Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol
Abstract
:1. Introduction
2. Results and Discussion
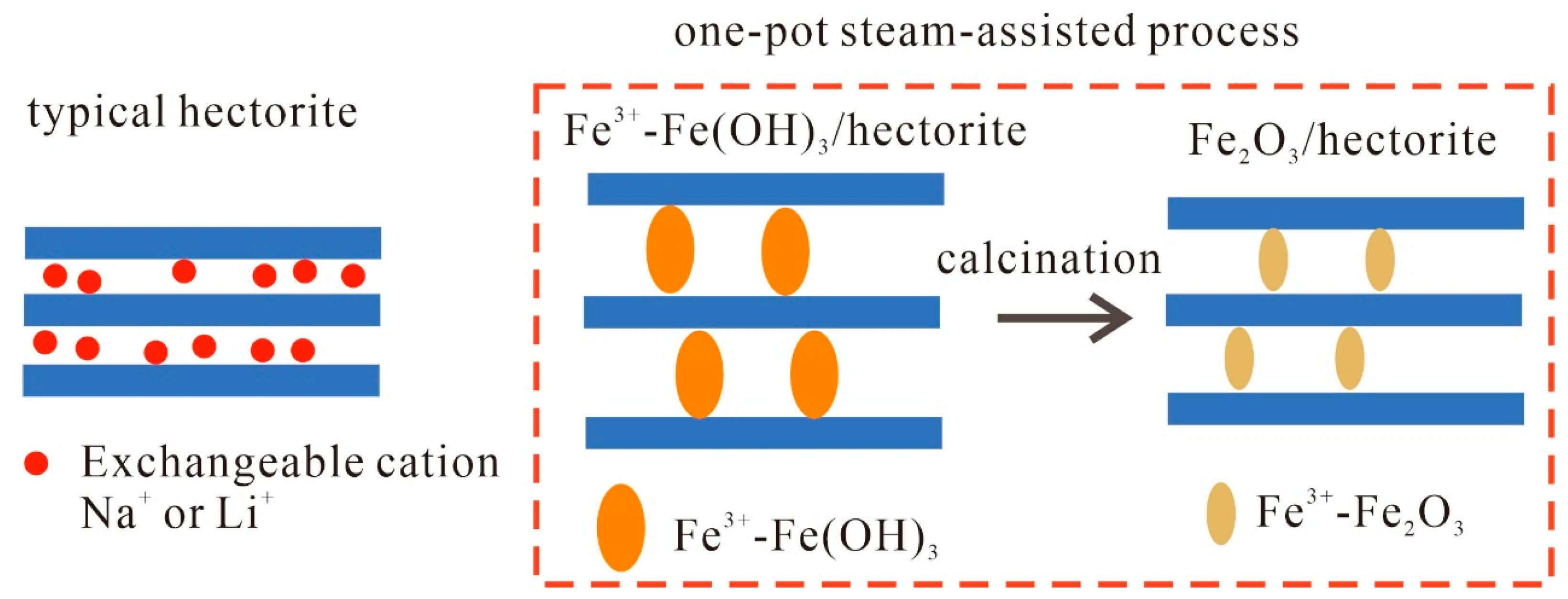
2.1. Steam Synthesis Reaction Mechanism
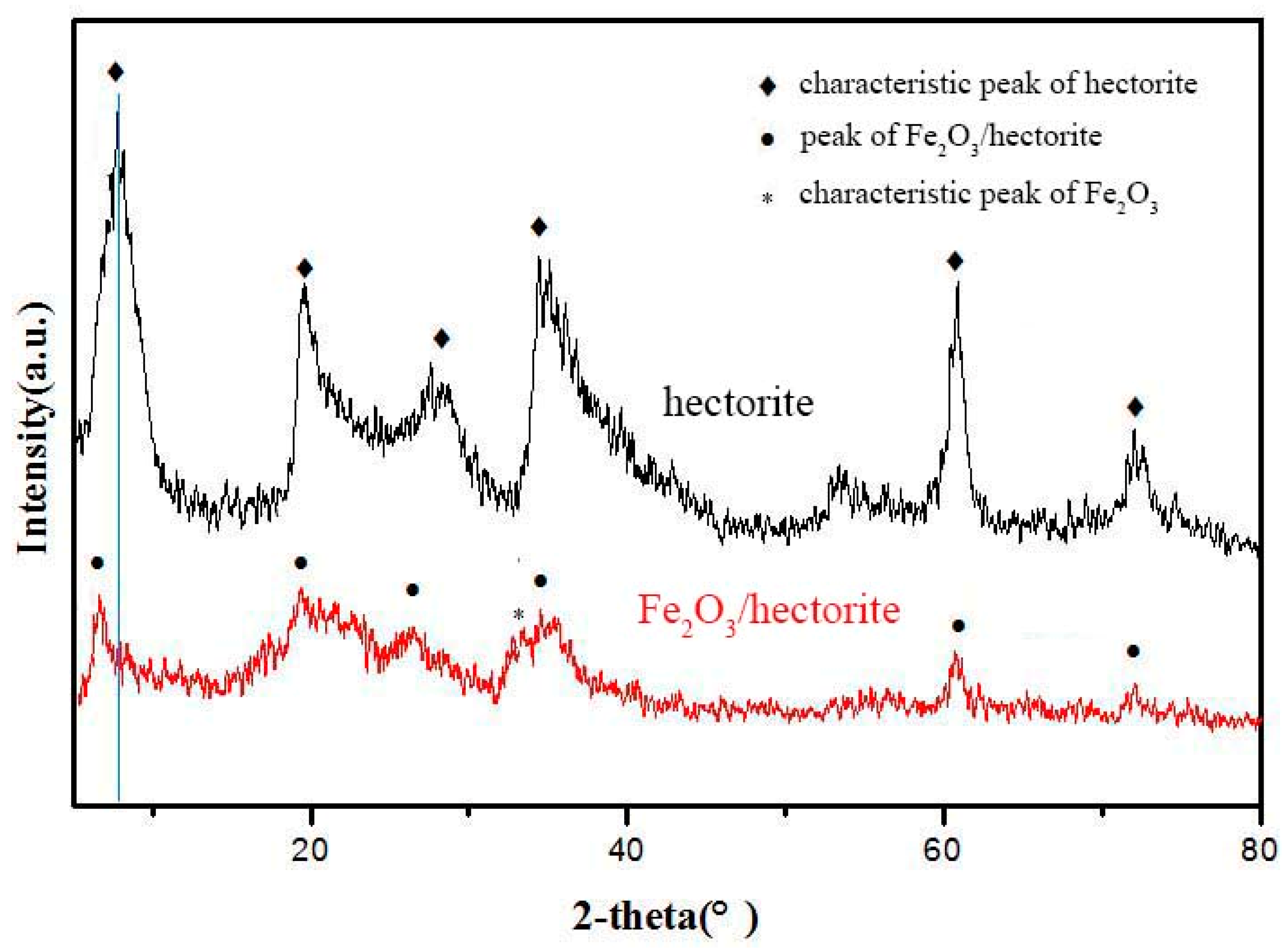
2.2. Characterization
2.3. Optimal Fenton Reaction Conditions
2.4. Stability of the Fe2O3/Hectorite Catalyst
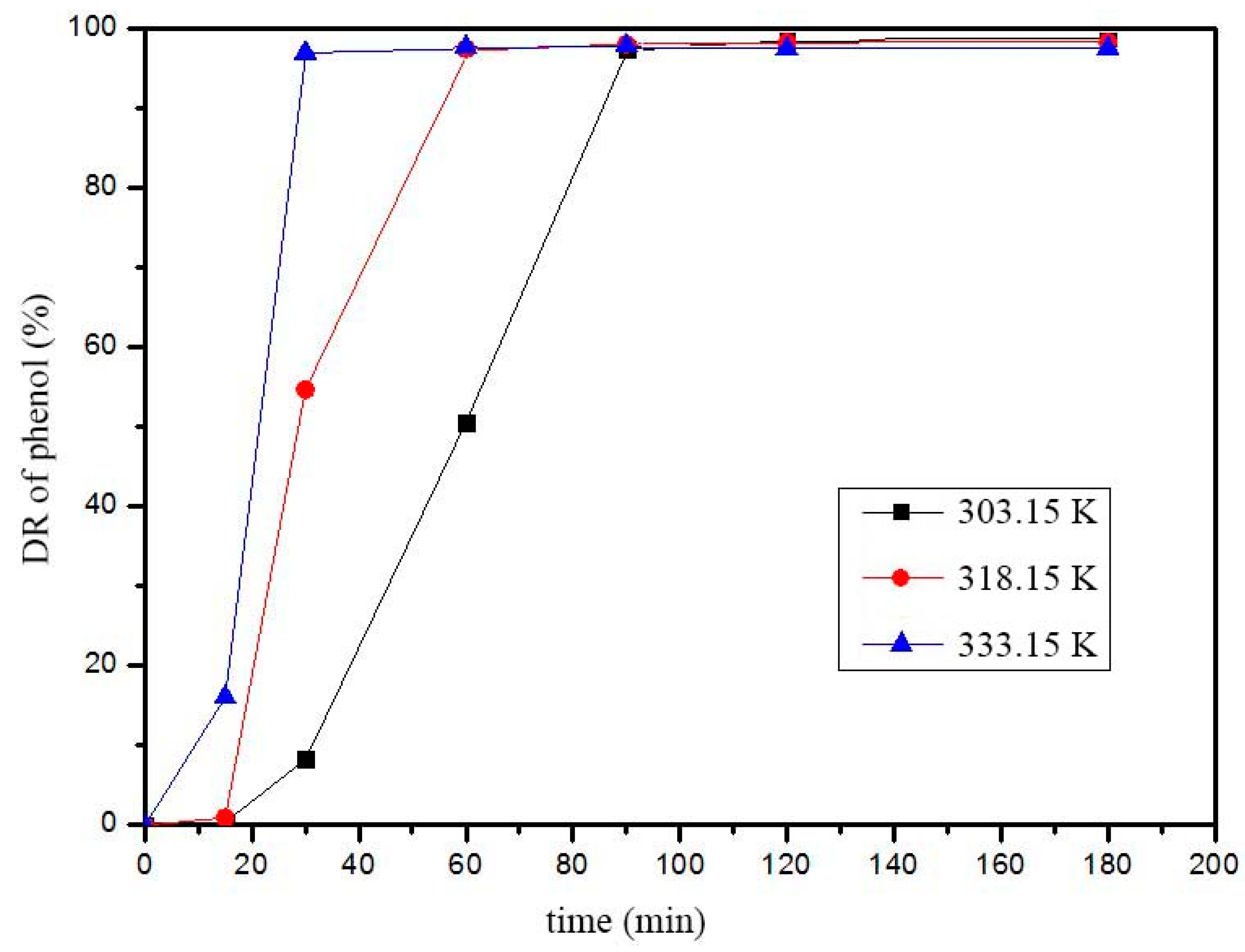
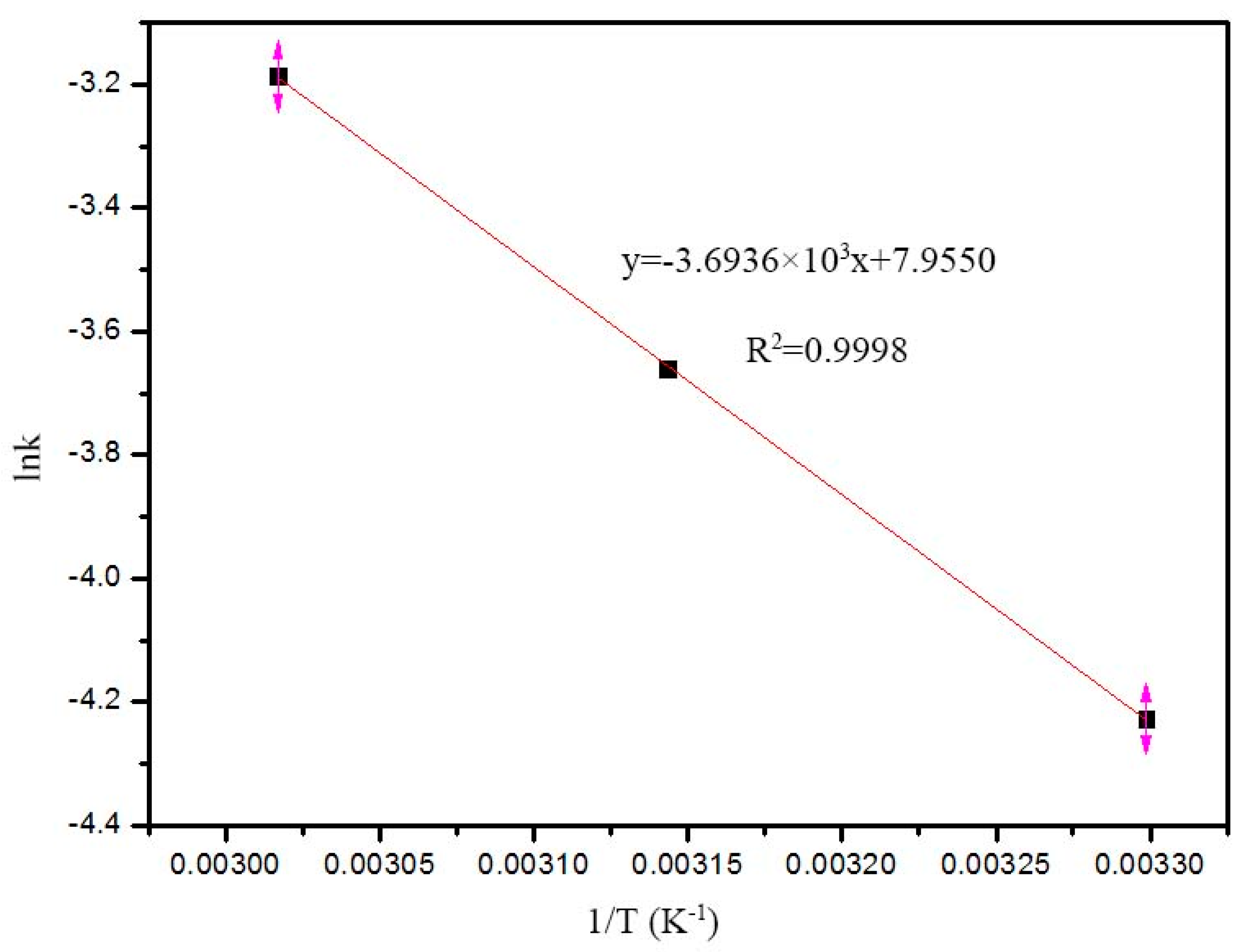
2.5. Fenton Reaction Kinetic
3. Materials and Methods
3.1. Materials
3.2. Synthesizing Fe2O3/Hectorite Catalyst
3.3. Characterizations
3.4. Fenton Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite Oxide Catalysts for Advanced Oxidation Reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Wang, Z.; Zhang, Q.; Yuan, D. Organic cocatalysts improved Fenton and Fenton-like processes for water pollution control: A review. Chemosphere 2024, 353, 141581. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Niu, Z.; Zhang, X.; Zhang, Y. Pollution characteristics and health risk of sixty-five organics in one drinking water system: PAEs should be prioritized for control. Chemosphere 2024, 350, 141171. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Sundarrajan, P.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.Y.; Meng, F.P.; Cui, H.W.; Lin, Y.F.; Wang, G.S.; Wu, J.Y. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Radovic, M.; Mitrovic, J.; Kostic, M.; Bojic, D.; Petrovic, M.; Najdanovic, S.; Bojic, A. Comparison of ultraviolet radiation/hydrogen peroxide, Fenton and photo-Fenton processes for the decolorization of reactive dyes. Hem. Ind. 2015, 69, 657–665. