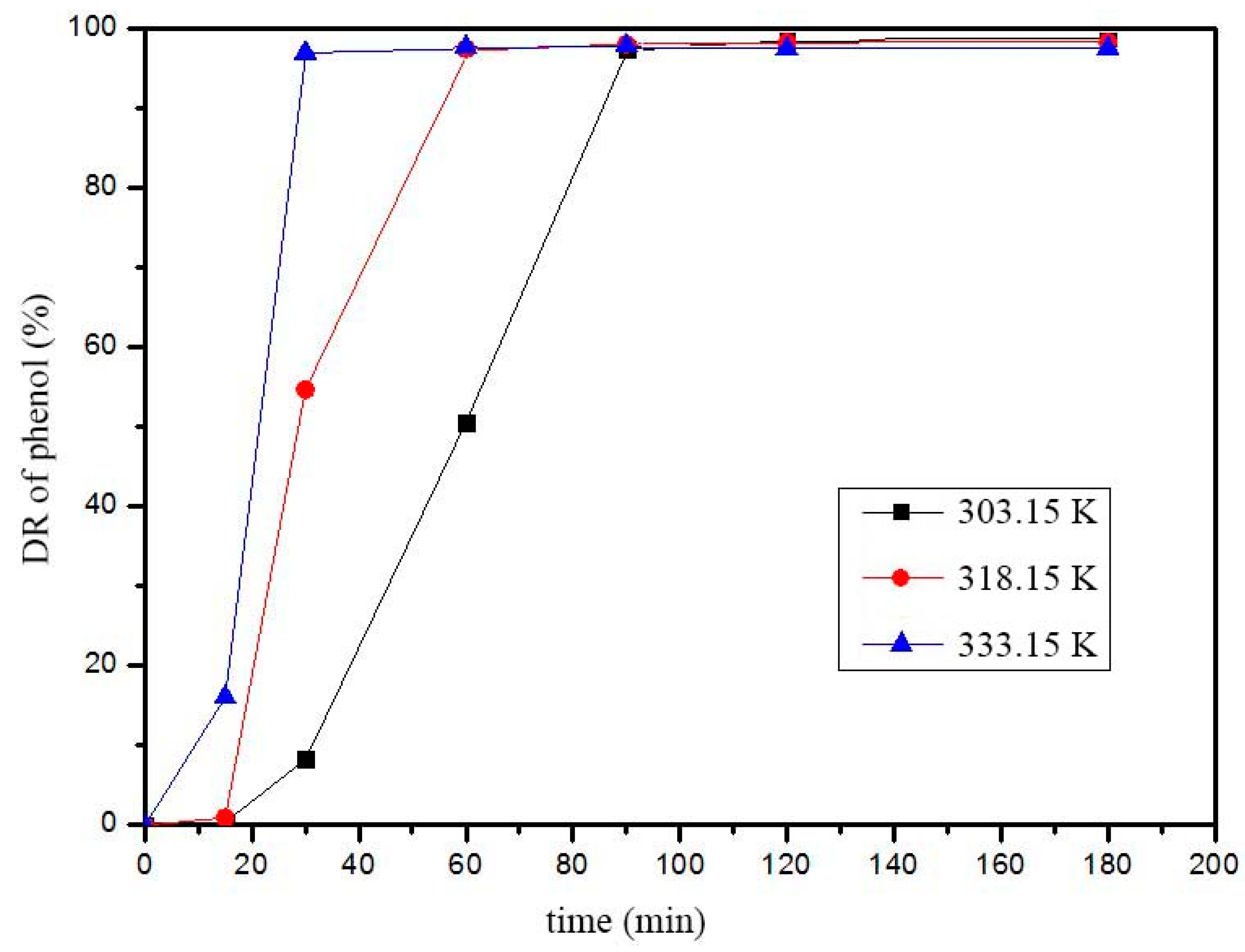
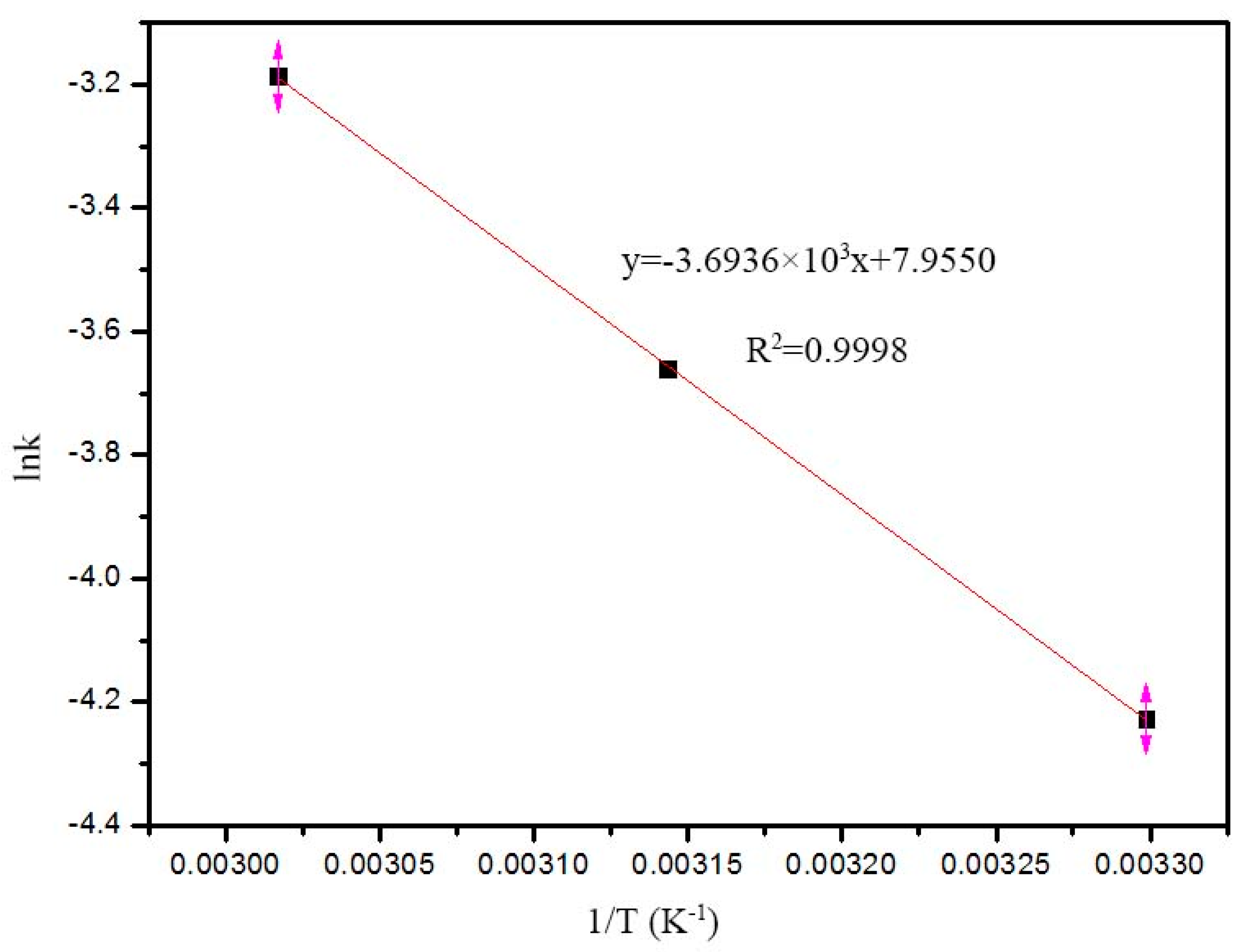
2.5. Fenton Reaction Kinetic
3. Materials and Methods
3.1. Materials
3.2. Synthesizing Fe2O3/Hectorite Catalyst
3.3. Characterizations
3.4. Fenton Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite Oxide Catalysts for Advanced Oxidation Reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Wang, Z.; Zhang, Q.; Yuan, D. Organic cocatalysts improved Fenton and Fenton-like processes for water pollution control: A review. Chemosphere 2024, 353, 141581. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Niu, Z.; Zhang, X.; Zhang, Y. Pollution characteristics and health risk of sixty-five organics in one drinking water system: PAEs should be prioritized for control. Chemosphere 2024, 350, 141171. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Sundarrajan, P.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.Y.; Meng, F.P.; Cui, H.W.; Lin, Y.F.; Wang, G.S.; Wu, J.Y. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Radovic, M.; Mitrovic, J.; Kostic, M.; Bojic, D.; Petrovic, M.; Najdanovic, S.; Bojic, A. Comparison of ultraviolet radiation/hydrogen peroxide, Fenton and photo-Fenton processes for the decolorization of reactive dyes. Hem. Ind. 2015, 69, 657–665. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Lyu, L.; Hu, C. Heterogeneous Fenton Catalytic Water Treatment Technology and Mechanism. Prog. Chem. 2017, 29, 981–999. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Jalali, H.; Naeimzadeh, F.; Gharibian, S. Efficient Removal of Diclofenac from Pharmaceutical Wastewater Using Impregnated Zeolite Catalyst in Heterogeneous Fenton Process. Phys. Chem. Res. 2019, 7, 37–52. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, Y.T.; Lee, J.; Xing, L. A review of application mechanism and research progress of Fe/montmorillonite-based catalysts in heterogeneous Fenton reactions. J. Environ. Chem. Eng. 2024, 12, 112152. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, H.; Ye, Z.H. Recent advances in the application of natural iron and clay minerals in heterogeneous electro-Fenton process. Curr. Opin. Electrochem. 2024, 46, 101495. [Google Scholar] [CrossRef]