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Lyu, L.; Hu, C. Heterogeneous Fenton Catalytic Water Treatment Technology and Mechanism. Prog. Chem. 2017, 29, 981–999. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Jalali, H.; Naeimzadeh, F.; Gharibian, S. Efficient Removal of Diclofenac from Pharmaceutical Wastewater Using Impregnated Zeolite Catalyst in Heterogeneous Fenton Process. Phys. Chem. Res. 2019, 7, 37–52. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, Y.T.; Lee, J.; Xing, L. A review of application mechanism and research progress of Fe/montmorillonite-based catalysts in heterogeneous Fenton reactions. J. Environ. Chem. Eng. 2024, 12, 112152. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, H.; Ye, Z.H. Recent advances in the application of natural iron and clay minerals in heterogeneous electro-Fenton process. Curr. Opin. Electrochem. 2024, 46, 101495. [Google Scholar] [CrossRef]
- Wang, J.L.; Tang, J.T. Fe-based Fenton-like catalysts for water treatment: Catalytic mechanisms and applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Shang, Y.; Kan, Y.J.; Xu, X. Stability and regeneration of metal catalytic sites with different sizes in Fenton-like system. Chin. Chem. Lett. 2023, 34, 108278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C.H.; Petit, S.; Zhang, H. Hectorite: Synthesis, modification, assembly and applications. Appl. Clay Sci. 2019, 177, 114–138. [Google Scholar] [CrossRef]
- Suman, K.; Joshi, Y.M. Microstructure and Soft Glassy Dynamics of an Aqueous Laponite Dispersion. Langmuir 2018, 34, 13079–13103. [Google Scholar] [CrossRef]
- Grigale-Sorocina, Z.; Birks, I. Hectorite and bentonite effect on water-based polymer coating rheology. Comptes Rendus Chim. 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Rolfe, B.; Schembri, M.A.; Cobbold, R.N.; Zhang, B.; Mahony, T.J.; Xu, Z.P. Clay nanoparticles co-deliver three antigens to promote potent immune responses against pathogenic Escherichia coli. J. Control. Release 2018, 292, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Carnevale, D.; Suss-Fink, G. Selective N-cycle hydrogenation of quinolines with sodium borohydride in aqueous media catalyzed by hectorite-supported ruthenium nanoparticles. J. Organomet. Chem. 2016, 821, 197–205. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.; Li, H.; Zhu, N.; Li, P.; Wu, J.; Wang, X.; Dang, Z. Synthesis and characterization of organo-montmorillonite supported iron nanoparticles. Appl. Clay Sci. 2010, 50, 330–336. [Google Scholar] [CrossRef]
- Manjanna, J. Preparation of Fe(II)-montmorillonite by reduction of Fe(III)-montmorillonite with ascorbic acid. Appl. Clay Sci. 2008, 42, 32–38. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 137, 47500. [Google Scholar] [CrossRef]