- Wang, J.L.; Tang, J.T. Fe-based Fenton-like catalysts for water treatment: Catalytic mechanisms and applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Shang, Y.; Kan, Y.J.; Xu, X. Stability and regeneration of metal catalytic sites with different sizes in Fenton-like system. Chin. Chem. Lett. 2023, 34, 108278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C.H.; Petit, S.; Zhang, H. Hectorite: Synthesis, modification, assembly and applications. Appl. Clay Sci. 2019, 177, 114–138. [Google Scholar] [CrossRef]
- Suman, K.; Joshi, Y.M. Microstructure and Soft Glassy Dynamics of an Aqueous Laponite Dispersion. Langmuir 2018, 34, 13079–13103. [Google Scholar] [CrossRef]
- Grigale-Sorocina, Z.; Birks, I. Hectorite and bentonite effect on water-based polymer coating rheology. Comptes Rendus Chim. 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Rolfe, B.; Schembri, M.A.; Cobbold, R.N.; Zhang, B.; Mahony, T.J.; Xu, Z.P. Clay nanoparticles co-deliver three antigens to promote potent immune responses against pathogenic Escherichia coli. J. Control. Release 2018, 292, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Carnevale, D.; Suss-Fink, G. Selective N-cycle hydrogenation of quinolines with sodium borohydride in aqueous media catalyzed by hectorite-supported ruthenium nanoparticles. J. Organomet. Chem. 2016, 821, 197–205. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.; Li, H.; Zhu, N.; Li, P.; Wu, J.; Wang, X.; Dang, Z. Synthesis and characterization of organo-montmorillonite supported iron nanoparticles. Appl. Clay Sci. 2010, 50, 330–336. [Google Scholar] [CrossRef]
- Manjanna, J. Preparation of Fe(II)-montmorillonite by reduction of Fe(III)-montmorillonite with ascorbic acid. Appl. Clay Sci. 2008, 42, 32–38. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 137, 47500. [Google Scholar] [CrossRef]
- De Leon, M.A.; Rodriguez, M.; Marchetti, S.G.; Sapag, K.; Faccio, R.; Sergio, M.; Bussi, J. Raw montmorillonite modified with iron for photo-Fenton processes: Influence of iron content on textural, structural and catalytic properties. J. Environ. Chem. Eng. 2017, 5, 4742–4750. [Google Scholar] [CrossRef]
- Munoz, H.J.; Blanco, C.; Gil, A.; Vicente, M.A.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Maitlo, G.; Shah, A.A.; Channa, I.A.; Kandhro, G.A.; Maitlo, H.A.; Bhatti, U.H.; Shah, A.; Memon, A.Q.; Jatoi, A.S.; et al. One pot menthol synthesis via hydrogenations of citral and citronellal over montmorillonite-supported Pd/Ni-heteropoly acid bifunctional catalysts. React. Kinet. Mech. Catal. 2019, 128, 917–934. [Google Scholar] [CrossRef]
- Shah, A.K.; Park, S.; Khan, H.A.; Bhatti, U.H.; Kumar, P.; Bhutto, A.W.; Park, Y.H. Citronellal cyclisation over heteropoly acid supported on modified montmorillonite catalyst: Effects of acidity and pore structure on catalytic activity. Res. Chem. Intermed. 2018, 44, 2405–2423. [Google Scholar] [CrossRef]
- Chen, H.; Long, Q.; Zhang, Y.; Yang, H.; Shu, J. Using red mud to prepare the iron-bearing catalyst for the efficient degradation of phenol in the Fenton-like process. Arab. J. Chem. 2024, 17, 105797. [Google Scholar] [CrossRef]
- Cheng, A.; He, Y.; Liu, X.; He, C. Honeycomb-like biochar framework coupled with Fe3O4/FeS nanoparticles as efficient heterogeneous Fenton catalyst for phenol degradation. J. Environ. Sci. 2024, 136, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; Lopez-Peinado, A.J.; Martin-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B-Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Elzatahry, A.A.; Chen, J.; Alghamdi, A.; Deng, Y. Recyclable Fenton-like catalyst based on zeolite Y supported ultrafine, highly-dispersed Fe2O3 nanoparticles for removal of organics under mild conditions. Chin. Chem. Lett. 2019, 30, 324–330. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Khanikar, N.; Bhattacharyya, K.G. Cu(II)-kaolinite and Cu(II)-montmorillonite as catalysts for wet oxidative degradation of 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. Chem. Eng. J. 2013, 233, 88–97. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Chi, L.; Chen, H.; Chen, C. Changes in activation energy and kinetics of heat-activated persulfate oxidation of phenol in response to changes in pH and temperature. Chemosphere 2017, 189, 86–93. [Google Scholar] [CrossRef] [PubMed]