- De Leon, M.A.; Rodriguez, M.; Marchetti, S.G.; Sapag, K.; Faccio, R.; Sergio, M.; Bussi, J. Raw montmorillonite modified with iron for photo-Fenton processes: Influence of iron content on textural, structural and catalytic properties. J. Environ. Chem. Eng. 2017, 5, 4742–4750. [Google Scholar] [CrossRef]
- Munoz, H.J.; Blanco, C.; Gil, A.; Vicente, M.A.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Maitlo, G.; Shah, A.A.; Channa, I.A.; Kandhro, G.A.; Maitlo, H.A.; Bhatti, U.H.; Shah, A.; Memon, A.Q.; Jatoi, A.S.; et al. One pot menthol synthesis via hydrogenations of citral and citronellal over montmorillonite-supported Pd/Ni-heteropoly acid bifunctional catalysts. React. Kinet. Mech. Catal. 2019, 128, 917–934. [Google Scholar] [CrossRef]
- Shah, A.K.; Park, S.; Khan, H.A.; Bhatti, U.H.; Kumar, P.; Bhutto, A.W.; Park, Y.H. Citronellal cyclisation over heteropoly acid supported on modified montmorillonite catalyst: Effects of acidity and pore structure on catalytic activity. Res. Chem. Intermed. 2018, 44, 2405–2423. [Google Scholar] [CrossRef]
- Chen, H.; Long, Q.; Zhang, Y.; Yang, H.; Shu, J. Using red mud to prepare the iron-bearing catalyst for the efficient degradation of phenol in the Fenton-like process. Arab. J. Chem. 2024, 17, 105797. [Google Scholar] [CrossRef]
- Cheng, A.; He, Y.; Liu, X.; He, C. Honeycomb-like biochar framework coupled with Fe3O4/FeS nanoparticles as efficient heterogeneous Fenton catalyst for phenol degradation. J. Environ. Sci. 2024, 136, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; Lopez-Peinado, A.J.; Martin-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B-Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Elzatahry, A.A.; Chen, J.; Alghamdi, A.; Deng, Y. Recyclable Fenton-like catalyst based on zeolite Y supported ultrafine, highly-dispersed Fe2O3 nanoparticles for removal of organics under mild conditions. Chin. Chem. Lett. 2019, 30, 324–330. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Khanikar, N.; Bhattacharyya, K.G. Cu(II)-kaolinite and Cu(II)-montmorillonite as catalysts for wet oxidative degradation of 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. Chem. Eng. J. 2013, 233, 88–97. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Chi, L.; Chen, H.; Chen, C. Changes in activation energy and kinetics of heat-activated persulfate oxidation of phenol in response to changes in pH and temperature. Chemosphere 2017, 189, 86–93. [Google Scholar] [CrossRef] [PubMed]




| Sample | SBET (m2/g) | Vtotal (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Hectorite | 335.6 | 0.2981 | 3.338 |
| Fe2O3/hectorite | 312.8 | 0.2580 | 3.404 |
| Level | pH | T (°C) | Dosage (g dm−3) |
|---|---|---|---|
| 1 | 3 | 30 | 0.1 |
| 2 | 5 | 45 | 0.3 |
| 3 | 7 | 60 | 0.5 |
| 4 | 9 | 75 | 0.7 |
| Number | pH | T (°C) | Dosage (g dm–3) | DR (%) |
|---|---|---|---|---|
| 1 | 1 (3) | 1 (30) | 1 (0.1) | 19.80 |
| 2 | 1 | 2 (45) | 2 (0.3) | 98.39 |
| 3 | 1 | 3 (60) | 3 (0.5) | 99.27 |
| 4 | 1 | 4 (75) | 4 (0.7) | 98.55 |
| 5 | 2 (5) | 1 | 2 | 6.49 |