| Sample | SBET (m2/g) | Vtotal (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Hectorite | 335.6 | 0.2981 | 3.338 |
| Fe2O3/hectorite | 312.8 | 0.2580 | 3.404 |
| Level | pH | T (°C) | Dosage (g dm−3) |
|---|---|---|---|
| 1 | 3 | 30 | 0.1 |
| 2 | 5 | 45 | 0.3 |
| 3 | 7 | 60 | 0.5 |
| 4 | 9 | 75 | 0.7 |
| Number | pH | T (°C) | Dosage (g dm–3) | DR (%) |
|---|---|---|---|---|
| 1 | 1 (3) | 1 (30) | 1 (0.1) | 19.80 |
| 2 | 1 | 2 (45) | 2 (0.3) | 98.39 |
| 3 | 1 | 3 (60) | 3 (0.5) | 99.27 |
| 4 | 1 | 4 (75) | 4 (0.7) | 98.55 |
| 5 | 2 (5) | 1 | 2 | 6.49 |
| 6 | 2 | 2 | 1 | 26.86 |
| 7 | 2 | 3 | 4 | 11.83 |
| 8 | 2 | 4 | 3 | 10.23 |
| 9 | 3 (7) | 1 | 3 | 11.55 |
| 10 | 3 | 2 | 4 | 26.30 |
| 11 | 3 | 3 | 1 | 7.40 |
| 12 | 3 | 4 | 2 | 12.47 |
| 13 | 4 (9) | 1 | 4 | 3.68 |
| 14 | 4 | 2 | 3 | 26.67 |
| 15 | 4 | 3 | 2 | 9.43 |
| 16 | 4 | 4 | 1 | 9.85 |
| Mean Values | pH | T (°C) | Dosage (g dm–3) |
|---|---|---|---|
| k1 | 79.00 | 10.38 | 15.98 |
| k2 | 13.85 | 44.55 | 31.69 |
| k3 | 14.43 | 31.98 | 36.93 |
| k4 | 12.41 | 32.77 | 35.09 |
| R | 66.59 | 34.17 | 20.95 |
| Cycles | DR (%) |
|---|---|
| 1 | 99.27 |
| 2 | 98.60 |
| 3 | 98.33 |
| 4 | 97.46 |
| 5 | 95.72 |
| T (K) | 303.15 | 318.15 | 333.15 |
|---|---|---|---|
| k | 0.0146 | 0.0257 | 0.0413 |
| R2 | 0.9033 | 0.9130 | 0.8656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, H.; Fu, X.; Chen, J. Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts 2024, 14, 521. https://doi.org/10.3390/catal14080521
Liu X, Xu H, Fu X, Chen J. Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts. 2024; 14(8):521. https://doi.org/10.3390/catal14080521
Chicago/Turabian StyleLiu, Xia, Haihui Xu, Xing Fu, and Jinyang Chen. 2024. "Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol" Catalysts 14, no. 8: 521. https://doi.org/10.3390/catal14080521
APA StyleLiu, X., Xu, H., Fu, X., & Chen, J. (2024). Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts, 14(8), 521. https://doi.org/10.3390/catal14080521