| 6 | 2 | 2 | 1 | 26.86 |
| 7 | 2 | 3 | 4 | 11.83 |
| 8 | 2 | 4 | 3 | 10.23 |
| 9 | 3 (7) | 1 | 3 | 11.55 |
| 10 | 3 | 2 | 4 | 26.30 |
| 11 | 3 | 3 | 1 | 7.40 |
| 12 | 3 | 4 | 2 | 12.47 |
| 13 | 4 (9) | 1 | 4 | 3.68 |
| 14 | 4 | 2 | 3 | 26.67 |
| 15 | 4 | 3 | 2 | 9.43 |
| 16 | 4 | 4 | 1 | 9.85 |
| Mean Values | pH | T (°C) | Dosage (g dm–3) |
|---|---|---|---|
| k1 | 79.00 | 10.38 | 15.98 |
| k2 | 13.85 | 44.55 | 31.69 |
| k3 | 14.43 | 31.98 | 36.93 |
| k4 | 12.41 | 32.77 | 35.09 |
| R | 66.59 | 34.17 | 20.95 |
| Cycles | DR (%) |
|---|---|
| 1 | 99.27 |
| 2 | 98.60 |
| 3 | 98.33 |
| 4 | 97.46 |
| 5 | 95.72 |
| T (K) | 303.15 | 318.15 | 333.15 |
|---|---|---|---|
| k | 0.0146 | 0.0257 | 0.0413 |
| R2 | 0.9033 | 0.9130 | 0.8656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, H.; Fu, X.; Chen, J. Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts 2024, 14, 521. https://doi.org/10.3390/catal14080521
Liu X, Xu H, Fu X, Chen J. Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts. 2024; 14(8):521. https://doi.org/10.3390/catal14080521
Chicago/Turabian StyleLiu, Xia, Haihui Xu, Xing Fu, and Jinyang Chen. 2024. "Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol" Catalysts 14, no. 8: 521. https://doi.org/10.3390/catal14080521
APA StyleLiu, X., Xu, H., Fu, X., & Chen, J. (2024). Steam-Assisted Synthesis of Hectorite Loaded with Fe2O3 and Its Catalytic Fenton Degradation of Phenol. Catalysts, 14(8), 521. https://doi.org/10.3390/catal14080521







